Introduction
Diabetic retinopathy (DR) is a microvascular
complication caused by diabetes. At present, the prevalence of
diabetes in China is approximately 5.6%. Half of the patients with
a course of 10–20 years have DR complications, and 100% of patients
with a course of >20 years are complicated by DR (1). DR is a chronic progressive irreversible
disease with a final outcome of complete loss of vision (2,3).
Abnormal insulin in diabetic patients leads to abnormal cell
metabolism, damage to the eye nerves, the blood-retinal barrier and
microcirculation, and causes fibroplasia and inadequate nutrition
supply to the eye, thus contributing to gradual impairment of
visual function (4). The
pathogenesis of DR is not yet clear, but studies have shown that a
variety of factors are associated with the incidence of DR, such as
abnormal glucose metabolism, increased blood viscosity, secretion
of growth hormone, angiogenesis factor and oxygen free radicals
(5). With the advancement of
medicine, treatment methods of DR are constantly improving, but so
far, almost all the treatments can only reduce the patient's
symptoms and slow down the disease process, but cannot reverse it
(6). Therefore, investigation of the
pathogenesis of DR helps to cure the root cause and look for ways
to really treat DR from the source.
CTGF is a connective tissue growth factor and a
member of the cysteine growth factor family (7), and its overexpression induces fibrosis,
which has been detected in the heart, liver, lung, blood vessels
and other tissues (8,9). Under pathological conditions, due to
its mitotic characteristics, CTGF is overproduced, which promotes
fibroblast proliferation and secretes more ECM, so as to induce
fibrosis. Vascular membrane decreases its toughness and is easy to
rupture, causing hematocele and retinal detachment. As a result,
the visual function is gradually lost and prognosis is poor
(10,11).
HO-1 is an oxygen stress-induced heme oxygenase.
After the cells are stimulated by stress, MAPK signaling pathway is
activated, promotes the translocation of Nrf2 into the nucleus,
induces the HO-1 expression, maintains heme concentration in the
body and produces biliverdin, CO, and Fe2+, all of which
have anti-oxidant and anti-inflammatory effects (12). ERK1/2 is an important signal
transduction media for the MAPK signaling pathway, and studies have
shown that its phosphorylation can promote CTGF expression
(13). Song et al (3) showed that anthocyanin protects retinal
cells from oxidative stress and inflammation caused by diabetes by
regulating Nrf 2/HO-1 signaling. Therefore, this study aims at
providing new ideas for a new and thorough treatment of DR through
investigating the expression and influence mechanism of CTGF and
HO-1 in DR rats.
Materials and methods
Experimental animals
One hundred and thirty male SD rats of healthy SPF
grade were provided by Shanghai SLAC laboratory Animal Co., Ltd.
(Shanghai, China) with a production license of SCXK (Shanghai)
2012–0002. Their age was 8 weeks old and their weight was 150±30 g.
The rats were kept in animal housing of SPF grade, with a
temperature of 20±2°C, humidity of 60±5% and simulated daytime 12 h
and night 12 h. They had free access to basic pellet diet and water
intake. The study was approved by the Hospitals Ethics Committee,
and the experimental procedures were in accordance with the
“Guidelines for the Protection and Use of Laboratory Animals”.
Materials
TRIzol kit (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA); RT-qPCR random primer (Sangon Biotech Co.,
Ltd., Shanghai, China); RNase inhibitor (Wuhan Huamei
Bioengineering Co., Ltd., Wuhan, China); reverse transcription kit
(Life Technologies; Thermo Fisher Scientific, Inc.); fluorescent
quantitative PCR kit (Shanghai ShineGene Molecular Biotech, Inc.,
Shanghai, China); H&E staining kit (Beyotime Institute of
Biotechnology, Haimen, China); TUNEL apoptosis kit (Beijing Jiamei
Niunuo Biotechnology Co., Ltd., Beijing, China).
Model building and group
processing
After being purchased, the rats were kept for one
week, adapted to the new laboratory environment, and randomly
divided into the DR and control groups, with 65 rats in each group.
Their body weights in the DR group were 135±31 g, and those in the
control group were 140±33 g. There was no significant difference in
the basic data such as weight and age between the two groups
(P>0.05). After feeding for 4 weeks, each rat was weighed and
the streptozotocin (STZ)/saline injection dose was calculated with
the rat body weights (kg) × 30 mg. Intraperitoneal injection of STZ
was performed in the DR group and saline in the control group. To
prevent wound infection, every rat received intramuscular injection
of penicillin 40,000 units per day for 3 days. Blood was taken from
the tail tip of rats for detection of blood glucose concentration
at the 5th day. The standard for successful modeling in the DR
group was that the blood glucose concentration was >16.7 mmol/l,
and the blood glucose and body weight of the rats were measured
monthly. The rats were kept conventionally until the 2nd, 4th and
6th month and sacrificed by excessive anesthesia with
intraperitoneal injection of 200 mg/kg pentobarbital sodium. A
total of 55 rats were included in the study. Eighteen rats with
successful modeling were sacrificed after the 2nd and 4th month of
feeding respectively, and the remaining 19 rats were all sacrificed
after the 6th month, and 21, 22 and 22 rats in the control group
were sacrificed at the same time.
Detection sample preparation
Complete eyeballs of the rats were taken after
sacrifice, and an eyeball of each rat was used to separate the lens
and vitreous from the eyecup using an eyeball ring shear. Paraffin
sections (4 µm) were prepared after 10% formaldehyde fixed eyecup
for HE staining and TUNEL apoptotic cell detection. The eyeball of
other rats was used to bluntly separate the retina using ophthalmic
microscopy for RT-qPCR detection of the expression of CTGF and
HO-1.
Expression of CTGF and HO-1 in rat
retina after RT-qPCR
TRIzol reagent was used to cleave and extract 100 mg
of retinal tissues, and the UV spectrophotometer to identify the
RNA concentration and purity. OD260/OD280 between 1.8 and 2.0
showed that the specimen purity passed. Preparing 25 µl reverse
transcription reaction system: 5X buffer 5 µl, MMLV (200 U/µl) 1
µl, 4X dNTP (10 mmol/l) 1.25 µl, RNasin (25 U/µl) 0.7 µl, RNase
free H2O added to 25 µl. The reverse transcription was
performed with PCR instrument at 42°C for 15 min and at 85°C for 5
sec. The synthesized cDNA amplified according to the prepared 25 µl
reaction system (SYBR Premix Ex Taq II 12.5 µl, upstream primer 1
µl, downstream primer 1 µl and 10X cDNA 2 µl) (Table I). The reaction process was a
three-step process: the first step was at 95°C for 30 sec, the
second step for a total of 40 cycles, each cycle included 95°C for
5 sec, 60°C for 30 sec and 72°C for 40 sec, and the third step at
72°C for 7 min. β-actin was used as internal reference, and primer
sequences were synthesized by Sangon Biotech Co., Ltd. The Ct
values obtained by PCR were analyzed and compared, and the relative
expression of CTGF and HO-1 was calculated. Experimental results
were analyzed using the 2−ΔΔCq method (14).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Upstream primers | Downstream
primers |
|---|
| β-actin |
5′-AACCCTAAGGCCAACAGTGAAAAG-3′ |
5′-TCATGAGGTAGTCTGTGAGGT-3′ |
| CTGF |
5′-CCGCCAACCGCAAGATT-3′ |
5′-CACGGACCCACCGAAGAC-3′ |
| HO-1 |
5′-GGAAGAGGAGATAGAGCGAAAC-3′ |
5′-AGAGGTCACCCAGGTAGCG-3′ |
H&E staining
Paraffin sections were deparaffinized and washed 5
times with 95, 75 and 50% ethanol, respectively. They were washed
with distilled water once, then placed in hematoxylin for 5 min and
washed with distilled water again after staining. Paraffin sections
were soaked in hydrochloric acid alcohol with a concentration of 1%
for 15 sec to differentiate, and in ammonia water with a
concentration of 1% for 20 sec back to blue after washed with clean
water, and in eosin alcohol for 1 min to stain after washed with
clean water. Paraffin sections were dehydrated with a low to high
concentration gradient of alcohol after being washed with clean
water, and soaked in xylene for 2 min for transparency. Histomount
was sealed and placed under a light microscope (Olympus, Shanghai,
China) for photography and observation.
TUNEL apoptotic cell detection
Paraffin sections were deparaffinized with xylene
and alcohol at a gradient concentration, soaked in 50X proteinase K
for 15 min at room temperature, and washed twice with PBS, and then
TUNEL reaction solution (50 µl) was added and incubated in a wet
box at 37°C incubator for 1 h. After drying the condensed drops of
water of the sample, 50 µl of POD conversion agent was added and
incubation was continued for 30 min. A total of 50 µl of DAB
solution was added and incubation was continued for 10 min. The
steps were the same as the H&E steps after the incubation,
staining, differentiation, back to blue, dehydration, transparency,
sealing and light microscopic observation.
The dark brown nucleus was considered to be an
apoptotic cell. Each specimen was selected to count the apoptotic
cells around the target area and in the center under the light
microscope (magnification, ×400). The ratio of the number of
apoptotic cells to the total number of cells in the visual field
was obtained and its average value was the apoptosis rate.
Statistical analysis
The SPSS20.0 [AsiaAnalytics (formerly SPSS China),
Shanghai, China] software package was used for processing. The
comparison of data between the two groups was performed using KS
non-parametric analysis test or t-test based on the distribution
characteristics. The comparison of data between groups was
performed using ANOVA analysis and Dunnetts post hoc test. The
comparison at different time-points in the group was the repeated
measures of variance analysis. The significance level is
α=0.05.
Results
Body weight and blood glucose
monitoring in rats
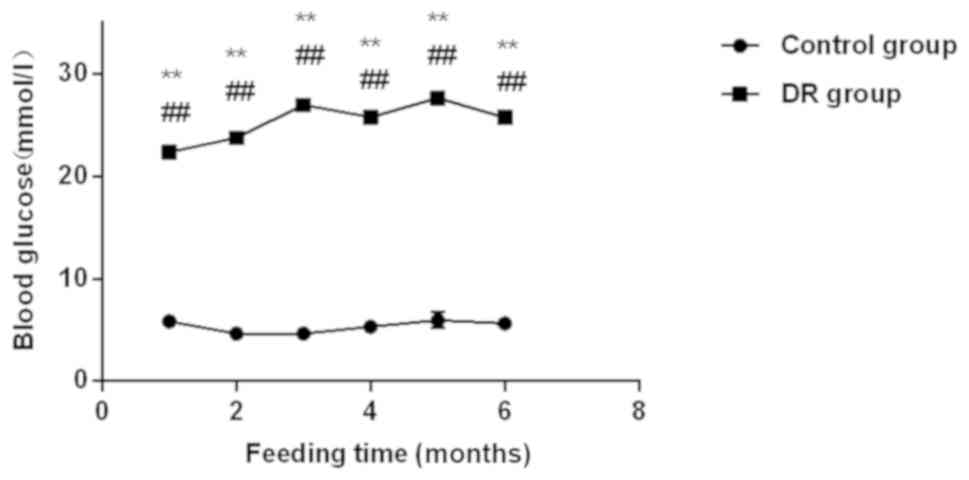
The experimental results showed that the blood
glucose levels in the DR group at the 1st, 2nd, 3rd, 4th, 5th, and
6th month were 22.36±5.37, 23.76±6.75, 26.96±7.64, 25.76±5.76,
27.65±7.32 and 25.75±5.75, respectively. Τhe blood glucose
concentrations in rats in the control group were 5.86±0.37,
4.65±0.57, 4.64±0.47, 5.32±0.63, 5.97±0.75 and 5.64±0.62,
respectively. Compared with the control group, the blood sugar
level of rats in the DR group significantly increased monthly,
while that in the control group was <6 mmol/l all the time,
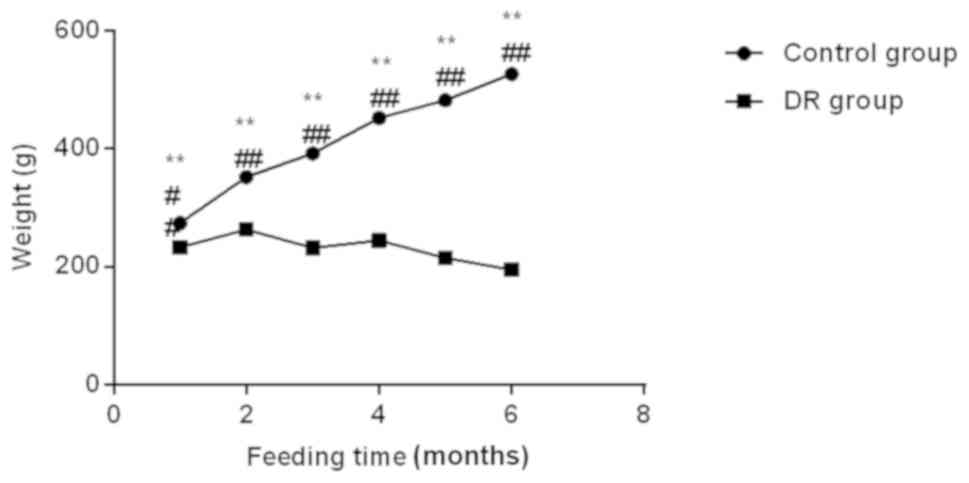
within the normal range (all P<0.01) (Fig. 1). The body weight of rats in the
control group increased steadily with the increasing age during the
breeding process (all P<0.01), while that of the DR group showed
a general trend of decline and the monthly body weight was lower
than that in the control group (P<0.01) (Fig. 2).
mRNA expression of CTGF and HO-1 in
rat retina
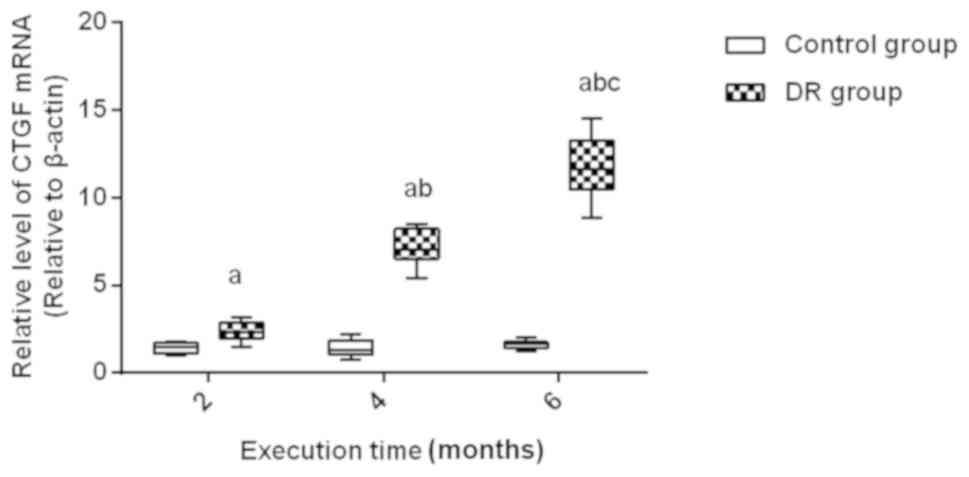
Through detection of the mRNA expression of CTGF and
HO-1 after the rats were sacrificed at the 2nd, 4th and 6th month,
it was indicated that the expression of CTGF mRNA in the DR rats at
the 2nd, 4th and 6th month was higher than that in the control
group at the same time (P<0.01); there was no significant
fluctuation in the expression of CTGF mRNA at the 2nd, 4th and 6th
month in the control group (P>0.05). The expression of CTGF mRNA
in the DR group gradually increased at the 2nd, 4th and 6th month
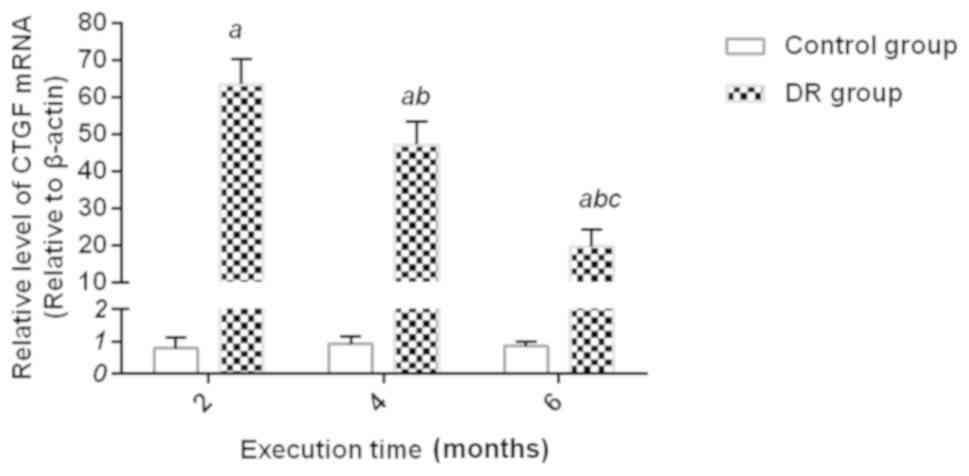
(all P<0.01) (Fig. 3).; there was
only a small amount of expression of HO-1 mRNA in retina in the
control group at the 2nd, 4th and 6th month, and there was no
statistical difference. The expression of HO-1 mRNA at the 2nd, 4th
and 6th month in the DR group was higher than that in the control
group, but its expression gradually decreased as time increased
(all P<0.01) (Fig. 4).
Results of H&E staining
In the control group, the retinal surface of the
rats was smooth, with clear layers, complete structure and regular
arrangement of cells at the 2nd, 4th and 6th month. In the DR
group, the layers of the retina were blurred and a few blood
vessels were seen to dilate at the 2nd month. In the DR group, the
inner membrane of the retina showed different sizes of swelling,
and the cells were irregularly arranged at the 4th month. In the DR
group, most of the blood vessels in the retina dilated and were
more obvious, the degree of membrane edema was more severe, and the
arrangement was very irregular and almost indistinguishable at the
6th month.
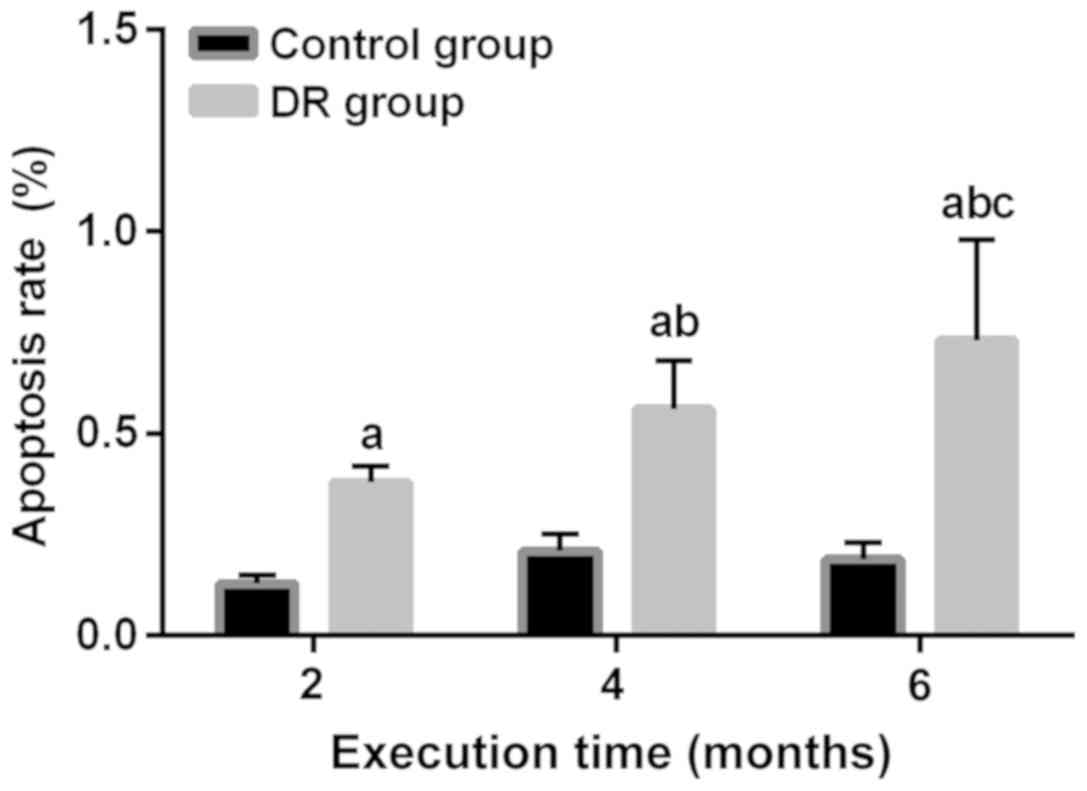
Apoptotic cells detection
Through apoptotic cells detection in rats at the
2nd, 4th and 6th month, it was found that the apoptotic rates of
rats in the DR group at the 2nd, 4th and 6th month were higher than
those in the control group at the same time (P<0.01). There was
no difference in expression of the apoptotic rate among the 2nd,
4th and 6th month in the control group (P>0.05). In addition,
the apoptotic rates in the DR group increased gradually at the 2nd,
4th and 6th month (all P<0.01) (Fig.
5).
Discussion
Diabetes is one of the three major diseases that
endanger human physical and mental health in the world today
(15). In recent years, with the
improvement of people's living standards and the changes of living
habits, the incidence of diabetes has been rising year after year,
and the number of DR patients has also been increasing each year
(16). DR is the result of a
combination of factors, and it is chronic, long-term, irreversible,
and widely damaging. Its various symptoms and mechanisms are
associated with hyperglycemia (17).
CTGF is extensively present in the human body, and it can
accelerate mitosis, favor cell proliferation, induce apoptosis and
have a chemotaxis effect (18). The
overexpression of CTGF in certain pathological conditions can lead
to a series of pathophysiological processes such as angiogenesis
and fibrosis (19). Oxidative stress
and high glucose environment can activate the HO-1 expression that
can upregulate anti-oxidation and anti-inflammatory factors, thus
protecting the retina tissue (20).
In this study, the rats were detected monthly for
blood glucose and body weight after successful modeling, the levels
of which in the DR group were consistently higher than those in the
control group and increased with time. This showed that the DR
model we built is successful and can be maintained until the end of
the study. Through detection of the mRNA expression of CTGF and
HO-1 after the rats were sacrificed at the 2nd, 4th and 6th month,
it was shown that those in the DR group at the 2nd, 4th and 6th
month were higher than those in the control group at the same time.
The expression of CTGF mRNA gradually increased at the 2nd, 4th and
6th month in the DR group. In the analysis of changes in CTGF serum
in proliferative DR patients, Baelde et al (21) also found that the CTGF expression
increased, which is consistent with this article; while the
expression of HO-1 mRNA gradually decreased as time increased.
Results of H&E staining also showed that with the passage of
time in the DR group, the retinal swelling degree became more and
more severe, the layers were more blurred, the structure
incomplete, the arrangement of cells irregular, and the blood
vessel dilatation more serious. Apoptotic cell detection showed
that the apoptotic rates of rats at the 2nd, 4th and 6th month in
the DR group were higher than those in the control group at the
same time, and they increased gradually at the 2nd, 4th and 6th
month in the DR group. Many studies have shown that HO-1 has
anti-oxidant and anti-inflammatory protective effects (3), but in this study, detection of the
expression in the DR group indicated that it was higher than that
in the control group. However, it gradually decreased over time.
This may be due to the fact that the HO-1 expression is low under
normal conditions, but it increases after oxidative stress and high
glucose stress and reaches the peak for a period of time. Over
time, this response to stress gradually decreases, so the
expression gradually decreases from the peak, and its protective
effect gradually weakens. When it is not enough to fight the
DR-inducing effect of other genes, the apoptosis of retinal cells
gradually increases. At the same time, as diabetes progresses, CTGF
gradually increases, more blood vessels are newly formed, fibrosis
is induced, and the retina gradually flakes off, exacerbating DR.
Sun et al (22) showed that
the combination of TGF-β1 and cerebroside carnosine injection can
increase the contents of CTGF and HO-1 in peripheral neuropathy
tissue of type 2 diabetes, so as to have a better therapeutic
effect. However, in this study, the increase of CTGF promoted the
occurrence of DR, because the mitogenicity of CTGF can indeed help
to repair tissue, but it promoted fibrosis at the same time, and in
the case of retinal tissue, which is particularly required for
toughness, this fibrosis undoubtedly damaged the structure and
function of the retina.
The conjecture on the role of HO-1 in the occurrence
and development of DR and the explanation for the contradiction
between the HO-1 expression and the apoptotic rate need to be
verified in future experiments, to observe the apoptosis rate of
diabetic patients and the retina situation after inhibiting the
HO-1 expression. Similarly, the role of CTGF and the above analysis
of the results also need similar experiments for verification, and
the specific mechanism of signaling pathway needs to be further
studied.
In conclusion, CTGF and HO-1 are associated with the
occurrence and development of DR in rats. CTGF may induce the
occurrence of DR and HO-1 may play a protective role in diabetic
retinal tissues. CTGF and HO-1 can be considered as targets for the
treatment of DR.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and CQ performed PCR. JZ and JX were responsible
for TUNEL assay. YH and JX helped with H&E staining. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Third People's Hospital of Changzhou (Changzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pedersen BK: Anti-inflammatory effects of
exercise: Role in diabetes and cardiovascular disease. Eur J Clin
Invest. 47:600–611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Welborn TA, Garcia-Webb P, Bonser A,
McCann V and Constable I: Clinical criteria that reflect C-peptide
status in idiopathic diabetes. Diabetes Care. 6:315–316. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song Y, Huang L and Yu J: Effects of
blueberry anthocyanins on retinal oxidative stress and inflammation
in diabetes through Nrf2/HO-1 signaling. J Neuroimmunol. 301:1–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NCD Risk Factor Collaboration (NCD-RisC):
Worldwide trends in diabetes since 1980: A pooled analysis of 751
population-based studies with 4.4 million participants. Lancet.
387:1513–1530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Q, Zhang F, Zhang X, Cheng R, Ma JX,
Yi J and Li J: Fenofibrate ameliorates diabetic retinopathy by
modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol
Cell Biochem. Dec 21–2017.(Epub ahead of print).
|
|
6
|
Keech AC, Mitchell P, Summanen PA, O'Day
J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson
E, et al FIELD study investigators, : Effect of fenofibrate on the
need for laser treatment for diabetic retinopathy (FIELD study): A
randomised controlled trial. Lancet. 370:1687–1697. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Henshaw FR, Boughton P, Lo L, McLennan SV
and Twigg SM: Topically applied connective tissue growth
factor/CCN2 improves diabetic preclinical cutaneous wound healing:
Potential role for CTGF in human diabetic foot ulcer healing. J
Diabetes Res. 2015:2362382015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu F, Tang W, Chen D, Li M, Gao Y, Zheng
H and Chen S: Expression of TGF-β1 and CTGF is associated with
fibrosis of denervated sternocleidomastoid muscles in mice. Tohoku
J Exp Med. 238:49–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montford JR and Furgeson SB: A new CTGF
target in renal fibrosis. Kidney Int. 92:784–786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klaassen I, van Geest RJ, Kuiper EJ, van
Noorden CJ and Schlingemann RO: The role of CTGF in diabetic
retinopathy. Exp Eye Res. 133:37–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Cai X, Yi B, Huang J, Wang J and
Sun J: Correlation of CTGF gene promoter methylation with CTGF
expression in type 2 diabetes mellitus with or without nephropathy.
Mol Med Rep. 9:2138–2144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Wang L, Tian XY, Liu L, Wong WT,
Zhang Y, Han QB, Ho HM, Wang N, Wong SL, et al: Unconjugated
bilirubin mediates heme oxygenase-1-induced vascular benefits in
diabetic mice. Diabetes. 64:1564–1575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YC, Chen BC, Yu CC, Lin SH and Lin
CH: miR-19a, −19b, and −26b mediate CTGF expression and pulmonary
fibroblast differentiation. J Cell Physiol. 231:2236–2248. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zinman B, Lachin JM and Inzucchi SE:
Empagliflozin, cardiovascular outcomes, and mortality in type 2
diabetes. N Engl J Med. 374:10942016.PubMed/NCBI
|
|
16
|
Pothineni NV and Mehta JL: Follow-up of
glycemic control and cardiovascular outcomes in type 2 diabetes. N
Engl J Med. 373:977–978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piette JD, Heisler M and Wagner TH:
Problems paying out-of-pocket medication costs among older adults
with diabetes. Diabetes Care. 27:384–391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Duan L, Guo T, Gao Y, Tian L, Liu
J, Wang S and Yang J: Downregulation of miR-30c promotes renal
fibrosis by target CTGF in diabetic nephropathy. J Diabetes
Complications. 30:406–414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu L, Zhao S, Liu S, Liu Q, Li F and Hao
J: PTEN regulates renal extracellular matrix deposit via increased
CTGF in diabetes mellitus. J Cell Biochem. 117:1187–1198. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Negi G, Nakkina V, Kamble P and Sharma SS:
Heme oxygenase-1, a novel target for the treatment of diabetic
complications: Focus on diabetic peripheral neuropathy. Pharmacol
Res. 102:158–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baelde HJ, Eikmans M, Lappin DW, Doran PP,
Hohenadel D, Brinkkoetter PT, van der Woude FJ, Waldherr R,
Rabelink TJ, de Heer E, et al: Reduction of VEGF-A and CTGF
expression in diabetic nephropathy is associated with podocyte
loss. Kidney Int. 71:637–645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun J, Zheng H, Qin X and Qi L: Effects of
immunocytokine combined with cattle encephalon glycoside and
ignotin on CTGF, HO-1 and NT-3 in patients with type 2 diabetic
peripheral neuropathy. Iran J Public Health. 46:1632–1638.
2017.PubMed/NCBI
|