Introduction
Despite major medical advances in recent decades,
acute myocardial infarction (AMI) remains one of the leading causes
of morbidity and mortality worldwide (1). Acute heart failure (AHF) is a common
serious complication of AMI. Due to its high rate of morbidity,
mortality and readmission, as well as the associated costs, AHF
represents a major socioeconomic challenge (2). Therefore, accurate evaluation of
cardiac function in the early stage of AMI and early treatment for
AHF are crucial for improving the prognosis of patients.
An autoimmune response against the myocardium may
contribute to the pathogenesis of dilated cardiomyopathy (DCM),
heart failure, myocarditis, rheumatic fever, idiopathic recurrent
pericarditis and atherosclerosis (3,4).
Autoantibodies against β1-adrenoreceptors
(β1R-AAbs) are amongst the most important autoantibodies
to cardiovascular receptors and have been proven to be associated
with myocardial enlargement and cardiac dysfunction. It is
currently unclear which functional effects of β1R-AAbs
are damaging to the heart during the pathogenesis of HF. The
prevalence of stimulating β1R-AAbs in healthy
individuals was determined to be low (<1%) when using the
screening strategy described by Jahns et al (5). Under physiological conditions, the
majority of cardiac antigens remain hidden from the immune system,
at least within the cell. However, under pathological conditions,
the cardiac antigens are more exposed on the cell surface, which
stimulates the production of autoantibodies. Furthermore, these
autoantibodies directed against key elements on the cell surface,
particularly autoantibodies that bind to and stimulate cardiac
β1-adrenoreceptors (β1-AR), may have an
important role in the initiation and/or progression of myocardial
remodeling. In vitro, β1R-AAbs were indicated to
have a positive chronotropic and inotropic effect on cardiomyocytes
(6). In addition, Gao et al
(7) demonstrated that
β1R-AAbs promoted apoptosis in neonatal rat
cardiomyocytes. It was previously demonstrated that
β1R-AAbs have an important role in the
pathophysiological process of chronic heart failure (CHF),
including DCM and ischemic cardiomyopathy (ICM) (8). Furthermore, Pei et al (9) observed that the positive rate of
β1R-AAbs was higher in patients with chronic and
systolic heart failure (SHF), which may serve as an independent
prognostic factor for sudden cardiac death (SCD) in patients with
CHF, including those with DCM and ICM. In addition, it has been
reported that patients with DCM positive for β1R-AAbs
had a higher incidence of serious ventricular arrhythmias and a
higher incidence of SCD compared to antibody-negative patients
(10). However, other studies
reported no association between β1R-AAbs and the
prognosis of ICM (11,12). Furthermore, studies on the changes of
β1R-AAbs in patients with acute SHF and diastolic heart
failure (DHF) are scarce and the association between
β1R-AAbs and prognosis for AMI patients remains
elusive.
The aim of the present study was to observe the
changes in plasma β1R-AAbs in patients with acute SHF
and DHF, and explore the association between these autoantibodies
and the prognosis of patients following AMI. The results may be of
value for early diagnosis and improve the prognosis of patients
after AMI.
Materials and methods
Study design and population
The present study included 126 consecutive patients
with AMI who were admitted to Beijing Chaoyang Hospital, Capital
Medical University (Beijing, China) between July and December 2012.
According to the heart function after AMI, the patients were
divided into three groups: Patients with SHF (n=33), with DHF
(n=49) and with normal heart function (n=44) following AMI. The
diagnosis of AMI was confirmed by at least two independent
professional cardiologists according to the Third Universal
Definition of Myocardial Infarction (13). The diagnosis of DHF was based on the
presence of heart failure symptoms, with left ventricular ejection
fraction (LVEF) >40% and left ventricular end diastolic volume
index (LVEDVI) <97 ml/m2, which met at least one of
the following conditions: i) Peak early diastolic transmitral
velocity (E)/diastolic velocities (E′) >15; ii) 8<E/E′<15
and N-terminal pro-brain natriuretic peptide (NT-proBNP) >220
pg/ml; and iii) E/E′>8 with E/late diastolic transmitral
velocity (E/A)<0.5, in combination with left atrial volume index
(LAVI) ≥40 ml/m2 or left ventricular mass index ≥149
g/m2 for males and ≥122 g/m2 for females
(14). The definition of SHF was the
presence of HF symptoms, with a reduction of LVEF <40%,
according to current guidelines of the European Society of
Cardiology (15).
The criteria for exclusion included any of the
following conditions: i) Mechanical complications after AMI,
including free wall rupture, ventricular septal perforation and
papillary muscle rupture; ii) cardiogenic shock; iii)
cardiomyopathy and valvular heart disease; iv) severe hepatic and
renal dysfunction (alanine aminotransferase ≥3 times the upper
limit of normal and serum creatinine ≥3 mg/dl); v) terminal disease
(e.g., terminal cancer) with an estimated survival time of <1
year; and vi) poor echocardiographic imaging results.
Routine clinical assessment
All patients enrolled in the present study underwent
coronary angiography and 86 patients underwent percutaneous
coronary intervention successfully, while 8 patients underwent
coronary artery bypass grafting. All participants were subjected to
physical examinations and answered a standardized questionnaire to
assess their medical history, current illness and intake of any
medications. After being admitted to the hospital, all patients
received standard coronary secondary prevention (including aspirin,
clopidogrel, angiotensin-converting enzyme inhibitors/angiotensin
receptor blockers, β-blockers and statins, unless these agents were
contraindicated). All of the baseline information was carefully
recorded.
Measurement of
β1R-AAbs
Blood samples were collected from the antecubital
vein using tubes containing EDTA within 24 h after the patients
were admitted to the Cardiac Care Unit. Within 2 h of collection,
the samples were centrifuged at 2,000 × g at 4°C for 10 min. Plasma
samples were stored at −80°C for analysis.
After blood sample collection was completed in
December 2012, the β1R-AAbs were detected in the
patients' plasma using a synthetic peptide corresponding to the
sequence of the second extracellular loop of the human
β1 receptor (amino acid sequence number,
β1:197-222:H-W-W-R-A-E-S-D-E-A-R-R-C-Y-N-D-P-K-C-C-D-F-V-T-N-R)
by ELISA.
The peptide was synthesized using the Merrifield
solid-phase method by the Biological Institute of the Chinese
Academy of Medical Sciences & Peking Union Medical College. The
purity of the peptides was determined by high-pressure liquid
chromatography on the automatic amino acid analyzer (Beckman
Instruments, Inc.). The procedures of ELISA were performed as
previously described by Nagatomo et al (16). The corresponding curves were used to
measure the sensitivity and specificity of the ELISA, for positive
and negative samples. All of the samples were measured twice by
ELISA to ensure the reliability of the results. The intra- and
inter-assay coefficient of variation was no >5%. The optical
density (OD) values were measured using a microplate reader
(Molecular Devices LLC) and the positive rate was determined with
positive/negative [P/N=(sample OD-blank OD)/blank OD]≥2.1 according
to a widely used method (9,17,18).
Assessment of heart function
All of the patients underwent routine
echocardiographic examination within 48 h after admission with the
use of a ultrasound device (Vivid5; GE Healthcare). Standard
transthoracic echocardiography was based on the recommendations of
the American Society of Echocardiography (ASE) guidelines (19). All studies were performed by an
experienced sonographer and interpreted by an experienced
physician.
Left atrial diameter, left ventricular end-systolic
diameter, left ventricular end-diastolic diameter and posterior
wall thickness were measured using M-mode tracing. Two-dimensional
(2D) imaging was performed in standard parasternal, apical four-
and two-chamber views. LVEF, LAVI, LVEDVI and left ventricular
end-systolic volume were measured according to the biplane
Simpson's method and then indexed to body surface area. According
to the ASE formula (19), LVM was
calculated by 2D liner LV measurements.
E and A were determined by pulse wave Doppler
imaging. E and E′ of the septal wall at the level of the mitral
annulus in the apical 4C view were recorded by pulse wave tissue
Doppler. The E/A ratio and E/E′ ratio were calculated.
Follow-up and endpoint events
Each patient was assigned to a designated study
investigator and patients were followed up in the first and fifth
year, or until the primary endpoint after initiation of the study.
The patients were followed up via outpatient visits or telephone
between January 2013 and January 2018. The primary endpoint events
were a composite of MACEs, including AMI, stroke, rehospitalization
for HF and death. All of the endpoint events were reviewed by
members of an independent committee, who were unaware of the
contents of the study and used pre-specified criteria.
Statistical analysis
Values are expressed as the mean ± standard
deviation of continuous variables, while categorical variables are
expressed as numbers and percentages. Continuous variables were
compared with the Kruskal-Wallis test and Dunn's test was used as a
post-hoc test following the Kruskal-Wallis test for comparing
between two groups. Pearson's χ2 test was used for
categorical variables. Receiver operating characteristics (ROC)
curves were constructed to evaluate the sensitivity and specificity
of all detection methods and evaluate their ability to diagnose SHF
after AMI. The association between the risk of MACEs and the
cardiovascular risk factors was assessed by univariate and
multivariate logistic regression. The results are expressed as the
univariate odds ratio (OR) with 95% CI and the OR was then adjusted
for age, sex, hypertension, dyslipidemia, obesity, diabetes and
smoking to assess the interdependence of the autoantibodies and
traditional cardiovascular risk factors. The effects of these
predictors on incidence for MACEs over 5 years were analyzed using
Kaplan-Meier survival curves. All tests were two-tailed and
P<0.05 was considered to indicate a statistical significance.
All statistical analyses were performed using IBM SPSS Statistical
software for Windows 22.0 (IBM Corp.).
Results
Clinical, hemodynamic and medical
characteristics at baseline
The baseline characteristics of the patients of the
present study are provided in Table
I. The heart rate was faster in patients with SHF after AMI
compared with that in patients with DHF of normal heart function
after AMI (P<0.001). Renal function was worse in patients with
SHF compared with that in the other two groups (P<0.05).
Patients with SHF after AMI were more likely to have diabetes
(P=0.003). As expected, left ventricular function was more impaired
in patients with SHF. A higher level of NT-proBNP and a lower LVEF
value were present in patients with SHF (P<0.05). Regarding the
other characteristics, there were no differences in age, sex,
systolic blood pressure, hypertension, hyperlipidemia, smoking,
type of AMI, revascularization, cTnI and medication among the three
groups (P>0.05).
 | Table I.Baseline data of the study
population. |
Table I.
Baseline data of the study
population.
| Variable | Normal | DHF | SHF | P-value |
|---|
| Age (years) | 63.3±7.9 | 66.9±11.1 | 67.9±13.6 | 0.063 |
| Male sex | 34 (77.3) | 30 (61.2) | 22 (66.7) | 0.246 |
| BMI
(kg/m2) | 25.5±2.9 | 25.5±2.4 | 25.9±2.9 | 0.749 |
| Heart rate
(bpm) | 73.4±11.0 | 71.3±11.9 |
87.2±16.4a,b | <0.001 |
| SBP (mmHg) | 123.3±17.4 | 133.3±24.6 | 128.5±27.1 | 0.198 |
| Risk factors |
|
|
|
|
|
Hypertension | 23 (52.3) | 34 (69.4) | 25 (75.8) | 0.073 |
|
Hyperlipidemia | 13 (29.5) | 8 (16.3) | 9 (27.3) | 0.282 |
|
Diabetes | 4 (9.1) | 19 (38.8) | 12 (36.4) | 0.003 |
|
Smoking | 19 (43.2) | 21 (42.9) | 13 (39.4) | 0.936 |
| Type of AMI |
|
|
|
|
|
STEMI | 27 (61.4) | 30 (61.2) | 20 (60.6) | 0.362 |
|
Anterior | 15 | 19 | 13 |
|
|
Inferior | 4 | 3 | 2 |
|
|
Inferior+right ventricle | 6 | 5 | 3 |
|
|
Inferior+posterior | 3 | 3 | 2 |
|
|
NSTEMI | 17 (38.6) | 19 (38.8) | 13 (39.4) | 0.362 |
|
Revascularization |
|
|
|
|
|
PCI | 32 (72.7) | 35 (71.4) | 19 (57.6) | 0.306 |
|
CABG | 1 (2.3) | 3 (6.1) | 4 (12.1) | 0.214 |
| Medication |
|
|
|
|
|
Aspirin | 43 (97.7) | 48 (98.0) | 30 (90.9) | 0.070 |
|
β-blockers | 31 (70.5) | 32 (65.3) | 24 (72.7) | 0.752 |
|
ACEI/ARB | 19 (43.2) | 30 (61.2) | 18 (54.5) | 0.216 |
|
Statin | 37 (84.1) | 45 (91.8) | 27 (81.8) | 0.362 |
| Heart function |
|
|
|
|
| LVEF
(%) | 61.0±10.6 | 57.6±7.7 |
35.1±7.6a,b | <0.001 |
|
NT-ProBNP (mg/dl) | 160.3±149.8 |
1510.3±2028.5c |
4092.0±4250.5a,b | <0.001 |
| Blood
parameters |
|
|
|
|
| Cr
(µmol/l) | 83.2±14.7 | 93.2±36.8 |
104.9±41.8a | 0.014 |
| UA
(µmol/l) | 317.8±81.7 | 308.2±105.8 | 333.0±115.4 | 0.572 |
| cTnI
(ng/ml) | 21.7±36.0 | 35.5±71.9 | 45.8±67.6 | 0.468 |
β1R-AAbs for early
diagnosis of SHF after AMI
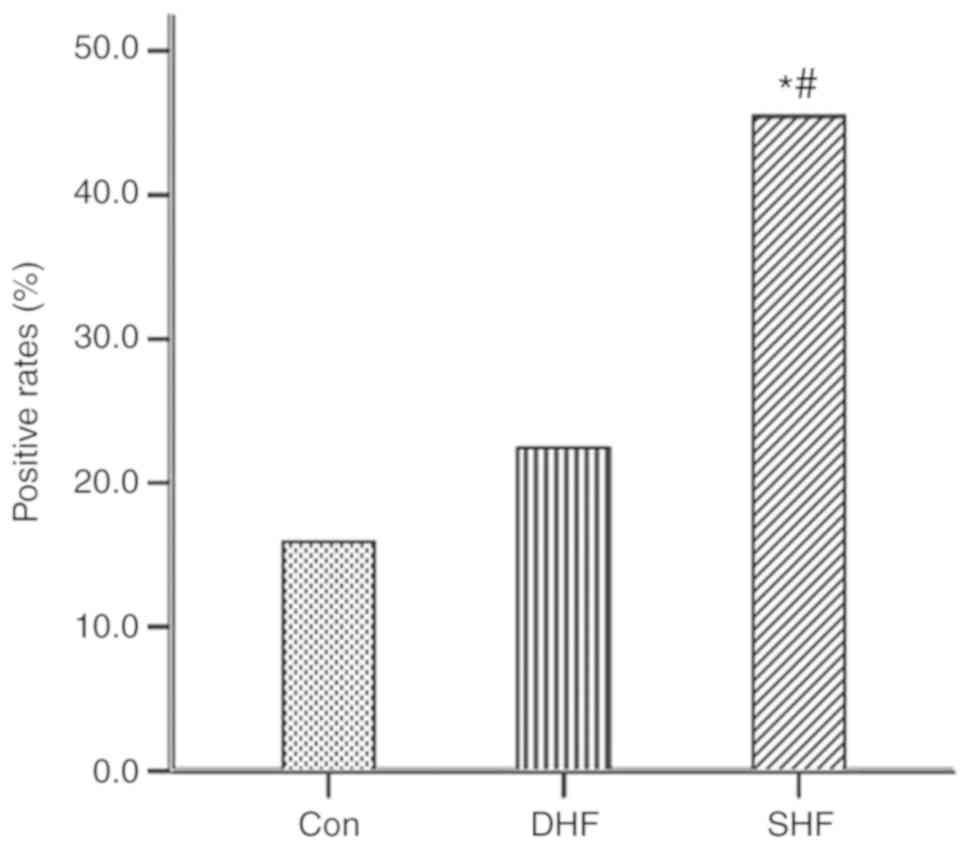
The positive rates of β1R-AAbs were 15/33
(45.5%) in the SHF group, 11/49 (22.4%) in the DHF group and 7/44
(15.9%) in the group with normal heart function after AMI. The
positive rates of β1R-AAbs were significantly higher in
patients with SHF compared with those in patients with other
diagnoses (P<0.05), but there was no significant difference
between the DHF and normal heart function groups (P>0.05), as
presented in Fig. 1.
Univariate and multivariate logistic regression was
used to assess whether β1R-AAbs acted synergistically
with other factors, including age, sex, hypertension, dyslipidemia,
obesity, diabetes and smoking. β1R-AAbs positivity was
significantly associated with an increased incidence of SHF after
AMI on univariate analysis (OR3.472; 95% CI: 1.474–8.179, P=0.004),
and this association was also statistically significant following
adjustment for the traditional cardiovascular risk factors
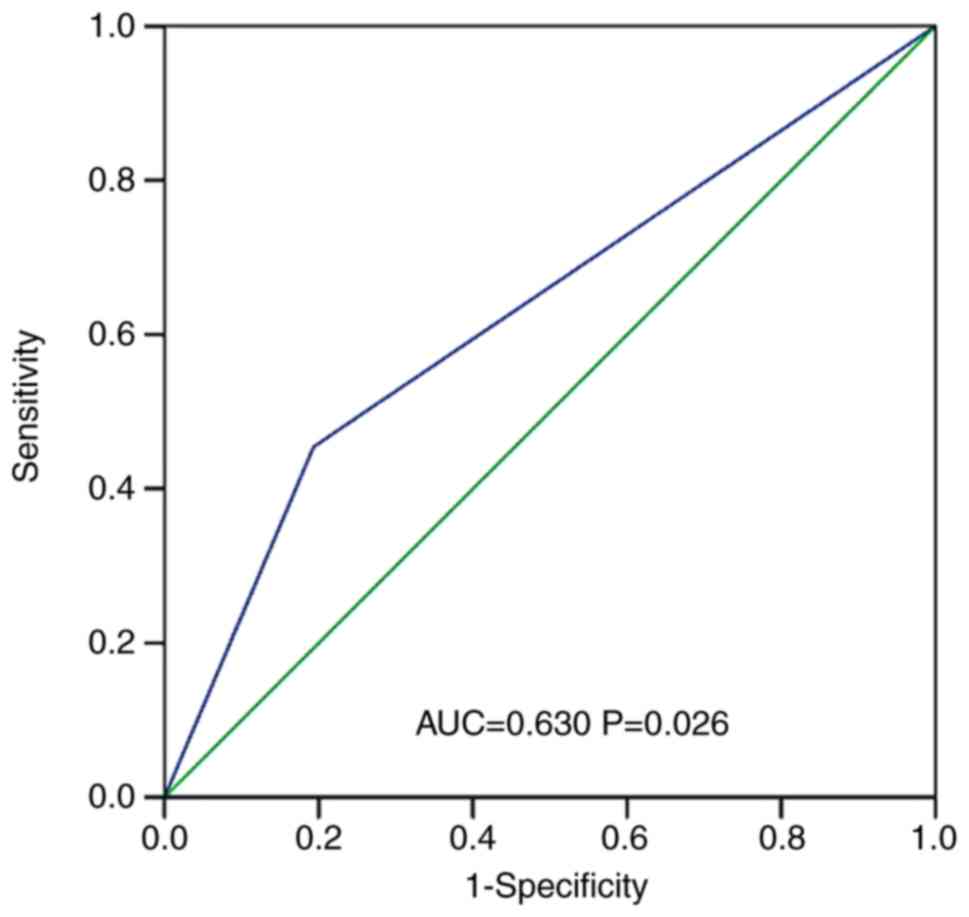
mentioned above (OR=4.791; 95% CI: 1.765–13.000, P=0.002). ROC
curve analyses indicated that β1R-AAbs exhibited good
accuracy for the diagnosis of SHF after AMI, with an area under the
curve of 0.630 (95% CI: 0.514–0.747, P=0.026), as presented in
Fig. 2.
Prognostic value of
β1R-AAbs for MACEs during 5-year follow-up
During the mean follow-up period of 51.0±15.4
months, 4/126 (3.2%) patients were lost in the fifth year. MACEs
were observed in 19/30 β1R-AAbs-positive patients
(63.3%) and in 38/92 β1R-AAbs-negative patients (41.3%);
the difference between these two groups was statistically
significant (P=0.036). On univariate analysis, antibody-positive
status was a predictive factor for MACEs in patients following AMI
(OR=2.455; 95% CI: 1.048–5.747, P=0.039). The univariately
predictive variables for MACEs of patients with different heart
function after AMI are provided in Table II. On multivariate logistic
regression analysis, LVEF and diabetes were identified as
independent predictors of 5-year primary endpoints following AMI,
but β1R-AAbs-positive status was not an independent
predictive factor, as presented in Table II.
 | Table II.Univariate and multivariate
predictors of 5-year major adverse cardiac events. |
Table II.
Univariate and multivariate
predictors of 5-year major adverse cardiac events.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
|
β1R-AAbs | 2.455 | 1.048–5.747 | 0.039 | 0.877 | 0.248–3.094 | 0.838 |
| Heart rate
(bpm) | 1.027 | 1.001–1.054 | 0.042 | 0.987 | 0.950–1.206 | 0.509 |
| Diabetes | 2.776 | 1.219–6.321 | 0.015 | 2.641 | 1.105–6.311 | 0.029 |
| LVEF (%) | 0.950 | 0.921–0.979 | 0.001 | 0.951 | 0.922–0.981 | 0.001 |
| NT-proBNP
(mg/dl) | 1.000 | 1.000–1.001 | 0.005 | 1.036 | 0.984–1.022 | 0.150 |
| Cr (µmol/l) | 1.016 | 1.003–1.028 | 0.015 | 0.996 | 0.978–1.015 | 0.706 |
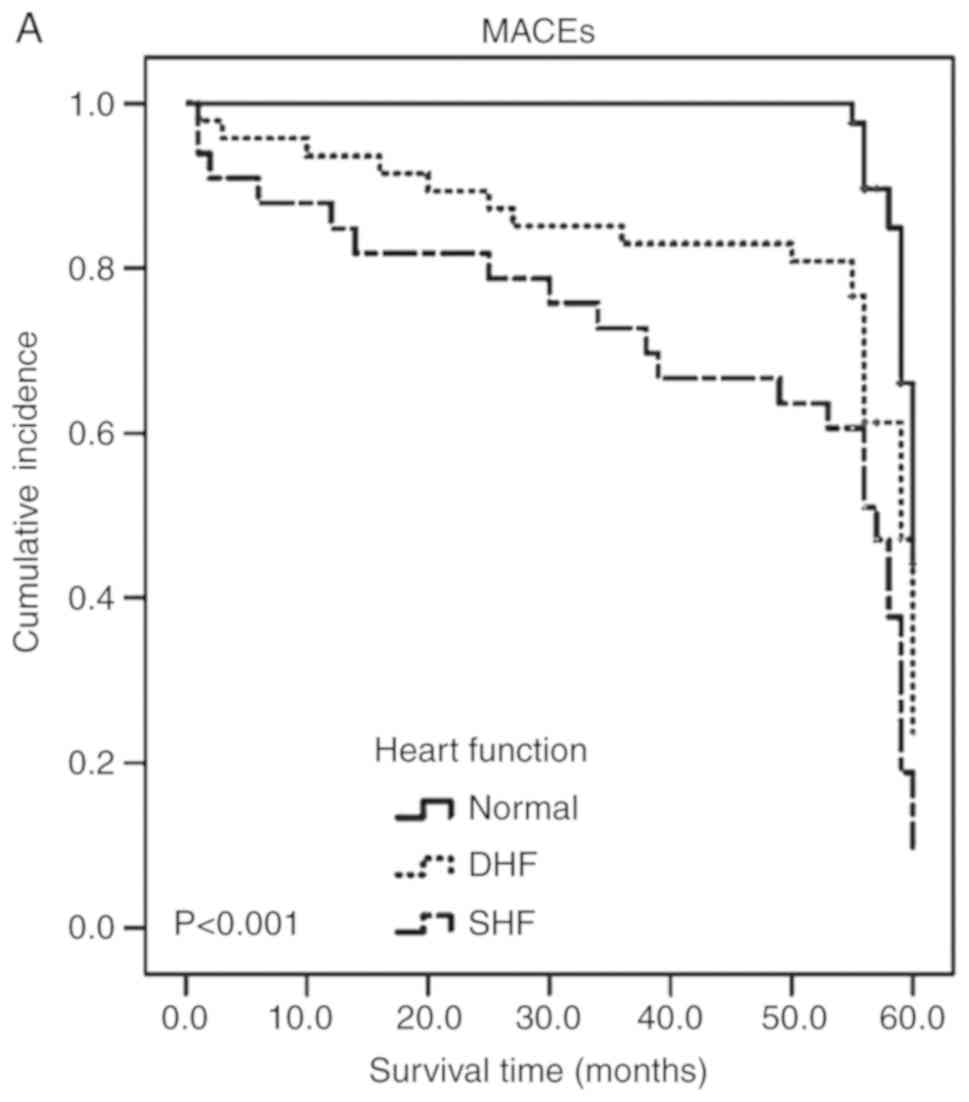
The adjusted Kaplan-Meier curves for MACEs in
patients following AMI are depicted in Fig. 3. Patients with SHF had the worst
5-year prognosis among the three groups (P<0.001; Fig. 3A). The 5-year prognosis was
significantly worse in patients with diabetes compared with that in
patients without diabetes (P<0.05; Fig. 3B). No significant difference was
observed in the 5-year prognosis between the antibody-positive and
-negative groups (P>0.05; Fig.
3C).
Discussion
The present single-center prospective study
evaluated the diagnostic and prognostic value of autoantibodies
that bind to and stimulate the human β1-AR in acute SHF,
DHF and normal heart function patients following AMI. The most
important results were as follows: First, the positivity rates of
β1R-AAbs were higher in patients with SHF after AMI
compared with those in patients with DHF and normal heart function
after AMI, but there was no difference between the DHF and normal
groups. Furthermore, β1R-AAbs may be a factor for
identifying patients with acute SHF after AMI. In addition,
positivity for β1R-AAbs appeared to be associated with
MACEs, but was not an independent predictor for the prognosis of
AMI patients, while a decreased LVEF and diabetes were determined
to be independent predictors.
Over the past 30 years, the autoimmune mechanisms
involved in cardiovascular disease have attracted extensive
attention. In 1989, Limas et al (20) discovered a substance in the serum of
patients with DCM that was able to inhibit ligand binding to a β-AR
distributed on myocardial cells of rats, which was suspected to be
an antibody. This substance was later proven to be a
β1R-AAb. β1R-AAbs have been reported to be a
common target for CHF caused by several autoantibody-associated
diseases, including DCM, Chagas disease and atrial fibrillation
(21–23). Previous studies by our group
indicated that, in patients with CHF arising from different causes,
the positive rates of β1R-AAbs were all significantly
higher compared with those of healthy subjects and were associated
with the severity of CHF (24,25). In
an animal study, Matsui et al (26) used synthetic peptides corresponding
to the sequence of the second extracellular loop of either the
human β1R or M2R to immunize rabbits monthly
for 1 year, and these peptides induced a marked enlargement of the
ventricles with thinning of the walls, identical to the changes of
DCM in humans. These results suggested that the levels of
β1R-AAbs did not depend on the diseases leading to HF,
but rather on the pathogenesis of HF itself. Based on the
above-mentioned results, the changes of β1R-AAbs in
patients with acute SHF and DHF were investigated by our group,
which have been rarely reported to date, to the best of our
knowledge. The mechanism of β1R-AAbs induction is
currently unknown, but it may either be due to the molecular
mimicry between AR and specific antigens or induced by exposure of
autoantigens to the immune system (27). Furthermore, it is unknown whether
certain patients have immune disorders or genetic defects that make
them more likely to produce β1R-AAbs, all of which
require further investigation.
Previous studies on β1R-AAbs have focused
on patients with chronic SHF (11,28,29), but
this has remained to be assessed in acute SHF, to the best of our
knowledge. The present study demonstrated that the positive rates
of β1R-AAbs were higher in patients with SHF following
AMI compared with those with DHF and normal heart function, while
there was no difference between the DHF and normal function groups.
In the present study, systolic cardiac insufficiency after AMI was
considered as acute cardiac insufficiency, characterized by the
decrease in LVEF due to AMI. The results suggested that
β1R-AAbs may participate in the pathogenesis of acute
SHF and cause myocardial damage and decreased heart function.
However, this is a hypothesis and it is not clear whether the
autoantibodies in heart disease are due to an improper autoimmune
response following heart injury, or if there is an increase in
primary autoantibodies without obvious cause or damage to the
heart. To the best of our knowledge, the present study was the
first to demonstrate that the presence of stimulating
β1R-AAbs affects the development of acute SHF.
Previous studies on β1R-AAbs mainly
focused on chronic SHF, but there are no reports on
β1R-AAbs in DHF during the early stages of AMI. In the
present study, the level of β1R-AAbs in patients with
DHF in the early stage of AMI was examined. The results
demonstrated that the rate of β1R-AAbs positivity in the
DHF group was not significantly different from that in the normal
heart function group, suggesting that β1R-AAbs were not
involved in the occurrence and development of DHF. The reason may
be that DHF is a compensatory condition in which the left ventricle
may increase in size to obtain normal ventricular filling and
cardiac volume. Its characteristics are decreased left ventricular
volume and increased end-diastolic pressure, with normal or
slightly decreased LVEF (30).
β1R-AAbs appear to mainly affect the myocardial
structure and function, leading to a decrease in, rather than just
pressure changes.
The mechanisms by which β1R-AAbs affect
cardiomyocytes, resulting in adverse cellular effects, are complex
and remain to be fully elucidated. Low concentrations of
β1R-AAbs were also determined in healthy subjects as a
product of natural immunity. Under pathological conditions,
functional β1-ARs are easily accessible targets
localized on the cell surface and the harmful potential of
autoantibodies depends on the significance of their targeting
function (31). β1R-AAbs
have been indicated to activate adenylate cyclase and then
moderately elevate second messenger cyclic AMP (32). In addition, it was observed that
β1R-AAbs, similar to isoproterenol receptor agonists,
activate protein kinase A to phosphorylate several phosphoproteins
in the cells (33). Prolonged
overstimulation of β1-ARs may lead to deterioration in
heart function and the underlying mechanism is considered to be the
induction of apoptosis by T-lymphocytes (34). β1R-AAbs exerted
pro-apoptotic effects with increased generation of phenyl glycidyl
ether. Comparatively, xamoterol is a true β1-AR agonist,
mimicking the effects of autoantibodies on atrial apoptosis in rats
(35). Furthermore,
β1R-AAbs may prolong the action potential duration and
increase the L-type Ca2+ current from the extracellular
compartment to the cytosol (36).
Ca2+ overload may be induced by permanent
β1-AR stimulation and a continuous receptor-associated
signaling cascade, leading to cell apoptosis and death (37). The above-mentioned processes are
biologically plausible mechanisms by which β1R-AAbs may
lead to acute SHF following AMI. β1R-AAbs may exert an
‘agonist activity’ on their target receptors, leading to myocardial
damage and cardiac dysfunction.
The prognostic value of β1R-AAbs was also
assessed, particularly for DCM. Störk et al (11) indicated that stimulation of
β1R-AAbs was an independent risk factor for all-cause
and cardiovascular mortality risk in DCM over a follow-up period of
>10 years. In line with this, another study reported that higher
levels of β1R-AAbs had a negative impact on the
prognosis of patients with DCM (38), as they were associated with a higher
risk of ventricular arrhythmias and SCD. It has also been reported
that the survival rate of patients with CHF significantly
deteriorated if β1R-AAbs >10 U/ml (39). Although β1R-AAbs are more
common in patients with DCM, they may also be detected in patients
with ICM. By contrast, they were not associated with the prognosis
of patients with ICM. In the present study, lower LVEF and the
presence of diabetes in patients following AMI were determined to
be independent predictors of 5-year prognosis, but
β1R-AAbs were not independent predictors for MACEs
during the 5-year follow-up of these patients. In addition, for the
40 patients with ICM (5 of whom tested positive for stimulated
β1R-AAbs), the presence of stimulated
β1R-AAbs was not associated with an increased risk of
all-cause mortality or cardiovascular-associated mortality during a
10-year follow-up (11), which was
expected, as the prognosis of patients with ICM may be affected by
other factors, including LVEF, rather than immunological risk
factors. However, since the number of patients in the present study
was small, it cannot be excluded with certainty that
β1R-AAbs do not influence the prognosis in ICM.
Several limitations should be taken into account
when interpreting the results of the present study. First, as in
all case-control studies, there was a possibility of selection
bias. Furthermore, the results may be biased, as the sample size of
the present study was small. An analysis with a larger sample size
is required to confirm the present results. In addition, while the
association between β1R-AAbs and SHF after AMI was
biologically reasonable, it should be pointed out that the
association was not necessarily causal. Further studies are
required to elucidate the causal role of these autoantibodies in
SHF. In addition, using β1R-AAbs alone as a diagnostic
factor may be not accurate. In the future, β1R-AAbs will
be combined with other diagnostic factors to further
comprehensively predict the occurrence of HF after AMI. Finally,
only serum β1R-AAbs were detected. Further studies on
the biological activities of autoantibodies, as well as their
receptors, in the plasma and myocardium are required.
In conclusion, the present study indicated that
levels of β1R-AAbs were significantly increased in
patients with acute SHF after AMI. However, the presence of
β1R-AAbs was not an independent prognostic factor of
MACEs in patients following AMI. In conclusion, β1R-AAbs
may be of value for early diagnostic of SHF in patients after AMI,
but they are not independent prognostic factors in such
patients.
Acknowledgements
The authors would like to express their appreciation
to Professor Xinchun Yang (Heart Center, Beijing Chaoyang Hospital)
for his valuable advice and assistance.
Funding
The present study was supported by grants from the
Natural Science Foundation of China (grant no. 81370340), the Basic
and Clinical Research Cooperative project of Capital Medical
University (grant no. 15JL04) and the Natural Science Foundation of
Capital Medical University (grant no. PYZ2018109).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding authors on reasonable
request.
Authors' contributions
XW was responsible for drafting the manuscript and
revising it critically for important intellectual content, and made
substantial contributions to analysis of data. MH and SH were
responsible for performing the study and the data analysis. YZ and
XX were responsible for collection of patient and laboratory data,
data interpretation and revision of the manuscript regarding
content. YW and CD performed the ELISA for β1R-AAbs. JZ
and HW performed the echocardiographic examination. JL and DH were
responsible for patient follow-up. MC and LZ conceived and designed
the study and were responsible for performing data collection,
analysis and interpretation. WZ and LX have substantial
contributions to conception and design of our study, analysis and
interpretation of data, and giving final approval of the version to
be published. All authors read and approved the final version of
the manuscript for publication.
Ethics approval and consent to
participate
The present study was performed in compliance with
the principles outlined in the Declaration of Helsinki and was
approved by the Ethics Committee and the Prescription and
Therapeutic Committee of Beijing Chao-Yang Hospital, Capital
Medical University (Beijing, China). All the patients provided
written informed consent prior to enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tang X, Liu P, Li R, Jing Q, Lv J, Liu L
and Liu Y: Milrinone for the treatment of acute heart failure after
acute myocardial infarction: A systematic review and meta-analysis.
Basic Clin Pharmacol Toxicol. 117:186–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Hsieh AF, Dharmarajan K, Masoudi
FA and Krumholz HM: National trends in heart failure
hospitalization after acute myocardial infarction for medicare
beneficiaries: 1998–2010. Circulation. 128:2577–2584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel PA and Hernandez AF: Targeting
anti-beta-1-adrenergic receptor antibodies for dilated
cardiomyopathy. Eur J Heart Fail. 15:724–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Generali E, Folci M, Selmi C and Riboldi
P: Immune-mediated heart disease. Adv Exp Med Biol. 1003:145–171.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jahns R, Boivin V, Siegmund C, Inselmann
G, Lohse MJ and Boege F: Autoantibodies activating human beta
1-adrenergic receptors are associated with reduced cardiac function
in chronic heart failure. Circulation. 99:649–654. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mobini R, Staudt A, Felix SB, Baumann G,
Wallukat G, Deinum J, Svensson H, Hjalmarson A and Fu M:
Hemodynamic improvement and removal of autoantibodies against
beta1-adrenergic receptor by immunoadsorption therapy in dilated
cardiomyopathy. J Autoimmun. 20:345–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao Y, Liu HR, Zhao RR and Zhi JM:
Autoantibody against cardiac beta1-adrenoceptor induces apoptosis
in cultured neonatal rat cardiomyocytes. Acta Biochim Biophys Sin
(Shanghai). 38:443–449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nussinovitch U and Shoenfeld Y: The
clinical significance of anti-beta-1 adrenergic receptor
autoantibodies in cardiac disease. Clin Rev Allergy Immunol.
44:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pei J, Li N, Chen J, Li X, Zhang Y, Wang
Z, Zhang P, Cao K and Pu J: The predictive values of
beta1-adrenergic and M2 muscarinic receptor autoantibodies for
sudden cardiac death in patients with chronic heart failure. Eur J
Heart Fail. 14:887–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwata M, Yoshikawa T, Baba A, Anzai T,
Mitamura H and Ogawa S: Autoantibodies against the second
extracellular loop of beta1-adrenergic receptors predict
ventricular tachycardia and sudden death in patients with
idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 37:418–424.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Störk S, Boivin V, Horf R, Hein L, Lohse
MJ, Angermann CE and Jahns R: Stimulating autoantibodies directed
against the cardiac beta1-adrenergic receptor predict increased
mortality in idiopathic cardiomyopathy. Am Heart J. 152:697–704.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nussinovitch U and Shoenfeld Y:
Intravenous immunoglobulin-indications and mechanisms in
cardiovascular diseases. Autoimmun Rev. 7:445–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thygesen K, Alpert JS, Jaffe AS, Simoons
ML, Chaitman BR, White HD; Writing Group on the Joint
ESC/ACCF/AHA/WHF Task Force for the Universal Definition of
Myocardial Infarction, ; Thygesen K, Alpert JS, White HD, et al:
Third universal definition of myocardial infarction. Eur Heart J.
33:2551–2567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paulus WJ, Tschöpe C, Sanderson JE,
Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De
Keulenaer G, Leite-Moreira AF, et al: How to diagnose diastolic
heart failure: A consensus statement on the diagnosis of heart
failure with normal left ventricular ejection fraction by the heart
failure and echocardiography associations of the European society
of cardiology. Eur Heart J. 28:2539–2550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dickstein K, Cohen-Solal A, Filippatos G,
McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van
Veldhuisen DJ, Atar D, Hoes AW, et al: ESC guidelines for the
diagnosis and treatment of acute and chronic heart failure 2008:
The Task Force for the diagnosis and treatment of acute and chronic
heart failure 2008 of the European society of cardiology. Developed
in collaboration with the heart failure association of the ESC
(HFA) and endorsed by the European society of intensive care
medicine (ESICM). Eur J Heart Fail. 10:933–989. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagatomo Y, Yoshikawa T, Kohno T,
Yoshizawa A, Baba A, Anzai T, Meguro T, Satoh T and Ogawa S: A
pilot study on the role of autoantibody targeting the
beta1-adrenergic receptor in the response to beta-blocker therapy
for congestive heart failure. J Card Fail. 15:224–232. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Segovia M, Ganzinelli S, Reina S, Borda E
and Sterin-Borda L: Role of anti-β1 adrenergic antibodies from
patients with periodontitis in cardiac dysfunction. J Oral Pathol
Med. 41:242–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Wang Y, Chen M, Zhao W, Wang X,
Wang H, Zhang Z, Zhang J, Xu L, Chen J, et al: The correlation
between peripartum cardiomyopathy and autoantibodies against
cardiovascular receptors. PLoS One. 9:e867702014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
American College of Cardiology Foundation
Appropriate Use Criteria Task Force: Society for Cardiovascular
Magnetic Resonance, ; Douglas PS, Garcia MJ, Haines DE, Lai WW,
Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Ward RP and
Weiner RB: ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/ SCCM/SCCT/SCMR 2011
appropriate use criteria for echocardiography. A report of the
American college of cardiology foundation appropriate use criteria
task force, American society of echocardiography, American heart
association, American society of nuclear cardiology, heart failure
society of America, heart rhythm society, society for
cardiovascular angiography and interventions, society of critical
care medicine, society of cardiovascular computed tomography, and
society for cardiovascular magnetic resonance endorsed by the
American college of chest physicians. J Am Coll Cardiol.
57:1126–1166. 2011.PubMed/NCBI
|
|
20
|
Limas CJ, Goldenberg IF and Limas C:
Autoantibodies against beta-adrenoceptors in human idiopathic
dilated cardiomyopathy. Circ Res. 64:97–103. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bornholz B, Hanzen B, Reinke Y, Felix SB,
Jahns R, Schimke I, Wallukat G and Boege F: Detection of
DCM-associated β1-adrenergic receptor autoantibodies requires
functional readouts or native human β1-receptors as targets. Int J
Cardiol. 202:728–730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cabral-Marques O and Riemekasten G:
Functional autoantibodies targeting G protein-coupled receptors in
rheumatic diseases. Nat Rev Rheumatol. 13:648–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yalcin MU, Gurses KM, Kocyigit D, Kesikli
SA, Ates AH, Evranos B, Yorgun H, Sahiner ML, Kaya EB, Oto MA, et
al: Elevated M2-muscarinic and β1-adrenergic receptor autoantibody
levels are associated with paroxysmal atrial fibrillation. Clin Res
Cardiol. 104:226–233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Hu D, Li J, Wu Y, Liu X and Yang
X: Autoantibodies against the myocardial beta1-adrenergic and
M2-muscarinic receptors in patients with congestive heart failure.
Chin Med J (Eng1). 115:1127–1131. 2002.
|
|
25
|
Zhang L, Hu D, Shi X, Li J, Zeng W, Xu L
and Cui L: Autoantibodies against the myocardium beta 1-adrenergic
and M2-muscarinic receptors in patients with heart failure.
Zhonghua Nei Ke Za Zhi. 40:445–447. 2001.(In Chinese). PubMed/NCBI
|
|
26
|
Matsui S, Fu ML, Katsuda S, Hayase M,
Yamaguchi N, Teraoka K, Kurihara T, Takekoshi N, Murakami E,
Hoebeke J and Hjalmarson A: Peptides derived from cardiovascular
G-protein-coupled receptors induce morphological cardiomyopathic
changes in immunized rabbits. J Mol Cell Cardiol. 29:641–655. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Becker NP, Goettel P, Mueller J, Wallukat
G and Schimke I: Functional autoantibody diseases: Basics and
treatment related to cardiomyopathies. Front Biosci (Landmark Ed).
24:48–95. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wallukat G, Nissen E, Morwinski R and
Müeller J: Autoantibodies against the beta- and muscarinic
receptors in cardiomyopathy. Herz. 25:261–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagatomo Y, McNamara DM, Alexis JD, Cooper
LT, Dec GW, Pauly DF, Sheppard R, Starling RC and Tang WH; IMAC-2
Investigators, : Myocardial recovery in patients with systolic
heart failure and autoantibodies against β1-adrenergic receptors. J
Am Coll Cardiol. 69:968–977. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gaasch WH: Deliberations on diastolic
heart failure. Am J Cardiol. 119:138–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bornholz B, Roggenbuck D, Jahns R and
Boege F: Diagnostic and therapeutic aspects of β1-adrenergic
receptor autoantibodies in human heart disease. Autoimmun Rev.
13:954–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du Y, Yan L, Wang J, Zhan W, Song K, Han
X, Li X, Cao J and Liu H: β1-Adrenoceptor autoantibodies from DCM
patients enhance the proliferation of T lymphocytes through the
β1-AR/cAMP/PKA and p38 MAPK pathways. PLoS One. 7:e529112012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du Y, Yan L, Du H, Wang L, Ding F, Quan L,
Cheng X, Song K and Liu H: β1-adrenergic receptor autoantibodies
from heart failure patients enhanced TNF-α secretion in RAW264.7
macrophages in a largely PKA-dependent fashion. J Cell Biochem.
113:3218–3228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du Y, Li X, Yu H, Yan L, Lau WB, Zhang S,
Qin Y, Wang W, Ma X, Liu H and Fu M: Activation of T lymphocytes as
a novel mechanism in Beta1-Adrenergic receptor autoantibody-induced
cardiac remodeling. Cardiovasc Drugs Ther. 33:149–161. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reina S, Ganzinelli S, Sterin-Borda L and
Borda E: Pro-apoptotic effect of anti-β1-adrenergic receptor
antibodies in periodontitis patients. Int Immunopharmacol.
14:710–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zuo L, Du Y, Ma J, Wang K, Zhao Y, Bai F,
Wu B, Ma X and Liu H: Pro-arrhythmic action of autoantibodies
against the second extracellular loop of β1-adrenoceptor and its
underlying molecular mechanisms. Int J Cardiol. 198:251–258. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wallukat G and Schimke I: Agonistic
autoantibodies directed against G-protein-coupled receptors and
their relationship to cardiovascular diseases. Semin Immunopathol.
36:351–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lazzerini PE, Capecchi PL, Guideri F,
Acampa M, Selvi E, Bisogno S, Galeazzi M and Laghi-Pasini F:
Autoantibody-mediated cardiac arrhythmias: Mechanisms and clinical
implications. Basic Res Cardiol. 103:1–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aso S, Yazaki Y, Kasai H, Takahashi M,
Yoshio T, Yamamoto K and Ikeda U: Anti-beta 1-adrenoreceptor
autoantibodies and myocardial sympathetic nerve activity in chronic
heart failure. Int J Cardiol. 131:240–245. 2009. View Article : Google Scholar : PubMed/NCBI
|