Introduction
The endothelium presents a single-cell lining on the
internal surface of blood vessels, cardiac valves, and numerous
body cavities. The vascular endothelium has been considered as a
multifunctional organ, which protects the vessel wall from the
vascular tone, vessel wall inflammation, and thrombosis resistance
(1), and the endothelium
participates in new vessel formation (2). Thus, the typical vascular function
needs to keep the integrity of the vascular endothelium and a
well-balanced release of numerous vasoactive substances (3). Endothelial dysfunction underlies the
pathogenesis of vascular disease and cardiovascular diseases, such
as coronary artery disease, coronary artery spasm, and
atherosclerosis (3–5). Endothelial dysfunction has always been
caused by reduced levels and adhesive function of circulating
endothelial progenitor cells, which accelerates
re-endothelialization (6,7). Previous studies have revealed that
angiogenesis is a physiological process involving the growth of new
blood vessels either from endothelial cell precursors or from the
pre-existing vasculature, and the processes are regulated by
various angiogenic growth factors, such as vascular endothelial
growth factor (VEGF) (8). By binding
to 1 of 3 cognate receptor tyrosine kinases (VEGF receptor 1–3),
VEGF has been regarded as the most vital cytokine in enhancing
endothelial cell growth. The VEGF-mediated signaling pathway
exhibits a vital role in maintaining the structure and function of
the vascular endothelium by promoting endothelial cell
proliferation (9,10).
Sevoflurane is a general anesthetic, and it has been
commonly used in the anesthesia of young children and infants
(11). Sevoflurane has exhibited
activity against oxidative stress, inflammation, and it has been
revealed to protect organs against stress-caused injury (12–14).
Sevoflurane pretreatment significantly inhibited TNF-α-induced
permeability and p38 MAPK activation in rat pulmonary microvascular
endothelial cells by decreasing ICAM-1 levels (15). Sevoflurane appears to offer a more
stable heart rate profile compared with either isoflurane or
desflurane (16). Notably,
sevoflurane increases HUVEC proliferation and adhesion, in addition
to the incorporation of tubular structures into endothelial
progenitor cells (17). However, the
effects and underlying mechanisms of sevoflurane on VEGF in human
endothelial cells have not been elucidated. In the present study,
the effects and molecular mechanisms of sevoflurane on the
proliferation of human umbilical vein endothelial cells (HUVECs)
were investigated.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from Gibco; Thermo Fisher Scientific, Inc. (cat. no.
C0155C). Cells were cultured in Medium 200 (cat. no. M200500)
supplemented with LSGS (cat. no. S00310; both from Gibco; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. HUVECs were digested with Trypsin/EDTA at the
appropriate confluency (~70–80%). Cells were cultured in an
incubator under normal conditions or with sevoflurane treatment (1
and 3%). Treatment with sevoflurane was performed according to a
previously reported method (18) and
was achieved by connecting the incubator with the sevoflurane
vaporizer (Abbott Laboratories) attached to the anesthetic machine
(Dräger). The infrared gas analyzer (Puritan-Bennett) was used to
monitor the sevoflurane concentration at the inflow and outflow
connectors.
Cell viability assay
Cell viability was performed by MTT assay (cat. no.
KA1606; Abnova). HUVECs were seeded in a 96-well plate at 2,000
cells/well under different conditions for 12, 24 48, and 72 h.
Reagent medium (15/80 µl per well) was added followed by incubation
for 4 h at 37°C. For the treatment with the VEGFA antibody, the
cells were incubated with the antibody (20 µM; cat. no. AF-493-NA;
R&D Systems) to chelate the effects of VEGFA in the culture
medium and the corresponding control antibody (20 µM; cat. no.
AB-108-C; R&D Systems) was applied as a control during the
exposure of sevoflurane. Then 100 µl of the solubilizer was added
to each well. OD570 nm was measured for each well on an absorbance
plate reader. The cell viability was calculated by the ratio of
OD570 at each time-point to OD570 at 0 h of each well and presented
as the percentage of the ratio.
Quantitative RT-PCR
Total RNA was extracted from HUVECs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). For mRNA
analysis, 2 µg total RNA was reverse-transcribed to cDNA using the
PrimeScript® reagent kit (Takara Bio, Inc.).
Quantitative PCR for mRNA was performed using SYBR-Green (Takara
Bio, Inc.) with an ABI 7900 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: Initial denaturation at 95°C for 3 min;
followed by 40 cycles of 95°C for 10 sec, 60°C for 5 sec and final
extension at 72°C for 10 sec. Quantification of the mRNA expression
was normalized to β-actin. The fold-change of the mRNA levels
relative to the control cells was calculated using the
2−ΔΔCq method (19). The
sequences of the human-specific primers were as follows: VEGFa
forward, 5′-CGAAGAGAAGAGACACATTG-3′ and reverse,
5′-GGATGGAGGAAGGTCAAC-3′; VEGFb forward, 5′-ACAGGACAGAGTTGGAAGA-3′
and reverse, 5′-GGAAGAGCCAGTTGTAAGAT-3′; VEGFc forward,
5′-TGTGTCCAGTGTAGATGAAC-3′ and reverse, 5′-TCTTCTGTCCTTGAGTTGAG-3′;
VEGFR1 forward, 5′-ACTCGTGGCTACTCGTTA-3′ and reverse,
5′-ACCTTGCTTCGGAATGATT-3′; VEGFR2 forward,
5′-ACTGTCATCCTTACCAATCC-3′ and reverse, 5′-CCTCCAACTGCCAATACC-3′
and VEGFR3 forward, 5′-ATGCGAATACCTGTCCTAC-3′ and reverse:
5′-GTTGCCGATGTGAATGAG-3′.
siRNA transfection
Cells subjected to siRNA transfection were firstly
cultured in half the volume of the culture medium and transfected
with 3 ng/ml siRNA (sense, 5′-GCAGCGACAAGGCAGACUAUU-3′ and
antisense, 5′-UAGUCUGCCUUGUCGCUGCGU-3′) or control siRNA (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) (Shanghai GenePharma Co., Ltd.) by
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.) for 8 h
according to the product protocol. After 8 h of incubation, the
other half of the culture medium was added, and cells were
incubated for another 40 h so that the total interference lasted
for 48 h. After 48 h of incubation, the culture medium was changed
to normal conditions without siRNA and subjected to further
experiments.
Western blotting
Cells from each group were scraped in RIPA lysis
buffer (Thermo Fisher Scientific, Inc.) with serine protease
inhibitor. Proteins (50 µg) were separated on 10%
SDS-polyacrylamide gels and electroblotted onto polyvinylidene
fluoride (PVDF) membranes (EMD Millipore). After incubating with
blocking buffer, the membranes were incubated overnight at 4°C with
the primary antibodies: Anti-VEGFR2 (dilution, 1:2,000; product
code ab2349), anti-phosphorylated (p)-VEGFR2 (phosphorylated at Tyr
1175; dilution, 1:2,000; product code ab194806) and anti-beta Actin
(dilution, 1:2,000; product code ab8226; all obtained from Abcam).
Next, the membranes were washed and incubated with appropriate
anti-rabbit HRP-conjugated secondary antibodies (dilution, 1:3,000;
product code ab6728, Abcam) at 37°C for 30 min. Protein bands were
detected using the SuperSignal West Pico Chemiluminescent Substrate
(Thermo Fisher Scientific, Inc.). The integrated optical density of
the detected protein band was normalized to the control band for
quantitative comparison by ImageJ (v. d1.47; National Institutes of
Health).
VEGFR inhibitor
Axitinib, a VEGFR inhibitor, was purchased from
Selleck Chemicals and was dissolved in DMSO at a stock
concentration of 10 mM. The inhibitor was diluted to an appropriate
final concentration in the culture medium.
Statistical analysis
All experiments were performed at least three times
with data expressed as the mean ± SEM or as otherwise stated.
Statistical analyses were performed with GraphPad Prism software
(version 7; GraphPad Software, Inc.). Group comparisons were
performed using one-way ANOVA with post hoc test using Dunnett's
test or Sidak's multiple comparisons test. The two-column
comparison was performed using Student's t-test. P<0.05 or
P<0.01 were considered to indicate a statistically significant
difference (*P<0.05 and **P<0.01, as indicated in the figures
and legends).
Results
Sevoflurane promotes the proliferation
of human umbilical vein endothelial cells (HUVECs)
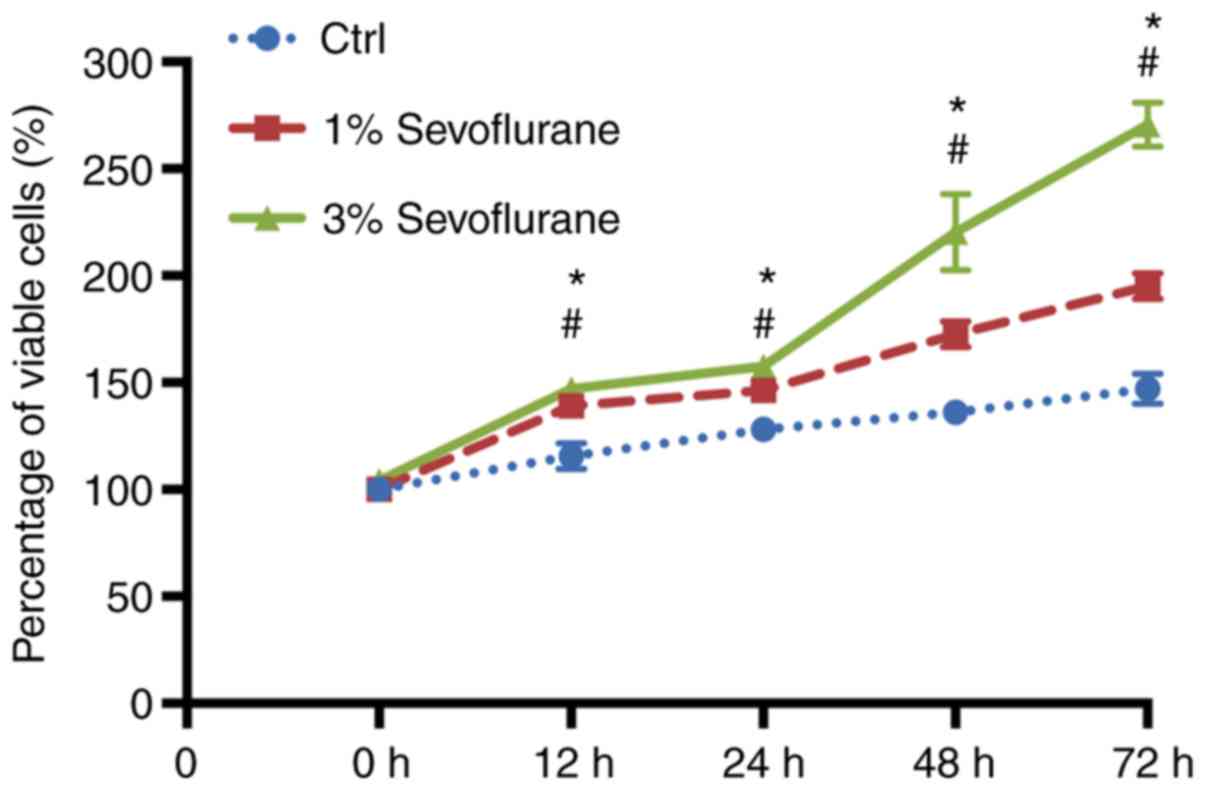
To investigate whether sevoflurane was able to
stimulate proliferation of HUVECs, the HUVECs were cultured in
different concentrations of sevoflurane. The MTT colorimetric assay
was used to determine HUVEC activity at different concentrations (1
and 3%, respectively) of sevoflurane at different time-points (12,
24, 48 and 72 h, respectively). The results revealed that
sevoflurane increased cell viability in a dose- and time-dependent
manner (Fig. 1).
Sevoflurane increases VEGF signaling
systems in HUVECs
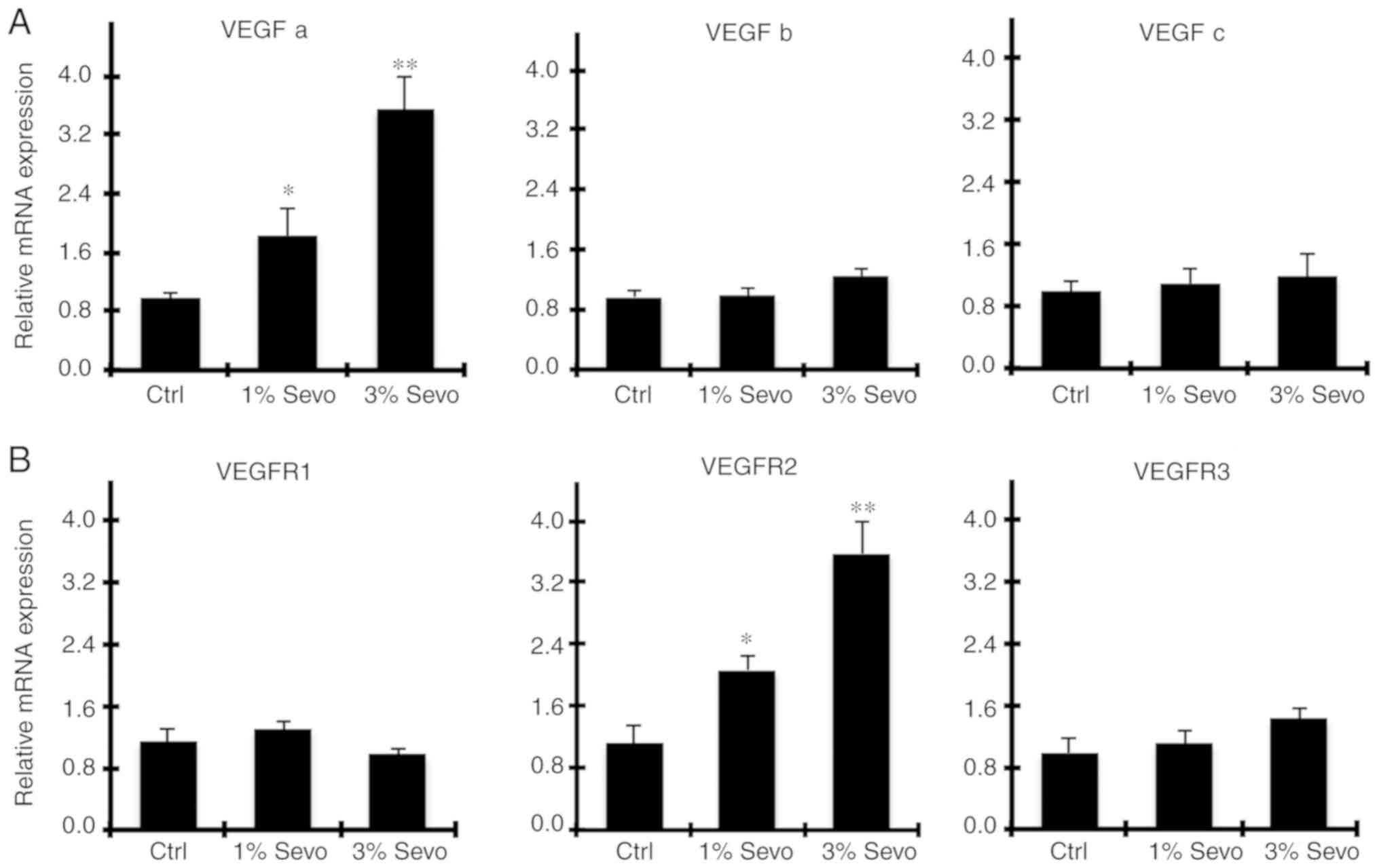
The VEGF pathway has been demonstrated to serve
essential roles in promoting HUVEC proliferation (5). The most well-known VEGFs and their
receptors (VEGFRs) include VEGFa, b, and VEGFR-1, 2, 3. Thus qPCR
was used to examine the expression levels of VEGFs and VEGFRs of
HUVECs treated with 1, and 3% sevoflurane, respectively. As
revealed in Fig. 2A, compared with
the control group, only the expression level of VEGFa was increased
after exposure to sevoflurane for 48 h, and the increase was in a
concentration-dependent manner (P<0.05; Fig. 2A). Similarly, VEGFR2 expression was
significantly increased after sevoflurane exposure (P<0.05;
Fig. 2B). These data indicated that
sevoflurane induced the expression of VEGFa, which may regulate
VEGFR2 and activation of the following signaling pathway.
VEGFα inhibition protects the
proliferation of HUVECs exposed to sevoflurane
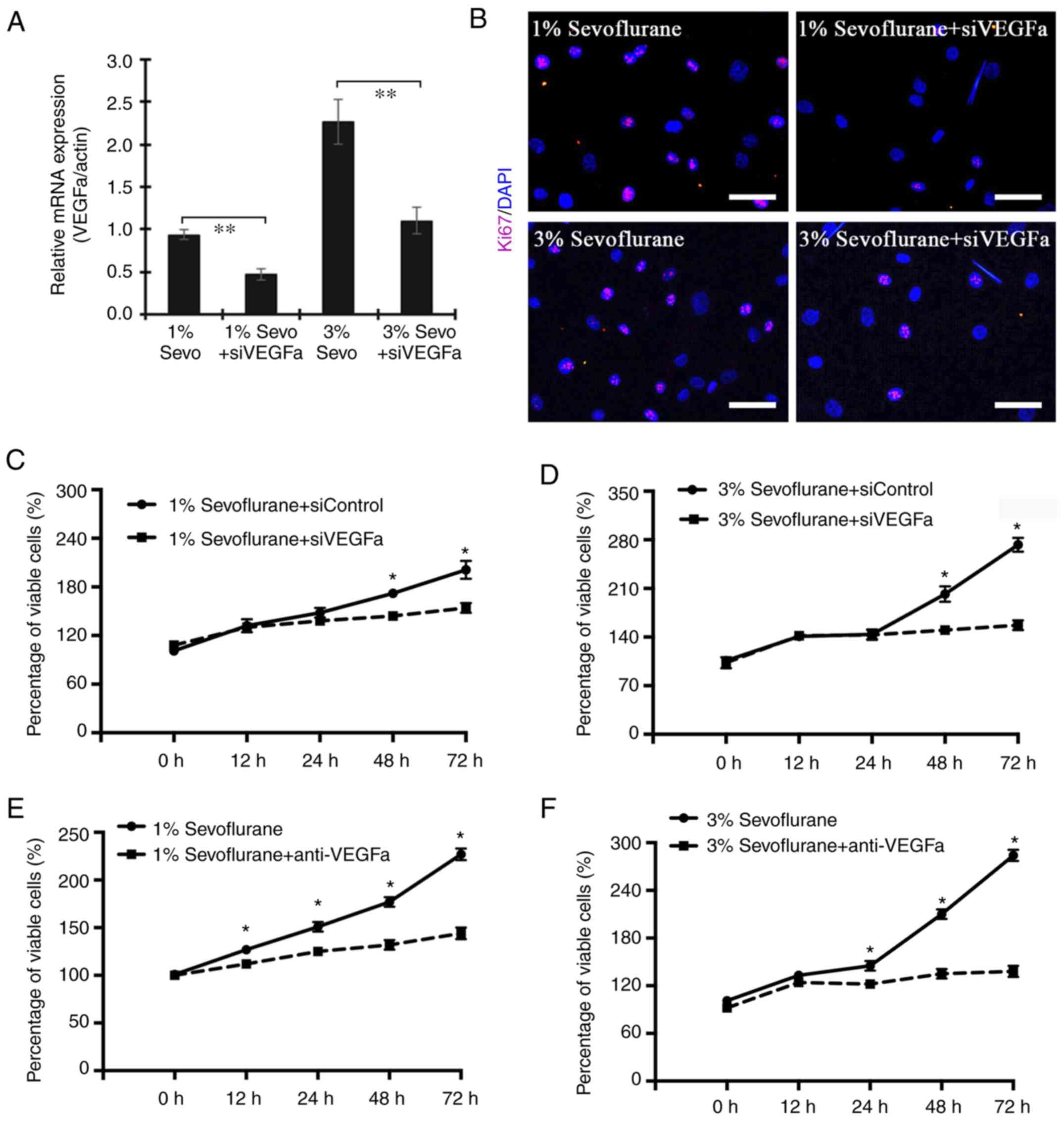
To explore whether VEGFa secreted by HUVECs is
involved in regulating the cell proliferation under sevoflurane
exposure, HUVECs in the presence of 1 and 3% sevoflurane were
transfected with VEGFa siRNA. The interference efficiency is
presented in Fig. S1. The data
revealed that silenced expression of VEGFa resulted in decreased
Ki67-positive HUVECs (Fig. 3A and
B). Moreover, the decreased expression of VEGFa exhibited a
similar effect on the viability of cells (Fig. 3C and D). To further assess whether
VEGFa was sufficient to target sevoflurane stimuli, a VEGFa
chelation experiment was performed in cell culture using anti-VEGFa
antibodies. As revealed in Fig. 3E and
F the proliferation of HUVECs under either 1 or 3% sevoflurane
medium was significantly blocked by the addition of anti-VEGFa
while the group receiving control IgG antibodies exhibited high
proliferation. These data indicated that the enhanced proliferation
of HUVECs due to the effect of sevoflurane was regulated by the
expression level VEGFa.
Sevoflurane promotes the proliferation
of HUVECs by activating the VEGF/VEGFR signaling pathway
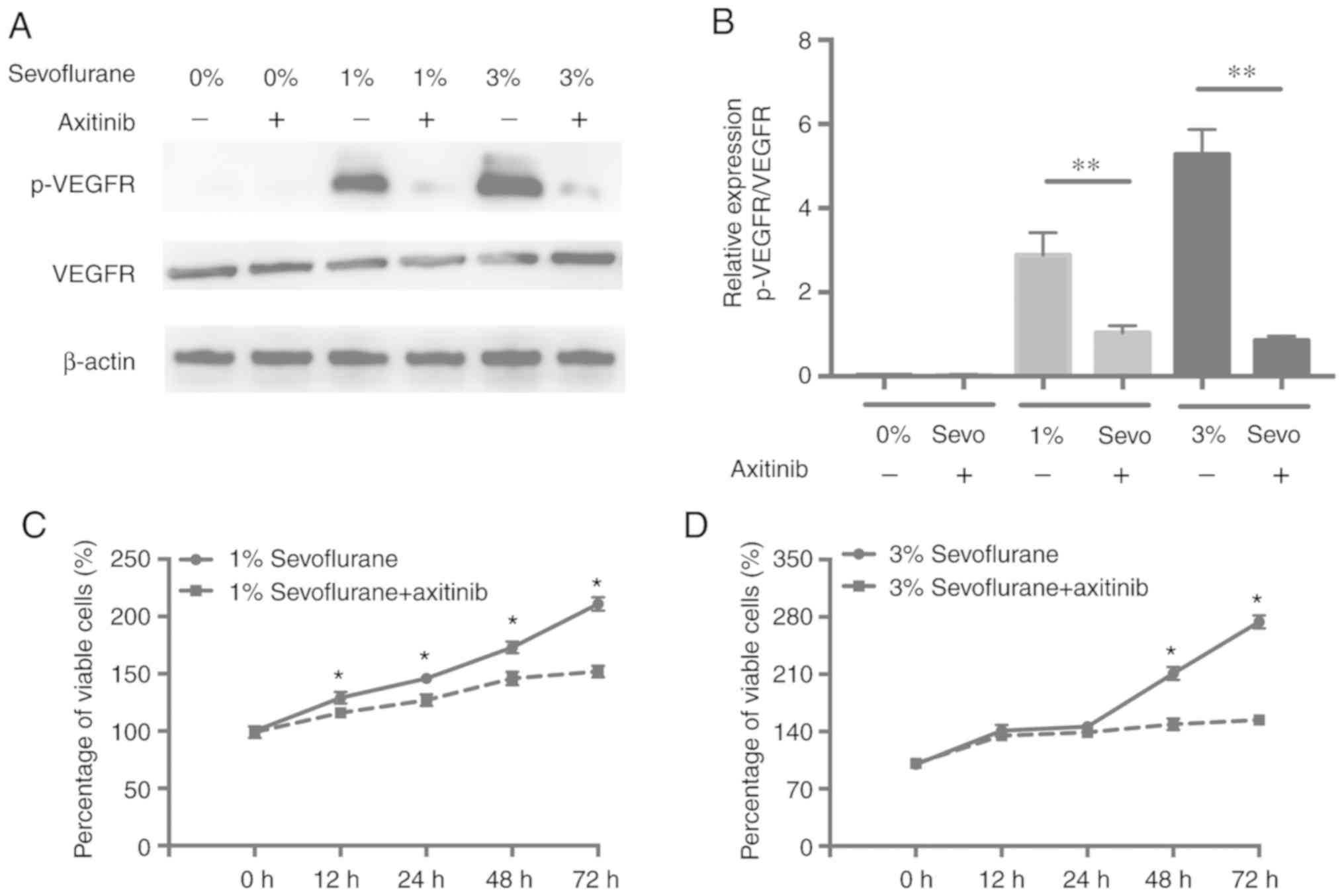
To investigate whether VEGF signaling was the
underlying mechanism of sevoflurane in enhancing HUVEC
proliferation, a small molecular VEGFR inhibitor, axitinib, was
used to block the VEGFR signaling pathway. Firstly, western
blotting was used to assess the levels of VEGFR and p-VEGFR after
exposure of HUVECs to different concentrations of Sevoflurane with
VEGFR inhibitor for 48 h. As revealed in Fig. 4A and B, the significant decrease of
the p-VEGFR level was observed in HUVECs exposed to axitinib and
sevoflurane when compared with cells treated with sevoflurane
alone. Notably, following the inhibition of the VEGFR pathway,
there was significantly decreased cell proliferation in the cells
treated with axitinib plus sevoflurane than with sevoflurane alone
(Fig. 4C and D), implying that
sevoflurane promotes the proliferation of HUVECs via activation of
the VEGF/VEGFR pathway.
Discussion
The endothelium is involved in new vessel formation
(2). The integrity of the vascular
endothelium and an appropriate release of numerous vasoactive
factors contribute to normal vascular physiology, and its
dysfunction may incur the pathogenesis of vascular disease and
cardiovascular diseases, such as coronary artery disease, coronary
artery spasm, and atherosclerosis (3–5,20,21). One
of the major therapeutic strategies for cardiovascular diseases is
to maintain vessel function and promote vessel regeneration
(4,5,20). In
the present study, it was revealed that sevoflurane significantly
increased cell viability and proliferation of HUVECs in a dose- and
time-dependent manner, indicating that sevoflurane may increase new
vessel formation resulting in attenuation of vascular disease.
The establishment and remodeling of the vascular
system are regulated by several secreted signaling molecules,
including VEGF, and their corresponding receptors. They mediate
downstream signaling that can result in the regulation of
endothelial cell number and function (8,22–24). The
VEGF gene undergoes alternative splicing to form 6 isoforms, VEGFa,
VEGFb, VEGFc, VEGFd, VEGFe, and VEGFf, where VEGFa is the most
biologically active among them (25). VEGF receptors are classified into
three groups, VEGFR1, VEGFR2, and VEGFR3 (26,27).
VEGF-A and VEGF-B always bind to VEGFR1, VEGFa and VEGFe frequently
bind to VEGFR2, whereas VEGFc and VEGFd mainly bind to VEGFR3
(28). Previous studies have
revealed that VEGFR1 [also known as Fms-related tyrosine kinase 1
(FLT1)] plays an essential role in vessel formation (10,29,30).
Depletion of VEGFR1 has been revealed to cause early embryonic
lethality at embryonic day 8.5 to 9 by enhancing endothelial cell
overgrowth and excessive VEGFR2 [also known as kinase insert domain
receptor, (KDR)] activation, which results in increased
hemangioblast commitment and disorganized vessel formation
(10,29,30).
This suggests that VEGFR1 negatively regulates VEGFR2 signaling by
sequestering VEGF and preventing it from binding to VEGFR2, which
is the primary receptor to modulating angiogenesis by elevating the
cell proliferation/survival, migration, and differentiation of the
endothelium (10,29–31).
VEGFR2-null mice die at embryonic day 8.5 to 9 by increasing the
defective blood-island formation and nearly absence of vasculature
(32,33). In addition, VEGFR3 (FLT-4), which may
be activated by VEGF-C, has been revealed to play a vital role in
establishing and maintaining lymphatic endothelial cells (34), and VEGFR3-mediated endothelial Snail
was revealed to regulate capillary branching morphogenesis in the
retina (35,36). Furthermore, clinical studies
demonstrated that VEGF inhibition was associated with
cardiovascular disease (37,38). It has been reported that sevoflurane
exhibits its protective effects on pulmonary microvascular
endothelial cells by downregulating ICAM-1 expression and
inhibiting TNF-α and p38 MAPK signaling (15). In the present study, it was revealed
that VEGF signaling could be activated by sevoflurane at a dose-
and time-dependent manner in HUVEC cells. This was consistent with
a previous study in which sevoflurane increased proliferation,
adhesion on HUVECs, and incorporation in tubular structures of
endothelial progenitor cells (17).
Moreover, sevoflurane significantly increased the mRNA expression
of VEGFa, however, sevoflurane did not affect VEGFb and VEGFc mRNA
expression. In addition, sevoflurane upregulated the expression of
VEGFR2 at the mRNA and protein levels, however it did not change
the mRNA expression of VEGFR1 and VEGFR3. Furthermore, sevoflurane
failed to elevate the VEGFR2 mRNA and protein expression when
VEGFR2 was inhibited by axitinib, an inhibitor of VEGF receptors.
These data indicated that sevoflurane could promote the
proliferation of HUVEC cells by activating the VEGFa/VEGFR2
signaling pathway.
In conclusion, sevoflurane may be a promising agent
against endothelium dysfunction-induced vascular disease by
activating the VEGFa/VEGFR2 signaling pathway.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW and CW performed majority of the experiments and
contributed to the manuscript preparation. MZ and AD assisted with
the animal experiments. RN performed the statistical analysis. JZ
supervised the experiments and reviewed the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davignon J and Ganz P: Role of endothelial
dysfunction in atherosclerosis. Circulation. 109:27–32. 2004.
View Article : Google Scholar
|
|
2
|
Yoder MC: Human endothelial progenitor
cells. Cold Spring Harbor Perspect Med. 2:a0066922012. View Article : Google Scholar
|
|
3
|
Chhabra N: Endothelial dysfunction-A
predictor of atherosclerosis. Inter J Med Update. 4:33–41.
2009.
|
|
4
|
Park KH and Park WJ: Endothelial
dysfunction: Clinical implications in cardiovascular disease and
therapeutic approaches. J Korean Med Sci. 30:1213–1225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rajendran P, Rengarajan T, Thangavel J,
Nishigaki Y, Sakthisekaran D, Sethi G and Nishigaki I: The vascular
endothelium and human diseases. Int J Biol Sci. 9:1057–1069. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Werner N, Junk S, Laufs U, Link A, Walenta
K, Bohm M and Nickenig G: Intravenous transfusion of endothelial
progenitor cells reduces neointima formation after vascular injury.
Circ Res. 93:e17–e24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang PH, Chen YH, Chen YL, Wu TC, Chen JW
and Lin SJ: Vascular endothelial function and circulating
endothelial progenitor cells in patients with cardiac syndrome X.
Heart. 93:1064–1070. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin JM, Zhao JY, Zhuang QC, Hong ZF and
Peng J: Xiongshao capsule promotes angiogenesis of HUVEC via
enhancing cell proliferation and up-regulating the expression of
bFGF and VEGF. Chin J Integr Med. 17:840–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carratelli CR, Paolillo R and Rizzo A:
Chlamydia pneumoniae stimulates the proliferation of HUVEC through
the induction of VEGF by THP-1. Int Immunopharmacol. 7:287–294.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hassel D, Cheng P, White MP, Ivey KN,
Kroll J, Augustin HG, Katus HA, Stainier DY and Srivastava D:
MicroRNA-10 regulates the angiogenic behavior of zebrafish and
human endothelial cells by promoting vascular endothelial growth
factor signaling. Circ Res. 111:1421–1433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Song X, Yuan T, He J, Wang X and
Wang Q: Effects of calpain on sevoflurane-induced aged rats
hippocampal neuronal apoptosis. Aging Clin Exp Res. 28:633–639.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hofstetter C, Boost KA, Flondor M,
Basagan-Mogol E, Betz C, Homann M, Muhl H, Pfeilschifter J and
Zwissler B: Anti-inflammatory effects of sevoflurane and mild
hypothermia in endotoxemic rats. Acta Anaesthesiol Scand.
51:893–899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herrmann IK, Castellon M, Schwartz DE,
Hasler M, Urner M, Hu G, Minshall RD and Beck-Schimmer B: Volatile
anesthetics improve survival after cecal ligation and puncture.
Anesthesiology. 119:901–906. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Julier K, da Silva R, Garcia C, Bestmann
L, Frascarolo P, Zollinger A, Chassot PG, Schmid ER, Turina MI, von
Segesser LK, et al: Preconditioning by sevoflurane decreases
biochemical markers for myocardial and renal dysfunction in
coronary artery bypass graft surgery: A double-blinded,
placebo-controlled, multicenter study. Anesthesiology.
98:1315–1327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun SX, Ge BX and Miao CH: Effects of
preconditioning with sevoflurane on TNF-α-induced permeability and
activation of p38 MAPK in rat pulmonary microvascular endothelial
cells. Cell Biochem Biophys. 61:123–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ebert TJ, Harkin CP and Muzi M:
Cardiovascular responses to sevoflurane: A review. Anesth Analg. 81
(Suppl 6):S11–S22. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vlad AM, Isvoranu G, Gilca M, Ceafalan L,
Surcel M, Stoian I and Manda G: Sevoflurane increases
proliferation, adhesion on HUVEC and incorporation in tubular
structures of endothelial progenitor cells. FASEB J.
29:LB5902015.
|
|
18
|
Yang Y, Hu R, Yan J, Chen Z, Lu Y, Jiang J
and Jiang H: Sevoflurane inhibits the malignant potential of head
and neck squamous cell carcinoma via activating the
hypoxiainducible factor-1α signaling pathway in vitro. Int J Mol
Med. 41:995–1002. 2018.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saffi MA, Furtado MV, Polanczyk CA,
Montenegro MM, Ribeiro IW, Kampits C, Haas AN, Rösing CK and
Rabelo-Silva ER: Relationship between vascular endothelium and
periodontal disease in atherosclerotic lesions: Review article.
World J Cardiol. 7:26–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meguro K, Iidaka T, Nakata M, Yamashita T,
Chinen T, Fujita M, Kikuchi T, Keida T and Ohira H: Regular
exercise habits and vascular endothelium function in patients with
cardiovascular diseases. Eur Heart J. 35:730–731. 2014.
|
|
22
|
Gerber HP, Vu TH, Ryan AM, Kowalski J,
Werb Z and Ferrara N: VEGF couples hypertrophic cartilage
remodeling, ossification and angiogenesis during endochondral bone
formation. Nat Med. 5:623–628. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dzietko M, Derugin N, Wendland MF, Vexler
ZS and Ferriero DM: Delayed VEGF treatment enhances angiogenesis
and recovery after neonatal focal rodent stroke. Transl Stroke Res.
4:189–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lopes RA, Neves KB, Tostes RC, Montezano
AC and Touyz RM: Downregulation of nuclear factor Erythroid
2-related factor and associated antioxidant genes contributes to
redox-sensitive vascular dysfunction in hypertension. Hypertension.
66:1240–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neves KB, Rios FJ, van der Mey L,
Alves-Lopes R, Cameron AC, Volpe M, Montezano AC, Savoia C and
Touyz RM: VEGFR (Vascular Endothelial Growth Factor Receptor)
inhibition induces cardiovascular damage via redox-sensitive
processes. Hypertension. 71:638–647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrara N, Carver-Moore K, Chen H, Dowd M,
Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ and Moore MW:
Heterozygous embryonic lethality induced by targeted inactivation
of the VEGF gene. Nature. 380:439–442. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carmeliet P, Ferreira V, Breier G,
Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A,
Harpal K, Eberhardt C, et al: Abnormal blood vessel development and
lethality in embryos lacking a single VEGF allele. Nature.
380:435–439. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi H and Shibuya M: The vascular
endothelial growth factor (VEGF)/VEGF receptor system and its role
under physiological and pathological conditions. Clin Sci (Lond).
109:227–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fong GH, Rossant J, Gertsenstein M and
Breitman ML: Role of the Flt-1 receptor tyrosine kinase in
regulating the assembly of vascular endothelium. Nature. 376:66–70.
1995. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roberts DM, Kearney JB, Johnson JH,
Rosenberg MP, Kumar R and Bautch VL: The vascular endothelial
growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1
(VEGFR-2) signaling during blood vessel formation. Am J Pathol.
164:1531–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang JX, Chen XL, Serizawa N,
Szyndralewiez C, Page P, Schröder K, Brandes RP, Devaraj S and
Török NJ: Liver fibrosis and hepatocyte apoptosis are attenuated by
GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol
Med. 53:289–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shalaby F, Rossant J, Yamaguchi TP,
Gertsenstein M, Wu XF, Breitman ML and Schuh AC: Failure of
blood-island formation and vasculogenesis in Flk-1-deficient mice.
Nature. 376:62–66. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ranayhossaini DJ, Rodriguez AI, Sahoo S,
Chen BB, Mallampalli RK, Kelley EE, Csanyi G, Gladwin MT, Romero G
and Pagano PJ: Selective Recapitulation of conserved and
Nonconserved regions of putative NOXA1 protein activation domain
confers isoform-specific inhibition of Nox1 oxidase and attenuation
of endothelial cell migration. J Biol Chem. 288:36437–36450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Makinen T, Jussila L, Veikkola T, Karpanen
T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H,
Nishikawa S, et al: Inhibition of lymphangiogenesis with resulting
lymphedema in transgenic mice expressing soluble VEGF receptor-3.
Nat Med. 7:199–205. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hogan BM, Herpers R, Witte M, Heloterä H,
Alitalo K, Duckers HJ and Schulte-Merker S: Vegfc/Flt4 signalling
is suppressed by Dll4 in developing zebrafish intersegmental
arteries. Development. 136:4001–4009. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park JA, Kim DY, Kim YM, Lee IK and Kwon
YG: Endothelial snail regulates capillary branching morphogenesis
via vascular endothelial growth factor receptor 3 expression. PLoS
Genet. 11:e10053242015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clarkin CE and Gerstenfeld LC: VEGF and
bone cell signalling: An essential vessel for communication? Cell
Biochem Funct. 31:1–11. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moslehi JJ: Cardiovascular toxic effects
of targeted cancer therapies. New Engl J Med. 375:1457–1467. 2016.
View Article : Google Scholar : PubMed/NCBI
|