Introduction
Natural defence compounds, such as the antibiotic
substance, allicin (diallylthiosulfinate), are produced by garlic
tissues when the cells are damaged (1–3). Allicin
can be easily synthesized in the laboratory by an oxidation of
diallyl disulphide (DADS) (4). The
substrate for allicin biosynthesis is S-allyl-L-cysteine
sulfoxide, an odourless non-toxic non-protein amino acid found in
the cytosol of garlic cells (5). The
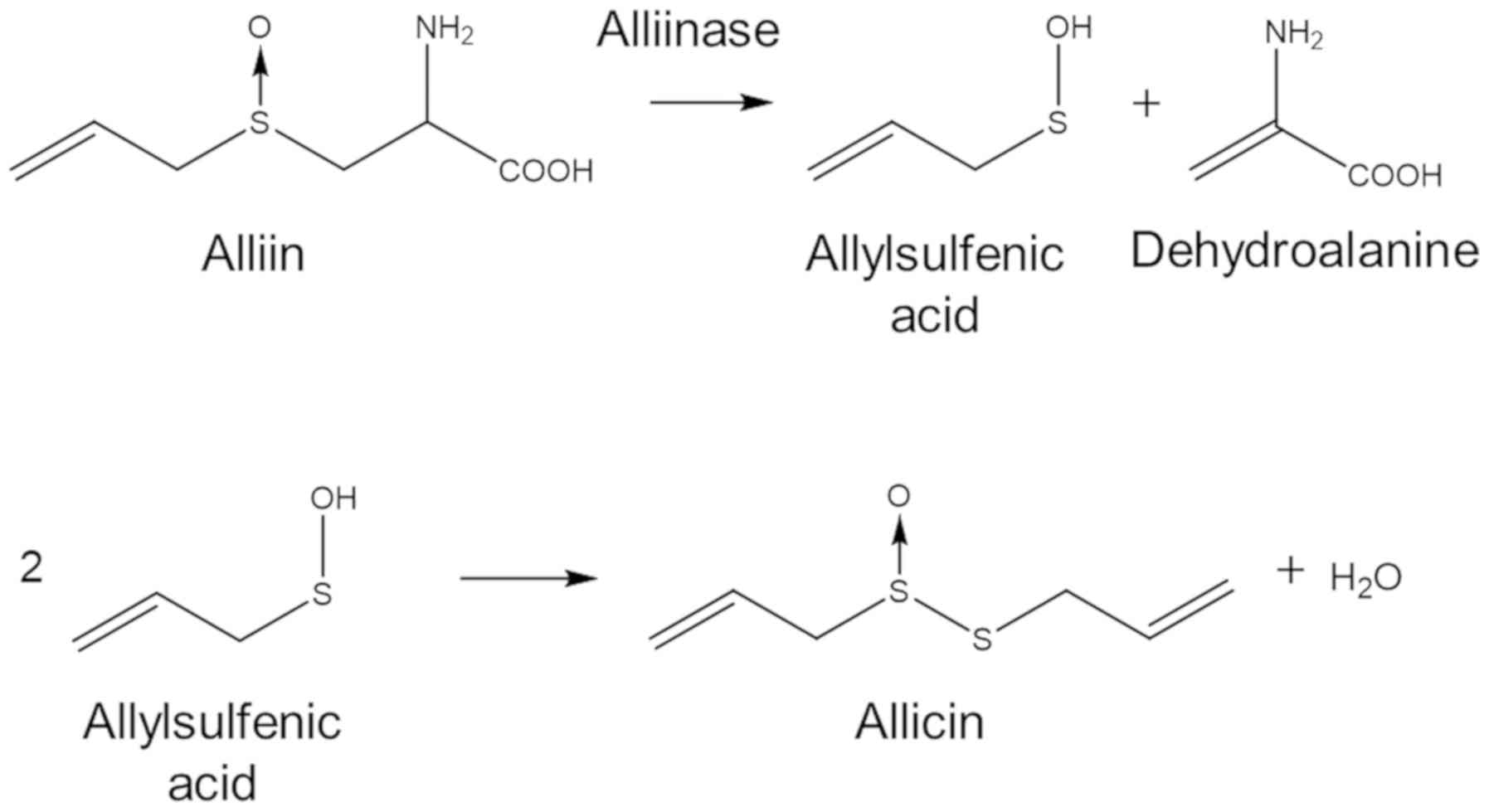
enzyme, alliin lyase (alliinase, E.C.4.4.1.4), is present in the
cell vacuole and hydrolyses alliin to dehydroalanine and
allylsulfenic acid. Two molecules of allylsulfenic acid condense
spontaneously to produce one molecule of allicin (Fig. 1).
Allicin crosses cell membranes easily and reacts
with thiol groups in glutathione (GSH) and accessible cysteines in
proteins to yield S-thioallylmercapto derivatives (6–10).
Allicin has been shown to have pronounced antimicrobial activities
against multiple drug-resistant (MDR) human lung pathogens
(11). The effects of allicin on
bacterial proteins have been well documented (10,12) and
a recent study identified a series of proteins
S-thioallylated by allicin in human cells in culture
(13). However, as allicin is
rapidly titrated out by GSH and metabolized in the human body, the
bioavailability of allicin per se following consumption is
generally low (14). Thus, any
attempts to use allicin to combat microbial infections encounter
the obstacle of achieving therapeutic concentration at the site of
infection, particularly if allicin is administered orally or is
injected into the blood (15,16). The
direct inhalation of allicin vapour or aerosols potentially offers
an immediate route for the treatment of lung pathogens which would
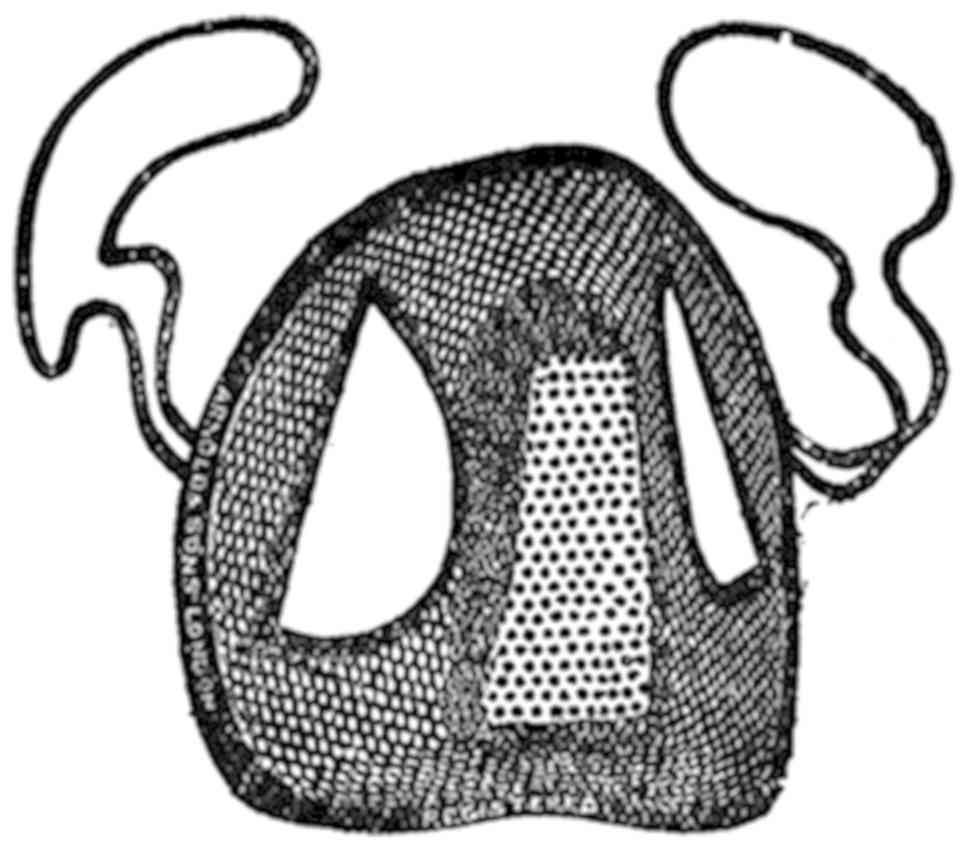
avoid these drawbacks. Indeed, there is a historical precedent from
the pre-streptomycin era reporting a high success rate for treating
tuberculosis in patients by inhaling the vapour from crushed garlic
pulp held in a specially designed inhaler (17) (Fig.
2). An enlightening summary of these studies has been reported
by Block (2). Moreover, allicin has
been shown to have antimicrobial activity against several human
lung pathogenic bacteria (11). The
development of allicin-inspired fluoroquinolones, such as
antibacterials against Enterococcus faecium, Staphylococcus
aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas
aeruginosa and Enterobacter spp. (ESKAPE) pathogens was
also recently reported (18).
The emergence of bacteria exhibiting multiple
resistance to antibiotics in current clinical use is an increasing
threat to the effective treatment of infectious diseases.
Frequently, resistance emerges rapidly following the discovery of
an antibiotic, particularly after its introduction into clinical
practice (19,20). There have been few new classes of
antibiotics discovered since the 1970s and novel antibiotics are
desperately required (21,22). MDR strains of lung pathogenic
bacteria, such as Pseudomonas aeruginosa, Streptococcus
pneumoniae, Staphylococcus aureus, Acinetobacter baumannii and
Mycobacterium tuberculosis have been reported and lung
infections are becoming increasingly difficult to treat (23–27). New
categories of antibiotics with multiple sites of action, such as
allicin, are particularly desirable as they are likely to render
the emergence of resistance more difficult.
Any novel antibiotic compounds require testing in
pre-clinical trials, which out of necessity, involve costly and
extensive animal studies. It is desirable to know as much as
possible about the behaviour of a test substance before progressing
to animal trials and in this regard, in this study, we designed and
constructed a test rig that accurately recapitulates the air-flow
through the life-size 2nd, 3rd, 4th and 5th bronchi of a human lung
in order to help characterize the behaviour of inhaled antibiotics.
The detailed construction and aerodynamic characteristics of
air-flow in this model have been previously reported (28). Herein, we report that using this
model, it is possible to demonstrate the dose-dependent efficacy of
gentamicin aerosols, and allicin aerosols and vapour. Moreover,
differences in the susceptibility of bacteria to the antibiotics
were demonstrated in the model and synergism for the inhibitory
effect to bacteria was revealed between ethanol and allicin in the
gas phase. Therefore, initial proof of principle for using the lung
model to predict the behaviour of antibiotics supplied to the lungs
has been shown, and it seems that some clinically relevant
questions can be addressed using the test rig prior to clinical
trials, which will reduce the need for some animal experiments.
Materials and methods
Allicin synthesis and application
Allicin was synthesized by the oxidation of DADS
with H2O2 as described previously (4).
Bacteria
The E. coli strain, MegaX DH10B T1R
(Invitrogen/Thermo Fisher Scientific), was transformed with either
the empty vector pRU1097 (=allicin-susceptible E. coli
strain) or with pRU1097 containing a 9 kb allicin
resistance-conferring genomic clone from an allicin-resistant
Pseudomonas fluorescens isolated from a garlic bulb
(=allicin-resistant E. coli strain) (29). As the pRU1097 vector (30) carries a gentamicin resistance gene as
a selectable marker, both transformant lines have high gentamicin
resistance.
Lung model
The construction details of the lung model have been
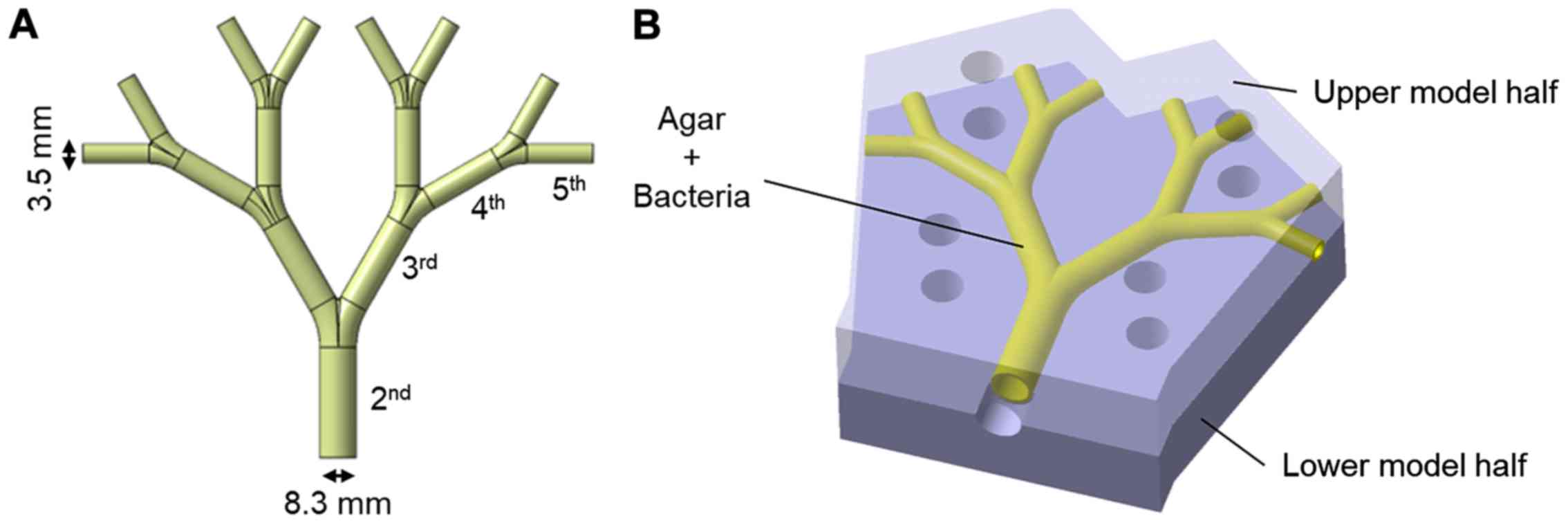
previously reported (28). The lung
model represents the 2nd to 5th generation (the trachea=0th
generation) of average life-size bronchial passages in a human lung
(Fig. 3A). The model is
approximately 10×10 cm. The inner coating of agar medium simulates
the epithelial surface of the bronchi and allows for the
incorporation of bacteria to simulate an infected lung. In the
agar-coated model, the internal diameter of the initial bronchus is
8.3 mm and the internal diameter of the 5th bronchus is 3.5 mm. The
agar-coated bronchi were prepared in two halves, which were
subsequently positioned together with the help of locating pins and
screw clamps to make the bronchial tubes.
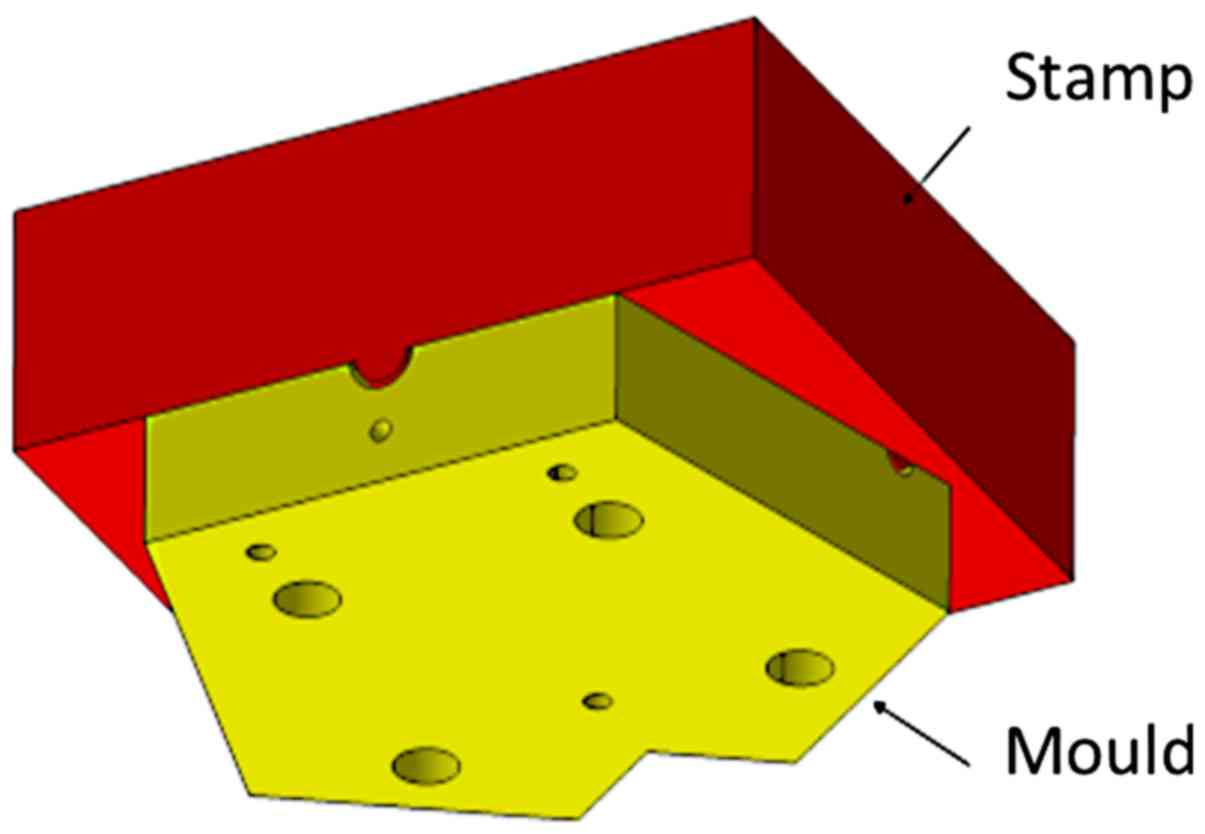
The casting process of the bronchial-surface coating
of bacteria-seeded agar was performed using a negative mould with a
positive stamp with an offset of 1 mm (Fig. 4). The mould and stamp were turned
from blocks of polyoxymethylene (POM) with a milling machine.
Before the agar surface was cast, the surfaces were sterilized by
UV light under a clean bench (Labgard® Class II
NU-437-500E, NuAir) for 20 min. The Luria-Bertani (LB) medium with
the addition of 1.5% (w/v) agar (Agar-Agar Kobe I, powdered, for
microbiology, Carl Roth) was autoclaved for 20 min at 121°C and
subsequently tempered in a water bath at 50°C prior to use. A 300
µl aliquot of bacterial suspension from a log phase culture grown
to an optical density of 0.2 at wavelength 600 nm
(OD600=0.2) was mixed with a 20 ml aliquot of agar
tempered to 50°C and was rapidly filled into the mould. Immediately
afterwards, the stamp was pushed down by the precise positioning of
dowel pins (Fig. 4). The stamp was
held for 5 min to ensure complete agar solidification.
Subsequently, the stamp was removed and excess medium at the inlet
and outlets was trimmed off with a scalpel to obtain sharp inlet
and outlet contours. This procedure was repeated for the other half
of the mould before both halves were assembled to yield the model
bronchial system.
The assembled agar-coated bronchial passages within
the upper and lower mould halves was then placed in the incubation
chamber and attached to the aerosol-generator and air supply
(Fig. 5). A TurboBOY SX with the
PARI LC SPRINT nebuliser nozzle attachment (Pari GmbH) was used to
generate the aerosol particles. According to the manufacturer, this
set-up produces aerosol particles with the mass median diameter
(MMD) of 3.5 µm with mass percentage below 5 µm 67% and total
output rate of 600 mg/min (measured with 0.9% NaCl solution and 20
l/min inspiratory flow). The nebulizer was attached to the
compressor and external air-supply and pressure gauge. The
air-supply was set to 6 l/min and the compressor delivered 5 l/min
and this provided in total, 11 l/min air-flow entering the lung
model via the long inlet tube with an internal diameter of 8.3 mm
(Fig. 5). The inlet tube connected
seamlessly with the agar-coated lung model and ensured a uniform
laminar air-flow into the model.
The temperature in the incubator chamber was
regulated by a copper heating plate with aluminium cooling fins
attached and on-off settings at 36.5 and 37.5°C, respectively. The
thermostat temperature sensor was located at the upper edge of the
long side of the incubator and air was circulated within the
chamber by means of a fan placed 1 cm from the heating plate. A 1.5
cm diameter air outlet from the incubation chamber was fitted with
a 0.45 µm bacteria filter.
Treatment
For aerosol treatment, allicin or gentamicin
solutions (diluted in deionised water) were used. The allicin
solutions were prepared from stock solutions and the concentrations
were confirmed by high-performance liquid chromatography (HPLC) as
previously described (4). For the
treatment with allicin vapour, allicin was diluted in 95% ethanol
and placed into a shallow plastic vessel to yield a 5
cm2 surface for evaporation at room temperature into the
gas phase. Following treatment, the residual volume of allicin
solution was measured and the concentration was determined by HPLC.
The quantity of allicin entering the lung model over the period of
the treatment could be calculated from the residual allicin
solution.
Visualization of bacterial growth and
growth inhibition
At the end of an experiment, and after the air-flow
was discontinued, the lung model assembly was taken out of the
incubation chamber and after covering with moist filter paper, was
wrapped in aluminium foil and incubated at 37°C for 18 h to allow
for bacterial growth. The apparatus was then dismantled and
separated, exposing the inner surfaces of the two halves of agar
tubes representing the bronchi. The halves were sprayed with 0.5%
3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT)
solution, covered with transparent polyethylene foil and incubated
at 37°C for 30 min. Metabolically active bacteria reduced the MTT
to dark coloured formazan and where bacteria were inhibited, the
agar remained pale.
Results
The detailed modelling of the test rig
has been previously described (28)
The qualitative axial velocity distribution through
the bronchi is shown as a spectral heatmap in Fig. 6. High air-flow velocities are at the
red end of the visible colour spectrum and low air-flow velocities
at the blue end. The highest velocities were at the inner surface
of the carinal surfaces after the branch points. Low velocity
‘shadow areas’ are also apparent, especially at the outer edge at
the first dichotomy.
Visualization of bacterial growth
A non-pathogenic E. coli strain (MegaX DH10B™
T1R transformed with empty pRU1097 vector) was used for the
characterization of the behaviour of the test rig in the
experiments reported in this study, as research with human
pathogens demands particularly stringent containment conditions in
specialized facilities. The feasibility of showing bacterial growth
and growth inhibition within the bronchi of the lung model by
spraying the surface with MTT was tested by killing bacteria with
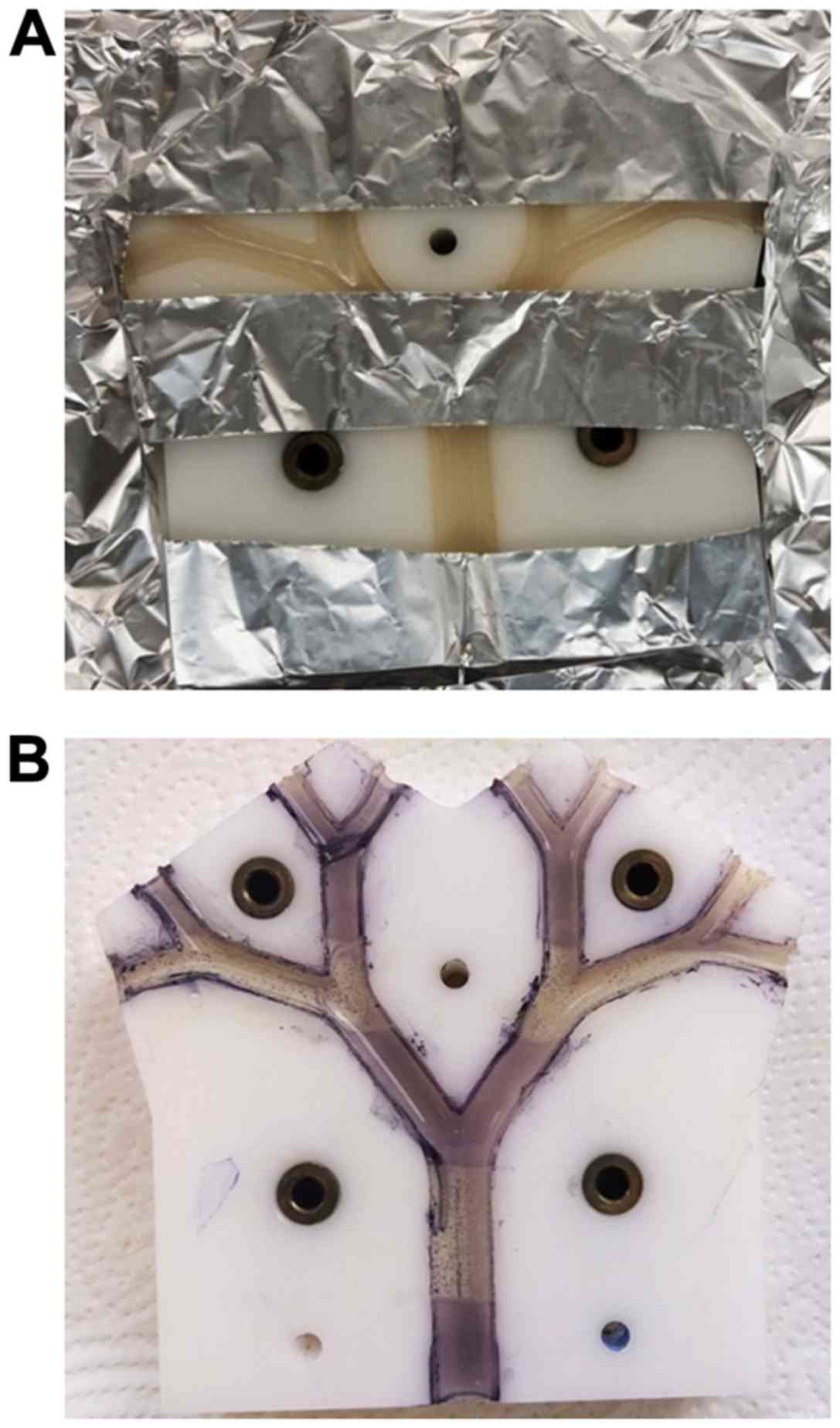
sterilizing UV radiation in a specific pattern (Fig. 7). The parts of the model shaded by
aluminium foil (Fig. 7A) to protect
them against UV-exposure exhibited purple-black colouration after
spraying with MTT, indicating the existence of metabolically active
bacteria. By contrast, the UV-exposed areas of the model where
bacteria had been killed, remained colourless (Fig. 7B). Moreover, the versatility of the
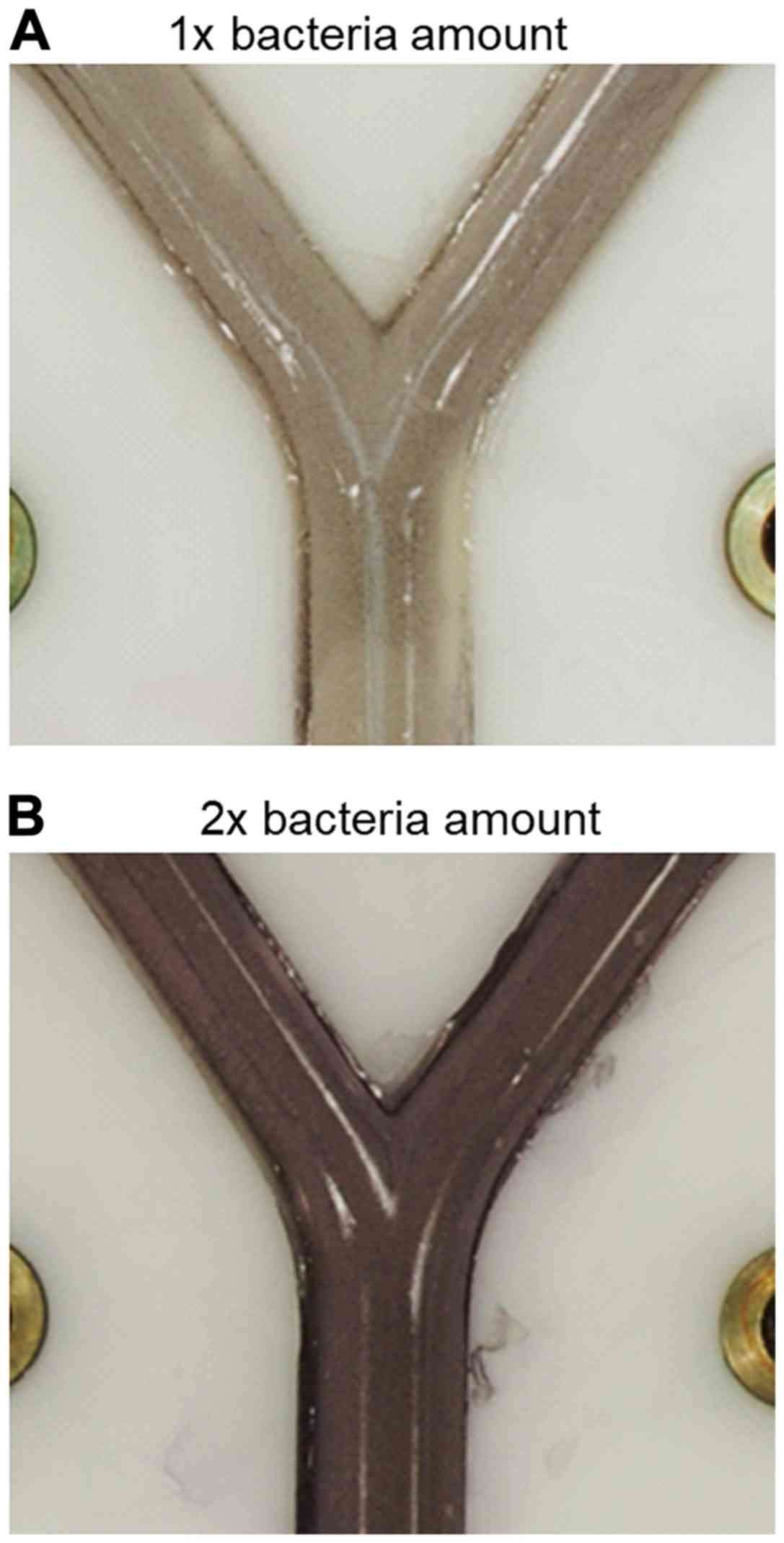
system was demonstrated by using various amounts of E. coli
suspension incorporated in the agar layer (300 or 600 µl of
suspension with OD600=0,2 per 20 ml LB-agar) and showing
that the cell density correlated with the intensity of colour
development after spraying with MTT solution (Fig. 8). This parameter can be optimized for
individual bacterial test species.
Aerosol deposition and dose-dependency
of bacterial inhibition with test antibiotics
Using an air-flow rate of 11 l/min and a 20 min flow
time as the standard, the effect of increasing the concentration of
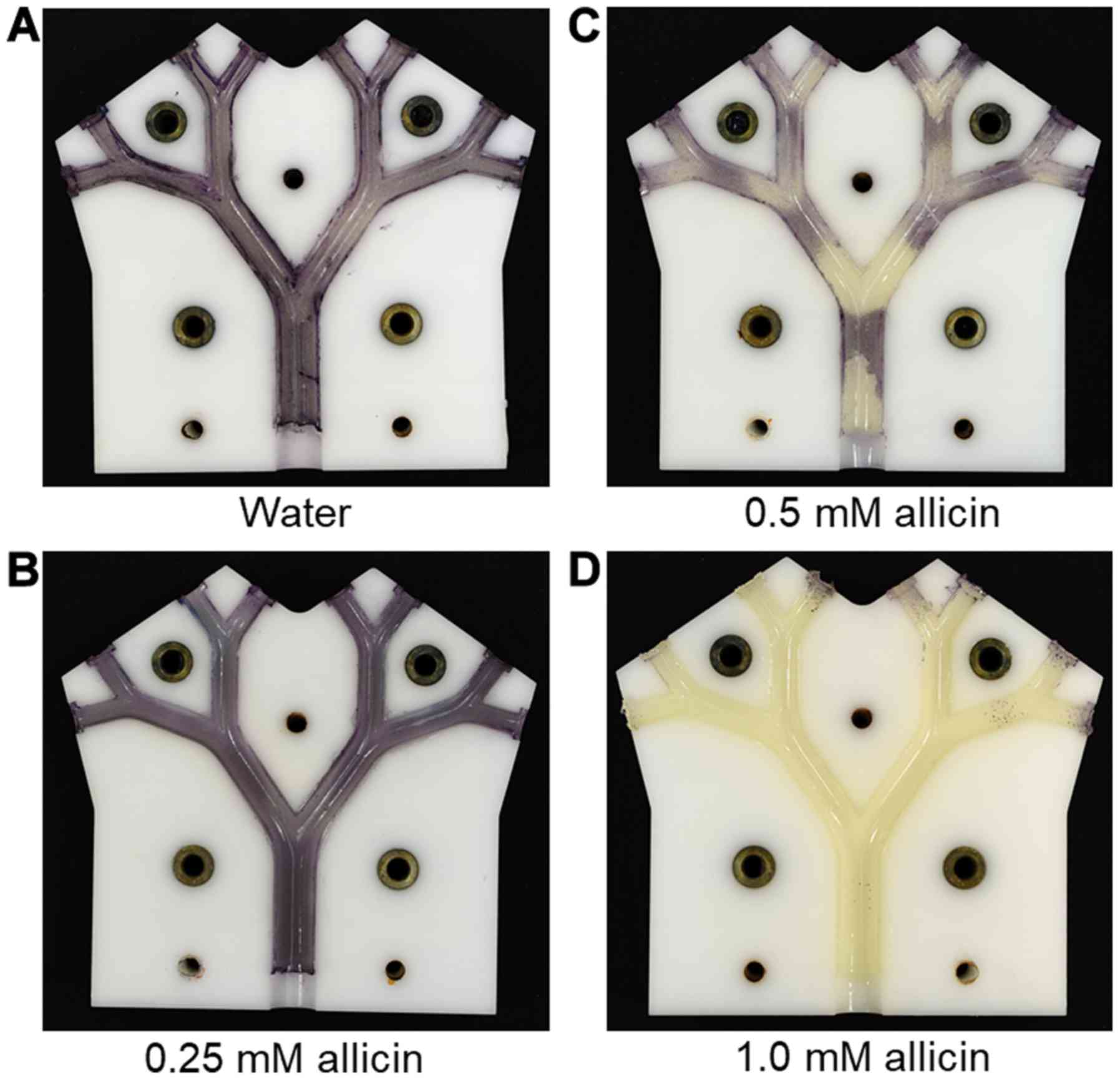
allicin solution in the aerosol was tested. At 0.25 mM allicin, no
growth inhibition of allicin-susceptible E. coli strain
(E. coli MegaX DH10B™ T1R transformed with empty pRU1097
vector) was detected; however, at 0.5 mM allicin, a clear
inhibition of bacterial growth on the bronchial surfaces at the
carinal branch points was observed (Fig.
9). This presumably reflects the areas of maximum aerosol
deposition and coincides with areas of locally high air-flow
velocities (Fig. 6). With an aerosol
of 1 mM allicin, the growth inhibition of allicin-susceptible E.
coli strain was complete on all the bronchial surfaces
throughout the model (Fig. 9). In
total, 8 ml of the allicin test solution were passed through the
model as an aerosol, meaning that the amounts of allicin aspirated
through the model were 0.33, 0.65 and 1.2 mg, respectively.
However, it still has to be investigated in further measurements
what proportion of the aerosol was deposited on the surfaces and
what proportion remained suspended in the transient air-flow.
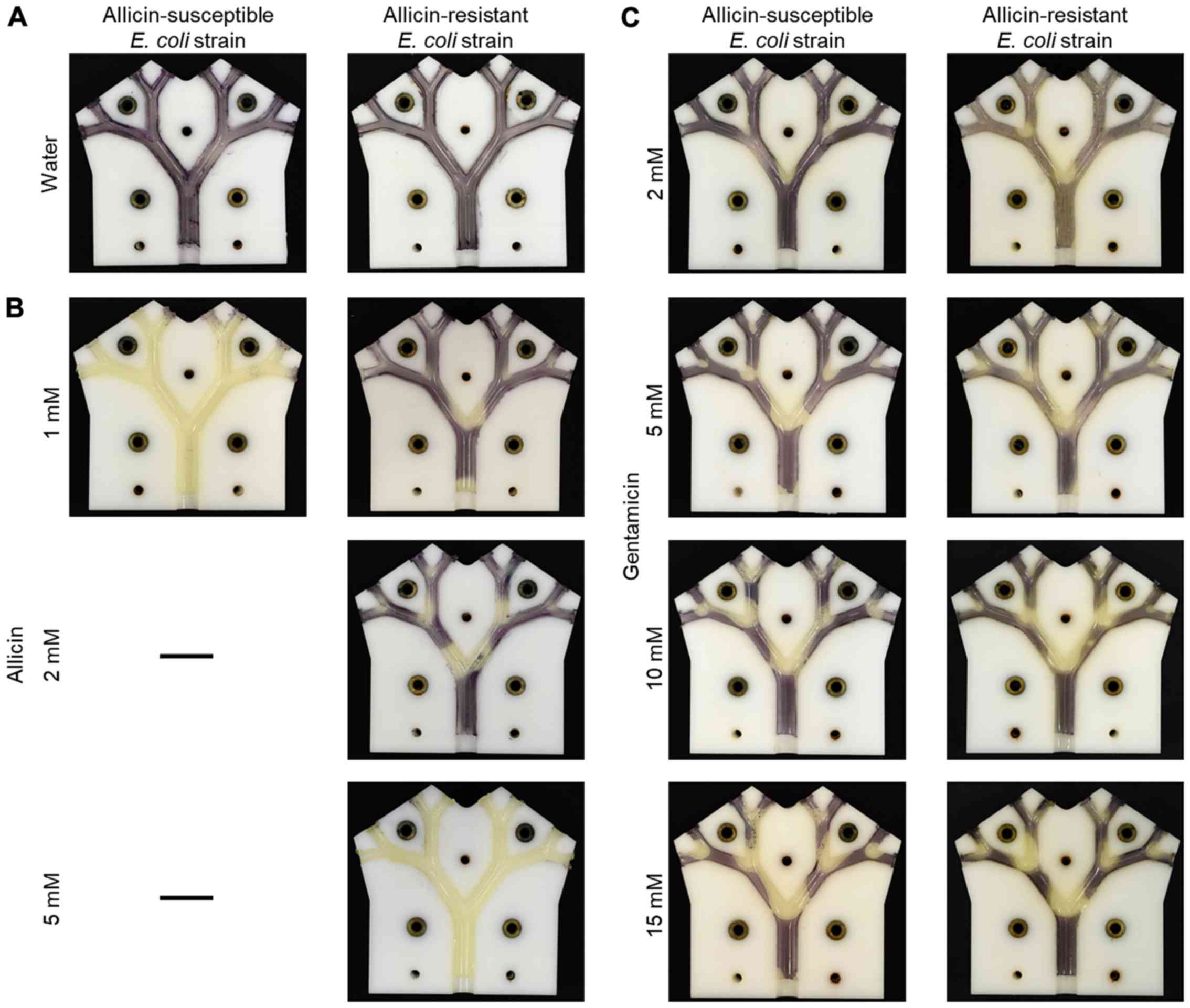
The usefulness of the lung model in highlighting the
differential resistance of bacteria to antibiotics is shown in
Fig. 10. The dose-dependency of
growth inhibition of the allicin-susceptible E. coli isolate
(MegaX DH10B™ T1R) with the empty pRU1097 vector (30) was compared to bacteria made resistant
to allicin by transforming E. coli with the recombinant
pRU1097 vector containing an allicin-resistance-conferring genomic
clone from an allicin-resistant Pseudomonas fluorescens
strain isolated from garlic (29).
The growth of the susceptible E. coli strain (empty vector
control) was completely inhibited throughout the lung model by a 1
mM allicin aerosol, whereas the allicin-resistant genomic clone
transformant exhibited only slight inhibition at the carinal
surfaces of the first branch point. With an aerosol of 2 mM
allicin, the inhibition of the resistant E. coli extended to
the carinal surfaces of the 2nd and 3rd branch points and at 5 mM
inhibition, it was complete over all the bronchial surfaces
(Fig. 10A and B). This differential
resistance was specific to allicin, as was shown by comparing the
inhibition of the two E. coli strains to gentamicin. The
pRU1097 vector carries gentamicin resistance as a selection marker
and both the empty vector and the E. coli genomic clone
transformants were equally susceptible to gentamicin aerosols over
the 2–15 mM range (Fig. 10C). This
observation, that the differential resistance of bacteria to
antibiotics can be demonstrated in the simulated bronchial
environment of the model, holds promise for the development of
allicin, either alone or in combination with other antibiotics, for
the treatment of actual lung diseases, including MDR strains.
Synergism between ethanol and allicin
vapour
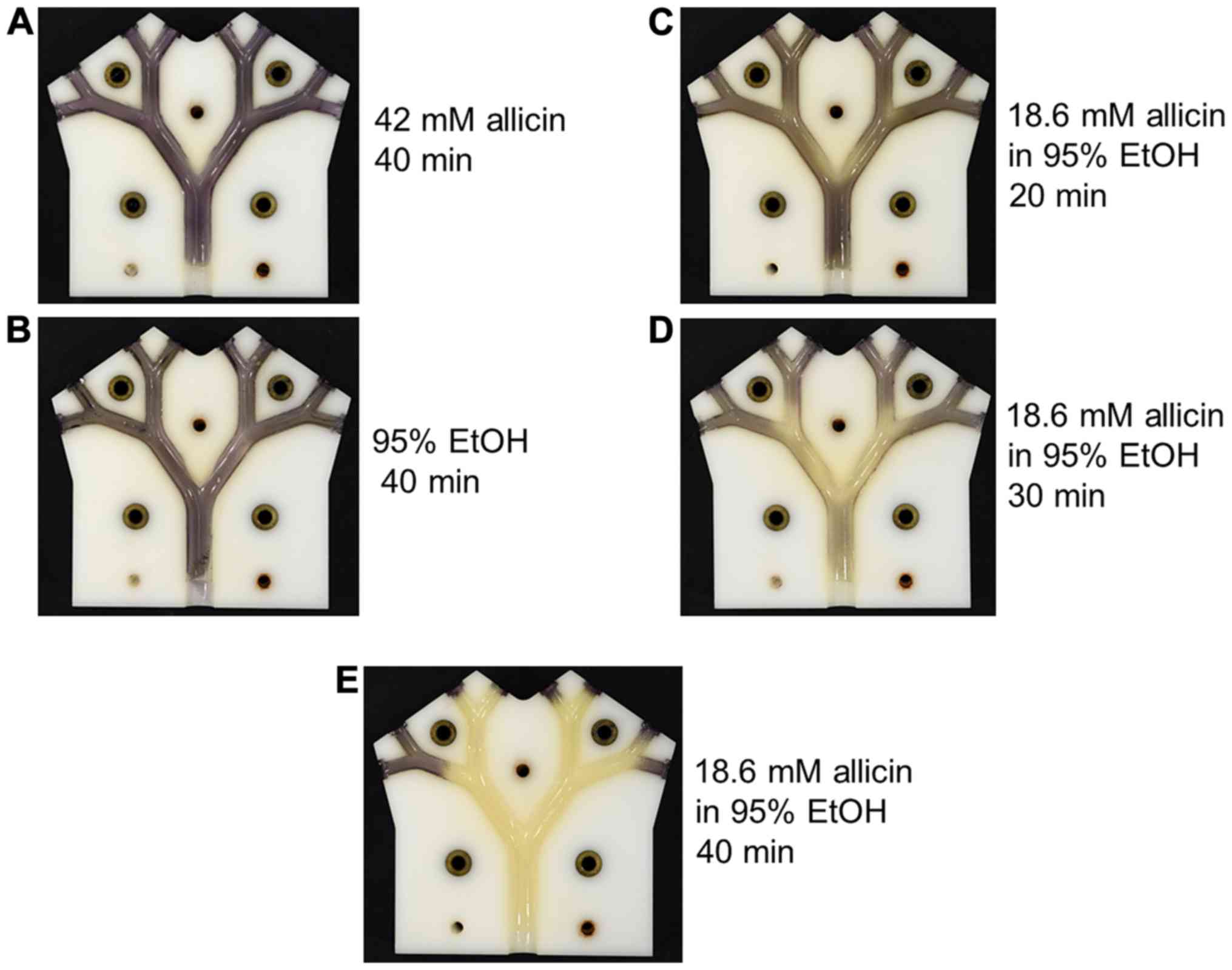
As allicin evaporates into the gas phase, an
alternative to supplying it as an aerosol via the pulmonary route,
would be to breathe in allicin molecules as a vapour. The efficacy
of allicin vapour against allicin-susceptible E. coli was
tested in the lung model. In these experiments, 2 ml of test
solution with an exposed surface area of 5 cm2 had air
passed over it through the lung model at 6 l/min. Neither the
vapour from 42 mM allicin in aqueous solution, nor from 95% (v/v)
ethanol caused any visible inhibition of E. coli growth
following 40 min of treatment (Fig. 11A
and B). The concentration and volume of the allicin solution
remaining at the end of the experiment were measured and the values
were used to calculate the amount of allicin, which had evaporated
into the gas flow during the experiment. In the case of the 42 mM
allicin solution, 1.27 mg of allicin had entered the gas phase and
had passed through the model in a 40-min period. By contrast, a
weaker 18.6 mM solution of allicin in 95% ethanol caused the
inhibition of E. coli growth after only 20 min ‘inhalation’
(Fig. 11C). In the latter case,
only 0.45 mg of allicin vapour had passed through the lung model.
The degree of inhibition of E. coli growth increased
progressively after 30 and 40 min of treatment with the vapour from
the 18.6 mM ethanolic allicin solution, and by 40 min inhibition,
was virtually complete (Fig. 11D and
E). The 30- and 40-min ‘inhalations’ corresponded to 0.9 mg and
maximally 6.0 mg, respectively, of allicin vapour passing through
the lung model. There is some uncertainty as to the amount of
allicin entering the vapour phase for the 40-min time point as
under these conditions, the remainder of the 2 ml of solution had
dried to a residue that could not be accurately quantified. The
value of a maximum 6.0 mg would be correct if all the allicin in
the original 2 ml solution had entered the vapour phase. Allicin
has a high relative molecular mass (Mr=162.28)
and is quite polar; thus, its vapour pressure (P) is
relatively low; however, it is not easy to determine as it
decomposes at the high temperatures needed to determine P
experimentally. The results for the 20- and 30-min ‘inhalation’
periods clearly demonstrated a pronounced synergism between allicin
and ethanol in the vapour phase in the inhibition of E. coli
growth.
Discussion
In the case of lung infections, the delivery of
antibiotics via the pulmonary rather than the oral route would seem
advantageous for a number of reasons. Most importantly, the
pulmonary route provides direct exposure of the antibiotic to the
pathogen and does not rely on assimilation into the body and
circulatory transport to achieve a therapeutic dose at the site of
treatment. Moreover, for a substance, such as allicin from garlic,
the oral route is in any case unrealistic as allicin will be very
rapidly titrated out by glutathione with which it reacts readily to
form S-allyl-mercapto-glutathione (6,7,14,31,32).
Antibiotics can be delivered directly into the lungs either as an
aerosol of droplets or as molecules in the gas phase as a vapour.
Aerosols would be required for most of the conventional antibiotics
as they are not volatile and do not enter the gas phase directly.
Allicin, however is volatile, as attested to by the odour, it
evaporates into the air by crushing or cutting garlic cloves.
Nevertheless, due to its relatively large size and high polarity,
its vapour pressure is not high. The efficacy of allicin aerosols
and allicin vapour against E. coli were investigated in this
study using the lung model. The ability of allicin and other
thiosulfinates to inhibit bacteria, including lung pathogenic
bacteria, as a vapour has been demonstrated in static Petri plate
assays (11,31). The development of allicin as an
antibiotic for clinical use is not completely straightforward, as
although it can be easily synthesized (4), is not stable in solution for long
periods of time unless it is frozen (33). Moreover, allicin is also inhibitory
to mammalian cell growth in a dose-dependent manner (11,34).
Furthermore, tissue burns from the inappropriate use of garlic
preparations in attempts to treat various conditions have been well
documented (2). Therefore, it
remains to be determined whether there is a sufficient degree of
differential toxicity between mammalian cells and the target
microbes in each given situation to warrant the use of allicin as a
treatment for infections. However, in this regard, it should be
remembered that cells cultured in vitro are likely to be
more sensitive than cells in vivo, where a continual supply
of GSH in bodily fluids will help to protect them against allicin
(11,31). For example, cells in the lung are
continually supplied with blood and bathed with epithelial lining
fluid (ELF) containing GSH in the mM range (35,36).
Moreover, by virtue of their function in oxygen uptake, lung cells
must be well protected against oxidative stress. With a substance,
such as allicin which has an oxidative mechanism of action, GSH
protects cells against the effects of allicin. Cells in culture,
with only a fixed charge of GSH in the medium, will probably be
more sensitive to allicin than cells in vivo which are
continually bathed with new GSH which titrates out allicin.
Bacteria colonizing the lung surface would be more immediately
exposed to allicin than the underlying epithelial cells and this
might be expected to contribute to a differential sensitivity of
epithelial cells and bacteria the in vivo situation in lung
disease (11). In this regard, the
historical report of Minchin of the successful treatment of
pulmonary tuberculosis with vapour from garlic pulp is significant
(17). It should be noted that
aerosols of allicin solutions could be potentially much more
damaging to lung tissue than allicin vapour and this aspect must be
carefully investigated before allicin can be used therapeutically.
These speculations aside, at some point, the efficacy of allicin
against lung pathogens must be tested in an animal model to address
these unknowns directly. However, a number of potential treatment
parameters can be determined in vitro using the lung test
rig in pre-clinical trials and can thus reduce the number of animal
experiments necessary.
A further difficulty for developing novel
antibiotics is the emergence of resistance. Even though an
antibiotic, such as allicin with multiple sites of action makes the
appearance of spontaneous resistance in microbes difficult, it does
not mean that it cannot occur, particularly under prolonged and
severe selection pressure. Thus, high resistance against allicin
has been found in some naturally occurring bacteria living in
association with garlic and presumably facing a high selection
pressure against allicin (29).
The test rig is scaled to cover the 2nd to 5th human
bronchial passages. It has been reported that this is the region of
the lung where pneumococcal pneumonia infections generally begin
(37). Thus, using appropriate test
bacteria, dosage regimes and aerosol deposition patterns which
would be likely to be successful in treating developing lung
infections could be determined before animal testing were
undertaken. In this study, non-pathogenic E. coli MegaX
DH10B™ T1R was used as a test bacterium to avoid the need for
special containment requirements for working with human pathogens;
however, it is envisaged that future tests with authentic pathogens
will be carried out as necessary.
Using the lung model the dose-dependent efficacy of
gentamicin and allicin aerosols against E. coli MegaX DH10B™
T1R was demonstrated (Figs. 9 and
10B and C). Furthermore, the model
proved its potential for determining dosage regimes for bacteria
exhibiting varying strengths of resistance to individual
antibiotics (Fig. 10B and C). In
these experiments, the E. coli strains used were isogenic
apart from the 9 kb allicin resistance-conferring genomic clone
from an allicin-resistant Pseudomonas fluorescens isolated
from a garlic bulb (29). E.
coli MegaX DH10B™ T1R transformants carrying this genomic clone
exhibited increased allicin resistance, although both E.
coli strains were equally susceptible to gentamicin. This
situation is clearly recapitulated in the results of the aerosol
dose-effectivity experiments shown in Fig. 10B and C, where it can clearly be
seen that a larger dose of allicin was needed to achieve the same
degree of inhibition of the resistant E. coli cells than the
susceptible cells. By contrast, both strains responded equally to
gentamicin. In this manner, treatment regimens tailored to
individual resistance profiles of lung pathogenic bacteria might be
optimized before progressing to animal trials.
Moreover, the potential of the model for
highlighting synergism was demonstrated for ethanol and allicin in
the gas phase (Fig. 11). Notably,
the historical publication by Minchin which documents the use of
garlic vapour to treat pulmonary tuberculosis in patient subjects,
describes the preparation used for inhalation as ‘3½ ounces of
succus allii sativi, 3½ drams sp. vini. and 1/2 dram of ol.
eucalyp. Citriodora’ (17).
In contemporary measurements, this would be 98 g of garlic pulp, 13
ml of ethanol and 1.85 ml of eucalyptus oil. Minchin states that
the ethanol helped to mask the garlic odour and preserved the
garlic pulp during storage, whereas the eucalyptus oil was solely
to mask the odour of the garlic. It is tempting to speculate that
the ethanol in Dr Minchin's preparations might have had an
unsuspected synergistic effect with allicin, similar to the one
observed with the lung model in this study, and enhanced the
inhibition of the mycobacteria in the lungs of his test subjects.
The use of the lung model to test for synergism between allicin and
other antibiotics, or other combinations of antibiotics not
including allicin, is a promising aspect of the model's potential
use.
There is certainly scope for future improvement and
further development of the basic lung model test rig so that it can
more closely resemble a real lung. For example, at present, the
test rig only allows a continual uni-directional air-flow and does
not simulate actual breathing during which air-flow accelerates,
then slows to a minimum and reverses direction as air is exhaled.
Incorporating such a breathing simulation pattern will be very
desirable in the future to more accurately model the exposure
pattern of bronchial surfaces to test substances. Other
improvements could be surface coatings allowing growth of lung
epithelial cells (38), with a long
term view to co-cultivation with pathogenic bacteria for a more
realistic simulation of the disease situation. Ideally, such
cultivated epithelial cells would be continually supplied with
blood and bathed with epithelial lining fluid (ELF), containing GSH
in the mM range (35,36). The technical difficulties which would
need to be overcome for this near-natural situation will certainly
not be trivial.
The present model could however, be easily adapted
to be coated with growth medium which is seeded with fungal spores.
With a suitable redox indicator, fungal growth inhibition by test
substances could also be characterized using the test rig.
Additionally, if test bacteria with a suitable bioluminescent
reporter system were used to seed the agar coating the lung model
surface, the quantitative response due to deposition of a test
substance could be indicated in the different parts of the model
and this would allow local deposition levels to be accurately
determined. This information could also be used to design dosage
parameters for any particular test substance as a prelude to animal
experiments.
Acknowledgements
The authors would like to gratefully acknowledge
Philipp Müller for Figs. 3, 4 and 6 and
Ulrike Noll for the proofreading of the manuscript.
Funding
Financial support from the RWTH Aachen University
(to JR, JB and AJS) is gratefully acknowledged. JR was supported by
an RFwN Ph.D. stipendium.
Availability of data and materials
All data generated or analysed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
JR, JB, PD, MK and contributed to the experimental
work, data acquisition and analysis. AJS contributed to the
conception and design of the experiments, data analysis and wrote
the manuscript. MCHG and WS contributed to the conception of the
study and critically revised the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cavallito CJ and Bailey JH: Allicin, the
antibacterial principle of Allium sativum. I. Isolation, physical
properties and antibacterial action. J Am Chem Soc. 66:1950–1951.
1944. View Article : Google Scholar
|
|
2
|
Block E: Garlic and the other Alliums. The
Lore and the Science (RSC Publishing); 2010
|
|
3
|
Borlinghaus J, Albrecht F, Gruhlke MC,
Nwachukwu ID and Slusarenko AJ: Allicin: Chemistry and biological
properties. Molecules. 19:12591–12618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albrecht F, Leontiev R, Jacob C and
Slusarenko AJ: An optimized facile procedure to synthesize and
purify allicin. Molecules. 22(pii): E7702017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cavallito CJ, Bailey JH and Buch J:
Allicin, the antibacterial principle of Allium sativum. III. Its
precursor and ‘essential oil of garlic’. J Am Chem Soc.
67:1032–1033. 1945. View Article : Google Scholar
|
|
6
|
Rabinkov A, Miron T, Konstantinovski L,
Wilchek M, Mirelman D and Weiner L: The mode of action of allicin:
Trapping of radicals and interaction with thiol containing
proteins. Biochim Biophys Acta. 1379:233–244. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rabinkov A, Miron T, Mirelman D, Wilchek
M, Glozman S, Yavin E and Weiner L: S-allylmercaptoglutathione: The
reaction product of allicin with glutathione possesses SH-modifying
and antioxidant properties. Biochim Biophys Acta. 1499:144–153.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miron T, Rabinkov A, Mirelman D, Wilchek M
and Weiner L: The mode of action of allicin: Its ready permeability
through phospholipid membranes may contribute to its biological
activity. Biochim Biophys Acta. 1463:20–30. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gruhlke MC, Hemmis B, Noll U, Wagner R,
Luehring H and Slusarenko AJ: The defense substance allicin from
garlic permeabilizes membranes of Beta vulgaris, Rhoeo discolor,
Chara corallina and artificial lipid bilayers. Biochim Biophys
Acta. 1850:602–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Müller A, Eller J, Albrecht F, Prochnow P,
Kuhlmann K, Bandow JE, Slusarenko AJ and Leichert LI: Allicin
induces thiol stress in bacteria through S-Allylmercapto
modification of protein cysteines. J Biol Chem. 291:11477–11490.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reiter J, Levina N, van der Linden M,
Gruhlke M, Martin C and Slusarenko AJ: Diallylthiosulfinate
(Allicin), a volatile antimicrobial from garlic (Allium sativum),
kills human lung pathogenic bacteria, including MDR strains, as a
vapor. Molecules. 22(pii): E17112017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loi VV, Huyen NTT, Busche T, Tung QN,
Gruhlke MCH, Kalinowski J, Bernhardt J, Slusarenko A and Antelmann
H: Staphylococcus aureus responds to allicin by global
S-thioallylation-role of the Brx/BSH/YpdA pathway and the
disulfide reductase MerA to overcome allicin stress. Free Radic
Biol Med. 139:55–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gruhlke MCH, Antelmann H, Bernhardt J,
Kloubert V, Rink L and Slusarenko AJ: The human allicin-proteome:
S-thioallylation of proteins by the garlic defence substance
allicin and its biological effects. Free Radic Biol Med.
131:144–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lawson LD and Hunsaker SM: Allicin
bioavailability and bioequivalence from garlic supplements and
garlic foods. Nutrients. 10(pii): E8122018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bjarnsholt T, Jensen PØ, Rasmussen TB,
Christophersen L, Calum H, Hentzer M, Hougen HP, Rygaard J, Moser
C, Eberl L, et al: Garlic blocks quorum sensing and promotes rapid
clearing of pulmonary Pseudomonas aeruginosa infections.
Microbiology. 151:3873–3880. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smyth AR, Cifelli PM, Ortori CA, Righetti
K, Lewis S, Erskine P, Holland ED, Givskov M, Williams P, Camara M,
et al: Garlic as an inhibitor of Pseudomonas aeruginosa quorum
sensing in cystic fibrosis-a pilot randomized controlled trial.
Pediatr Pulmonol. 45:356–362. 2010.PubMed/NCBI
|
|
17
|
Minchin WC: A study in tubercle virus,
polymorphism and the treatment of tuberculosis and lupus with Oleum
alii. 3rd. Bailliere, Tindall & Cox; London: pp. 1101927
|
|
18
|
Sheppard JG and Long TE: Allicin-inspired
fluoroquinolones as antibacterials against ESKAPE pathogens. Bioorg
Medic Chem Lett. 26:5545–5549. 2016. View Article : Google Scholar
|
|
19
|
Högberg LD, Heddini A and Cars O: The
global need for effective antibiotics: Challenges and recent
advances. Trends Pharmacol Sci. 31:509–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ventola CL: The antibiotic resistance
crisis: Part 1: Causes and threats. P T. 40:277–283.
2015.PubMed/NCBI
|
|
21
|
Powers JH: Antimicrobial drug
development-the past, the present, and the future. Clin Microbiol
Infect. 10 (Suppl 4):S23–S31. 2004. View Article : Google Scholar
|
|
22
|
Silver LL: Challenges of antibacterial
discovery. Clin Microbiol Rev. 24:71–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muto CA, Jernigan JA, Ostrowsky BE, Richet
HM, Jarvis WR, Boyce JM and Farr BM: SHEA guideline for preventing
nosocomial transmission of multidrug-resistant strains of
Staphylococcus aureus and Enterococcus. Infect Control Hosp
Epidemiol. 24:362–386. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Georg AM, Jones PM and Middleton PG:
Cystic fibrosis infections: Treatment strategies and prospects.
FEMS Microbiol Lett. 300:153–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davies J and Davies D: Origins and
evolution of antibiotic resistance. Microbiol Mol Biol Rev.
74:417–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Croucher NJ, Harris SR, Fraser C, Quail
MA, Burton J, van der Linden M, McGee L, von Gottberg A, Song JH,
Ko KS, et al: Rapid pneumococcal evolution in response to clinical
interventions. Science. 331:430–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dini C, Fabbri A and Geraci A: The
potential role of garlic (Allium sativum) against the multi-drug
resistant tuberculosis pandemic: A review. Ann Ist Super Sanita.
47:465–473. 2011.PubMed/NCBI
|
|
28
|
Dörner P, Müller PM, Reiter J, Gruhlke MC,
Slusarenko AJ, Schröder W and Klaas M: Feasibility of a
surface-coated lung model for the quantification of active agent
deposition for preclinical studies. bioRxiv. doi:
10.1101/639245.
|
|
29
|
Borlinghaus J, Bolger A, Schier C, Vogel
A, Gruhlke MCH and Slusarenko AJ: Plant-microbe co-evolution:
allicin resistance in a Pseudomonas fluorescens strain
(PfAR-1) isolated from garlic. bioRxiv. 769265doi:
https://doi.org/10.1101/769265.
|
|
30
|
Karunakaran R, Mauchline TH, Hosie AH and
Poole PS: A family of promoter probe vectors incorporating
autofluorescent and chromogenic reporter proteins for studying gene
expression in Gram-negative bacteria. Microbiology. 151:3249–3256.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leontiev R, Hohaus N, Jacob C, Gruhlke MCH
and Slusarenko AJ: A comparison of the antibacterial and antifungal
activities of thiosulfinate analogues of allicin. Sci Rep.
8:67632018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horn T, Bettray W, Slusarenko AJ and
Gruhlke MCH: S-allylmercaptoglutathione is a substrate for
glutathione reductase (E.C. 1.8.1.7) from Yeast (Saccharomyces
cerevisiae). Antioxidants. 7(pii): E862018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koch HP and Lawson LD: Garlic: The science
and therapeutic application of Allium sativum L. and related
species. Williams & Wilkins; Baltimore, MD: 1996
|
|
34
|
Gruhlke MC, Nicco C, Batteux F and
Slusarenko AJ: The effects of allicin, a reactive sulfur species
from garlic, on a selection of mammalian cell lines. Antioxidants.
6(pii): E12016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cantin AM, North SL, Hubbard RC and
Crystal RG: Normal alveolar epithelial lining fluid contains high
levels of glutathione. J Appl Physiol. 63:152–157. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rahman I: Inflammation and the regulation
of glutathione level in lung epithelial cells. Antioxid Redox
Signal. 1:425–447. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van der Poll T and Opal SM: Pathogenesis,
treatment, and prevention of pneumococcal pneumonia. Lancet.
374:1543–1556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lüngen AE, Bezela S, Kniebs C, Cornelissen
CG, Jockenhoevel S and Thiebes AL: Differentiation and evaluation
of respiratory epithelial cells on polycarbonate urethane
nonwovens. Pneumologie. 73:1172019.
|