Introduction
Bitter perception has a critical role in host
defense, as it functions as a warning signal against the ingestion
of toxic substances (1). The
recognition of bitter substances is mediated by a family of G
protein-coupled receptors called type 2 taste receptors (TAS2Rs),
which are prominently expressed in the tongue and include >20
members. However, recent investigations revealed that TAS2Rs are
widely expressed in extra-oral tissues, including the gastric and
intestinal mucosa, respiratory tract, bladder, pancreas, testes and
central nervous system (2,3), indicating TAS2Rs may perform numerous
other functions other than perception of bitter taste.
Based on their agonist spectra, TAS2Rs may be
grouped into broadly, narrowly, and intermediately tuned receptors
(4); TAS2R10, TAS2R14 and TAS2R46
are broadly tuned receptors (5) and
each recognizes about one-third of the bitter components tested so
far (6). As such, these broadly
tuned receptors may considerably contribute to the overall bitter
tasting ability and it may be speculated that each of them may have
numerous biological functions for different bitter substances that
may exert various effects in the human body. Recent investigations
on TAS2R10 confirmed this speculation. TAS2R10 induces the
relaxation of smooth muscles of the ileum; this action may be
mediated by nitric oxide and BKCa channels (7). TAS2R10 also dilates the airways by
inhibiting airway smooth muscle calcium oscillations and calcium
sensitivity (8). Furthermore,
TAS2R10 was reported to mediate relaxation of vascular smooth
muscles (9). Of note, TAS2R10 also
has a tumor suppressor role in human neuroblastoma cells (10).
Based on the aforementioned studies, the present
study hypothesized that TAS2R10 has multiple biological functions.
To prove this hypothesis, a bioinformatics analysis of genes
positively co-expressed with TAS2R10 was performed using 60,000
Affymetrix expression arrays and 5,000 TCGA datasets. The analysis
indicated that TAS2R10 is mostly involved in biological activities,
including ‘positive regulation of biological process’, ‘cellular
protein metabolic process’, ‘protein modification process’,
‘cellular protein modification process’ and ‘cellular component
assembly’. The most prominent terms in the category cellular
component were ‘Spt-Ada-Gcn5 acetyltransferase (SAGA)-type complex’
and ‘SAGA complex’. The major terms in the category molecular
function were ‘hexosaminidase activity’, ‘cytoskeletal adaptor
activity’, ‘cyclin binding’ and ‘β-N-acetylhexosaminidase
activity’. Of note, it was indicated that TAS2R10 may be involved
in ‘ubiquitin-mediated proteolysis’, which may provide a key
direction for investigating the functions of TAS2R10 in the future.
TAS2R10 was also suggested to be linked to human diseases, i.e.
‘Salmonella infection’. To the best of our knowledge, the present
study was the first to comprehensively reveal the potential
biological roles of TAS2R10 and provide crucial insights into the
notion that this gene may have critical roles in addition to the
perception of bitterness.
Materials and methods
Collection of expression data and
pre-processing
The gene expression data were collected and
pre-processed as described previously (11). The required data were retrieved from
the European Bioinformatics Institute database (https://www.ebi.ac.uk/) and incorporated CEL files
were pre-processed using the robust multichip average normalization
method. The value 0.25 of the standard deviation level was used as
the cutoff to guarantee for high-quality data and to capture
significantly correlated transcriptome information. TAS2R10 was
performed using 60,000 Affymetrix expression arrays and 5,000 TCGA
datasets.
Biological pathway analysis
Gene Ontology (GO) function and Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathway analyses were performed
(11). Analysis of biological
pathways was performed for the genes that exhibited the highest
correlation with TAS2R10 expression levels. The associated
parameters used were identical to those of a previous study
(11).
Statistical analysis
The statistical analysis was performed as described
previously (11). Pearson
correlation analysis was performed to assess the correlation
between TAS2R10 and other genes and the P-values were unmodified.
The q-value package in R was utilized to implement multiple testing
corrections. Genes with q-values of <0.05 were considered as
significant co-expressed genes of TAS2R10.
Results
TAS2R10 may be involved in a variety
of biological functions according to GO analysis
To reveal the potential functions of TAS2R10, a big
data bioinformatics analysis, namely a positive co-expression
analysis using 60,000 Affymetrix expression arrays and 5,000 TCGA
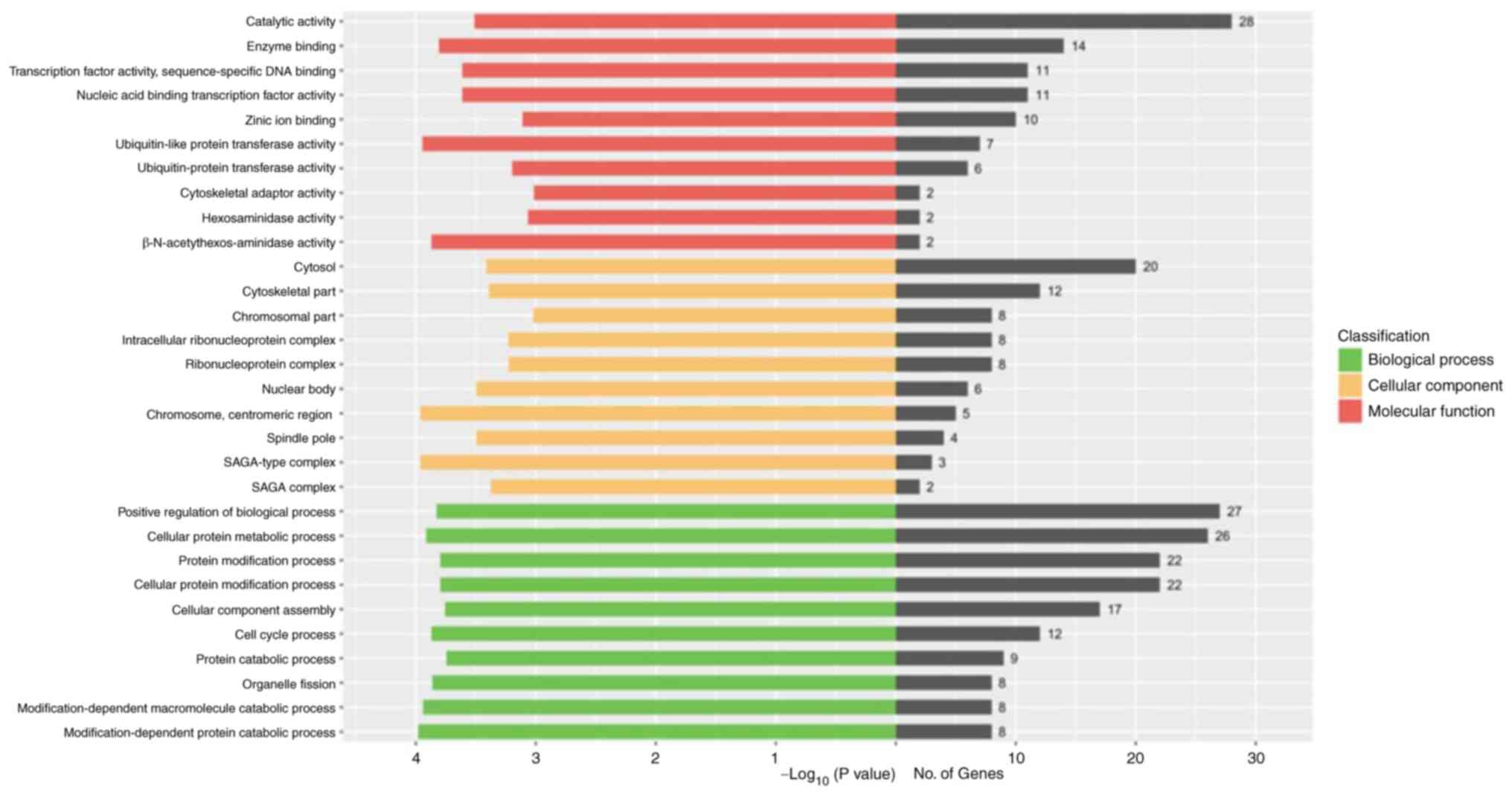
data sets, was performed. As indicated in Fig. 1, GO enrichment analysis indicated
that TAS2R10 may be involved in a myriad of functional terms in the
category biological process, and the top five processes were
‘positive regulation of biological process’, ‘cellular protein
metabolic process’, ‘protein modification process’, ‘cellular
protein modification process’ and ‘cellular component assembly’.
The top 5 enriched functional terms in the category cellular
component included ‘cytosol’, ‘cytoskeletal part’, ‘chromosomal
part’, ‘intracellular ribonucleoprotein complex’ and
‘ribonucleoprotein complex’. The top five terms in the category
molecular functions were ‘catalytic activity’, ‘enzyme binding’,
‘transcription factor activity’, ‘sequence-specific DNA binding’,
‘nucleic acid binding transcription factor activity’ and ‘zinc ion
binding’.
To provide further information on the functions of
TAS2R10, the top 20 items were revealed by searching the databases
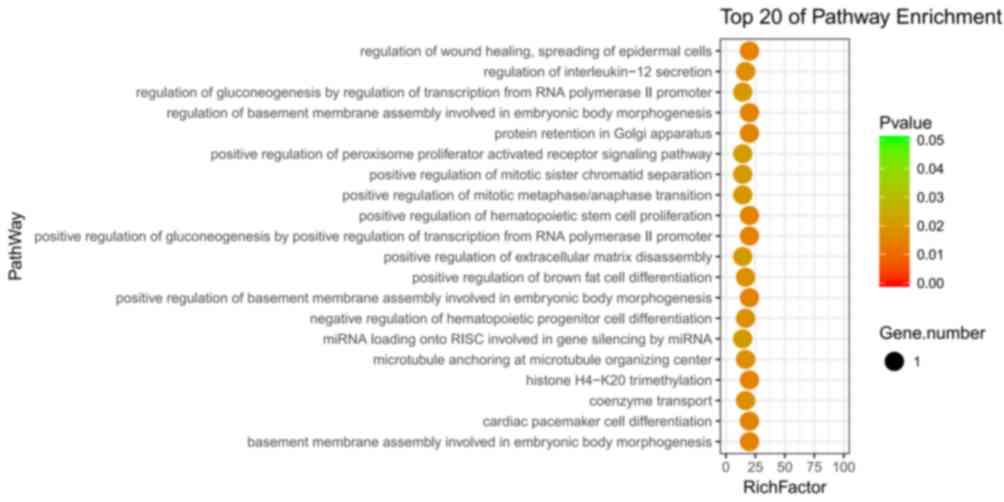
based on the GO analysis. Fig. 2
provides the top 20 biological process terms obtained using
enrichment analysis. The top 20 biological processes included
‘regulation of wound healing’, ‘spreading of epidermal cells’,
‘regulation of interleukin-12 secretion’, ‘regulation of
gluconeogenesis by regulation of transcription from RNA polymerase
II promoter’, ‘regulation of basement membrane assembly involved in
embryonic body morphogenesis’, ‘protein retention in Golgi
apparatus’, ‘positive regulation of peroxisome proliferator
activated receptor signaling pathway’, ‘positive regulation of
mitotic sister chromatid separation’, ‘positive regulation of
mitotic metaphase/anaphase transition’, ‘positive regulation of
hematopoietic stem cell proliferation’, ‘positive regulation of
gluconeogenesis by positive regulation of transcription from RNA
polymerase II promoter’, ‘positive regulation of extracellular
matrix disassembly’, ‘positive regulation of brown fat cell
differentiation’, ‘positive regulation of basement membrane
assembly involved in embryonic body morphogenesis’, ‘negative
regulation of hematopoietic progenitor cell differentiation’,
‘microRNA (miRNA) loading onto retinoid inducible serine
carboxypeptidase involved in gene silencing by miRNA’, ‘microtubule
anchoring at microtubule organizing center’, ‘histone H4-K20
trimethylation’, ‘coenzyme transport’, ‘cardiac pacemaker cell
differentiation’ and ‘basement membrane assembly involved in
embryonic body morphogenesis’ (Fig.
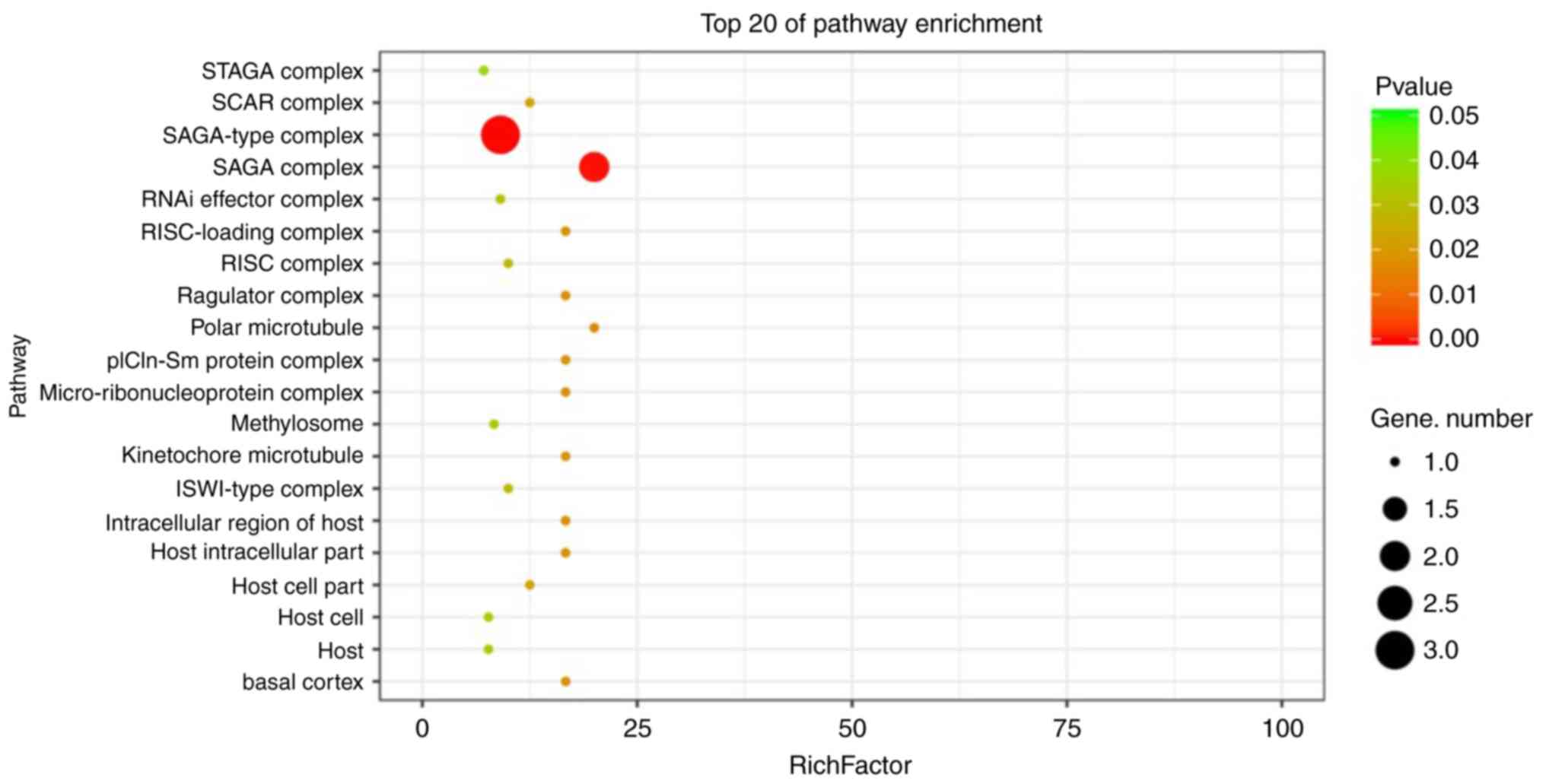
2). The most prominent terms in the category cellular component
were ‘SAGA-type complex’ and ‘SAGA complex’ (Fig. 3). The molecular functions covered
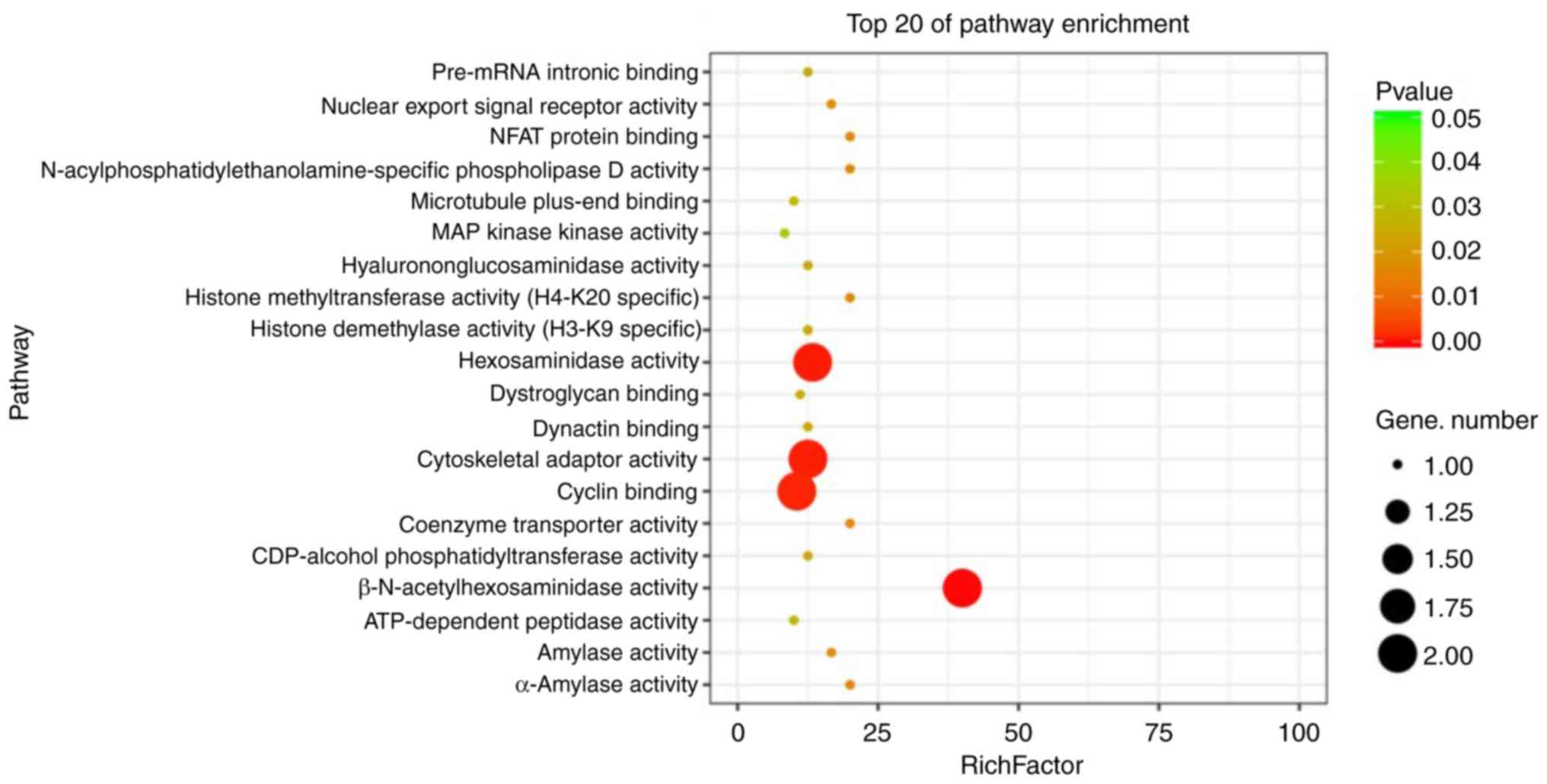
‘hexosaminidase activity’, ‘cytoskeletal adaptor activity’, ‘cyclin
binding’ and ‘β-N-acetylhexosaminidase activity’ (Fig. 4). Furthermore, in order to confirm
the results of the bioinformatics analysis, the expression of
TAS2R10 was examined in different human cell lines, HeLa, TPC1,
CAPAN-2, HEK293, HEPG2, A549, Caco-2, MCF7 and RT4 (Bena Culture
Collection) by reverse transcription-quantitative (RT-q)PCR and
western blot analysis (Fig. S1). It
was indicated that TAS2R10 was mainly expressed in the cell lines
HeLa, TPC1 and CAPAN-2 at different levels, suggesting that TAS2R10
may have different functions in different tissue types. It may
perform biological functions in the tissues of uterus, thyroid and
pancreas, and exhibit no functions in other tissues, kidney, liver,
lung, colon, breast, and bladder.
TAS2R10 may take part in various
signaling pathways according to KEGG analysis
To further identify the pathways involving TAS2R10,
a KEGG analysis was performed based on the genes positively
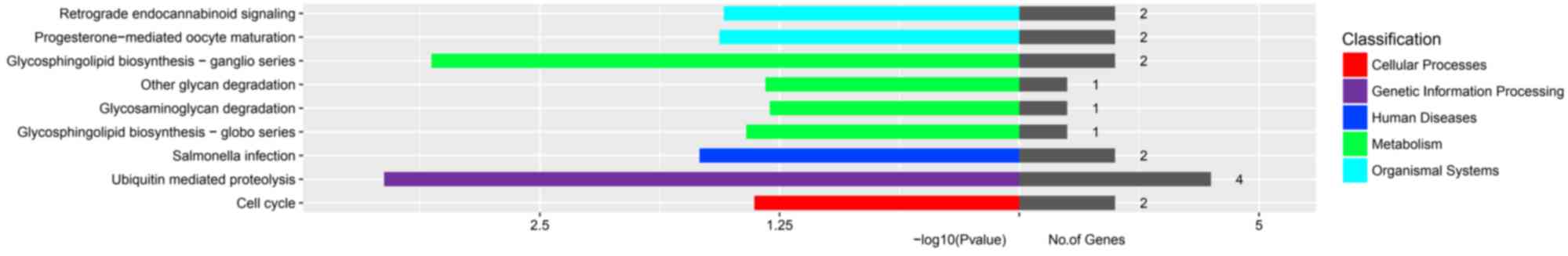
co-expressed with TASR10 (Fig. 5).
In the item ‘organismal systems’, TAS2R10 was positively associated
with ‘retrograde endocannabinoid signaling’ and
‘progesterone-mediated oocyte maturation’. In the item ‘genetic
information processing’, TAS2R10 was positively linked to
‘ubiquitin-mediated proteolysis’. In addition, TAS2R10 was
indicated to be involved in numerous metabolic activities,
including ‘glycosphingolipid biosynthesis-ganglio series’, ‘other
glycan degradation’, ‘glycosaminoglycan degradation’ and
‘glycosphingolipid biosynthesis-globo series’. Of note, TAS2R10 was
indicated to be involved in certain pathogenic processes/human
diseases, specifically ‘Salmonella infection’. These functional
pathways are not only associated with the tongue but also are
involved in extra-oral functions.
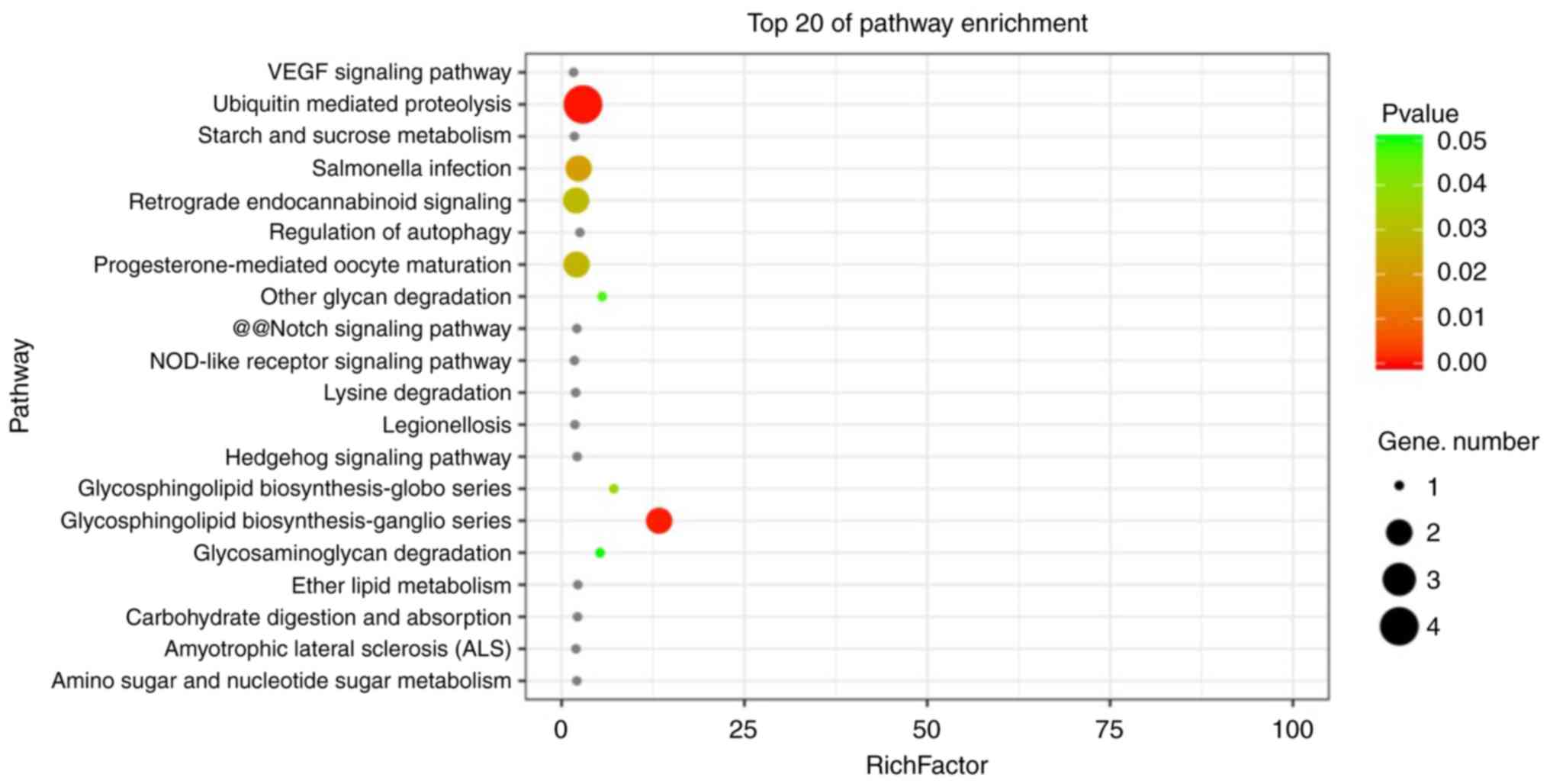
To provide further information on the signaling
pathways of TAS2R10, an enrichment analysis was used to determine
the top 20 pathways (Fig. 6). The
top five pathways are ‘ubiquitin mediated proteolysis’, ‘Salmonella
infection’, ‘retrograde endocannabinoid signaling’,
‘progesterone-mediated oocyte maturation’ and ‘glycosphingolipid
biosynthesis-ganglio series’. Among these pathways, ‘ubiquitin
mediated proteolysis’ ranked first, followed by ‘glycosphingolipid
biosynthesis-ganglio series’. For ubiquitin-mediated pathway, the
potential targets for TAS2R10 include anaphase promoting complex
subunit 5 (ANAPC5) and ubiquitin protein ligase E3B were predicted.
Furthermore, in order to confirm the results of the bioinformatics
analysis, the association of TAS2R10 and ANAPC5 in human thyroid
was examined by using RT-qPCR (Fig.
S2). ANAPC5 is required for the proper ubiquitination function
(12). It was indicated that the
expression levels of TAS2R10 were significantly associated with
those of ANAPC5, suggesting TAS2R10 may be involved in the
ubiquitin pathway.
Discussion
Bitterness sensing is mediated by the TAS2R family
occurring in the tongue (1). The
wide expression of TAS2Rs in tissues other than the tongue has been
reported in recent years (2,3). Thus, TAS2Rs were postulated to have
extra-oral biological roles. However, at present, functional
investigations are limited and scattered. In the present study, a
comprehensive investigation of the potential functions of TAS2R10
was performed by positive co-expression analysis using 60,000
Affymetrix expression arrays and 5,000 TCGA datasets. Various
noteworthy results were obtained. First, TAS2R10 may be involved in
various biological activities beyond the perception of bitterness
with a focus on protein modification and metabolic processes. The
present experimental results confirmed that TAS2R10 was mainly
expressed in the cell lines HeLa, TPC1 and CAPAN-2, suggesting that
TAS2R10 may be involved in different biological activities in
different tissues. Furthermore, major GO terms in the category
cellular component were ‘SAGA-type complex’ and ‘SAGA complex’. In
addition, ‘ubiquitin mediated proteolysis’ is a typical pathway
involving TAS2R10. Finally, TAS2R10 may be involved in ‘Salmonella
infection’. With regard to the ubiquitin pathway, the potential
targets for TAS2R10 include ANAPC5 and ubiquitin protein ligase E3B
based on the present bioinformatics analysis. ANAPC5 is a ubiquitin
ligase that controls cell cycle progression through ubiquitination
(12). In the present study, the
association of TAS2R10 and ANAPC5 in human thyroid was confirmed by
RT-qPCR. These results indicate that TAS2R10 may be involved in the
ubiquitin pathway through ANAPC5.
TAS2R10 is known to be a broadly tuned bitter
receptor (5), and it was able to
recognize approximately one-third of the bitter components tested
thus far (6). It may be speculated
that TAS2R10 is able to perform numerous biological functions.
Recent studies have indicated other functions of TAS2R10 than
bitterness-sensing (10). However,
only two functions have been reported. One function of TAS2R10 is
that it is able to induce the relaxation of smooth muscles of the
ileum (7), airway (8) and blood vessels (9). In the present study, it was observed
that TAS2R10 features cytoskeletal adaptor activity and is also
associated with hexosaminidase activity. These activities are
linked to the role of smooth muscles. The second function reported
for TAS2R10 was its tumor-suppressor role (10). In the present study, TAS2R10 was
indicated to be involved in ‘cellular processes of cell cycle’ and
may thus exert a regulatory function in cancer.
In conclusion, through positive co-expression
analysis using 60,000 Affymetrix expression arrays and 5,000 TCGA
datasets, the present study not only confirmed the previously
reported functions but also revealed novel roles of TAS2R10. It was
observed that TAS2R10 is associated with ‘ubiquitin mediated
proteolysis’ and ‘Salmonella infection’, which may serve as a
reference for detailed investigation of the functions of TAS2R10 in
the future. To the best of our knowledge, the present study was is
the first to comprehensively investigate the biological functions
of TAS2R10, providing crucial insight into the concept that this
gene may have critical roles other than bitterness sensing.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported and the publication
costs were covered in part by grants from Specialty Feature
Construction Project of Pudong Health and Family Planning
Commission of Shanghai (grant no. PWZzb2017-22), Key Discipline
Construction Project of Pudong Health and Family Planning
Commission of Shanghai (grant no. PWZxk2017-07), Key Specialty
Construction Project of Pudong Health and Family Planning
Commission of Shanghai (grant no. PWZzk2017-29), Key Disciplines
Group Construction Project of Pudong Health and Family Planning
Commission of Shanghai (grant no. PWZxq2017-17), Pudong New Area
Science and Technology Commission (grant nos. PKJ2019-Y21,
PKJ2016-Y03 and PKJ2018-Y08), and Shanghai Health and Family
Planning Commission (grant no. 201640177), Outstanding Leaders
Training Program of Pudong Health Bureau of Shanghai (grant no.
PWRl2018-02).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
HZ, MG and XL conceived and designed the study; SH
and ZZ collected the data; XZ, CS, XW and CZ performed the
analysis; LR, LL and JM wrote the manuscript and performed the
experiments All of the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Punan Hospital of Pudong New District (Shanghai,
China). All of the patients provided written informed consent to
participate in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li D and Zhang J: Diet shapes the
evolution of the vertebrate bitter taste receptor gene repertoire.
Mol Biol Evol. 31:303–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kook JH, Kim HK, Kim HJ, Kim KW, Kim TH,
Kang KR, Oh DJ and Lee SH: Increased expression of bitter taste
receptors in human allergic nasal mucosa and their contribution to
the shrinkage of human nasal mucosa. Clin Exp Allergy. 46:584–601.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colombo M, Trevisi P, Gandolfi G and Bosi
P: Assessment of the presence of chemosensing receptors based on
bitter and fat taste in the gastrointestinal tract of young pig. J
Anim Sci. 90 (Suppl 4):S128–S130. 2012. View Article : Google Scholar
|
|
4
|
Behrens M and Meyerhof W: Bitter taste
receptor research comes of age: From characterization to modulation
of TAS2Rs. Semin Cell Dev Biol. 24:215–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowak S, Di Pizio A, Levit A, Niv MY,
Meyerhof W and Behrens M: Reengineering the ligand sensitivity of
the broadly tuned human bitter taste receptor TAS2R14. Biochim
Biophys Acta Gen Subj. 1862:2162–2173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyerhof W, Batram C, Kuhn C, Brockhoff A,
Chudoba E, Bufe B, Appendino G and Behrens M: The molecular
receptive ranges of human TAS2R bitter taste receptors. Chem
Senses. 35:157–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jing F, Liu M, Yang N, Liu Y, Li X and Li
J: Relaxant effect of chloroquine in rat ileum: Possible
involvement of nitric oxide and BKCa. J Pharm Pharmacol.
65:847–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan X and Sanderson MJ: Bitter tasting
compounds dilate airways by inhibiting airway smooth muscle calcium
oscillations and calcium sensitivity. Br J Pharmacol. 171:646–662.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manson ML, Safholm J, Al-Ameri M, Bergman
P, Orre AC, Swärd K, James A, Dahlèn SE and Adner M: Bitter taste
receptor agonists mediate relaxation of human and rodent vascular
smooth muscle. Eur J Pharmacol. 740:302–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo Y, Kim YS, Lee KE, Park TH and Kim Y:
Anti-cancer stemness and anti-invasive activity of bitter taste
receptors, TAS2R8 and TAS2R10, in human neuroblastoma cells. PLoS
One. 12:e01768512017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen M, Wang J, Luo Y, Huang K, Shi X, Liu
Y, Li J, Lai Z, Xue S, Gao H, et al: Identify Down syndrome
transcriptome associations using integrative analysis of microarray
database and correlation-interaction network. Hum Genomics.
12:22018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schreiber A, Stengel F, Zhang Z, Enchev
RI, Kong EH, Morris EP, Robinson CV, da Fonseca PC and Barford D:
Structural basis for the subunit assembly of the anaphase-promoting
complex. Nature. 470:227–232. 2011. View Article : Google Scholar : PubMed/NCBI
|