Introduction
Diabetes mellitus (DM) is a metabolic disease
characterized by hyperglycemia, and its chronic complications can
cause patient death and disability (1). There are about 415 million patients
with DM worldwide, and the number has been increasing annually
(2). Currently, about 193 million of
the patients have not been diagnosed. Chronic DM that is untreated
induces many complications and the incidence rates are still on the
rise even after blood glucose is controlled (3). The long-term development of DM causes
pathological changes of vascular structure, and then leads to
microvascular and macrovascular complications (4). Previous findings have shown that DM is
an independent risk factor for cerebral infarction (CI). The
development of CI is closely related to severe metabolic
dysfunction, and early CI is caused by arterial occlusion (5,6). The
mortality of patients with diabetes mellitus complicated with
cerebral infarction (DMCI) is very high. The pathological basis of
the disease is atherosclerosis and the pathological change is
thrombosis (7). Therefore, the
timely detection and control of macroangiopathy is of great
significance to prevent and for treatment of DMCI.
Carotid intima-media thickness (CIMT) is an early
manifestation of atherosclerosis, and changes in its value reflect
the severity of atherosclerosis. It is practical, easy to operate,
and conducive to assessing the risk of cerebrovascular diseases
(8). Homocysteine (Hey) level plays
an important role in the development and progression of DM. Its
high level causes atherosclerosis and cardio-cerebrovascular
diseases through damaging vascular endothelium, inducing
thrombosis, and promoting the proliferation of vascular smooth
muscle cells (9). DM is a chronic
low-grade inflammation and is characterized by the excessive
secretion of interleukin-1β (IL-1β) and other pro-inflammatory
cytokines, which enhances inflammatory signals and then results in
complications such as vasculopathy (10,11).
Previous studies have reported that systolic blood pressure (SBP),
diastolic blood pressure (DBP), high-density lipoprotein
cholesterol (HDL-C), and low-density lipoprotein cholesterol
(LDL-C) are major risk factors for CI (12–14).
However, there are currently few studies on the diagnostic values
of Hey, IL-1β, and fasting blood glucose (FBG) levels for DMCI, or
whether IL-1β is a risk factor for CI.
Therefore, the roles and significance of Hey, IL-1β,
and FBG levels in patients with DMCI were explored in this
study.
Patients and methods
General information
Altogether 124 patients with DMCI (the DMCI group)
and 103 patients with DM (the DM group) admitted to the People's
Hospital of Liuhe District of Nanjing from February 2016 to January
2019 were enrolled in this study. The DMCI group consisted of 65
males and 59 females aged 46–78 years with an average age of
60.9±9.7 years. The DM group consisted of 53 males and 50 females
aged 43–75 years with an average age of 60.1±9.5 years. A further
100 healthy controls undergoing physical examinations during the
same period (the HC group) were also enrolled. The group comprised
59 males and 41 females aged 39–70 years with an average age of
58.4±8.6 years. The research subjects were informed and signed an
informed consent form. This study did not violate ethics or
morality and was approved by the Ethics Committee of the People's
Hospital of Liuhe District (Nanjing).
Inclusion and exclusion criteria
Inclusion criteria for the study were: patients in
the DMCI and DM groups met the diagnostic criteria for DM from the
American Diabetes Association (ADA) in 2017 (15), and their diabetes was type II
diabetes mellitus. Patients in the DMCI group met the diagnostic
criteria for CI from the American Heart Association (AHA) / the
American Stroke Association (ASA) in 2018 (16), and their CI was confirmed by cranial
imaging, MRI, or CT. The age range of the patients in the two
groups were >38 years, but <78 years, and they had complete
clinical data. Exclusion criteria were: those complicated with
severe hepatic and renal dysfunction, cardiogenic CI, heart
failure, thyroid dysfunction, diabetic nephropathy, autoimmune
diseases, severe infections, or malignant tumors; those with
cognitive disorders or mental illness; those who had taken
immunosuppressants or anti-inflammatory drugs in the previous one
month. The inclusion and exclusion criteria were applied to the
DMCI and DM groups. The healthy controls were in the HC group.
CIMT examination
Carotid artery ultrasound was performed using a
Philips E33 Color Doppler Ultrasonic Diagnosis Apparatus
(Koninklijke Philips Electronics N.V). All subjects were examined
by the same physician. The subjects laid down for 15-min rest and
then their necks were fully exposed. Next, the common carotid
artery, internal carotid artery, and carotid bifurcation were
successively examined at 7.5 MHz. The vertical distance between the
lumen intima and the media-adventitia, which was measured at 1 cm
before and after the carotid bifurcation, was the CIMT value. The
value was measured 3 times to obtain the average value.
Detection of markers
The subjects fasted for 3 h. In the morning the next
day, their venous blood (5 ml) was extracted, placed in vacuum
blood collection tubes, and centrifuged at 1,450 × g for 10 min at
4°C with a radius of 10 cm, so as to separate and store the upper
layer of the serum for later use. The levels of serum Hey
(enzymatic cycling method) and FBG (glucose oxidase method)
(Beijing Bioassay Technologies Co., Ltd.; batch no. 301, 401) were
detected by the AU5800 fully automatic biochemical analyzer
(Beckman Coulter, Inc.). The reference ranges of Hey and FBG were
5–15 µmol/l and 3.33–5.55 mmol/l, respectively. The detection was
carried out with reference to the instructions of the corresponding
kits and instruments. Serum IL-1β level was detected by
enzyme-linked immunosorbent assay (ELISA) (17), with steps carried out with reference
to the instructions of human IL-1β ELISA kit [Gelatin & Protein
Co., Ltd., batch no. JK-(a)-4956]. Standard wells, sample wells to
be tested, and blank control wells (without samples and
enzyme-labeled reagents) were set up. The standard wells in the
enzyme-labeled plate that was coated were accurately added with
standard substances (50 µl), while the sample wells to be tested
were added with sample diluent (40 µl) and then the samples to be
tested (10 µl) (the final dilution of the sample was 5 times).
After that, the plate was coated, incubated at 37°C for 30 min, and
then patted dry after liquid in the wells was discarded. The plate
was repeatedly washed 5 times. Each well, except the blank control
wells, was added with the enzyme-labeled reagents (50 µl), coated,
and then incubated at 37°C for 30 min. Next, each well was added
with substrate A and then substrate B (50 µl each), and colored at
37°C for 10 min in the dark after the substrates were mixed well.
Finally, each well was added with stop solution (50 µl) to cease
the reaction. Optical density (OD) values of each well were
sequentially measured at 450 nm using a SpectraMax
iD5-multifunctional microplate reader (Molecular Devices), to
calculate the IL-1β level.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used for statistical
analysis. Enumeration data were expressed by the number of
cases/percentage [n (%)], and the comparison of the data between
groups was analyzed by Chi-square test. Measurement data were
expressed by mean ± standard deviation (mean ± SD), and comparison
of the data between groups was analyzed by independent samples
t-test. One-way ANOVA was used for the comparison of means between
multiple groups. LSD t-test was used for pairwise comparison
between groups. Pearson's test was used to analyze the correlations
of Hey, IL-1β, and FBG levels with CIMT value. Receiver operating
characteristic (ROC) curves were plotted to calculate the area
under the curve (AUC), to determine the cut-off values of Hey,
IL-1β, and FBG for diagnosing DMCI, and to calculate their
sensitivity and specificity. Multivariate Logistic regression was
used to analyze risk factors for DMCI. P<0.05 indicates a
statistically significant difference.
Results
General information
There were significant differences between the DMCI,
DM, and HC groups in terms of hemoglobin Alc (HbAlc), SBP, DBP,
HDL-C, LDL-C, triglyceride (TG), apolipoprotein AI (apoAI), and
apolipoprotein B (apoB) (P<0.05), but not in sex, age, body mass
index (BMI), years of smoking, history of drinking, place of
residence, coronary heart disease (CHD), platelet (PLT) count, and
total cholesterol (TC) (P>0.05). There were significant
differences between the DMCI and DM groups in terms of SBP, DBP,
HDL-C, LDL-C, TG, apoAI, and apoB (P<0.05), not in course of
disease, HbAlc, the use of insulin, and CHD (P>0.05) (Table I).
 | Table I.General information [n (%)]/(mean ±
SD). |
Table I.
General information [n (%)]/(mean ±
SD).
| Categories | DMCI group
(n=124) | DM group (n=103) | HC group (n=100) |
t/F/χ2 | P-value |
|---|
| Sex |
|
|
| 1.398 | 0.497 |
| Male | 65 (52.42) | 53 (51.46) | 59 (59.00) |
|
|
|
Female | 59 (47.58) | 50 (48.54) | 41 (41.00) |
|
|
| Age (years) | 60.9±9.7 | 60.1±9.5 | 58.4±8.6 | 2.035 | 0.132 |
| BMI
(kg/m2) | 25.46±2.64 | 26.04±2.49 | 25.28±2.63 | 2.426 | 0.090 |
| Course of disease
(years) | 12.3±2.5 | 11.9±2.1 | – | 1.289 | 0.199 |
| HbAlc (%) |
10.26±2.59a |
10.12±2.96a | 4.93±0.83 | 174.600 | <0.001 |
| Use of insulin |
|
|
| 0.153 | 0.696 |
| Yes | 80 (64.52) | 69 (66.99) | – |
|
|
| No | 44 (35.48) | 34 (33.01) | – |
|
|
| History of
smoking |
|
|
| 0.643 | 0.725 |
|
Yes | 45 (36.29) | 42 (40.78) | 36 (36.00) |
|
|
| No | 79 (63.71) | 61 (59.22) | 64 (64.00) |
|
|
| Years of
smoking | 6.8±2.7 | 6.4±2.1 | 6.2±1.8 | 2.056 | 0.130 |
| History of
drinking |
|
|
| 0.344 | 0.842 |
|
Yes | 48 (38.71) | 43 (41.75) | 38 (38.00) |
|
|
| No | 76 (61.29) | 60 (58.25) | 62 (62.00) |
|
|
| Place of
residence |
|
|
| 2.129 | 0.349 |
|
Yes | 91 (73.39) | 70 (67.96) | 77 (77.00) |
|
|
| No | 33 (26.61) | 33 (32.04) | 23 (23.00) |
|
|
| CHD |
|
|
| 0.753 | 0.385 |
|
Yes | 11 (8.87) | 6 (5.83) | – |
|
|
| No | 113 (91.13) | 97 (94.17) | – |
|
|
| SBP (mmHg) |
151.23±21.86a,b |
143.59±21.83a | 109.52±7.15 | 114.000 | <0.001 |
| DBP (mmHg) |
85.73±13.67a,b |
79.41±6.52a | 76.05±8.49 | 25.710 | <0.001 |
| PLT (xb
109/l) | 147.19±61.26 | 146.75±71.53 | 153.47±61.58 | 0.349 | 0.706 |
| HDL-C (mmol/l) |
1.16±0.19a,b |
1.27±0.13a | 1.39±0.29 | 10.610 | <0.001 |
| LDL-C (mmol/l) |
3.06±0.59a,b |
2.76±0.43a | 2.59±0.54 | 22.910 | <0.001 |
| TC (mmol/l) | 4.63±0.83 | 4.49±0.81 | 4.38±0.71 | 2.826 | 0.061 |
| TG (mmol/l) |
1.47±0.32a,b |
1.35±0.21a | 1.23±0.57 | 10.520 | <0.001 |
| apoAI (g/l) |
1.01±0.19a,b |
1.16±0.23a | 1.41±0.38 | 59.850 | <0.001 |
| apoB (g/l) |
0.98±0.32a,b |
0.88±0.19a | 0.78±0.25 | 16.030 | <0.001 |
Levels of Hey, IL-1β, and FBG and CIMT
value
The levels of Hey, IL-1β, and FBG and the CIMT value
in the DMCI and DM groups were significantly higher than those in
the HC group (P<0.001). The levels and the value in the DMCI
group were significantly higher than those in the DM group
(P<0.001) (Fig. 1).
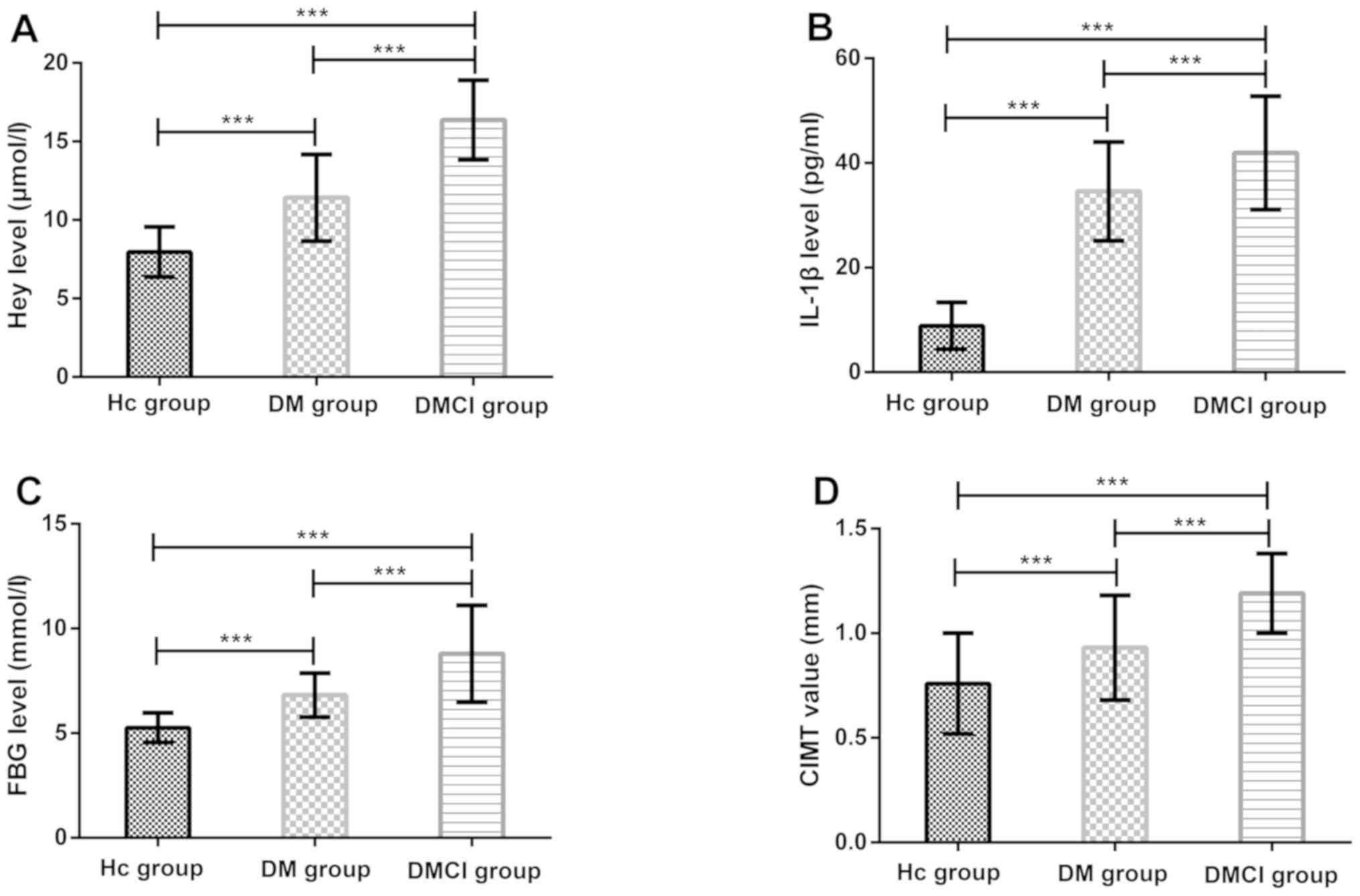 | Figure 1.Comparison of levels of Hey, IL-1β,
and FBG and CIMT value. (A) Comparison of Hey level between the HC,
DM, and DMCI groups. (B) Comparison of IL-1β level between the HC,
DM, and DMCI groups. (C) Comparison of FBG level between the HC,
DM, and DMCI groups. (D) Comparison of CIMT value between the HC,
DM, and DMCI groups. ***P<0.001. HC, healthy controls; DM,
diabetes mellitus; DMCI, diabetes mellitus complicated with
cerebral infarction; Hey, homocysteine; IL-1β, interleukin-1β; FBG,
fasting blood glucose; CIMT, carotid intima-media thickness. |
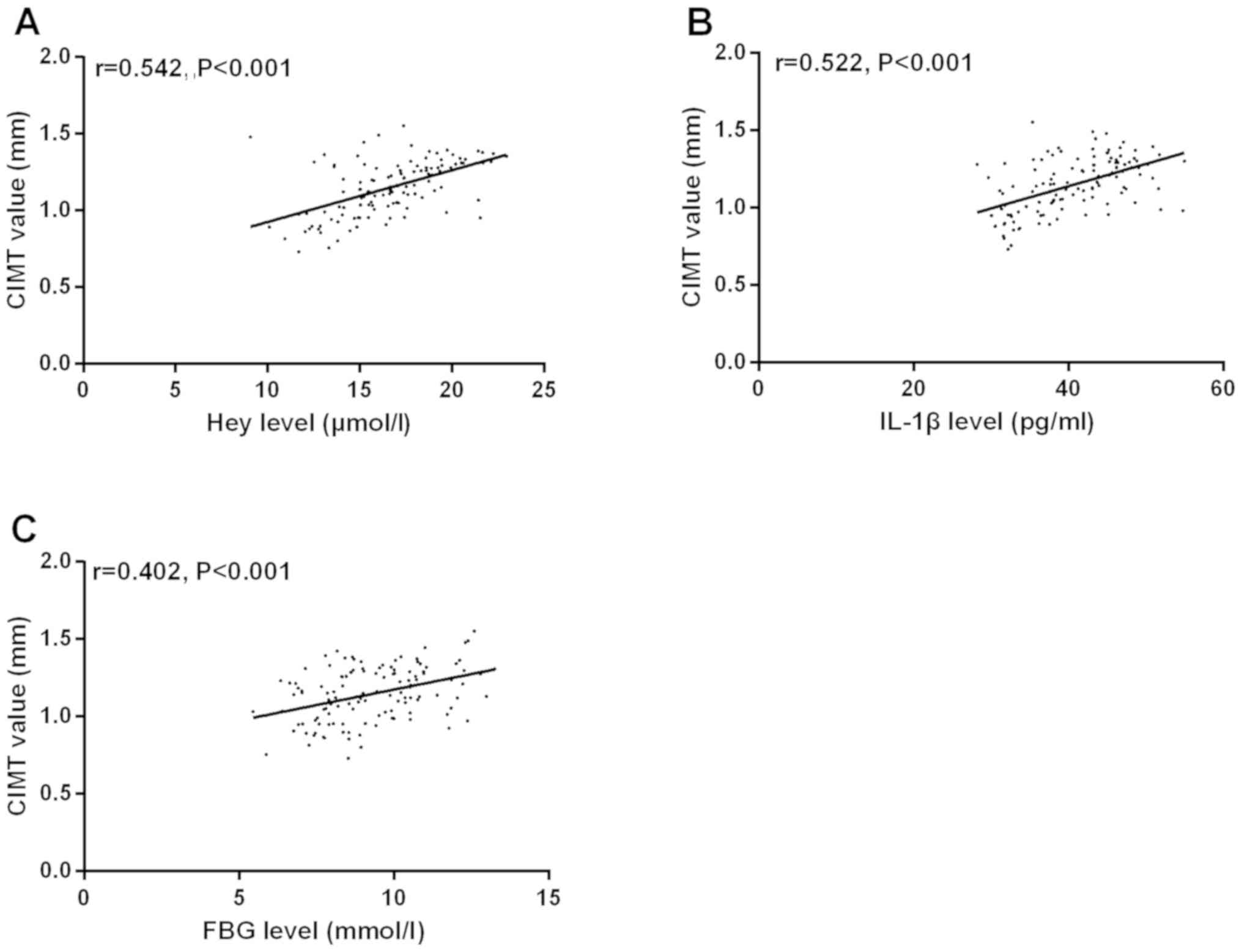
Correlations of Hey, IL-1β, and FBG
levels with CIMT value in DMCI group
According to the Pearson's correlation analysis, Hey
level was positively correlated with CIMT value (r=0.542,
P<0.001). IL-1β level was positively correlated with CIMT value
(r=0.522, P<0.001). FBG level was positively correlated with
CIMT (r=0.402, P<0.001) (Fig.
2).
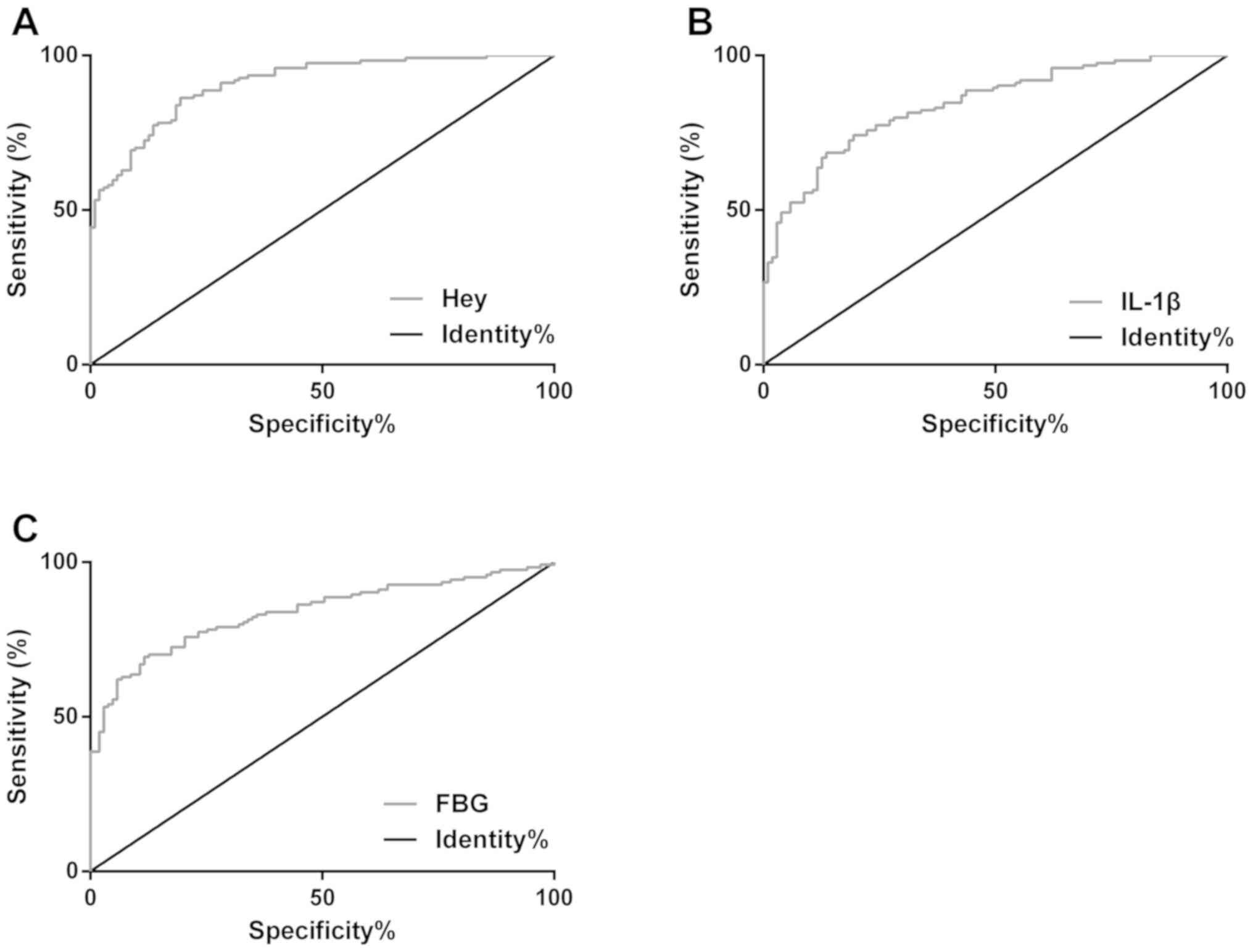
Diagnostic values of Hey, IL-1β, and
FBG levels for DMCI
The ROC curves of Hey, IL-1β, and FBG levels for
diagnosing DMCI were plotted. The AUC of Hey for the diagnosis was
0.909 (95% CI, 0.872–0.945) and the cut-off value was 13.53 µmol/l,
with the sensitivity of 86.29 and the specificity of 80.58%. The
AUC of IL-1β was 0.842 (95% CI, 0.793–0.892) and the cut-off value
was 38.67 pg/ml, with the sensitivity of 68.55 and the specificity
of 86.41%. The AUC of FBG was 0.837 (95% CI, 0.784–0.889) and the
cut-off value was 7.89 pg/ml, with the sensitivity of 69.35 and the
specificity of 88.35% (Table II and
Fig. 3).
 | Table II.Diagnostic values of Hey, IL-1β, and
FBG levels for DMCI. |
Table II.
Diagnostic values of Hey, IL-1β, and
FBG levels for DMCI.
| Indicators | AUC | 95% CI | Standard error | Cut-off | Sensitivity
(%) | Specificity
(%) |
|---|
| Hey | 0.909 | 0.872–0.945 | 0.018 | 13.53 (µmol/l) | 86.29 | 80.58 |
| IL-1β | 0.842 | 0.793–0.892 | 0.025 | 38.67 (pg/ml) | 68.55 | 86.41 |
| FBG | 0.837 | 0.784–0.889 | 0.027 | 7.89
(mmol/l) | 69.35 | 88.35 |
Multivariate logistic regression
analysis of risk factors for DMCI
The multivariate Logistic regression analysis was
performed on factors with differences between the DMCI and DM
groups. The results showed that SBP (P=0.007), DBP (P=0.009), HDL-C
(P=0.001), LDL-C (P=0.041), Hey (P<0.001), IL-1β (P=0.003), FBG
(P=0.049), and CIMT (P=0.006) were independent risk factors for
DMCI (P<0.05) (Tables III and
IV).
 | Table III.Assignment of multivariate Logistic
regression analysis. |
Table III.
Assignment of multivariate Logistic
regression analysis.
| Factors | Variables | Assignment |
|---|
| SBP (mmHg) | X1 | A continuous
variable |
| DBP (mmHg) | X2 | A continuous
variable |
| HDL-C (mmol/l) | X3 | A continuous
variable |
| LDL-C (mmol/l) | X4 | A continuous
variable |
| TG (mmol/l) | X5 | A continuous
variable |
| apoAI (g/l) | X6 | A continuous
variable |
| apoB (g/l) | X7 | A continuous
variable |
| Hey (µmol/l) | X8 | A continuous
variable |
| IL-1β (pg/ml) | X9 | A continuous
variable |
| FBG (mmol/l) | X10 | A continuous
variable |
| CIMT (mm) | X11 | A continuous
variable |
 | Table IV.Multivariate logistic regression
analysis of risk factors for DMCI. |
Table IV.
Multivariate logistic regression
analysis of risk factors for DMCI.
| Factors | B | Standard error | Wals | P-value | HR (95% CI) |
|---|
| SBP (mmHg) | 0.127 | 0.047 | 7.362 | 0.007 | 1.135
(1.036–1.245) |
| DBP (mmHg) | 0.062 | 0.024 | 6.865 | 0.009 | 1.064
(1.016–1.115) |
| HDL-C (mmol/l) | −6.458 | 1.988 | 10.555 | 0.001 | 0.002
(0.000–0.077) |
| LDL-C (mmol/l) | 1.16 | 0.687 | 2.852 | 0.041 | 3.191
(0.830–12.267) |
| TG (mmol/l) | 1.473 | 1.184 | 1.549 | 0.213 | 4.363
(0.429–44.394) |
| apoAI (g/l) | −2.839 | 1.67 | 2.890 | 0.089 | 0.058
(0.002–1.543) |
| apoB (g/l) | 0.359 | 0.210 | 2.930 | 0.087 | 1.432
(0.949–2.159) |
| Hey (µmol/l) | 0.788 | 0.159 | 24.718 | <0.001 | 2.199
(1.612–3.001) |
| IL-1β (pg/ml) | 0.163 | 0.054 | 9.031 | 0.003 | 1.177
(1.058–1.309) |
| FBG (mmol/l) | 0.311 | 0.161 | 3.721 | 0.049 | 1.364
(0.995–1.871) |
| CIMT (mm) | 4.267 | 1.563 | 7.456 | 0.006 | 71.281
(3.333–1524.23) |
Discussion
DM is the main cause of macroangiopathy and its
incidence has been increasing year by year. With the improvement of
living standards and the development of economy, increasing number
of young people have developed DM (18). The pathological basis of CI is
atherosclerotic plaques, which lead to luminal stenosis, then
microthrombus, and finally hypoxia and ischemia of the brain tissue
(19). As a long-term chronic
result, DMCI has no obvious clinical signs and symptoms during this
period (20). Therefore, to diagnose
DMCI in time and analyze its risk factors is of great
significance.
CIMT predicts the risk of coronary artery stenosis
in asymptomatic patients. Related to the severity of
atherosclerosis, its value is significantly higher in patients with
type II DM than that in healthy people (21). As an intermediate metabolite of
sulfur amino acids, Hey activates the coagulation system and
promotes deposition on vascular walls, thus affecting vasodilation
(22). IL-1β is a pro-inflammatory
cytokine that plays an important role during atherothrombosis.
IL-1β-mediated inflammation functions in the deterioration of islet
β cell function, atherogenesis, and plaque progression (23). In this study, the levels of Hey,
IL-1β, and FBG and the CIMT value in the DMCI and DM groups were
significantly higher than those in the HC group; the levels and the
value in the DMCI group were significantly higher than those in the
DM group. This indicates that patients with DMCI have severe
atherosclerosis, and that Hey, IL-1β, and FBG are involved in the
development and progression of DMCI. Moreover, Hey, IL-1β, and FBG
levels were positively correlated with CIMT value, which shows that
the three levels may affect CIMT, so Hey, IL-1β, and FBG are
expected to be indicators for evaluating the severity of DMCI. In a
study by Matsumoto et al (24), the CIMT value in the DMCI group is
significantly higher than that in the CI group. The probability of
CI in patients with DM increases by 1.8 times every time CIMT
increases by 0.1 mm. HbAlc and age are independently related to
CIMT. In a study by Eikelboom et al (25), Hey is closely associated with the
pathological changes of arteries, and its level is positively
correlated with vascular stenosis and arteriosclerosis, so high Hey
level is an independent risk factor for CI. In a study by Huang
et al (26), large glucose
fluctuations are related to CI and the poor short-term prognosis of
patients with the disease. The results of this study showed that
SBP, DBP, HDL-C, LDL-C, Hey, FBG, and CIMT were independent risk
factors for DMCI, which is similar to the findings of previous
studies (27–29). Whether IL-1β can be used as an
independent risk factor for DMCI has been rarely studied. According
to a previous study, IL-1β release in the cerebral cortex of
Fischer rats increases after permanent middle cerebral artery
occlusion, while blocking IL-1β with anti-IL-1β antibody reduces
the scope of CI, and relieves neurological and behavioral
dysfunction (30). Thus, IL-1β may
play an important role in CI. The results of the present study
showed that IL-1β was an independent risk factor for DMCI. The
massive release of Hey damages the vascular endothelial function
and unbalances the coagulation and fibrinolytic systems, thereby
causing a prethrombotic state (31).
Vascular inflammatory responses accelerate the formation of
unstable plaques. Hyperglycemia causes acidosis of brain cells
through lactic acid accumulation during cerebral ischemia. It also
causes brain cell damage through the destruction of mitochondrial
function, the promotion of free radical generation, and the
enhancement of lipid peroxidation (32,33).
Therefore, the development and progression of DMCI may be the
result of synergy between Hey, IL-1β, and hyperglycemia. There are
currently few studies on the diagnostic values of Hey, IL-1β, and
FBG levels for DMCI. According to the ROC curves in this study, the
three levels had good predictive values for DMCI, and the cut-off
values of the three for diagnosing the disease were 13.53 µmol/l,
38.67 pg/ml, and 7.89 mmol/l, respectively. The observation of the
cut-off values was helpful to identify DM and DMCI. Therefore, Hey,
IL-1β, and FBG levels can be monitored and intervened, which is
conducive to reducing cervical atherosclerosis, delaying the
progression of atherosclerosis, and preventing DMCI.
This study confirms the roles of Hey, IL-1β, and FBG
in the development and progression of DMCI, but it still has
deficiencies. The changes in the three levels during treatment were
not observed. Basic experiments were not carried out. The
regulatory mechanisms of Hey, IL-1β, and FBG in the development and
progression of DMCI were not fully explored. These deficiencies
need to be supplemented in future studies to corroborate the
conclusions of this study.
In summary, patients with DMCI have severe
atherosclerosis. Hey, IL-1β, and FBG are involved in the
development and progression of DMCI, so they can be used as
predictive markers for the disease. Hey, IL-1β, FBG, and CIMT are
independent risk factors for patients with DMCI.
Acknowledgements
Not applicable.
Funding
This study was supported by Value of Grass-roots
Hospital's Green Channel in the Treatment of Acute Cerebral
Infarction of The Project Fund of Nanjing Health Committee (no.
YKK17241).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZD was the major contributor in writing the
manuscript and carried out all experiments. YJ analyzed and
interpreted the patient general data. QF and AQ performed ELISA. LX
and JL were responsible for analysis of the observation indicators.
All the authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the People's Hospital of Liuhe District of Nanjing. Patients who
participated in this research, signed an informed consent and had
complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Setoodeh A, Didban A, Rabbani A,
Sayarifard A, Abbasi F, Sayarifard F and Hoseinzade F: The effect
of metformin as an adjunct therapy in adolescents with type 1
diabetes. J Clin Diagn Res. 11:SC01–SC04. 2017.PubMed/NCBI
|
|
2
|
Chawla A, Chawla R and Jaggi S:
Microvasular and macrovascular complications in diabetes mellitus:
Distinct or continuum? Indian J Endocrinol Metab. 20:546–551. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel A, MacMahon S, Chalmers J, Neal B,
Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et
al ADVANCE Collaborative Group, : Intensive blood glucose control
and vascular outcomes in patients with type 2 diabetes. N Engl J
Med. 358:2560–2572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu D, Wu C and Zhong Y: The association
between paraoxonase 1 activity and the susceptibilities of diabetes
mellitus, diabetic macroangiopathy and diabetic microangiopathy. J
Cell Mol Med. 22:4283–4291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Güven A, Hancili S, Karatoprak EY and
Tasel B: Symptomatic cerebral infarction in a child with severe
diabetic ketoacidosis. J Pediatr Endocrinol Metab. 27:1001–1004.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao L and Hu FX: α-Lipoic acid treatment
of aged type 2 diabetes mellitus complicated with acute cerebral
infarction. Eur Rev Med Pharmacol Sci. 18:3715–3719.
2014.PubMed/NCBI
|
|
7
|
Zhang B, Wang D, Ji TF, Shi L and Yu JL:
Overexpression of lncRNA ANRIL up-regulates VEGF expression and
promotes angiogenesis of diabetes mellitus combined with cerebral
infarction by activating NF-κB signaling pathway in a rat model.
Oncotarget. 8:17347–17359. 2017.PubMed/NCBI
|
|
8
|
Wang SW, Liu Z and Shi ZS: Non-coding RNA
in acute ischemic stroke: Mechanisms, biomarkers and therapeutic
targets. Cell Transplant. 27:1763–1777. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin T, Liu JC, Chang LY and Shen CW:
Association of C-reactive protein and homocysteine with subclinical
coronary plaque subtype and stenosis using low-dose MDCT coronary
angiography. Atherosclerosis. 212:501–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Konya H, Miuchi M, Satani K, Matsutani S,
Yano Y, Tsunoda T, Ikawa T, Matsuo T, Ochi F, Kusunoki Y, et al:
Asymmetric dimethylarginine, a biomarker of cardiovascular
complications in diabetes mellitus. World J Exp Med. 5:110–119.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glaser NS, Ghetti S, Casper TC, Dean JM
and Kuppermann N; Pediatric Emergency Care Applied Research Network
(PECARN) DKA FLUID Study Group, : Pediatric diabetic ketoacidosis,
fluid therapy, and cerebral injury: The design of a factorial
randomized controlled trial. Pediatr Diabetes. 14:435–446. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan X, Lo EH and Wang X: Effects of
minocycline plus tissue plasminogen activator combination therapy
after focal embolic stroke in type 1 diabetic rats. Stroke.
44:745–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mohan V, Cooper ME, Matthews DR and Khunti
K: The standard of care in type 2 diabetes: Re-evaluating the
treatment paradigm. Diabetes Ther. 10 (Suppl 1):1–13. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osei E, den Hertog HM, Berkhemer OA,
Fransen PS, Roos YB, Beumer D, van Oostenbrugge RJ, Schonewille WJ,
Boiten J, Zandbergen AA, et al MR CLEAN pretrial investigators, :
Increased admission and fasting glucose are associated with
unfavorable short-term outcome after intra-arterial treatment of
ischemic stroke in the MR CLEAN pretrial cohort. J Neurol Sci.
3711–5. (201)2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
American Diabetes Association: 8.
Pharmacologic approaches to glycemic treatment: Standards of
Medical Care in Diabetes-2018. Diabetes Care. 41 (Suppl 1):S73–S85.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Powers WJ, Rabinstein AA, Ackerson T,
Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk
BM, Hoh B, et al American Heart Association Stroke Council, : 2018
guidelines for the early management of patients with acute ischemic
stroke: A guideline for healthcare professionals from the American
Heart Association/American Stroke Association. Stroke. 49:e46–e110.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charrad R, Berraïes A, Hamdi B, Ammar J,
Hamzaoui K and Hamzaoui A: Anti-inflammatory activity of IL-37 in
asthmatic children: Correlation with inflammatory cytokines TNF-α,
IL-β, IL-6 and IL-17A. Immunobiology. 221:182–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joung B, Lee JM, Lee KH, Kim TH, Choi EK,
Lim WH, Kang KW, Shim J, Lim HE, Park J, et al KHRS Atrial
Fibrillation Guideline Working Group, : 2018 Korean guideline of
atrial fibrillation management. Korean Circ J. 48:1033–1080. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Ding J, Leng X, Pu Y, Huang LA, Xu
A, Wong KSL, Wang X and Wang Y: Guidelines for evaluation and
management of cerebral collateral circulation in ischaemic stroke
2017. Stroke Vasc Neurol. 3:117–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCoy CE, Langdorf MI and Lotfipour S:
American Heart Association/American Stroke Association Deletes
Sections from 2018 Stroke Guidelines. West J Emerg Med. 19:947–951.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun YP, Cai YY, Li HM, Deng SM, Leng RX
and Pan HF: Increased carotid intima-media thickness (CIMT) levels
in patients with type 1 diabetes mellitus (T1DM): A meta-analysis.
J Diabetes Complications. 29:724–730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neumiller JJ and Umpierrez GE: 2018
Standards of care update: Pharmacologic approaches to glycemic
management in people with type 2 diabetes. Diabetes Spectr.
31:254–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
American Diabetes Association: Standards
of medical care in diabetes-2018 abridged for primary care
providers. Clin Diabetes. 36:14–37. 2018.PubMed/NCBI
|
|
24
|
Matsumoto K, Sera Y, Nakamura H, Ueki Y
and Miyake S: Correlation between common carotid arterial wall
thickness and ischemic stroke in patients with type 2 diabetes
mellitus. Metabolism. 51:244–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eikelboom JW, Hankey GJ, Anand SS,
Lofthouse E, Staples N and Baker RI: Association between high
homocyst(e)ine and ischemic stroke due to large- and small-artery
disease but not other etiologic subtypes of ischemic stroke.
Stroke. 31:1069–1075. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang J, Zhang X, Li J, Tang L, Jiao X and
Lv X: Impact of glucose fluctuation on acute cerebral infarction in
type 2 diabetes. Can J Neurol Sci. 41:486–492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang N, Lin M, Wang BG, Zeng WY, He YF,
Peng HY, Zeng J, Wu ZY and Zhong Y: Low level of low-density
lipoprotein cholesterol is related with increased hemorrhagic
transformation after acute ischemic cerebral infarction. Eur Rev
Med Pharmacol Sci. 20:673–678. 2016.PubMed/NCBI
|
|
28
|
You S, Zhong C, Xu J, Han Q, Zhang X, Liu
H, Zhang Y, Shi J, Huang Z, Xiao G, et al: LDL-C/HDL-C ratio and
risk of all-cause mortality in patients with intracerebral
hemorrhage. Neurol Res. 38:903–908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang XL, Fu HJ, Yang GR, Wan G, Li D, Zhu
LX, Xie RR, Lv YJ, Zhang JD, Li YL, et al Beijing Communities
Diabetes Study Group, : The effects of cardiovascular risk factor
combined anti-platelet therapy and the risk of cerebrovascular
events in patients with T2DM in an urban community over 96-months
follow-up: The Beijing communities diabetes study 19. Diabetes Res
Clin Pract. 144:236–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kirkman MS, Mahmud H and Korytkowski MT:
Intensive blood glucose control and vascular outcomes in patients
with type 2 diabetes mellitus. Endocrinol Metab Clin North Am.
47:81–96. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu W, Guan Y, Xu K, Fu XJ, Lei XF, Lei LJ,
Zhang ZQ, Cheng Y and Li YQ: Plasma homocysteine levels predict the
risk of acute cerebral infarction in patients with carotid artery
lesions. Mol Neurobiol. 53:2510–2517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fayfman M, Pasquel FJ and Umpierrez GE:
Management of hyperglycemic crises: Diabetic ketoacidosis and
hyperglycemic hyperosmolar state. Med Clin North Am. 101:587–606.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Olamoyegun M, Ibraheem W, Iwuala S, Audu M
and Kolawole B: Burden and pattern of micro vascular complications
in type 2 diabetes in a tertiary health institution in Nigeria. Afr
Health Sci. 15:1136–1141. 2015. View Article : Google Scholar : PubMed/NCBI
|