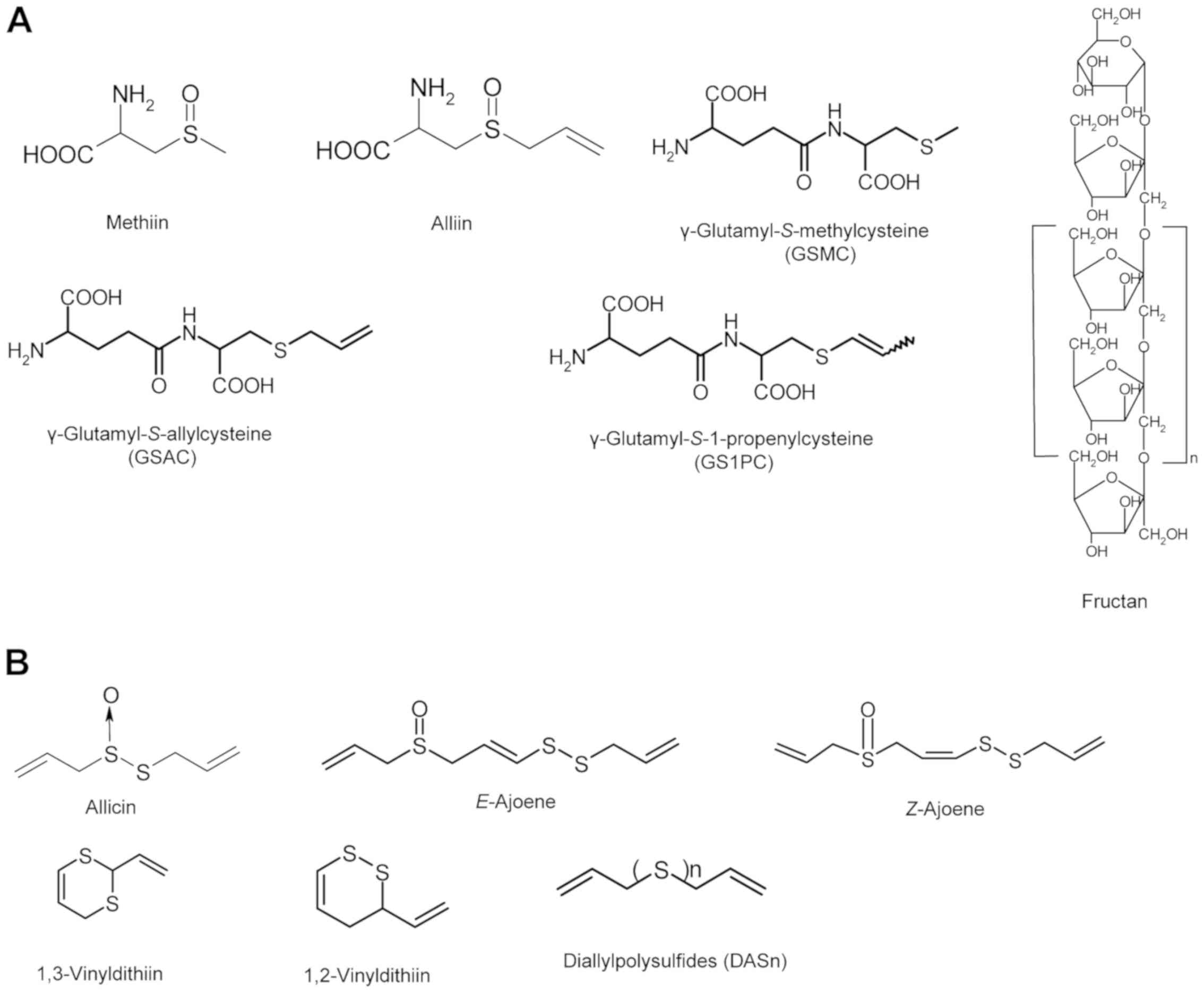
Introduction
Allium plants incorporate the ultimate
inorganic source, sulfate (SO42-), fixed as L-cysteine
molecule and produce several other organic sulfur compounds via
various biosynthetic pathways, including glutamylation and
glycylation to yield glutathione (GSH), deglycylation to yield
γ-glutamyl-S-alk(en)-ylcysteines, and S-oxygenation
and deglutamylation to yield S-alk(en)ylcysteine sufloxides
(Fig. 1A) (1–3). The
biosynthesis and accumulation of these sulfur-containing compounds
become the most active in one to two months prior to the harvest
(4). Other constituents, such as
common amino acids, carbohydrates, proteins and lipids, are also
actively produced and accumulate during the same period when garlic
bulbs grow rapidly (4). The
resulting mature garlic is harvested from early summer through
mid-summer to be used for various purposes, such as in foods,
seasonings, medicinal treatments and others (4).
In the manufacturing of garlic products, raw garlic
is processed through various methods, such as crushing, slicing,
freeze-drying, heating, soaking, macerating, steam distillation,
etc. Some constituents in raw garlic change to other compounds by
processing, and the physical and chemical properties of preparation
depend on the processing methods (5–7). Garlic
oil, a yellow-brown-colored liquid with a sharp characteristic
smell, is produced by the steam distillation method and its
constituents are mainly composed of diallylpolysulfides (DASn)
(Fig. 1B) (5,7). Oil
macerate, a liquid form with a characteristic smell, is produced by
the soaking of garlic in vegetable oil, and its constituents
include unique sulfur compounds, ajoenes and vinyldithiins
(Fig. 1B) (5,7). Black
garlic, mainly being supplied as a whole garlic bulb with an outer
skin, is produced by the heating of garlic at 70–80°C for several
months under high humidity conditions, and its constituents are
claimed to contain Maillard reaction products and polyphenolic
compounds (8,9).
Another unique garlic preparation, aged garlic
extract (AGE), is produced by the soaking of garlic in a
water/ethanol mixture, and is thus subjected to natural
extraction/aging for >10 months at room temperature. Its
constituents include both hydrophilic and hydrophobic compounds,
such as S-allylcysteine (SAC), S−1-propenylcysteine
(S1PC), S-allylmercaptocysteine (SAMC),
Nα-(1-deoxy-D-fructos-1-yl)-L-arginine (Fru-Arg), DASn and
phenolic compounds (Fig. 1C and E)
(10–12). The biological properties of garlic
have been evaluated by several researchers and garlic has been
shown to have useful biological activities for the promotion of
human health (6,10,11,13). In
addition, some characteristic constituents found in raw garlic,
such as alliin, γ-glutamyl-S-allylcysteine (GSAC) and
γ-glutamyl-S−1-pro-penylcysteine (GS1PC), are also found in
AGE (6,13). These diverse constituents found in
AGE are thought to be produced by the processing conditions
involving both physical and chemical factors, such as room
temperature, a near neutral pH, a suitable balance of
hydrophilicity and hydrophobicity for promoting chemical/enzymatic
reactions, suitable/sufficient processing time, etc. The compounds
present in AGE can be produced via various and complex reactions.
In particular, the complex chemistry of sulfur-containing compounds
is considered to contribute mostly to the diversity of the
constituents found in AGE. In order to reveal the production
mechanisms of characteristic compounds found in AGE during the
aging process, the model reaction and the laboratory experiments
mimicking the aging process were conducted under the hypothesis of
production mechanisms of identified compounds leading from the
analytical results of key compounds fluctuations.
In this review, we present the changes of some
characteristic compounds found in AGE during the aging process, and
discuss their production mechanisms involving various chemical and
enzymatic reactions. We also discuss associations between products
and their precursor materials.
S-Alk(en)ylcysteines
Garlic accumulates S-alk(en)ylcysteine
sulfoxides (SACOs; methiin, alliin and isoalliin) and
γ-glutamyl-S-alk(en)ylcysteines [GSAkCs;
γ-glutamyl-S-methylcysteine (GSMC), GSAC and GS1PC] as its
characteristic sulfur constituents. GSAkCs abundantly exist in
garlic cloves during the dormant period, from the time after
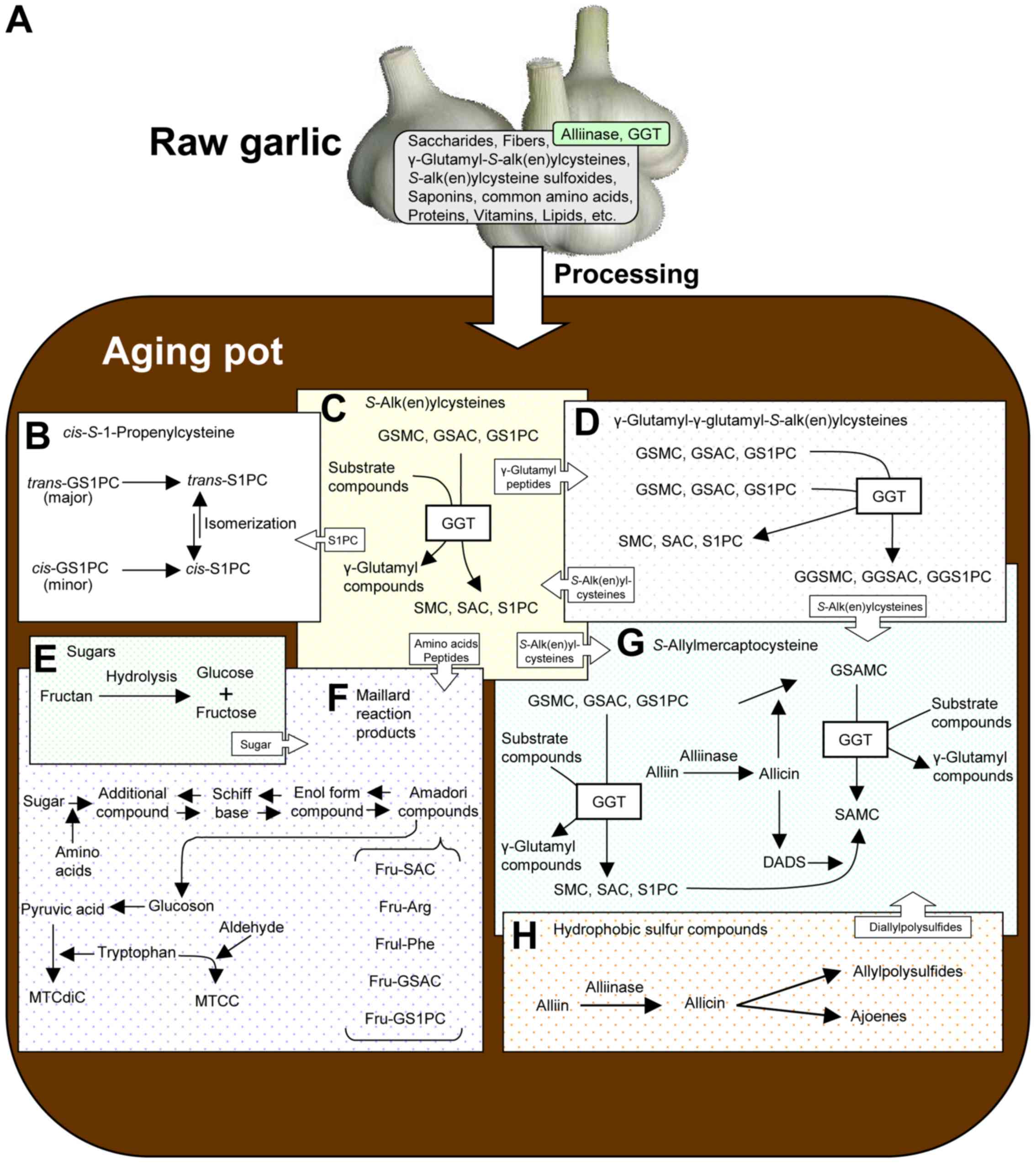
harvest in summer to before sprouting in autumn (Figs. 1A and 2A) (1,4).
However, the contents of these constituents decease when garlic
cloves sense a decrease in environmental temperature, which acts as
a stimulation signal to break the dormancy period, and they are
gradually transformed into SACOs (13,14).
Furthermore, deoxy forms of SACOs [S-methylcysteine (SMC),
SAC and trans-S1PC] are hardly detected in raw garlic.
Although only a trace amount of SAC is detected in raw garlic, the
content of this compound increases during the processing of garlic
(13,14). The same phenomenon is observed for
the generation of SMC and trans-S1PC. These compounds are
produced from GSAkCs through the hydrolysis reaction by endogenous
γ-glutamyltranspeptidase (GGT) in garlic that undergoes changes
when chopped/sliced garlic is soaked into aqueous alcohol for
>10 months, i.e., during the aging process (Figs. 1A, and 2A
and C). GGT activity in AGE is detectable even after 300 days,
although its activity is weak (10).
Sun et al reported that GGT activity derived from garlic was
significantly enhanced by glucose (Glc), aspartic acid (Asp) and
cysteine (Cys) (15). Saccharides in
raw garlic are mainly fructans that are composed of 1–2 glycosyl
linkage polymer of fructose (Fru) with the reduction end of Glc
(Fig. 1A). Raw garlic contains
almost no free Glc; however, its content in AGE gradually increases
with the progress of aging by the hydrolysis of fructan, including
the release of Glc in the reduction end of fructan. The Asp content
in raw garlic has been shown to be 0.2–6.1 mg/g-dry-weight
(13). These constituents can
promote the activity of GGT during the aging process.
In previous studies, when SMC, SAC and
trans-S1PC were orally administered to experimental animals,
they were rapidly absorbed, and exhibited excellent
bioavailability, remaining stable in body fluids (16,17). The
beneficial effects of these compounds have been documented by a
number of researchers (13,18–24). The
bioavailability of the oxide form of SAC, alliin, has been shown to
be <17% in a pharmacokinetic experiment using the radioisotope
compound (25). Allicin, a
transformation product of alliin, subsequently generates various
compounds, such as DASn, ajoenes, vinyldithiins, etc. They have a
low absorption rate and remain unstable in the body (25–28). The
overall yields of these hydrophobic compounds are small as they
transform into various compounds and their source compound in raw
garlic is only alliin, i.e., two molecules of alliin transform into
one molecule of allicin, diallyldisulfide (DADS) and dithiin,
respectively, two molecules of ajoene are produced from three
molecules of alliin, and these compounds produced subsequently
transform into other compounds (13). On the other hand, one molecule of SAC
is produced from one molecule of GSAC, and SMC and S1PC are
produced in the same manner (13).
Although produced S-alk(en)ylcysteines are generally stable
in AGE, some portion of them are used to produce the other
compounds, such as cis-S1PC, SAMC and Amadori compounds as
described in the sections below.
The endogenous enzyme GGT which produces
S-alk(en)yl-cysteines is considered to be one of key factors
for the diversity of constituents in AGE. The generation of
water-soluble cysteine derivatives during the aging process may
contribute to the promotion of human health.
cis-S−1-Propenylcysteine
The chemical and biological properties of S1PC have
not been evaluated for a long period of time, although S1PC was
found to be present in garlic preparations in the early 1960s
(29). Recently, the fluctuation of
its cis- and trans-form in AGE has been reported
(30). S1PC is an isomer of SAC that
is known to be produced from GSAC by enzymatic reaction (Fig. 2C) (13). Garlic abundantly contains the
trans-form of GS1PC, a plausible precursor compound of S1PC
(13,30,31). The
content of cis-GS1PC in raw garlic has been shown to be
<50% of the cis-S1PC content in AGE on a molar basis,
although the content of cis-S1PC gradually increases during
the aging period (30,31). The difference in content between the
precursor and product suggests that cis-S1PC is produced not
only from the precursor compound, γ-glutamyl peptide containing
cis form of S1PC, but also from the other compounds
(30,31). The production mechanism of
cis-S1PC was previously investigated with the hypothesis
that ‘cis-S1PC is produced via isomerization of
trans-form’ (30,31). The model reaction to confirm the
isomerization of S1PC was previously conducted using cis-
and trans-form of S1PC organochemically synthesized on the
chronological analysis of nuclear magnetic resonance (NMR) spectrum
changes. While only one propenyl group form of signals was observed
in each test sample at day 0, both proton signals of cis-
and trans-S1PC in the test substances at day 30 were
detected with almost the same intensities (31). According to the results of
chronological evaluation, the isomerization reaction was confirmed
to occur reversibly (Fig. 2B).
Divalent sulfides have an electron-releasing conjugative effect
when unsaturated group or carbonium ion directly binds to sulfur
(32,33). This causes the cleavage of the π bond
between C1 and C2 in the unsaturated group. Although a part of
cis-GS1PC in raw garlic was considered to be hydrolyzed by
GGT to produce cis-S1PC during the aging process,
cis-GS1PC exhibits a slight increase rather than a sharp
decline, similar to a trans-form (31). According to the results of S1PC
isomerization experiments and the analysis of cis-GS1PC
fluctuation during the aging process, some portion of
cis-GS1PC in AGE also seems to be produced from
trans-GS1PC. Although the biological properties of
cis-S1PC have not yet been reported, the advances being made
in garlic chemistry may soon precisely clarify these properties,
including the associations between the biological activities and
structure difference of cis- and trans-form.
Furthermore, the balance of cis- and trans-form
content may serve as a marker of the aging process, as the content
of cis-form compound in fresh garlic is very small compared
to that of trans-form; however, its amount increases with
time.
γ-Glutamyl-γ-glutamyl-S-alk(en)ylcysteines
Raw garlic accumulates the dipeptides,
γ-glutamyl-S-alk(en)-ylcysteines as sulfur storage molecules
that are precursor compounds of S-alk(en)ylcysteines/SACOs
(e.g., SMC, SAC, S1PC, methiin, alliin and isoalliin) in the
agricultural field (1,6,13). The
contents of these dipeptides in AGE decrease with the increasing
S-alk(en)ylcysteine content (Fig.
2C) (6,13,30,31).
Nakamoto et al confirmed that the mass signal [M+H]+ =
420.1435, which was likely tripeptides,
γ-glutamyl-γ-glutamyl-S-allylcysteine (GGSAC) or
γ-glutamyl-γ-glutamyl-cis/trans-S−1-propenylcysteine
(cis/trans-GGS1PC), based on its elemental
composition on the analysis of AGE (34). They isolated and identified the peak
as the GGSAC using preparative high-performance liquid
chromatography (HPLC), liquid chromatography/high resolution mass
spectrometry (LC-HRMS) and NMR comparison with authentic chemically
synthesized compounds.
γ-Glutamyl-γ-glutamyl-S-methylcysteine (GGSMC) and
cis/trans-GGS1PC were isolated and identified in the
same manner as GGSAC isolation/identification (34). Although GGSMC has been found in
Vigna radiata seeds and Vigna mungo seeds (35), there are no reports available to date
(at least to the best of our knowledge) of the identification of
γ-glutamyl-γ-glutamyl tripeptides GGSMC in Allium plants,
whereas other types of γ-glutamyl tripeptides, GSH and its
derivatives, S-methylglutathione and
S-(β-carboxypropyl)-glutathione, have been confirmed as
intermediate compounds in the biosynthesis pathway of SACOs
(Fig. 1C) (6,13).
Furthermore, the isolation and identification of GGSAC and
cis/trans-GGS1PC were not previously reported (at
least to the best of our knowledge) before the recently published
study by Nakamoto et al (34).
Nakamoto et al hypothesized that
γ-glutamyl-γ-glutamyl tripeptides were produced from two molecules
of γ-glutamyl dipeptides by the endogenous GGT in garlic during the
aging period, and conducted the model reactions using synthesized
γ-glutamyl dipeptides and a protein fraction derived from garlic
having GGT activity, which were inhibited by the GGT inhibitor
(34). The production of
γ-glutamyl-γ-glutamyl tripeptides was also confirmed in laboratory
experiments mimicking the aging process, and GGT inhibitor
suppressed the production of these compounds (Fig. 2D) (34). Both cis-GGS1PC and
trans-GGS1PC were identified and the proportion of their
content in AGE was approximately cis/trans = 10/90
(%) that was similar to the ratio of cis/trans-S1PC
at aging at 16–22 months (30,31).
According to the above-mentioned report concerning the production
mechanism of cis-S1PC in AGE, the cis form of GGS1PC
can be produced from the trans form by an isomerization
reaction during the aging process, as the contents of the
cis-GS1PC in raw garlic are much lower than those in AGE
(13,31,34). The
study by Nakamoto et al suggested that γ-glutamyl
tripeptides are produced via the transfer of
γ-glutamyl-S-alk(en)ylcysteines to glutamic acid in other
γ-glutamyl-S-alk(en)ylcysteines by an endogenous catalyst in
garlic, which helps to simultaneously produce
γ-glutamyl-γ-glutamyl-S-alk(en)ylcysteines and
S-alk(en)ylcysteines during the aging process. Furthermore,
the aging process in an aqueous alcoholic solution can provide the
conditions under which to produce γ-glutamyl tripeptides that are
formed by endogenous GGT in raw garlic.
To date, at least to the best of our knowledge,
there is no report available concerning the biological properties
of three γ-glutamyl tripeptides above. Kasai et al suggested
that GGSMC is a useful index compound for the quality control of
imported Vigna radiata seeds and Vigna mungo seeds
(35). These three compounds may be
the same as a marker/index compound for the aging process using an
aqueous alcoholic solution, as the contents of these compounds in
raw garlic are <10–20% of the corresponding amount in AGE, since
these compounds are generated mainly during the aging process
(34).
S-Allylmercaptocysteine
SAMC is formed in AGE as a characteristic
hydrophilic compound, and its biological properties have been
evaluated (13,19). However, this compound is not detected
in fresh whole garlic, and only a trace amount is found when garlic
is crushed (approximately 0.01 µmol/g fresh garlic) (13). SAMC is chemically synthesized by the
reaction between allicin and Cys that proceeds very rapidly, even
at room temperature (13,36). Allicin is rapidly and abundantly
produced from the precursor, alliin, when raw garlic is crushed or
sliced; however, Cys is not detectable in whole raw garlic and
crushed garlic (13). Fujii et
al analyzed the chronological fluctuation of the SAMC content
during the aging process and found that its content gradually
increased during the aging process (37). Furthermore, allicin disappears in the
early stage of the aging process. These findings indicate that SAMC
in AGE can be produced without Cys during the aging process. This
is different from the production mechanism in the textbook
involving the reaction between allicin and Cys.
Matsutomo et al demonstrated that SAMC was
produced by the reaction between SAC/S1PC and DADS using model
reactions, and the amount of production was temperature-dependent
(e.g., 25°C << 40°C << 60°C) (30). Since the aging process normally
occurs at room temperature, the contribution of the reaction
involving DADS/SAC/S1PC towards total SAMC production may be
minimal (Fig. 2G).
Matsutomo et al also demonstrated the
presence of γ-glutamyl-S-allylmercaptocysteine (GSAMC) in
AGE by LC-HRMS analysis, a putative precursor of SAMC, similar to
GSAC for a precursor of SAC and GS1PC for a precursor of S1PC
(Fig. 1C). GSAMC is not detectable
in raw garlic (30), although its
content in AGE reaches a maximum level within one month and then
gradually decreases (37). Based on
these fluctuations of GSAMC and SAMC in AGE, Fujii et al
hypothesized that GSAMC is produced from GSAC/GS1PC in the early
stages of the aging process, and that SAMC is produced from GSAMC
by endogenous GGT as an alternative mechanism of SAMC production
(37). They conducted model
reactions and laboratory experiments mimicking the aging process
using GSAC, GS1PC, alliin, allicin, garlic protein fraction (GPF)
having GGT activity and raw garlic cloves (37). The production of GSAMC and SAMC was
observed in the combination of GS1PC/allicin/GPF and
GSAC/allicin/GPF, and SAMC production was inhibited by GGT
inhibitor. GGT inhibitor also suppressed SAMC production in
laboratory experiments mimicking the aging process using raw
garlic.
According to the above-mentioned observations, the
production of SAMC during the aging process is postulated as
follows: First, when garlic is soaked in an aqueous alcoholic
solution, alliin is transformed to allicin by alliinase, and GSAMC
is produced by the reaction between allicin and GSAC/GS1PC, and
finally SAMC is produced from GSAMC by GGT (Fig. 2G). Thus, it is suggested that GSAMC
and SAMC in AGE are produced through the combination of chemical
and enzymatic reactions during the aging process. The two
production pathways of SAMC mentioned above differ from the ones in
the textbook, and suggest that SAMC can be made without endogenous
Cys during aging process. Furthermore, SAMC production involves
plural reaction pathways that provide several raw materials by the
chemical and enzymatic reactions. This is not a simple reaction,
such as the condensation of two raw materials, Cys and allicin.
SAMC has been reported to possess useful biological
properties, such as hepatoprotective effects, anti-ototoxicity and
anticancer effects (38–41). However, SAMC quickly disappeared when
it was mixed with blood (26).
Recently, Yang et al reported the metabolism of SAMC using
LC-MS/MS, and revealed that its half-life in rat plasma was <5
min and its clearance was within 30 min (42). These results suggest that SAMC has
strong affinity to proteins in plasma or that the metabolite of
SAMC may be an active molecule.
Hydrophobic sulfur compounds
Following the discovery of allicin and the
alliin-allinase system in the early 1940s, the scientific knowledge
in the garlic chemistry, particularly that relating to the chemical
and biological properties of hydrophobic compounds, has rapidly
expanded (7,43). Allicin has always played a central
role in garlic chemistry; however, this compound has chemical
characteristics of low stability and high reactivity (26,27).
Allicin transforms into various sulfur compounds, such as DASn,
ajoenes and vinyldithiins (Figs. 1B
and 2H) (7). Transformation products have also been
widely investigated in terms of their biological and chemical
properties by several researchers (13,43).
These include characteristic constituents in the lipophilic
products, such as garlic oil and oil macerate product. AGE is
characterized by water-soluble constituents and its content of
lipophilic substances is very low (7,10). Among
these hydrophobic compounds in AGE, DASn are main lipophilic
constituents and play an important role in producing one of the
characteristic sulfur compounds, SAMC. Matsutomo et al
performed model reactions to confirm the production mechanism of
SAMC using DADS, SAC and S1PC (Fig. 2G
and H) (30). The production of
SAMC was observed in the model reaction mixtures of DADS/SAC and
DADS/S1PC, and its production was enhanced in a
temperature-dependent manner. These results indicate that some
hydrophobic volatile compounds in AGE are condensed into a
hydrophilic compound through the production of SAMC by the reaction
with SAC/S1PC during the aging process. The appropriate balance of
hydrophilicity and hydrophobicity in AGE may provide the proper
condition for the production of SAMC.
Maillard reaction products
The amino-carbonyl reaction, namely, the Maillard
reaction, is known as a chemical reaction between reducing sugars
and the amino group in amino acids and peptides. This reaction
occurs non-enzymatically and yields browning, characteristic
flavors and tastes to food materials (44). Although raw garlic contains abundant
polysaccharides and amino acids, the contents of reducing
monosaccharides, such as Glc, galactose and Fru, are very low
(Fig. 2E and F). Ryu et al
isolated and identified Fru-Arg as a Maillard reaction product in
AGE that was produced by the reaction between Glc and Arg (Fig. 1E) (11). Arg is the most abundant common amino
acid in raw garlic, although free Glc is not detectable. Although
Fru-Arg is not detectable within four months of aging, its content
gradually increases with the further progression of aging, as Glc
is supplied into the container in which the aging process occurs by
the degradation of fructan, having Glc at the reducing end (please
see section 8 below entitled ‘Sugars’). Matsutomo et al
isolated and identified the ‘Fru-Arg’ type of sulfur-containing
Amadori compounds, such as
Nα-(1-deoxy-D-fructos-1-yl)-S-allylcysteine
(Fru-SAC),
Nα-(1-deoxy-D-fructos-1-yl)-γ-glu-tamyl-S-allylcysteine
(Fru-GSAC) and
Nα-(1-deoxy-D-fructos-1-yl)-γ-glutamyl-S−1-propenyl-cysteine
(Fru-GS1PC) in AGE (Fig. 1E)
(45). These sulfur-containing
Amadori compounds are characteristic compounds in AGE as they are
produced during the aging process from GSAC and GS1PC that are
characteristic constituents in A. sativum L.
Ide et al reported the identification of four
Maillard reaction products, namely (1R, 3S)-1-methyl-1, 2, 3,
4-tetrahydro-β-carbo-line-3-carboxylic acid [(1R, 3S)-MTCC], (1S,
3S)-1-methyl-1, 2, 3, 4-tetrahydro-β-carboline-3-carboxylic acid
[(1S, 3S)-MTCC], (1R, 3S)-1-methyl-1, 2, 3,
4-tetrahydro-β-carboline-1, 3-dicarboxylic acid [(1R, 3S)-MTCdiC],
(1S, 3S)-1-methyl-1, 2, 3, 4-tetrahydro-β-carboline-1,
3-dicarboxylic acid [(1S, 3S)-MTCdiC] that were produced by the
reaction between tryptophan and acetaldehyde/pyruvic acid (Figs. 1E and 2F) (46).
These compounds differ from the type of ‘Fru-Arg’. Small amounts of
these four compounds were found in four month-aging material, and
their contents were then gradually increased. Raw garlic contains
tryptophan, while acetaldehyde and pyruvic acid are not detectable.
Acetaldehyde may be produced from ethanol added and pyruvic acid is
generated by the degradation of alliin and deoxyglucoson produced
by the Maillard reaction (46). When
the aging process begins, the degradation of alliin immediately
occurs, and the production/degradation of deoxyglucoson progresses
later than the degradation of alliin. Therefore, these four
compounds may be generated earlier than Fru-Arg production, while
the degradation of polysaccharides and the production/degradation
of deoxyglucoson are also necessary for providing pyruvic acid.
Reactive oxygen species (ROS) play an important
role in the induction of various diseases. AGE has been shown to
have antioxidant activities, including the scavenging of ROS, and
its sulfur-containing constituents are mainly responsible for these
antioxidant activities (47). Ryu
et al and Ide et al evaluated the antioxidant
activity of their isolated Maillard reaction products by
H2O2 scavenging activity, and found that the
antioxidant activity of (1S,3S)-MTCdiC was particularly more potent
than that of ascorbic acid (11,46).
Zhou et al reported that Fru-Arg attenuated
neuroinflammatory responses and Nrf2-mediated oxidative stress
response in microglial cells (48).
Oxidative stress in neuron cells induces brain dysfunction,
resulting in severe symptoms, such as cognitive impairment and
Alzheimer's disease. These results indicate that the aging process
generates the various antioxidant compounds via the Maillard
reaction.
Sugars
Carbohydrates are the most abundant components in
raw garlic, which are approximately 80% of the dry weight (8,49). Among
these, the main constituent is a water-soluble
fructo-polysaccharide termed fructan, which has the structure of an
inulin type 2→1 linkage and Glc molecule at the reducing end
(13,49). Fructan is the main carbohydrate
storage compound and may play a role in osmotic regulation and in
cold resistance (50). Its amount in
raw garlic is >60% of the dry weight (49). The other carbohydrate constituents
are the monosaccharides, Glc and Fru, disaccharide sucrose and the
polysaccharides, galactan, galacturonan, xyloglucan and pectin
(13,51). Fructan is gradually hydrolyzed into
the monosaccharides, Fru and Glc, during the aging process
(Fig. 2E). The Glc content in AGE
has been shown to be 3.3%, indicating that a number of fructans
remained without hydrolysis (11).
On the other hand, black garlic is produced by continuous heating
at 70–80°C under high humidity conditions for several months;
subsequently, fructan is completely hydrolyzed into monosaccharide,
and the resulting product becomes sweet, wet and sticky (8,9,52). The contents/balance of
monosaccharides and polysaccharides affects the development of the
product formulation through changes in physical properties, such as
viscosity and density in liquid form, promoting the dissolution of
slightly soluble substances in liquid form, the binding ability of
the powder in solid form, the active water content in solid form,
and so on. Furthermore, the monosaccharide Fru content affects the
taste in the final product, which contributes to the sweetness.
It is well known that sugars react with amino acids
to produce the Maillard reaction products (44). Although the amount of reducing
monosaccharides is low in raw garlic, its content during the aging
process gradually increases with the progression of the aging
process, and the content of Maillard reaction products fluctuates
in a synchronized manner (please see the section 7 above entitled
‘Maillard reaction products’). Fructans and the degradation
products of fructans may characterize AGE both physically and
chemically.
Saponins
Saponin is a general term for compounds in natural
products that produce foam and possess surface-active properties as
they consist of a fat-soluble aglycone linked to one or more
water-soluble carbohydrate chains. A number of plants contain
saponins, and in particular, ginsenoside in ginseng and
glycyrrhizin in liquorice are well known as useful saponins for
human health. In their chemical structures, they are divided into
two groups, triterpenoid saponins and steroidal saponins.
Furthermore, steroidal saponins are divided into two forms, namely
spirostanol and furostanol. Although Smoczkiewicz et al
suggested in the early of 1980s that garlic contains saponins
having a structure of steroidal glycoside, the isolation and
structural determination of these had not yet been accomplished
(53). Towards the end of the 1980s,
Matsuura et al successfully isolated novel furostanol
glycosides termed proto-eruboside-B and satiboside-B from a crude
glycoside fraction that was prepared from methanol extract of
frozen garlic (Fig. 1D) (54,55).
Furthermore, they found that the combination of freezing and
extraction with methanol was effective by the suppression of
glucosidase activity in order to isolate genuine glycosides from
raw materials. That is, no spirostanol glycosides were observed in
the extract with the combination treatment of freezing and
extraction with methanol. However, spirostanol glycosides were
observed in the methanol extract of garlic without freezing
treatment.
In the steroidal saponins isolated from garlic,
spirostanol types have several biological activities and effects,
such as antimicrobial activities (54), anticancer activities (56), inhibitory effects on blood
coagulability (57), promoting
effects on fibrinolysis (57) and
cholesterol-lowering effects (58).
However, genuine glycosides furostanol types do not have these
activities. According to previous studies (54–58), the
processing method involving the reaction with endogenous enzymes in
garlic may be crucial for the production of the useful biological
activities.
A. ampeloprasum L. (common name: Elephant
garlic) is often used as garlic. This plant is commonly known as
wild leek, while its appearance is very similar to A.
sativum L. in its stalk, leaves, cloves and bulb, apart from
the size of the clove and bulb. A. ampeloprasum L. also
contains spirostanol glycoside having agigenin as an aglycone
(56). On the other hand, the
sapogenin part of spirostanol glycoside derived from garlic is
β-chlorogenin. Itakura et al successfully established the
convenient analytical method of TLC to distinguish A.
sativum L. and A. ampeloprasum L. using the difference
of aglycones in their saponins (59). Furthermore, their method can
distinguish A. sativum L. from other Allium plants,
such as A. cepa (onion), A. porrum (leek), A.
fistulosum (Welsh onion), and so on, even they are processed
into commercial products (56,59).
Compounds of which the production mechanisms
have not been elucidated
The existence of two sulfanyl-alanines,
3-allyltrisulfanyl-alanine (3ATSA) and 4-allyltetrasulfanyl-alanine
(4ATSA), was confirmed by the LC-HRMS analysis of AGE: 3ATSA,
calculated [M+H]+ = 226.0025, observed [M+H]+ = 226.0021; 4ATSA,
calculated [M+H]+ = 257.9745, observed [M+H]+ = 257.9744 (Fig. 1C). 3ATSA has been reported as a novel
sulfur compound derived from garlic having a potent anti-yeast
activity, which is generated when garlic is heated at 120°C for 30
min and completely disappears following 90 min of heating, while
its content in heated garlic and production mechanisms have not
been described (60). The MS signal
of 3ATSA is not detectable in 4-month-old AGE; however, its content
gradually increases in the subsequent aging process, while the
aging process occurs at room temperature. Although the content of
4ATSA is lower than that of 3ATSA, it gradually increases with
aging. According to the fluctuation during the aging process, it
may be produced by the reaction between S-alkenylcysteines
and DASn, such as SAMC production mechanisms (please see sections 5
and 6 above, entitled ‘S-Allylmercaptocysteine’ and
‘Hydrophobic sulfur compounds’, respectively).
Two GSH derivatives, S-allylglutathione
(SAG) and S-allylmercaptoglutathione (SAMG), were also
confirmed by the LC-HRMS analysis of AGE, yielding mass signals of
theoretical elemental composition of each compound: SAG, calculated
[M+H]+ = 348.1224, observed [M+H]+ = 348.1219; SAMG, calculated
[M+H]+ = 380.0945, observed [M+H]+ = 380.0940 (Fig. 1C). SAG is thought to exist in raw
garlic as an intermediate on alliin synthesis pathway involving
S-alk(en)ylation of Cys residue, deglycinylation of GSH,
S-oxygenation of SAC moiety and deglutamylation of
γ-glutamylpeptide, while its source in the allyl group is unknown
(13). SAG in AGE may be the
remaining part not being used for alliin production. Rabinkov et
al charactrerized the chemical and biological properties of
SAMG that was organochemically synthesized using allicin and GSH
(61). To date, to the best of our
knowledge, there is no report available to indicate that raw garlic
contains SAMG. S-Allylsulfanylation on GSH/SAG could occur
similar to SAMC production; however, the production mechanisms of
SAMG are unknown.
Ban et al reported the induction of colon
cancer apoptosis by a unique heterocyclic compound, thiacremonone,
derived from heated garlic (Fig. 1E)
(62). This compound is known to be
related to pigment and aroma in soy sauce, miso, beer, heated
garlic and heated onion, which is produced by the Maillard reaction
between Cys/cystine (Cys2) and Fru (63). This compound in AGE has been
investigated by LC/LC-MS analysis and its content has been found to
be lower than a few micrograms per dry-material base of AGE. Fru is
abundantly produced by hydrolysis of fructan during the aging
process, while the small amount is present in raw garlic (please
see section 8 above entitled ‘Sugars’). Since Cys and Cys2 are not
detectable in raw garlic (13);
thiacremonone in AGE could be produced by the Maillard reaction
between Fru and some of Cys derivatives, such as SAC, S1PC and
SAMC. However, at the present time, its production mechanism during
the aging process are unclear.
Matsutomo et al isolated and identified
coniferyl alcohols and dilignol drivatives,
(−)-(2R,3S)-dihydrodehydrodiconiferyl alcohol (DDDC),
(+)-(2S,3R)-dehydrodiconiferyl alcohol (DDC),
erythro-guaiacylglycerol-β-O-4′-coniferyl ether and
threo-guaiacylglycerol-β-O-4′-coniferyl ether in AGE by HPLC under
the activity-guided fractionation of antioxidant activity (Fig. 1D) (12). These compounds exhibit a high
antioxidant activity. Yamakawa et al examined the effects of
DDDC and DDC on primary human coronary artery smooth muscle cells,
and only DDC inhibited alkaline phosphatase activity in cells
(64). The production mechanisms of
these phenolic compounds are unclear. Ichikawa et al
isolated and identified six phenolic compounds, namely
trans-coumaric acid, trans-ferulic acid,
N-trans-coumaroyloctopamine,
N-trans-feruloyloctopamine, guaiacylglycerol-β-ferulic acid
and guaiacylglycerol-β-caffeic acid methyl ether, as an antioxidant
derived from garlic skin (65).
According to their report, DDC and DDDC may be degradation products
of lignans in garlic skin or cell walls.
Kodera et al isolated and identified a
unique phenolic compound,
3-hydroxy-5-methoxy-6-methyl-2-n-pentyl-4H-pyran-4-one,
named allixin (Fig. 1D) (66). This compound is classified as
phytoalexin that is produced by plants when they are subjected to
stress from their environment, and is the first phytoalexin
isolated from garlic (66–68). This compound exhibits weak
antimicrobial activity (66), potent
antitumor activity in vivo/in vitro (69) and neurotrophic activity (70). Phytoalexins are synthesized de
novo by plants at harmed areas by pathogen infection, or
physical/chemical stress. Kodera et al induced this compound
using several physical/chemical stresses; however, the details of
the biosynthesis of this compound are not clear (66–68).
Allixin in AGE may be produced before the aging process or at the
very early period of aging.
Conclusion
The constituents in raw garlic can change into
various compounds depending on the processing method and
conditions. The aging process produces both hydrophilic and
hydrophobic substances by the enzymatic and chemical reactions,
including transformation, hydrolysis, isomerization, the Maillard
reaction, etc. During the aging process, some hydrophobic volatile
compounds reacted with hydrophilic compounds to produce useful
constituents in AGE, indicating that the aging process can condense
even volatile compounds and these reactions lead to a greater
diversity of constituents in AGE. Aging is a superior processing
method as some of the constituents in AGE have been found to
possess useful biological properties which are beneficial to human
health.
Acknowledgements
The authors would like to express their deep
gratitude to Dr Takami Oka of Wakunaga Pharmaceutical Co., Ltd.,
for his helpful advice, encouragement and critical reading of the
manuscript.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YK designed the study, researched the literature,
performed the analysis of the data in sections 2, 3, 4, 5 and 10,
and drafted the manuscript. MK and MN also designed the study,
researched the literature, performed the analysis of the data in
sections 5 and 6, and drafted the manuscript. TM also designed the
study, researched the literature, performed the analysis of the
data in sections 3, 4, 5 and 10, and drafted the manuscript. All
author have reviewed and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lancaster JE and Show ML: γ-Glutamyl
peptides in the biosynthesis of S-alk(en)yl-L-cysteine
sulphoxides (flavor precursors) in Allium. Phytochemistry.
28:455–460. 1989. View Article : Google Scholar
|
|
2
|
Yoshimoto N, Yabe A, Sugino Y, Murakami S,
Sai-Ngam N, Sumi S, Tsuneyoshi T and Saito K: Garlic γ-glutamyl
transpeptidases that catalyze deglutamylation of biosynthetic
intermediate of alliin. Front Plant Sci. 5:7582015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshimoto N, Onuma M, Mizuno S, Sugino Y,
Nakabayashi R, Imai S, Tsuneyoshi T, Sumi S and Saito K:
Identification of a flavin-containing S-oxygenating
monooxygenase involved in alliin biosynthesis in garlic. Plant J.
83:941–951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuura H, Inagaki M, Maeshiga K, Ide N,
Kajimura Y and Itakura Y: Changes in contents of γ-glutamyl
peptides and fructan during growth of Allium sativum. Platna
Med. 62:70–71. 1996. View Article : Google Scholar
|
|
5
|
Block E: The chemistry of garlic and
onions. Sci Am. 252:114–119. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kodera Y, Ushijima M, Amano H, Suzuki JI
and Matsutomo T: Chemical and Biological Properties of
S−1-Propenyl-L-Cysteine in Aged Garlic Extract. Molecules.
22:E5702017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Block E: The organosulfur chemistry of the
genus Allium-implication for organic sulfur chemistry. Angew
Chem Int Ed Engl. 31:1135–1178. 1992. View Article : Google Scholar
|
|
8
|
Kimura S, Tung YC, Pan MH, Su NW, Lai YJ
and Cheng KC: Black garlic: A critical review of its production,
bioactivity and application. Yao Wu Shi Pin Fen Xi. 25:62–70.
2017.
|
|
9
|
Martínez-Casas L, Lage-Yusty M and
López-Hernández J: Changes in aromatic profile, sugars and
bioactive compounds when purple garlic is transformed into black
garlic. J Agric Food Chem. 65:10804–10811. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kodera Y, Matsuura H, Sumiyoshi H and Sumi
SI: Chapter 30. Garlic Chemistry: chemical and biological
properties of sulfur-containing compounds derived from garlic. ACS
symposium series 851, Food Factors in Health Promotion and Disease
Prevention. Shahadi F, Ho CT, Watanabe S and Osawa T: American
Chemical Society; Washington DC: pp. 346–357. 2003, View Article : Google Scholar
|
|
11
|
Ryu K, Ide N, Matsuura H and Itakura I:
Nα-(1-deoxy-D-fructos-1-yl)-L-arginine, an antioxidant
compound identified in aged garlic extract. J Nutr. 136 (Suppl
3):972S–976S. 2006.
|
|
12
|
Matsutomo T, Stark TD and Hofmann T: In
vitro activity-guided identification of antioxidants in aged garlic
extract. J Agric Food Chem. 61:3059–3067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koch HP and Lawson LD: Garlic: The science
and therapeutic application of Allium Sativum L and related
species. Williams & Wilkins; 1996
|
|
14
|
Ichikawa M, Ide N and Ono K: Changes in
organosulfur compounds in garlic cloves during storage. J Agric
Food Chem. 54:4849–4854. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Hu J, Wang W, Zhang B and Shen Y:
Characterization of γ-glutamyltranspeptidases from dormant garlic
and onion bulbs. Food Sci Nutr. 7:499–505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amano H, Kazamori D, Itoh K and Kodera Y:
Metabolism, excretion, and pharmacokinetics of
S-allyl-L-cysteine in rats and dogs. Drug Metab Dispos.
43:749–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amano H, Kazamori D and Itoh K:
Pharmacokinetics and N-acetylation metabolism of
S-methyl-L-cysteine and trans-S−1-propenyl-L-cysteine
in rats and dogs. Xenobiotica. 46:1017–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amano H, Kazamori D and Itoh K: Evaluation
of the effects of S-allyl-L-cysteine,
S-methyl-L-cysteine, trans-S−1-propenyl-L-cysteine,
amd their N-acetylated and S-oxidized metabolites on
human CYP activities. Biol Pharm Bull. 39:1701–1707. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amagase H: Clarifying the real bioactive
constituents of garlic. J Nutr. 136 (Suppl 3):716S–725S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colín-González AL, Santana RA, Silva-Islas
CA, Chánez-Cárdenas ME, Santamaría A and Maldonado PD: The
antioxidant mechanisms underlying the aged garlic extract- and
S-allylcysteine-induced protection. Oxid Med Cell Longev.
2012:9071622012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukushima S, Takada N, Wanibuchi H, Hori
T, Min W and Ogawa M: Suppression of chemical carcinogenesis by
water-soluble organosulfur compounds. J Nutr. 136 (Suppl
3):1049S–1053S. 2006.
|
|
22
|
Suzuki J, Yamaguchi T, Natsutomo T, Amano
H, Morihara N and Kodera Y: S−1-Propenylcysteine promotes
the differentiation of B cell into IgA-producing cells by the
induction of Erk1/2-dependent Xbp1 expression in Peyer's patches.
Nutrition. 32:884–889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki JI, Kodera Y, Miki S, Ushijima M,
Takashima M, Matsutomo T and Morihara N: Anti-inflammatory action
of cysteine derivative S−1-propenylcysteine by inducing
MyD88 degradation. Sci Rep. 8:141482018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsutomo T, Ushijima M, Kodera Y,
Nakamoto M, Takashima M, Morihara N and Tamura K: Metabolomic study
on the antihypertensive effect of S−1-propenylcysteine in
spontaneously hypertensive rats using liquid chromatography coupled
with quadrupole-Orbitrap mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 1046:147–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pushpendran CK, Devasagayam TP, Chintalwar
GJ, Banerji A and Eapen J: The metabolic fate of [35S]-diallyl
disulphide in mice. Experientia. 36:1000–1001. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lawson LD and Wang ZJ: Pre-hepatic fate of
the organosulfur compounds derived from garlic (Allim
sativum). Planta Med. 59:688A–689A. 1993. View Article : Google Scholar
|
|
27
|
Freeman F and Kodera Y: Garlic chemistry:
Stability of S-(2-propyl) 2-propen-1-sulfinothioate
(allicin) in blood, solvents, and stimulated physiological fluids.
J Agric Food Chem. 43:2332–2338. 1995. View Article : Google Scholar
|
|
28
|
Amagase H, Petesch BL, Matsuura H, Kasuga
S and Itakura Y: Intake of garlic and its bioactive components. J
Nutr. 131 (Suppl 3):955S–962S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sugii M, Suzuki T and Nagasawa S:
Isolation of (−)S-propenyl-L-cysteiene from garlic. Chem
Abstr. 59:65091963.
|
|
30
|
Matsutomo T and Kodera Y: Development of
an analytical method for sulfur compounds in aged garlic extract
with the use of a post-column high performance liquid
chromatography method with sulfur-specific detection. J Nutr.
146:450S–455S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kodera Y, Matsutomo T and Itoh K: The
evidence for the production mechanism of
cis-S−1-propenylcysteine in aged garlic extract based on a
model reaction approach using its isomers and deuterated solvents.
Planta Med Lett. 2:e69–e72. 2015. View Article : Google Scholar
|
|
32
|
Oae S, Tamagaki S and Kunieda N:
Stereoelectronic effect of sulfur group. Organic Sulfur Chemistry.
Oae S: Kagakudojin; Kyoto: pp. 41–81. 1982
|
|
33
|
Oae S, Ohno A and Tagaki W: Acid-catalyzed
hydrogen-deuterium exchange reaction of deuterated anisole,
thioanisole and benzene. Bull Chem Soc Jpn. 35:681–683. 1962.
View Article : Google Scholar
|
|
34
|
Nakamoto M, Fujii T, Matsutomo T and
Kodera Y: Isolation and identification of three γ-glutamyl
tripeptides and their putative production mechanism in aged garlic
extract. J Agric Food Chem. 66:2891–2899. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kasai T, Shiroshita Y and Sakamura S:
γ-Glutamyl peptides of Vigna radiata seeds. Phytochemistry.
25:679–682. 1986. View Article : Google Scholar
|
|
36
|
Albrecht F, Leontiev R, Jacob C and
Slusarenko AJ: An optimized facile procedure to synthesize and
purify allicin. Molecules. 22:7702017. View Article : Google Scholar
|
|
37
|
Fujii T, Matsutomo T and Kodera Y: Changes
of S-allylmercaptocysteine and
γ-glutamyl-S-allylmercaptocysteine contents and their
putative production mechanisms in garlic extract during the aging
process. J Agric Food Chem. 66:10506–10512. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sumioka I, Matsura T and Yamada K:
Therapeutic effect of S-allylmercaptocysteine on
acetaminophen-induced liver injury in mice. Eur J Pharmacol.
433:177–185. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiao J, Guo R, Fung ML, Liong EC, Chang
RC, Ching YP and Tipoe GL: Garlic-derived
S-allylmercaptocysteine ameliorates nonalcoholic fatty liver
disease in a rat model through inhibition of apoptosis and
enhancing autophagy. Evid Based Complement Alternat Med.
2013:6429202013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uzun L, Kokten N, Cam OH, Kalcioglu MT,
Ugur MB, Tekin M and Acar GO: The effect of garlic derivatives
(S-allylmercaptocysteine, diallyl disulfide, and
S-allylcysteine) on gentamicin induced ototoxicity: An
experimental study. Clin Exp Otorhinolaryngol. 9:309–313. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu X, Jiang X, Li A, Sun Y, Liu Y, Sun X,
Feng X, Li S and Zhao Z: S-Allylmercaptocysteine suppresses
the growth of human gastric cancer xenografts through induction of
apoptosis and regulation of MAPK and PI3K/Akt signaling pathways.
Biochem Biophys Res Commun. 491:821–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang M, Dong Z, Jiang X, Zhao Z, Zhang J,
Cao X and Zhang D: Determination of S-allylmercaptocysteine
in rat plasma by LC-MS/MS and its application to a pharmacokinetics
study. J Chromatogr Sci. 56:396–402. 2018. View Article : Google Scholar
|
|
43
|
Block E: Garlic and Other Alliums: The
Lore and the Science. The Royal Society of Chemistry; Cambridge:
2010
|
|
44
|
Rizzi GP: The Maillard Reaction in Foods.
Maillard Reactions in Chemistry, Food, and Health. Labuza TP,
Reineccius GA, Monnier VM and Baynes JW: The Royal Society of
Chemistry; Cambridge: pp. 11–19. 1994
|
|
45
|
Matsutomo T, Stark TD and Hofmann T:
Targeted screening and quantitative analyses of antioxidant
compounds in aged-garlic extract. Eur Food Res Technol.
244:1803–1814. 2018. View Article : Google Scholar
|
|
46
|
Ide N, Ichikawa M, Ryu K, Yoshida J,
Sasaoka T, Sumi S and Sumiyoshi H: Chapter 22. Antioxidants in
processed garlic: Tetrahydro-β-carboline derivatives in aged garlic
extract. ACS Symposium Series 851. Food Factors in Health Promotion
and Disease Prevention. Shahidi F, Ho CT, Watanabe S and Osawa T:
American Chemical Society; Washington, DC: pp. 250–263. 2003,
View Article : Google Scholar
|
|
47
|
Imai J, Ide N, Nagae S, Moriguchi T,
Matsuura H and Itakura Y: Antioxidant and radical scavenging
effects of aged garlic extract and its constituents. Planta Med.
60:417–420. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou H, Qu Z, Mossine VV, Nknolise DL, Li
J, Chen Z, Cheng J, Greenlief CM, Mawhinney TP, Brown PN, et al:
Proteomic analysis of the effects of aged garlic extract and its
FruArg component on lipopolysaccharide-induced neuroinflammatory
response in microglial cells. PLoS One. 9:e1135312014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fenwick TE and Hanley AB: Critical review
in food science and nutrition. The genus Allium. Part 2.
Furia TE: CRC Press; Boca Raton, FL: pp. p2841985
|
|
50
|
Darbyshire B and Henry RJ: Differences in
fructan content and synthesis in some Allium species. New Phytol.
87:249–256. 1981. View Article : Google Scholar
|
|
51
|
Ohsumi C and Hayashi T: The
oligosaccharide unit of xyloglucan in the cell wall of bulbs of
onion, garlic, and their hybrid. Plant Cell Physiol. 35:963–967.
1994.PubMed/NCBI
|
|
52
|
Kang OJ: Physicochemical Characteristics
of Black Garlic after Different Thermal Processing Steps. Prev Nutr
Food Sci. 21:348–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Smoczkiewicz MA, Nitschke D and Wieladek
H: Microdetermination of steroid and triterpene saponin glycosides
in various plant materials I. Allium species. Mikrochim Acta.
78:43–53. 1982. View Article : Google Scholar
|
|
54
|
Matsuura H, Ushiroguchi T, Itakura Y,
Hayashi N and Fuwa T: A frostanol glycoside from garlic, bulbs of
Allium sativum L. Chem Pharm Bull (Tokyo). 36:3659–3663.
1988. View Article : Google Scholar
|
|
55
|
Matsuura H, Ushiroguchi T, Itakura Y and
Fuwa T: Further studies on steroidal glycosides from bulbs, root
and leaves of Allium sativum L. Chem Pharm Bull (Tokyo).
37:2741–2743. 1989. View Article : Google Scholar
|
|
56
|
Matsuura H: Phytochemistry of garlic
horticultural and processing procedures. Neutraceuticals: Designer
Foods III. Garlic, Soy and Licorice. Lachance PA: Food and
Nutrition Press; Trumbull, CT: pp. 55–69. 1997
|
|
57
|
Peng JP, Chen H, Qiao YQ, Ma LR, Narui T,
Suzuki H, Okuyama T and Kobayashi H: Two new steroidal saponins
from Allium sativum and their inhibitory effects on blood
coagulability. Yao Xue Xue Bao. 31:607–612. 1996.PubMed/NCBI
|
|
58
|
Slowing K, Ganado P, Sanz M, Ruiz E,
Beecher C and Tejerina T: Study of garlic extract and fractions on
cholesterol plasma levels and vascular reactivity in
cholesterol-fed rats. J Nutr. 131 (Suppl 3):994S–999S. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Itakura Y, Ichikawa M, Mori Y, Okino R,
Udayama M and Morita T: How to distinguish garlic from other Allium
vegetables. J Nutr. 131 (Suppl 3):963S–967S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kang SS, Lim DR and Kyung KH:
3-(Allyltrisulfanyl)-2-aminopropanoic acid, a novel nonvolatile
water-soluble antimicrobial sulfur compound in heated garlic. J Med
Food. 13:1247–1253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rabinkov A, Miron T, Mirelman D, Wilchek
M, Glozman S, Yavin E and Weiner L:
S-Allylmercaptoglutathione: The reaction product of allicin
with glutathione possesses SH-modifying and antioxidant properties.
Biochim Biophys Acta. 1499:144–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ban JO, Yuk DY, Woo KS, Kim TM, Lee US,
Jeong HS, Kim DJ, Chung YB, Hwang BY, Oh KW and Hong JT: Inhibition
of cell growth and induction of apoptosis via inactivation of
NF-kappaB by a sulfurcompound isolated from garlic in human colon
cancer cells. J Pharmacol Sci. 104:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Furusawa R, Goto C, Satoh M, Nomi Y and
Murata M: Formation and distribution of
2,4-dihydroxy-2,5-dimethyl-3(2H)-thiophenone, a pigment, an
aroma and a biologically active compound formed by the Maillard
reaction, in foods and beverages. Food Funct. 4:1076–1081. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yamakawa T, Matsutomo T, Hofmann T and
Kodera Y: Aged garlic extract and one of the constituent,
(+)-(2S,3R)-dehydrodiconiferyl alcohol, inhibits alkaline
phosphatase activity induced by inflammation factors in human
vascular smooth muscle cells. Food Nutr Sci. 5:177–184. 2014.
|
|
65
|
Ichikawa M, Ryu K, Yoshida J, Ide N,
Kodera Y, Sasaoka T and Rosen RT: Identification of six
phenylpropanoids from garlic skin as major antioxidants. J Agric
Food Chem. 51:7313–7317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kodera Y, Matsuura H, Yoshida S, Sumida T,
Itakura Y, Fuwa T and Nishino H: Allixin, a stress compound from
garlic. Chem Pharm Bull (Tokyo). 37:1656–1658. 1989. View Article : Google Scholar
|
|
67
|
Kodera Y, Ayaba M, Ogasawara K and Ono K:
Allixin induction and accumulation by light irradiation. Chem Pharm
Bull (Tokyo). 49:1636–1637. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kodera Y, Ayaba M, Ogasawara K, Yoshida S,
Hayashi N and Ono K: Allixin accumulation with long-term storage of
garlic. Chem Pharm Bull (Tokyo). 50:405–407. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nishino H, Nishino A, Takayasu J, Iwashima
A, Itakura Y, Kodera Y, Matsuura H and Fuwa T: Antitumor-promoting
activity of allixin, a stress compound produced by garlic. Cancer
J. 3:20–21. 1990.
|
|
70
|
Moriguchi T, Matsuura H, Itakura Y,
Katsuki H, Saito H and Nishiyama N: Allixin, a phytoalexin produced
by garlic, and its analogues as novel exogenous substances with
neurotrophic activity. Life Sci. 61:1413–1420. 1997. View Article : Google Scholar : PubMed/NCBI
|