Introduction
Abdominal cocoon (AC) is a rare clinical entity that
is infrequently reported, with the majority of cases occurring
post-operatively (1). Abdominal
cocoon is an abdominal disease characterized by a fibrous membrane
enveloping part or all of the organs of the abdominal pelvis;
covering of the small intestine is the most common presentation.
Named for its resemblance to a cocoon, the condition is also termed
peritoneal fibrosis, peritoneal sclerosis, calcified peritonitis
and encapsulated peritoneal sclerosis (1). Clinically, this disease has no specific
symptoms or abnormal laboratory diagnostic indicators; often, acute
abdominal pain, an abdominal mass or incomplete intestinal
obstruction are the first symptoms identified in hospital. AC is a
rare peritoneal disease, the pathogenesis of which remains to be
determined. It is difficult to make a definite pre-operative
diagnosis, thus AC is often misdiagnosed or omitted (2). Correct pre-operative diagnosis and
early treatments may reduce complications, but also reduce
mortality (2,3). Imaging examinations, especially CT, can
facilitate pre-operative diagnosis, but few studies have discussed
AC's imaging features and the diagnostic value of radiological
imaging. The authors of the present study analyzed the clinical
data and imaging results of nine patients who were diagnosed with
AC from laparotomy and histopathological examinations between
January 1991 and January 2018 in The Second Affiliated Hospital of
Soochow University. The authors also reviewed the relevant
literature to investigate the imaging characteristics, clinical
symptoms and treatments of AC in order to improve understanding of
the condition, which may help in the selection of suitable
detection methods and treatment protocols.
Materials and methods
Data review
The case files of patients with AC were extracted
from The Second Affiliated Hospital of Soochow University between
January 1991 and January 2018. Data included clinical
manifestations, imaging examinations, diagnoses and treatments of
the nine patients with AC, and were reviewed in detail. Patients
inclusion criteria were as follows: i) Surgical treatment and
complete pathological data; ii) CT scan and enhanced scan completed
prior to surgery; iii) Patients had no previous history of
abdominal surgeries, peritonitis, tuberculosis or peritoneal
dialysis, autoimmune diseases or prolonged drug intake. Patients
exclusion criteria were: Patients who received CT scan without an
enhanced CT. All patients were followed up (at 3, 6 and 12 months
after surgery) by telephone or using the outpatient service.
Radiological imaging
X-ray and sonographic examination
Plain abdominal X-ray and ultrasonography were
performed before surgery as routine examinations. A barium meal was
performed in patients with or without partial intestinal
obstruction, and a barium follow through was observed at different
times (60 and 90 min) after drinking barium.
CT examination
CT scans and subsequent contrast-enhanced CT scans
were performed with a 64 detector row helical CT scanner in all
cases. For the patients with or without partial intestinal
obstruction, 4% mannitol was orally administered 1 h before the
examination to fill the gastrointestinal tract.
Surgery
A laparotomy was performed 24 h after helical CT in
all nine cases. All samples were histopathologically examined. The
pathological examination was performed under an optical microscope
with a magnification of ×100. Fixation was performed using 10%
formalin at room temperature for 6–8 h. Staining was performed
using hematoxylin-eosin at 40°C for ~30 min. The thickness of the
tissue sections was 4 µm.
Results
Patient characteristics
The current study included a total of nine patients
with a mean age of 43 years (range, 25–64 years), which included
five men and four women. All patients had no previous history of
abdominal surgeries, peritonitis, tuberculosis or peritoneal
dialysis, autoimmune diseases or prolonged drug intake. All
patients showed recurrent abdominal pain and distention, which was
more apparent after meals, six cases had episodes of intestinal
obstruction, abdominal distention, colicky abdominal pain, nausea
and vomiting and five cases had a non-tender, soft, smooth mass
upon abdominal palpation and the boundary of the mass was not sharp
(Table I).
 | Table I.Clinical data of nine cases of
abdominal cocoon. |
Table I.
Clinical data of nine cases of
abdominal cocoon.
| Clinical data | Number of
cases | Ratio (%) |
|---|
| Sex |
|
|
|
Male | 5 | 55.56 |
|
Female | 4 | 44.44 |
| Clinical
symptoms |
|
|
|
Abdominal pain and
detention | 9 | 100 |
|
Intestinal obstruction | 6 | 66.67 |
|
Abdominal mass | 5 | 55.56 |
Imaging
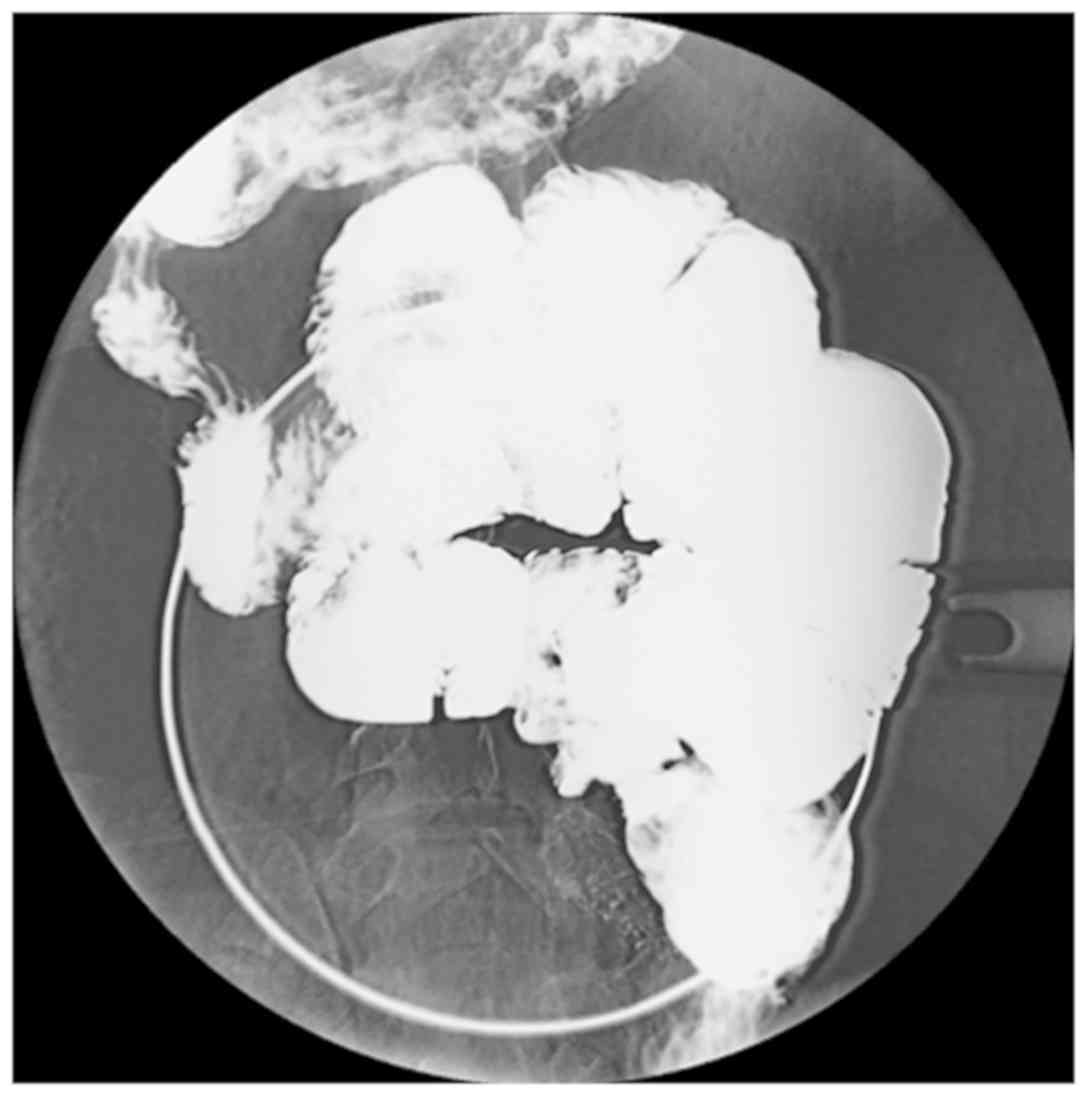
The plain abdominal X-ray examination identified
that six out of nine cases presented with dilated small-bowel loops
with air-fluid levels in the small intestine. A total of three
cases provided normal findings. Following the barium meal
examination revealed that seven of the nine patients had a
cauliflower sign, which consisted of disorderly arranged and
bunched bowels that congregated in a single area. When pressing the
clustered bowel loops, they remained in a constant position
(Fig. 1; Table II). A total of six cases presented
with dilated small-bowel loops with air-fluid levels and the
small-bowel transit time was delayed. However, the mucous membrane
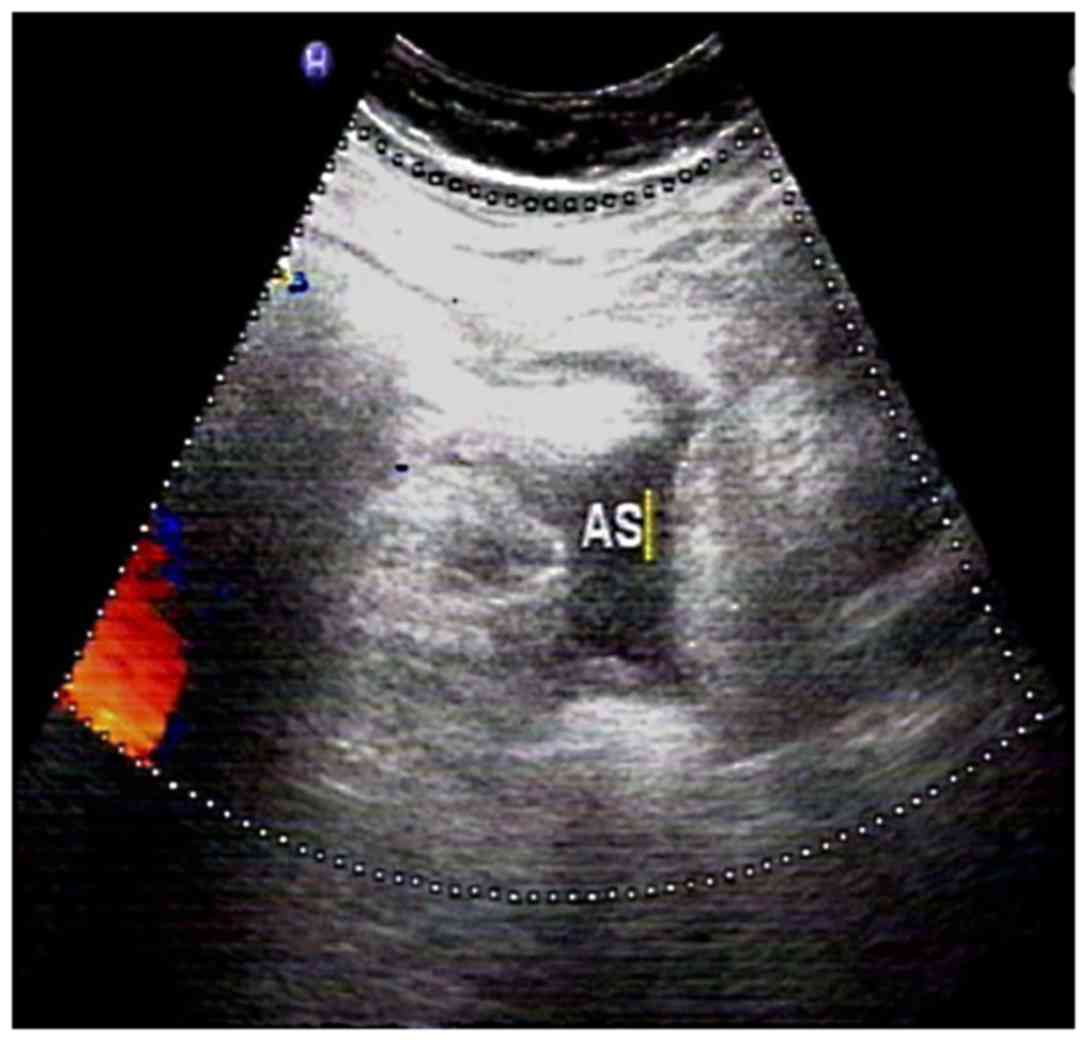
of the small intestine was normal. Sonographic examination
identified that each patient had a mass within the echogenic bowels
and a number of cases had masses within the liquid anechoic area
(Fig. 2).
 | Table II.Imaging in patients with abdominal
cocoon. |
Table II.
Imaging in patients with abdominal
cocoon.
| Imaging technique
and finding | Number of
cases | Ratio (%) |
|---|
| X-ray |
|
|
|
Dilatation of small
intestine | 6 | 66.67 |
|
Intestinal air-fluid
levels | 6 | 66.67 |
|
Cauliflower sign | 7 | 77.78 |
| CT |
|
|
| Soft
tissue wraps around the small intestine | 6 | 66.67 |
|
Small-bowel loops encased by
the sac | 2 | 22.22 |
| Part of
colons encased by the sac | 1 | 11.11 |
|
Peritoneal thickening | 7 | 77.78 |
| Bottle
Gourd sign | 6 | 66.67 |
| Bowel
wall thickening | 2 | 22.22 |
|
Mesenteric hydrops | 2 | 22.22 |
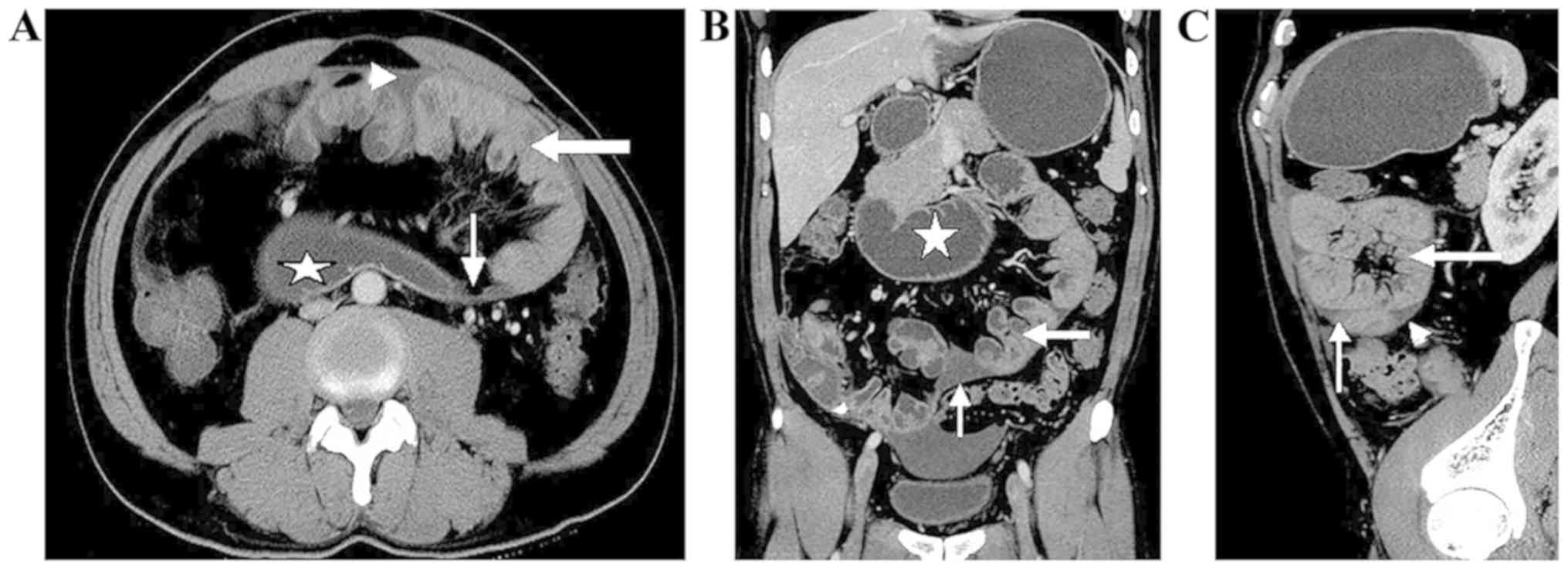
CT interpretation
An appropriate amount (1,000–1,500 ml) of oral 4%
mannitol liquid was used as the contrast medium that filled the
gastrointestinal tract prior to the CT scan, and helped to identify
clustered intestinal loops encased by a membrane-like sac. In six
cases, the small-bowel loops congregated in the middle-lower
abdomen, and were encased by a soft-tissue density mantle. Partial
small-bowel loops were encased by the sac in two cases and the
small bowel was rarely out of the sac. A section of the colon was
covered in one case. A small amount of fluid was identified between
the sac and these encapsulated bowel loops (Fig. 3; Table
II). A thick (2 mm) mild-moderate enhancing membrane surrounded
the bowel, and the wall of the enhancing membrane was
well-distributed in seven cases. The corresponding mesenteric
structure had developmental abnormalities with centralized
mesenteric vessels, and the omentum majus appeared hypoplastic or
absent. A Bottle Gourd sign was noted in six cases. A Bottle Gourd
sign is the dilatation of the second and third part of the duodenum
with encasement of distal duodenum and jejunal loops (4). A total of two cases out of nine
presented with strangulated intestinal obstruction. The CT findings
included a thickened intestine wall, which either could not be
enhanced or could be enhanced slightly, and mesenteric hydrops when
the mesenteric fat gained density. Ascitic fluid was identified in
six of nine patients.
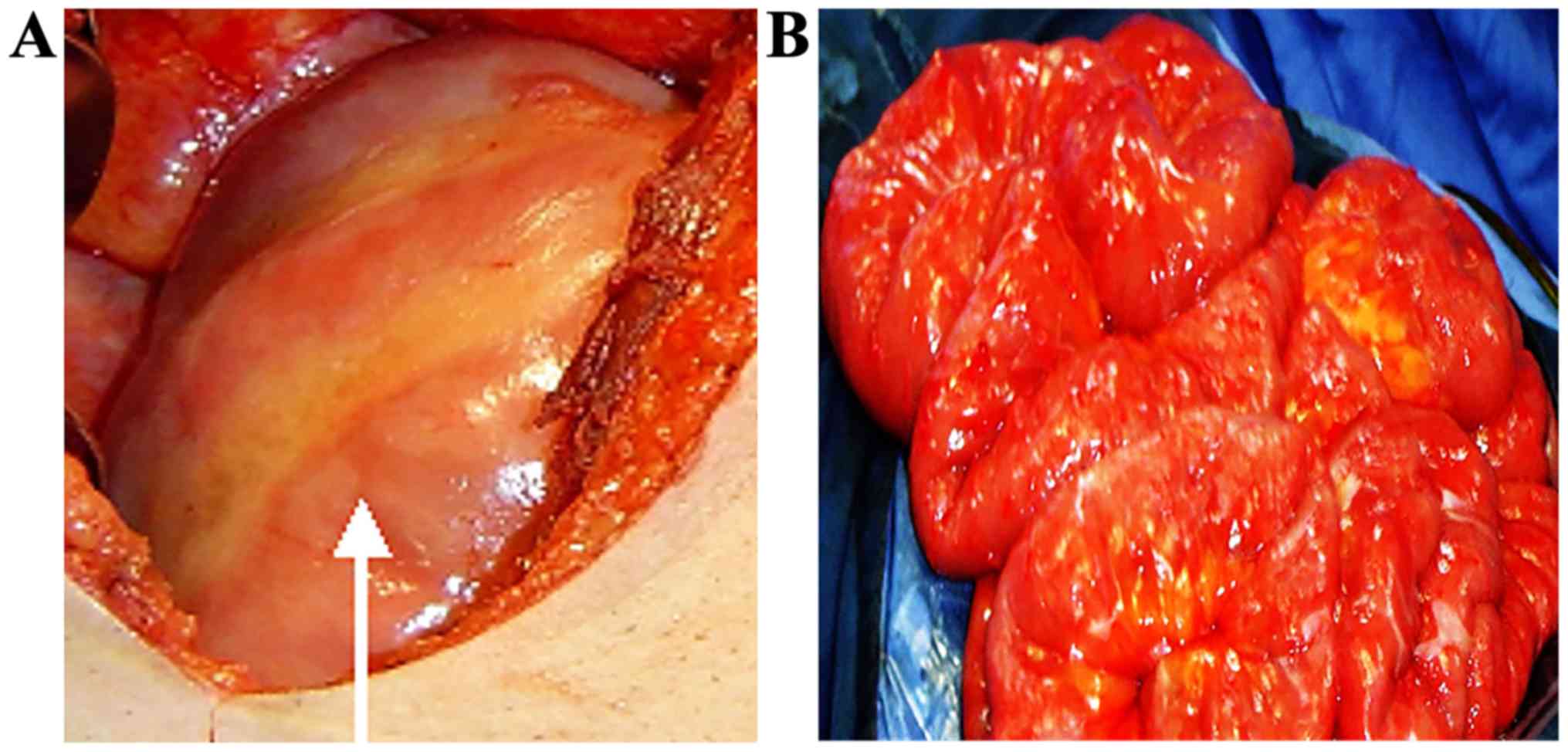
Surgical interpretation
Upon opening the abdomen, all or part of the small
bowel and the colon were revealed to be enclosed by a whitish
cocoon-like sac (Fig. 4A). The small
bowel was shortened and the intestine was either lacking or missing
entirely, or in the abdominal cavity. During surgery it was
observed that the sac's position, size and the scope of involved
intestine were consistent with CT findings. Part of the tough
well-distributed membrane-like tissues adhered to the wall of
abdomen, which was 2-mm thick and involved encased intestine loops.
The tissues congregated to the center of the abdomen with the
intestine loops adhering to each other and to the sac. Dilated
proximal bowel loops were observed in six cases, two of which had
strangulated intestinal obstruction with the presence of dark
purple thickening and swelling of the necrotic bowel wall. A small
volume of flaxen or red liquid was observed in the sac, and
edematous mesenteric and bloody dialysis was effluent in the
abdominal cavity. Homologous mesenteric vessels with preternatural
distribution thickened and centralized. The greater omentum
appeared hypoplastic or absent.
Surgical treatment
Adherent areas in most cases were loose and easy to
separate. After excision of the cocoon-like fibrous tissue, the
adhesions among bowel loops, between loops and the sac wall or
between the sac and abdominal wall were dissected allowing the
encapsulated intestines to be freed (Fig. 4B), and the intestine was rearranged.
Resection of the necrotic bowel was also required. Sufficient
postoperative drainage was performed after rinsing the abdominal
cavity repeatedly. All patients were followed up (3, 6 and 12
months after surgery) by telephone or using the outpatient service,
and the prognosis was good, with patients being asymptomatic.
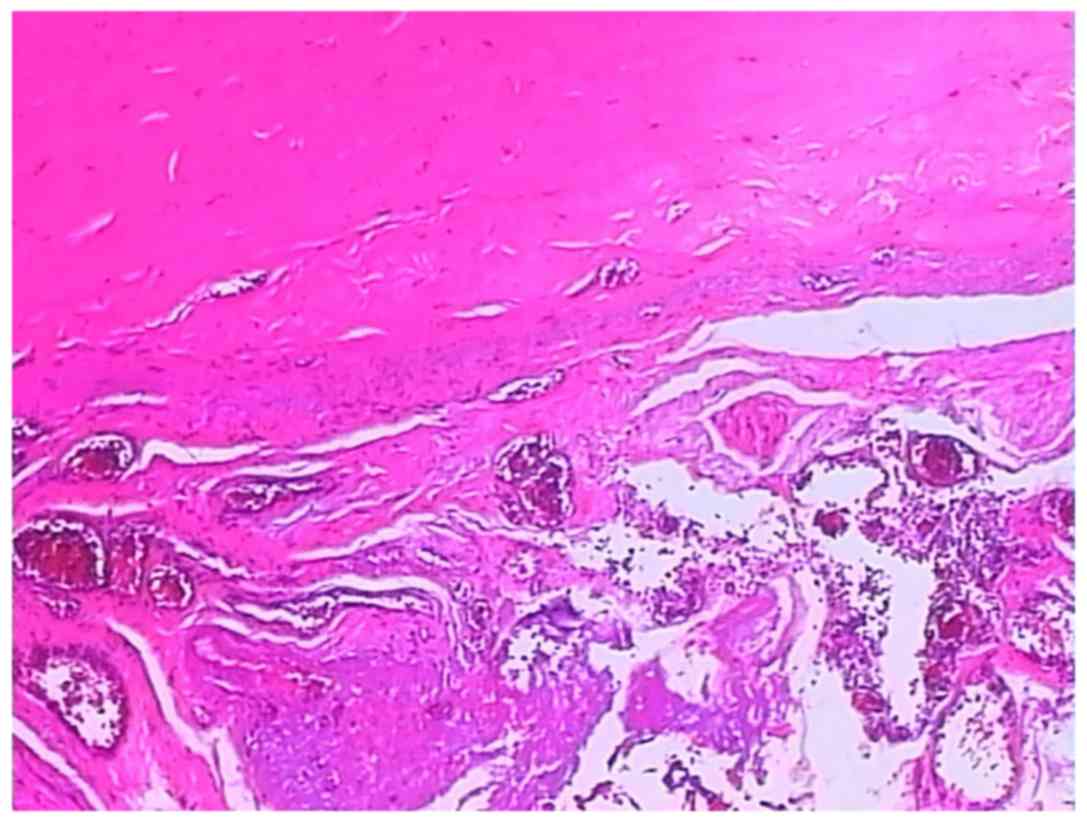
Pathological examination
The microbiological examination of the opalescent
membrane showed proliferation of fibroconnective and adipose tissue
with a chronic inflammatory reaction accompanied by degeneration or
necrosis and formation of form granulation tissue (Fig. 5). The dark purple wall of the
necrotic bowel thickened and swelled with the hemorrhagic contents
leaking into the enteric cavity. Microscopically, the bowel wall
was observed to bleed, necrose and exhibit the inflammatory
reaction.
Discussion
The observation that abdominal organs were partially
or totally encased in a fibrous membrane and consisted of multiple
internal adhesions was first reported by Owtschinnikow (5) in 1907, in a study entitled ‘Peritonitis
chronic fibrosa incapsulata’. However, the condition has also been
described as peritoneal fibrosis, peritoneal sclerosis, calcified
peritonitis and encapsulated peritoneal sclerosis (1). The abdominal cocoon was first named in
1978 by Foo et al (6). The
fibrous membrane surrounds the small bowel, but it occasionally
extends to include the colon, stomach or other organs (7). However, it rarely encases all abdominal
organs. The authors of the current study hypothesized that the
abdominal cocoon may reflect the morphological symptoms of this
condition more directly as the fibrous sac is not limited to the
small intestine, and a number of cases in the literature (4,8,9) also lack the inflammatory reaction and
calcification.
AC is a rare peritoneal disease, the pathogenesis of
which remains to be determined. AC can be divided into an
idiopathic and a secondary form (10). The idiopathic form has been reported
to be association with congenital dysplasia (11). Causes of the secondary form include
chronic peritoneal dialysis, intra-abdomen foreign body
stimulation, serious and chronic peritonitis, autoimmune disease,
intake of the β-blocker practolol, bacteria proof filter use, liver
transplantation, tuberculous inflammation, ventriculoperitoneal and
peritoneovenous shunts and carcinoid syndrome (10,12–17). All
these factors can lead to peritonitis, leading to a decrease in
mesothelial cells, a sustained expression of mesothelial metastatic
growth factor and the production of a large number of extracellular
matrix products, which increase the exudation of protein fiber and
peritoneal fibroblasts hyperplasia, and lead to the formation of
the fibrous sac (18). Peritoneal
dialysis-related AC may be due to the dialysis solution and its
metabolites damaging the peritoneum, which can lead to the
subcutaneous area of the peritoneal mesothelium thickening and
expanding (19). Repeated infections
can cause peritoneal damage, which can impair the normal
physiological function of the peritoneum, and undergoes three
stages of turbidity, deformation and fibrosis (3). In the current study, the nine included
cases did not exhibit peritonitis, peritoneal dialysis or prolonged
drug intake. The patients had a wide range of age distribution and
majority were male. Therefore, it was considered that these
patients had experienced abnormal congenital development, which
supports the congenital etiology of AC.
The clinical presentation of AC syndrome mostly
occurs as acute abdominal pain. The incidence of acute abdominal
pain in the current study was 100%. The main clinical
manifestations included signs of bowel function and peritoneum
physiological function disorder, and the fibrous sac also lead to
bowel function disorder, decreased reabsorption and weakened
enterokinesia, which caused nausea, vomiting, abdominal distension,
the disappearance of bowel tone, abdominal pain, abdomen or pelvic
masses, intestine obstruction, weight loss and blunt abdominal wall
trauma-induced intra-cocoon bleeding (2,3,10,20).
Peritoneal fibrosis lead to ascites by blocking the lymphatic
vessels, which is a nonspecific symptom (21). The most common manifestation of the
disease is small bowel obstruction, which is characterized by
complete or partial obstruction (16,17,22) and
this was observed in approximately two-thirds of the cases in the
current study. Signs of bowel function disorder were not only
related to the encapsulated intestine but also the damaged vessels,
vas lymphatica and nerve plexus of the bowel wall muscular layer.
AC may lead to infertility in female patients as the fibrous
membrane encapsulates the fallopian tubes, which restricts its
movement, blocks the fimbriated extremities and makes it difficult
for the ovum to travel the fallopian tubes, however the uterus and
ovaries appear normal (20).
Clinical diagnosis of AC is undertaken based on
signs of bowel function and peritoneum physiological function
disorder (10–12). The signs of AC are nonspecific, so it
is difficult to make a definite preoperative diagnosis (2,20,23). The
majority of cases are diagnosed during a laparotomy (20) Patients presenting with recurrent
episodes of abdominal pain, abdominal distension, unexplained
chronic mechanical intestinal obstruction and soft abdominal mass
may have AC. Weakened peritoneal transport function, anemia and
c-reactive protein levels can act as a clinical reference, but have
no specific value. An elevated WBC count and C-reactive protein
level, hypoalbuminemia and anemia may be detected in patients with
AC (16).
The majority of studies assessing the imaging of AC
are case reports (2,7,14,16,21,24–27).
Plain abdominal X-ray has been demonstrated to exhibit no
diagnostic specificity, and only indicated dilated small-bowel
loops with air-fluid levels and peritoneal calcification in
previous studies (10,28). In the current study, a total of six
cases (66.67%) exhibited small intestinal air-fluid levels and
there were no cases of peritoneal calcification. Barium meal
examination revealed the cauliflower sign, and seven cases (77.78%)
exhibited this characteristic in the current study. Sonographic
examination revealed cystic echoes in the bowel sac with occasional
identification of the sac wall and sac effusion. Reports about MRI
findings of AC are rare, therefore it is unknown if MRIs have any
value in the diagnosis of AC until more cases are accumulated in
the future. CT scans can indicate a distinctive manifestation of
AC, which is of important value for diagnosis (3). With CT findings, a definite
preoperative diagnosis is more likely. These manifestations
include: i) The small-bowel loops amassing in a certain area of the
abdomen and the intestine is rarely seen in other areas of the
peritoneal cavity; ii) the clustered bowel loops are surrounded by
a sac-like structure in a typical cocoon pattern; iii) the wall of
the sac is complete or incomplete, and well- or poorly distributed.
iv) Intestine loops in the sac may adhere to each other and the
wall causing it to thicken; v) small amounts of encapsulated
effusion in the sac are visible; vi) corresponding mesenteric
vessels are centralized with abnormal running and distribution,
mesenteric fat gains density and the greater omentum is hypoplastic
or absent; vii) cocoon-like membrane and the wall of the intestine
occasionally show calcification; viii) lymph nodes swell
reactively; ix) CT findings of AC with the complication of
intestinal obstruction; x) intestine loops encased by the sac and
secondary adhesion being the main cause of the obstruction. In the
current study, a total of six patients (66.67%) showed proximal
intestinal dilatation, normal or poor intestinal dilatation and the
distal intestine was normal or empty. Bowel ischemia is a
manifestation of a strangulated intestinal obstruction. And the
Bottle Gourd sign, cauliflower sign are important radiological
findings and they were identified in approximately two-thirds of
cases.
Enhanced CT is an effective way to observe bowel
mesenteric ischemia and necrosis, and has high sensitivity and
accuracy (3). CT findings of bowel
ischemia include: i) Bowel wall annular thickening; ii) abnormal
enhancement of the bowel wall; iii) bowel wall bleeding; and iv)
mesenteric effusion, mesenteric vessels thickening and fuzziness.
In the current study, characteristic CT appearances of AC include
clustered bowel loops encased by a thickened sac that are
accompanied by the accumulation of mesenteric vessels, abnormal
distribution and the hypoplasia or absent omentum majus.
Stafford-Johnson et al (9)
indicated that calcification of the intestinal frenum and
peritoneum were more characteristic of AC, but none of the cases in
the current study showed peritoneum calcification. In the past, AC
could only be definitively diagnosed after surgery. However,
combined with clinical and imaging reports, the current study
performed preoperative diagnosis using CT scans, which allow for a
reliable diagnostic method. Using this method increases the
understanding of the disease and serves an important role in
assisting surgical treatments (4,10,25).
The common characteristics of peritoneal
morphological changes are mesothelium loss and interstitium
thickening within the peritoneum (20). A thickened interstitium may be
cellulous (possibly fibroblasts) or acellular (collagen
deposition). Collagen fibers, inflammatory cells and abnormal
morphological vessels have also been previously observed, with
focal mesothelial cells, lymphocytes and reactive hyperplasia lymph
nodes with or without plasmacytes (29,30).
AC should be identified due to peritoneal
encapsulation (3), which is a rare
type of abnormal congenital development. Cleland (31) first reported, in 1868, that the
peritoneal membrane is divided from the yolk sac as it is drawn
into the embryonic abdominal cavity during the twelfth week of
pregnancy, and malrotation of the midgut and a vascular anomaly may
result in AC. The majority of patients with AC are asymptomatic,
and a few cases exhibit show intermittent abdominal pain, with
acute intestinal obstruction occurring in a number of infant
patients. Peritoneal encapsulation characteristically presents with
part or all of the small bowel being enveloped in an accessary
peritoneal sac, the wall of which is formed by the omentum and
mesocolon. CT imaging of the small intestine enveloped in the
peritoneum and existing omentum are diagnostic. Histologically, the
sac of PE is the crystalloid peritoneum, which is normal, has no
fibrosis and no adhesion with the intestine. However, AC often
presents with omental dysplasia or absence, and the sac is formed
of thickened collagen and fibrous tissue, which may be accompanied
by nonspecific chronic inflammation (26,32,33). In
addition, AC also needs to be identified with tuberculosis,
peritoneal mesothelioma and peritoneal pseudomyxoma (27,32,33).
The present study demonstrated that clinical
symptoms might manifest iteratively for patients who accept
conservative treatments. Therefore, the contention is that surgical
intervention is an effective treatment (2,20,28,34),
especially for those with intestinal obstruction or an abdominal
mass. Laparoscopy is a useful tool for a definitive diagnosis and
treatment protocol for AC (20). The
therapeutic principle of AC is lysis of adhesions and removal of
the membrane (2,25). In the current study, the treatment
was effective by excising the thickened cocoon-like membrane,
thereby freeing intestinal adhesions and enveloping bowl loops, as
well as relieving intestinal obstruction and removing the necrotic
intestine. During surgery, the adhesion between the sac and the
surrounding structure, between the sac and the intestine and
between the intestinal tube and the intracapsular intestine was
easier to remove, but extensive separation should be avoided so as
not to completely excise the fibrous membrane (20). This may result in intestinal serosal
injury due to intestinal rupture or postoperative adhesion
obstruction (7). For the cases
secondary to chronic bacterial or chemical peritonitis, the
condensing fibrous adhesion throughout the intestine makes
separation difficult (35), which
requires avoidance of intestinal vessel damage to lessen ischemia
or necrosis of the intestine. For those patients, whose condition
involves wide-ranging bowel or serious adhesions that cannot be
separated, rearrangement of the intestinal position is necessary to
prevent postoperative adhesion and obstruction (36). However, a number of authors
hypothesize that intestinal arrangement will greatly increase the
difficulty of the operation for patients with postoperative
adhesion (10). The method used to
manage the appendix has been debated and where additional
appendectomy is necessary is determined depending on the
appendiceal position, in relation to the sac and whether
inflammation may emerge (20,28).
As the current study was retrospective study, there
may have been unavoidable selection bias. Additionally, the sample
size was small. Further expansion of the sample size is required in
future studies.
In summary, preoperative CT examination serves an
important role in making a definitive diagnosis, understanding the
sac and complications that can occur, and selecting the most
suitable treatments for AC. CT scans can help to avoid excising the
peritoneum, which can lead to the intestine being accidentally cut,
or resecting a mass of encapsulated small bowel believing it is a
tumor, which will lead to short-intestine syndrome (24). To prevent the postoperative adhesion
and the sac reformation, the peritoneal cavity can be
intraoperatively filled with anti-adhesion agents (2,37),
including sodium hyaluronate or medium molecular dextran.
Postoperatively, drugs, including neostigmine, which promotes
enterokinesia and recovery of bowel function, or hydrocortisone,
which inhibits the generation of cellulose, may be useful. For
recurrent bowel obstruction, surgical complications are the major
cause of mortality, followed by intestinal leakage or
short-intestine syndrome (38). For
recurrent bowel obstruction, the majority of cases can be cured by
conservative treatment as reoperation is difficult and
complications are more likely to occur.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RY designed the study, performed the research,
analyzed the data and wrote the article. YY performed the research
and analyzed the data. XN designed the study. GF made significant
contributions to data acquisition, data analysis and
interpretation, and made critical and important revisions to the
manuscript. GF reviewed and approved the manuscript for
publication. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all study
participants and ethical approval for this study was obtained from
the local research ethics committee of The Second Affiliated
Hospital of Soochow University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Solmaz A, Tokoçin M, Arıcı S, Yiğitbaş H,
Yavuz E, Gülçiçek OB, Erçetin C and Çelebi F: Abdominal cocoon
syndrome is a rare cause of mechanical intestinal obstructions: A
report of two cases. Am J Case Rep. 16:77–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia J, Xie W, Chen L and Liu D: Abdominal
cocoon with early postoperative small bowel obstruction: A case
report and review of literature in China. Medicine (Baltimore).
97:e111022018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singhal M, Krishna S, Lal A, Narayanasamy
S, Bal A, Yadav TD, Kochhar R, Sinha SK, Khandelwal N and Sheikh
AM: Encapsulating peritoneal sclerosis: The abdominal cocoon.
Radiographics. 39:62–77. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gorsi U, Gupta P, Mandavdhare HS, Singh H,
Dutta U and Sharma V: The use of computed tomography in the
diagnosis of abdominal cocoon. Clin Imaging. 50:171–174. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Owtschinnikow P.J.: Peritonitis chronica
fibrosa incapsulata. Arch für Klinische Chirurgie. 83:623–634.
1907.
|
|
6
|
Foo KT, Ng KC, Rauff A, Foong WC and
Sinniah R: Unusual small intestinal obstruction in adolescent
girls: The abdominal cocoon. Br J Surg. 65:427–430. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeniay L, Karaca CA, Calışkan C, Fırat O,
Ersin SM and Akgün E: Abdominal cocoon syndrome as a rare cause of
mechanical bowel obstruction: Report of two cases. Ulus Travma Acil
Cerrahi Derg. 17:557–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tarzi RM, Lim A, Moser S, Ahmad S, George
A, Balasubramaniam G, Clutterbuck EJ, Gedroyc W and Brown EA:
Assessing the validity of an abdominal CT scoring system in the
diagnosis of encapsulating peritoneal sclerosis. Clin J Am Soc
Nephrol. 3:1702–1710. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stafford-Johnson DB, Wilson TE, Francis IR
and Swartz R: CT appearance of sclerosing peritonitis in patients
on chronic ambulatory peritoneal dialysis. J Comput Assist Tomogr.
22:295–299. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Danford CJ, Lin SC, Smith MP and Wolf JL:
Encapsulating peritoneal sclerosis. World J Gastroenterol.
24:3101–3111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fei X, Yang HR, Yu PF, Sheng HB and Gu GL:
Idiopathic abdominal cocoon syndrome with unilateral abdominal
cryptorchidism and greater omentum hypoplasia in a young case of
small bowel obstruction. World J Gastroenterol. 22:4958–4962. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma V, Mandavdhare HS, Rana SS, Singh
H, Kumar A and Gupta R: Role of conservative management in
tubercular abdominal cocoon: A case series. Infection. 45:601–606.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown EA, Bargman J, van Biesen W, Chang
MY, Finkelstein FO, Hurst H, Johnson DW, Kawanishi H, Lambie M, de
Moraes TP, et al: Length of time on peritoneal dialysis and
encapsulating peritoneal sclerosis-position paper for ISPD: 2017
update. Perit Dial Int. 37:362–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takebayashi K, Sonoda H, Shimizu T, Ohta
H, Ishida M, Mekata E, Endo Y, Tani T and Tani M: Successful
surgical approach for a patient with encapsulating peritoneal
sclerosis after hyperthermic intraperitoneal chemotherapy: A case
report and literature review. BMC Surg. 14:572014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaur S, Doley RP, Chabbhra M, Kapoor R and
Wig J: Post trauma abdominal cocoon. Int J Surg Case Rep. 7C:64–65.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KW, Cho CW, Lee N, Lee S, Kim JM, Choi
GS, Kwon CH, Joh JW and Lee SK: Encapsulating peritoneal sclerosis
in liver transplant recipients: A report of 2 cases. Ann Surg Treat
Res. 92:164–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salamone G, Atzeni J, Agrusa A and Gulotta
G: A rare case of abdominal cocoon. Ann Ital Chir.
84:2013.PubMed/NCBI
|
|
18
|
Koak Y, Gertner D, Forbes A and Ribeiro
BF: Idiopathic sclerosing peritonitis. Eur J Gastroenterol Hepatol.
20:148–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Izumotani T, Ishimura E, Yamamoto T,
Otoshi T, Okuno S, Inaba M, Kim M and Nishizawa Y: Correlation
between peritoneal mesothelial cell cytology and peritoneal
histopathology with respect to prognosis in patients on continuous
ambulatory peritoneal dialysis. Nephron. 89:43–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Wang JJ, Hu WX, Zhang MC, Liu XY, Li
Y, Cai GF, Liu SL and Yao XQ: Diagnosis and treatment of 26 cases
of abdominal cocoon. World J Surg. 41:1287–1294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gurleyik G, Emir S and Saglam A: The
abdominal cocoon: A rare cause of intestinal obstruction. Acta Chir
Belg. 110:396–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ceulemans LJ, Deferm NP, Deferm S,
Willaert RAV, Deferm JT and Vanhoenacker FM: Unusual cause of
mechanical ileus: Abdominal cocoon syndrome. J Belg Soc Radiol.
100:362016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng Y, Qu L, Li J, Wang B, Geng J and
Xing D: Abdominal cocoon accompanied by multiple peritoneal loose
body. Medicine (Baltimore). 96:e61852017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim MC, Chotai NC and Giron DM: Idiopathic
sclerosing encapsulating peritonitis: A rare cause of subacute
intestinal obstruction. Case Rep Med. 2016:82068942016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aliyev V, Yagi S, Hammad A, Badawy A,
Sasaki Y, Masano Y, Yamamoto G, Kamo N, Taura K, Okajima H, et al:
Sclerosing encapsulating peritonitis after living-donor liver
transplantation: A case series, Kyoto experience. Ann Hepatobiliary
Pancreat Surg. 22:144–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mbanje C, Mazingi D, Forrester J and
Mungazi SG: Peritoneal encapsulation syndrome: A case report and
literature review. Int J Surg Case Rep. 41:520–523. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tombak MC, Apaydin FD, Colak T, Duce MN,
Balci Y, Yazici M and Kara E: An unusual cause of intestinal
obstruction: Abdominal cocoon. AJR Am J Roentgenol. 194:W176–W178.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Allam H, Al Yahri O, Mathew S, Darweesh A,
Suliman AN, Abdelaziem S, Khairat M, Toro A and Di Carlo I: The
enigma of primary and secondary encapsulating peritoneal sclerosis.
BMC Surg. 16:812016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Braun N, Alscher DM, Fritz P, Edenhofer I,
Kimmel M, Gaspert A, Reimold F, Bode-Lesniewska B, Ziegler U,
Biegger D, et al: Podoplanin-positive cells are a hallmark of
encapsulating peritoneal sclerosis. Nephrol Dial Transplant.
26:1033–1041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lopez-Anton M, Lambie M, Lopez-Cabrera M,
Schmitt CP, Ruiz-Carpio V, Bartosova M, Schaefer B, Davies S, Stone
T, Jenkins R, et al: miR-21 promotes fibrogenesis in peritoneal
dialysis. Am J Pathol. 187:1537–1550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cleland: On an abnormal arrangement of the
peritoneum, with remarks on the development of the mesocolon. J
Anat Physiol. 2:201–206. 1868.PubMed/NCBI
|
|
32
|
Jagdale A, Prasla S and Mittal S:
Abdominal cocoon-A rare etiology of intestinal obstruction. J
Family Med Prim Care. 6:674–676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Acar T, Kokulu İ, Acar N, Tavusbay C and
Hacıyanlı M: Idiopathic encapsulating sclerosing peritonitis. Ulus
Cerrahi Derg. 31:241–243. 2015.PubMed/NCBI
|
|
34
|
Li Y, Li N, Zhu WM, Gong JF, Zhang W, Gu
LL, Zuo LG and Li JS: Surgical treatment for idiopathic abdominal
cocoon. Zhonghua wai ke za zhi. 51:139–141. 2013.(In Chinese).
PubMed/NCBI
|
|
35
|
Basu A, Sukumar R, Sistla SC and Jagdish
S: ‘Idiopathic’ abdominal cocoon. Surgery. 141:277–278. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rajagopal AS and Rajagopal R: Conundrum of
the cocoon: Report of a case and review of the literature. Dis
Colon Rectum. 46:1141–1143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jaber S, Dulaijan K, Sadoun M, Moghazy K
and El-Said M: Post-traumatic intra-cocoon mesenteric tear: A case
report. Case Rep Gastroenterol. 5:206–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawaguchi Y, Kawanishi H, Mujais S, Topley
N and Oreopoulos DG: Encapsulating peritoneal sclerosis:
Definition, etiology, diagnosis, and treatment. International
Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration
Management in Peritoneal Dialysis. Perit Dial Int. 20 (Suppl
4):S43–S55. 2000.PubMed/NCBI
|