Introduction
Garlic (Allium sativum L.) is well-recognized
as a botanical resource for health maintenance, largely due to its
active components with antioxidant properties (1). However, fresh garlic causes indigestion
and its odor is a possible social deterrent (2). Fresh garlic soaked in 15–20% aqueous
ethanol for >10 months at room temperature produces aged garlic
extract (AGE), which is odorless and contains rich antioxidant
chemicals that prevent oxidative damage (3,4). It has
been demonstrated that AGE can reduce oxidative damage by
scavenging reactive oxygen species (ROS) and inhibiting the
formation of lipid peroxides (5). In
addition, there is evidence to indicate that AGE can reduce the
risk of cardiovascular and cerebrovascular diseases, and possibly
inhibit carcinogenesis (6–12). One of the major bioactive components
in AGE is S-allylcysteine (SAC), a stable organosulfur compound
with a thiol group (13). SAC also
possesses antioxidant properties and can neutralize excessive
electrophilic species (14).
Apart from SAC, AGE also contains a carbohydrate
derivative, N-α-(1-deoxy-D-fructos-1-yl)-L-arginine
(FruArg). FruArg is an Amadori rearrangement product from the
amino-carbonyl (Maillard) reaction of AGE, which is a non-enzymatic
browning reaction occurring between amino acids and sugars
(15,16). FruArg was first isolated from the
non-saponin fraction of Korean red ginseng, as a principle
antioxidant species with potent effects on lowering blood pressure
and improving microcirculation (17,18). The
antioxidant potential of FruArg has been demonstrated in an in
vitro model of atherosclerosis (19), where FruArg was shown to efficiently
block copper(II)-induced low density lipoprotein (LDL) oxidation.
Moreover, was demonstrated FruArg that inhibited, in a
dose-dependent manner, the release of peroxides by macrophages
exposed to oxidized LDL (19). Such
activities are attributed to the metal-chelating and
peroxide-scavenging capabilities of FruArg. Previous studies by our
group also demonstrated the ability of both AGE and FruArg to
reduce nitric oxide (NO) production in lipopolysaccharide
(LPS)-stimulated murine microglial BV-2 cells in a
concentration-dependent manner (20,21).
Subsequently, we applied quantitative proteomic analysis by
two-dimensional differential in-gel electrophoresis (2D-DIGE)
integrated with liquid chromatography tandem mass spectrometry
(LC-MS/MS) to profile the proteome patterns and to determine the
effects of AGE and FruArg on LPS-stimulated BV-2 cells (20). Furthermore, we investigated gene
regulation and associated signaling pathways using the
RNA-sequencing (RNA-Seq) approach coupled with machine learning
based bioinformatics analysis (21).
Importantly, in another study on mice using the sensitive
ultra-high performance liquid chromatography-tandem mass
spectrometry (UPLC-MS/MS) protocol, we obtained evidence that
FruArg can pass through the blood-brain barrier in mice (22). The ability of FruArg to penetrate the
blood-brain barrier in subnanomolar concentrations suggests its
potential to be studied as a therapeutic compound.
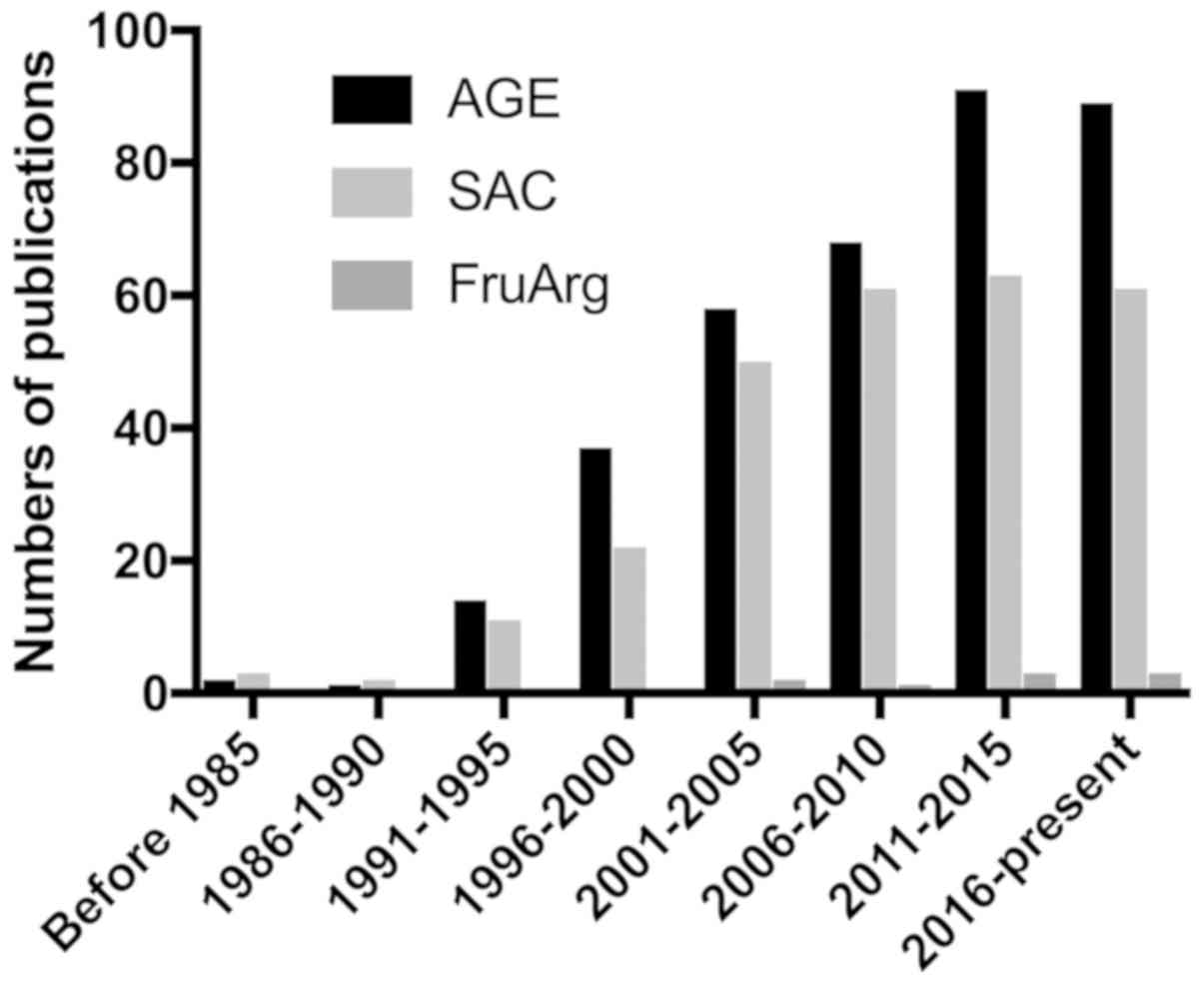
Currently, over 340 publications from PubMed are
related to studies on AGE. SAC has also been explored in numerous
research articles (approximately 270). However, there are currently
only 9 studies available on FruArg (Fig.
1), at least to the best of our knowledge. In this review, we
summarize the neuroprotective effects of garlic bioactive
components, including AGE, SAC and FruArg, respectively, in three
separate sections.
Neuroprotective effects of AGE
AGE is commercially available and has been widely
used in studies investigating its beneficial effects on health
(3,19,23).
Emerging evidence suggests that AGE attenuates the oxidative damage
and neuroinflammation underlying a variety of neurological
diseases, such as Alzheimer's disease (AD) as well as other
age-related neurodegenerative disorders (14). In earlier studies, AGE was found to
protect against cellular damage occurring due to the toxic effects
of β-amyloid peptide (Aβ) in vitro (24,25).
Another study also demonstrated the suppressive effects of AGE on
both the Aβ load and the numbers of Aβ plaques in the brains of
amyloid precursor protein (APP) transgenic (Tg) mice as compared to
untreated Tg mice used as controls (26). Mechanistically, AGE can inhibit
caspase-3 activation, and can counteract the toxicity of Aβ
(27,28). Additionally, in relation to the
pathogenesis of AD, AGE can reduce tau hyperphosphorylation with a
decreased activity of glycogen synthase kinase (GSK)-3β (26). Furthermore, the neuroprotective
effects of AGE on related cognitive dysfunction have also been
explored. The oral administration of AGE has been shown to improve
spatial recognition memory in cognitively impaired rats induced by
Aβ42. The increased expression of synaptic proteins may underlie
the improved cognitive functions (29). This effect was also accompanied by
the attenuation of the loss of cholinergic neurons and
neuroinflammation via the suppression of microglial activation and
the secretion of interleukin (IL)-1β (30,31).
Neuroinflammation plays an important role in the pathogenesis of a
number of neurodegenerative disorders (32,33).
Microglial cells are the resident immune cells in
the central nervous system (CNS). After being exposed to brain
injuries, stress or infections, microglial cells are the first line
of defense that are capable of conferring resilience against
oxidative stress and neuroinflammatory responses. Moreover,
microglial cells can also become activated and release inflammatory
mediators, such as ligands of Toll-like receptors (TLRs), IL-1β,
tumor necrosis factor-α (TNF-α), reactive oxygen species (ROS) and
NO (34,35). A number of botanical compounds with
antioxidant properties have been explored to target
neuroinflammation. There is evidence to indicate that AGE can exert
potent neuroprotective effects via the amelioration of
pro-inflammatory responses (36,37).
The effects of AGE on neuroinflammation and
oxidative stress have been extensively investigated (38). AGE can reduce the activation of
microglia and astrocytes [by immune-detection of the respective
ionized calcium-binding adapter molecule 1 (Iba1) and Glial
fibrillary acidic protein (GFAP) expression levels], and can thus
mitigate neuroinflammatory responses (13,39).
Additionally, AGE can reduce the expression levels of
neuroinflammatory mediators, including IL-1β, nuclear factor
(NF)-κB, TLR4, nuclear factor erythroid 2-related factor 2 (Nrf2)
and heme oxygenase 1 (HO-1) in microglial and endothelial cells
and/or Aβ-induced neuroinflammation in rats (21,30,40). AGE
has also been found to alleviate oxidative stress by scavenging
ROS, inhibiting LDL oxidation, enhancing antioxidant signaling
[such as superoxide dismutase (SOD), glutathione peroxidase (GPX)
and glutathione (GSH)] and mitigating lipid peroxidation
[malondialdehyde (MDA)] (41–45). Our
previous study also demonstrated the protective effects of AGE on
ROS and NO production from L-arginine by NO synthase (NOS)
(20). Excessive NO can undergo
oxidative-reductive reactions and cause cellular damage by
endangering reactive nitrogen species (RNS) (46). Nitrosative stress elicited by NO and
RNS has been implicated in the pathogenesis of neurodegenerative
diseases, such as AD, Parkinson's disease and traumatic brain
injury (TBI) (47–52). Previous studies by our group
demonstrated that AGE can attenuate NO production in microglial
BV-2 cells (20,21). Specifically, treatment with AGE
(0.1–1%) or FruArg (2–5 mM) for 20 h was found to significantly
reduce NO production in a dose-dependent manner in BV2 cells
against LPS-induced neuroinflammation (20). Additionally, AGE was found to be
capable of regulating both marker protein and gene expression
levels, such as caspase-3, IL-6, NF-κB, HO-1, SOD2 and
peroxiredoxin-1 (PRDX1), against LPS-induced neuroinflammation
(21). By using quantitative
proteomics and RNA-Seq analysis, it is possible to unbiasedly
identify key canonical pathways associated with LPS stimulation,
including TLR signaling, IL-6 signaling, IL-10 signaling, tumor
necrosis factor receptor 2 (TNRF2) signaling, 14-3-3-mediated
signaling, superoxide radical degradation, pentose phosphate
pathway (oxidative branch) and Nrf2-mediated oxidative stress
response through AGE treatment (20,21). Our
findings are consistent with those of others demonstrating that AGE
is capable of reducing the oxidative-nitrosative stress,
alleviating neuroinflammation and exerting neuroprotective effects
against the pathogenesis of neurodegenerative diseases (37).
Neuroprotective effects of SAC: A major
active component of AGE
SAC is formed by the catabolism of
γ-glutamyl-S-allylcysteine. The antioxidant effects of SAC in the
nervous system have been reported (53). Indeed, numerous neuroprotective
effects of AGE mentioned above are derived from its multipotent
phytochemicals, of which SAC is a major component. The amount of
bioactive SAC from AGE increases during the aging process of the
garlic, and SAC plays important roles as an antioxidant (37). SAC has been found to destabilize Aβ
fibrils in vitro (54).
Aβ-induced hippocampal neurodegeneration and neuronal cell death
can be prevented by SAC treatment, which is mediated through the
caspase-12-dependent pathway and SAC can attenuate endoplasmic
reticulum stress (41). SAC can also
inhibit apoptosis by regulating caspase-3 activity via
phosphoinositide-3-kinase (PI3K), protein kinase B (PKB/Akt) and
c-Jun N-terminal kinase (JNK) signaling (41,53).
Research has also indicated that SAC can protect against
1-methyl-4-phenylpyridinium (MPP) toxicity associated with reduced
lipid peroxidation and superoxide radical production. As a result,
SAC is able to improve the MPP-induced locomotor impairment in a
mouse model of Parkinson's disease (PD) (55). Researchers have shown the protective
effects of SAC on other neurobehavioral outcomes. The oral
administration of SAC has been shown to protect against LPS-induced
cognitive deficits in rats, and to improve spatial recognition
memory in the Y-maze, the discrimination ratio in the novel object
discrimination task, and the retention and recall in the passive
avoidance test (13). SAC has been
shown to alleviate the spatial recognition memory and cognitive
deficits associated with novelty recognition and passive avoidance
in rats (56). These studies
identified the reducing effects of SAC on acetylcholinesterase
activity, the lipid peroxidation marker, MDA, as well as its
promoting effects on the levels of antioxidant system markers,
including SOD, catalase and reduced GSH in the hippocampal brain
subregion. Data have also demonstrated that SAC can modulate NF-κB,
TLR-4 and TNF-α, and prevent the reduction of Nrf2 and HO-1
(13,56,57). Due
to its anti-inflammatory properties, a recent study used SAC for
the treatment of experimental autoimmune encephalomyelitis in mice.
That study demonstrated that SAC not only regulated TNF-α, IL-17
and matrix metalloprotease 9 levels, but was also capable of
attenuating inflammatory cell infiltration, axonal demyelination
and axonal loss in the lumbar spinal cord (58). Additionally, another study used allyl
sulfide in aging mice and demonstrated that it ameliorated gut
dysbiosis and memory functions associated with lncRNA-Hotair
regulation and H3K27ac regulatory activity (59). Taken together, these neuroprotective
effects, namely the reduction of oxidative stress and inflammatory
responses may be the key underlying benefits for SAC against
neurodegeneration.
FruArg: A potential alternative compound
with which to mitigate neuroinflammation
FruArg, also known as fructosyl arginine, is
fractioned from AGE as a bioactive compound and can be chemically
synthesized (23). FruArg is
identified in AGE at a concentration range of 2.1–2.4 mmol/l. The
antioxidant activity of FruArg is as potent as that of ascorbic
acid, considering its hydrogen peroxide scavenging activity
(23,42). FruArg was first studied, along with
AGE and SAC, in the treatment of sickle cell anemia by inhibiting
the formation of dense cells in vitro (60). Since the year 2000, researchers have
indicated the anti-tumor effects of FruArg through its ability to
inhibit cancer cell proliferation and adhesion (61). However, there is limited information
available regarding the neuroprotective effects of FruArg.
Therefore, to the best of our knowledge, we were the first to have
studied the effects of FruArg on LPS-induced inflammatory responses
in BV2 microglial cells (14,20,21).
Our previous studies demonstrated that FruArg reduced LPS-induced
NO production in a concentration-dependent manner (20,21). The
ability of FruArg to mitigate neuroinflammation was investigated
using quantitative proteomics and RNA-Seq analysis. We demonstrated
that FruArg alleviated oxidative stress and neuroinflammatory
responses with similar potency as AGE. In fact, both AGE and FruArg
shared a large number of regulated protein and gene markers, with
distinct profiles in those differentially expressed targets by
bioinformatics analyses of the differential levels of the targets
in their proteomes and transcriptomes, respectively, affecting the
scales of the signaling pathways. Consistently, FruArg was found to
alter the Nrf2-mediated oxidative stress response, 14-3-3-mediated
signaling, superoxide radical degradation, glutathione
biosynthesis, TLR, IL-10, TNRF2 and IL-6 signaling pathways. Of
note, several signaling pathways on protein and gene levels
regulated by FruArg were also shared with AGE treatment, supporting
that FruArg contributed to certain anti-inflammatory effects of
AGE. Additionally, we also identified that FruArg treatment
triggered the protein-protein interactions associated with
immunological disease, and cellular assembly and organization,
cellular movement, cell death and survival and cell morphology
(20,21).
Apart from an in vitro analysis showing the
anti-inflammatory potential of FruArg, researchers have also
examined whether FruArg can be delivered to the brain for the
treatment of neurological disorders. The blood-brain barrier is
known to block >98% of all small molecular drug candidates
entering the brain, thus causing a major issue for drug therapy
development (62). Therefore, it is
critical that we previously demonstrated that FruArg could pass
through the blood-brain barrier in subnanomolar concentrations in
mice (22). Using the UPLC-MS/MS
detection protocol, the results demonstrated that, in spite of
being highly hydrophilic, FruArg could be absorbed and appear in
the blood circulation following an intraperitoneal injection. The
pharmacokinetics analysis determined the elimination rate in plasma
samples (fraction of FruArg that is removed from the body per unit
time) was 6.91×10-2/min, with a half-life (t1/2) of 10.03 min,
clearance (Cl) of 1.8×10-4 l·min, and volume distribution (VD) of
2.63×10-3 l. Ongoing studies by our research group aim to utilize
ribosome profiling (Ribo-Seq) technology by profiling the mRNA
activation to analyze such neuroprotective effects of FruArg in the
same in vitro model. This could help us to provide a
systematic and quantitative method, data profiling and
visualization to further elucidate the molecular and genetic
mechanisms of the neuroprotective effects of FruArg. Considering
that our studies have provided a basis for the anti-inflammatory
properties of FruArg, future studies are warranted to include
in-depth investigations to examine the mechanisms whereby FruArg
can exert its neuroprotective effects, for the prevention and
treatment of neurological disorders.
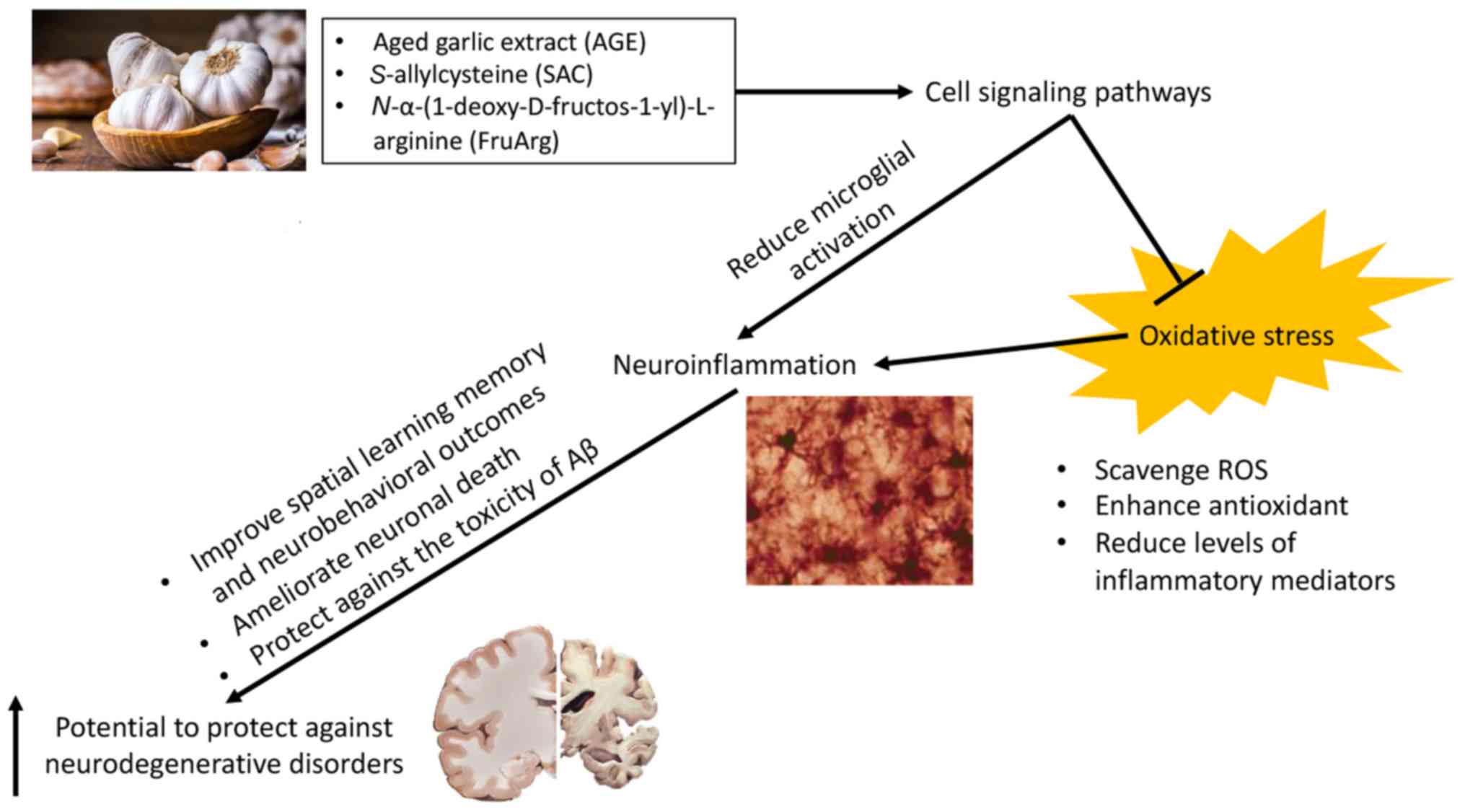
Conclusions
In conclusion, the neuroprotective effects of garlic
components, including AGE, SAC and FruArg on oxidative stress,
neuroinflammation and neurodegeneration were discussed herein
(Fig. 2). The health benefits of
AGE, SAC and FruArg are supported by extensive research on various
experimental paradigms. Of importance, our studies demonstrated
that FruArg mitigates LPS-induced neuroinflammatory responses and
that it is capable of penetrating the blood-brain barrier. This
constitutes important information which can provide a promising
basis and may open new avenues for promoting brain resilience for
health maintenance against neuroinflammation and neurodegeneration
from neurological disorders.
Acknowledgements
Not applicable.
Funding
This publication was made possible by the funding of
the Department of Pathology and Anatomical Sciences research fund
at the University of Missouri School of Medicine (to ZG), as well
as by grant no. P50AT006273 from the National Center for
Complementary and Integrative Health (NCCIH), the Office of Dietary
Supplements (ODS), and the National Cancer Institute (NCI). Its
contents are solely the responsibility of the authors and do not
necessarily represent the official views of the NIEHS, NCCIH, ODS,
NCI, or the National Institutes of Health.
Availability of data and materials
Not applicable.
Authors' contributions
HS, JC and ZG wrote the manuscript with significant
scientific contributions and concept inputs from VVM, CMG, KF and
GYS. All authors have reviewed and approved the current version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chung LY: The antioxidant properties of
garlic compounds: Allyl cysteine, alliin, allicin, and allyl
disulfide. J Med Food. 9:205–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heber D: The stinking rose: Organosulfur
compounds and cancer. Am J Clin Nutr. 66:425–426. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borek C: Antioxidant health effects of
aged garlic extract. J Nutr. 131 (Suppl 3):1010S–1015S. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kyo E, Uda N, Kasuga S and Itakura Y:
Immunomodulatory effects of aged garlic extract. J Nutr. 131 (Suppl
3):1075S–1079S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morihara N, Hayama M and Fujii H: Aged
garlic extract scavenges superoxide radicals. Plant Foods Hum Nutr.
66:17–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borek C: Garlic reduces dementia and
heart-disease risk. J Nutr. 136 (Suppl 3):810S–812S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathew B and Biju R: Neuroprotective
effects of garlic a review. Libyan J Med. 3:23–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katsuki T, Hirata K, Ishikawa H, Matsuura
N, Sumi S and Itoh H: Aged garlic extract has chemopreventative
effects on 1,2-dimethylhydrazine-induced colon tumors in rats. J
Nutr. 136 (Suppl 3):847S–851S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka S, Haruma K, Yoshihara M, Kajiyama
G, Kira K, Amagase H and Chayama K: Aged garlic extract has
potential suppressive effect on colorectal adenomas in humans. J
Nutr. 136 (Suppl 3):821S–826S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varshney R and Budoff MJ: Garlic and Heart
Disease. J Nutr. 146:416S–421S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsumoto S, Nakanishi R, Li D, Alani A,
Rezaeian P, Prabhu S, Abraham J, Fahmy MA, Dailing C, Flores F, et
al: Aged garlic extract reduces low attenuation plaque in coronary
arteries of patients with metabolic syndrome in a prospective
randomized double-blind study. J Nutr. 146:427S–432S. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ried K, Travica N and Sali A: The effect
of aged garlic extract on blood pressure and other cardiovascular
risk factors in uncontrolled hypertensives: The AGE at Heart trial.
Integr Blood Press Control. 9:9–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zarezadeh M, Baluchnejadmojarad T,
Kiasalari Z, Afshin-Majd S and Roghani M: Garlic active constituent
s-allyl cysteine protects against lipopolysaccharide-induced
cognitive deficits in the rat: Possible involved mechanisms. Eur J
Pharmacol. 795:13–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu Z, Mossine VV, Cui J, Sun GY and Gu Z:
Protective Effects of AGE and Its Components on Neuroinflammation
and Neurodegeneration. Neuromolecular Med. 18:474–482. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Finot P: The Maillard reaction in food
processing, human nutrition and physiology. Birkäuser Verlag;
Basel, Switzerland: 1990, View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martins SI, Jongen WM and Van Boekel MA: A
review of Maillard reaction in food and implications to kinetic
modelling. Trends Food Sci Technol. 11:364–373. 2000. View Article : Google Scholar
|
|
17
|
Yukinaga M, Yinan Z, Takeshi T, Kenji K
and Hiromichi O: Isolation and Physiological Activites of a New
Amino Acid Derivative from Korean Red Ginseng. J Ginseng Res.
18:204–211. 1994.
|
|
18
|
Kitao T, Kon K, Nojima K, Takaku T, Maeda
N and Okuda H: Effect of components in non-saponin fraction of red
ginseng on microcirculation. J Tradit Med (Toyama). 12:294–295.
1995.
|
|
19
|
Ide N, Lau BH, Ryu K, Matsuura H and
Itakura Y: Antioxidant effects of fructosyl arginine, a Maillard
reaction product in aged garlic extract. J Nutr Biochem.
10:372–376. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou H, Qu Z, Mossine VV, Nknolise DL, Li
J, Chen Z, Cheng J, Greenlief CM, Mawhinney TP, Brown PN, et al:
Proteomic analysis of the effects of aged garlic extract and its
FruArg component on lipopolysaccharide-induced neuroinflammatory
response in microglial cells. PLoS One. 9:e1135312014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song H, Lu Y, Qu Z, Mossine VV, Martin MB,
Hou J, Cui J, Peculis BA, Mawhinney TP, Cheng J, et al: Effects of
aged garlic extract and FruArg on gene expression and signaling
pathways in lipopolysaccharide-activated microglial cells. Sci Rep.
6:353232016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnson MC, Song H, Cui J, Mossine VV, Gu
Z and Greenlief CM: Development of a Method and Validation for the
Quantitation of FruArg in Mice Plasma and Brain Tissue Using
UPLC-MS/MS. ACS Omega. 1:663–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ryu K, Ide N, Matsuura H and Itakura Y: N
alpha-(1-deoxy-D-fructos-1-yl)-L-arginine, an antioxidant compound
identified in aged garlic extract. J Nutr. 131 (Suppl 3):972S–976S.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Selassie M, Griffin B, Gwebu N and Gwebu
ET: Aged garlic extract attenuates the cytotoxicity of beta-amyloid
on undifferentiated PC12 cells. In Vitro Cell Dev Biol Anim.
35:369–370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng Q, Buz'Zard AR and Lau BH:
Neuroprotective effect of garlic compounds in amyloid-beta
peptide-induced apoptosis in vitro. Med Sci Monit. 8:BR328–BR337.
2002.PubMed/NCBI
|
|
26
|
Chauhan NB: Effect of aged garlic extract
on APP processing and tau phosphorylation in Alzheimer's transgenic
model Tg2576. J Ethnopharmacol. 108:385–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jackson R, McNeil B, Taylor C, Holl G,
Ruff D and Gwebu ET: Effect of aged garlic extract on caspase-3
activity, in vitro. Nutr Neurosci. 5:287–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marín N, Romero B, Bosch-Morell F,
Llansola M, Felipo V, Romá J and Romero FJ: Beta-amyloid-induced
activation of caspase-3 in primary cultures of rat neurons. Mech
Ageing Dev. 119:63–67. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ray B, Chauhan NB and Lahiri DK: Oxidative
insults to neurons and synapse are prevented by aged garlic extract
and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg
mouse model. J Neurochem. 117:388–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nillert N, Pannangrong W, Welbat JU,
Chaijaroonkhanarak W, Sripanidkulchai K and Sripanidkulchai B:
Neuroprotective effects of aged garlic extract on cognitive
dysfunction and neuroinflammation induced by β-amyloid in rats.
Nutrients. 9:92017. View Article : Google Scholar
|
|
31
|
Thorajak P, Pannangrong W, Welbat JU,
Chaijaroonkhanarak W, Sripanidkulchai K and Sripanidkulchai B:
effects of aged garlic extract on cholinergic, glutamatergic and
GABAergic systems with regard to cognitive impairment in Aβ-induced
rats. Nutrients. 9:92017. View Article : Google Scholar
|
|
32
|
Gelders G, Baekelandt V and Van der Perren
A: Linking Neuroinflammation and Neurodegeneration in Parkinson's
Disease. J Immunol Res. 2018:47842682018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ransohoff RM: How neuroinflammation
contributes to neurodegeneration. Science. 353:777–783. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Graeber MB and Streit WJ: Microglia:
Biology and pathology. Acta Neuropathol. 119:89–105. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: Uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun AY, Wang Q, Simonyi A and Sun GY:
Botanical phenolics and brain health. Neuromolecular Med.
10:259–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colín-González AL, Santana RA, Silva-Islas
CA, Chánez-Cárdenas ME, Santamaría A and Maldonado PD: The
antioxidant mechanisms underlying the aged garlic extract- and
S-allylcysteine-induced protection. Oxid Med Cell Longev.
2012:9071622012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ray B, Chauhan NB and Lahiri DK: The ‘aged
garlic extract’: (AGE) and one of its active ingredients
S-allyl-L-cysteine (SAC) as potential preventive and therapeutic
agents for Alzheimer's disease (AD). Curr Med Chem. 18:3306–3313.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takechi R, Pallebage-Gamarallage MM, Lam
V, Giles C and Mamo JC: Nutraceutical agents with anti-inflammatory
properties prevent dietary saturated-fat induced disturbances in
blood-brain barrier function in wild-type mice. J
Neuroinflammation. 10:732013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hiramatsu K, Tsuneyoshi T, Ogawa T and
Morihara N: Aged garlic extract enhances heme oxygenase-1 and
glutamate-cysteine ligase modifier subunit expression via the
nuclear factor erythroid 2-related factor 2-antioxidant response
element signaling pathway in human endothelial cells. Nutr Res.
36:143–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kosuge Y, Koen Y, Ishige K, Minami K,
Urasawa H, Saito H and Ito Y: S-allyl-L-cysteine selectively
protects cultured rat hippocampal neurons from amyloid
beta-protein- and tunicamycin-induced neuronal death. Neuroscience.
122:885–895. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ide N and Lau BH: Aged garlic extract
attenuates intracellular oxidative stress. Phytomedicine.
6:125–131. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ide N and Lau BH: Garlic compounds
minimize intracellular oxidative stress and inhibit nuclear
factor-kappa b activation. J Nutr. 131 (Suppl 3):1020S–1026S. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dillon SA, Burmi RS, Lowe GM, Billington D
and Rahman K: Antioxidant properties of aged garlic extract: An in
vitro study incorporating human low density lipoprotein. Life Sci.
72:1583–1594. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thomson M, Al-Qattan KK, Js D and Ali M:
Anti-diabetic and anti-oxidant potential of aged garlic extract
(AGE) in streptozotocin-induced diabetic rats. BMC Complement
Altern Med. 16:172016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Calabrese V, Mancuso C, Calvani M,
Rizzarelli E, Butterfield DA and Stella AM: Nitric oxide in the
central nervous system: Neuroprotection versus neurotoxicity. Nat
Rev Neurosci. 8:766–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Raichle ME and Snyder AZ: A default mode
of brain function: A brief history of an evolving idea. Neuroimage.
37:1083–1090; discussion 1097–1099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Giasson BI, Ischiropoulos H, Lee VM and
Trojanowski JQ: The relationship between oxidative/nitrative stress
and pathological inclusions in Alzheimer's and Parkinson's
diseases. Free Radic Biol Med. 32:1264–1275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Danielson SR and Andersen JK: Oxidative
and nitrative protein modifications in Parkinson's disease. Free
Radic Biol Med. 44:1787–1794. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Besson VC, Croci N, Boulu RG, Plotkine M
and Marchand-Verrecchia C: Deleterious poly(ADP-ribose)polymerase-1
pathway activation in traumatic brain injury in rat. Brain Res.
989:58–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Besson VC: Drug targets for traumatic
brain injury from poly(ADP-ribose)polymerase pathway modulation. Br
J Pharmacol. 157:695–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chauhan NB and Mehla J: Ameliorative
Effects of Nutraceuticals in Neurological Disorders. Bioactive
Nutraceuticals and Dietary Supplements in Neurological and Brain
Disease. Watson RR and Preedy VR: Academic Press; San Diego, CA:
pp. 245–260. 2015, View Article : Google Scholar
|
|
54
|
Gupta VB and Rao KS: Anti-amyloidogenic
activity of S-allyl-L-cysteine and its activity to destabilize
Alzheimer's beta-amyloid fibrils in vitro. Neurosci Lett.
429:75–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rojas P, Serrano-García N, Medina-Campos
ON, Pedraza-Chaverri J, Maldonado PD and Ruiz-Sánchez E:
S-Allylcysteine, a garlic compound, protects against oxidative
stress in 1-methyl-4-phenylpyridinium-induced parkinsonism in mice.
J Nutr Biochem. 22:937–944. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Baluchnejadmojarad T, Kiasalari Z,
Afshin-Majd S, Ghasemi Z and Roghani M: S-allyl cysteine
ameliorates cognitive deficits in streptozotocin-diabetic rats via
suppression of oxidative stress, inflammation, and
acetylcholinesterase. Eur J Pharmacol. 794:69–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shi H, Jing X, Wei X, Perez RG, Ren M,
Zhang X and Lou H: S-allyl cysteine activates the Nrf2-dependent
antioxidant response and protects neurons against ischemic injury
in vitro and in vivo. J Neurochem. 133:298–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zeinali H, Baluchnejadmojarad T, Fallah S,
Sedighi M, Moradi N and Roghani M: S-allyl cysteine improves
clinical and neuropathological features of experimental autoimmune
encephalomyelitis in C57BL/6 mice. Biomed Pharmacother. 97:557–563.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Behera J, Kelly KE and Tyagi N: Altered
Non-Coding RNA-Histone Acetylation Regulatory Circuit Is Associated
With Cognitive Impairment via Gut Dysbiosis in Aging Mice. The
FASEB J. 33:714.32019.
|
|
60
|
Ohnishi ST and Ohnishi T: In vitro effects
of aged garlic extract and other nutritional supplements on sickle
erythrocytes. J Nutr. 131 (Suppl 3):1085S–1092S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mossine VV, Chopra P and Mawhinney TP:
Interaction of tomato lycopene and ketosamine against rat prostate
tumorigenesis. Cancer Res. 68:4384–4391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Banks WA: From blood-brain barrier to
blood-brain interface: New opportunities for CNS drug delivery. Nat
Rev Drug Discov. 15:275–292. 2016. View Article : Google Scholar : PubMed/NCBI
|