Introduction
MicroRNAs (miRNAs/miRs) are small, non-coding RNA
molecules that negatively regulate gene expression either by
messenger RNA (mRNA) degradation, or translational repression via
binding to the 3′-untranslated region (3′-UTR) of target genes.
miRNA dysfunction can play an important role in tumorigenesis and
tumor progression. miR200c is an important member of the miR200
family, which functions as a tumor suppressor and is aberrantly
expressed in various types of malignant tumors, including breast
(1,2), gastric (3,4),
colorectal (5) and ovarian cancer
(6), clear cell renal cell carcinoma
(7) and prostate cancer (8). Furthermore, miR200c has been shown to
be a key regulator of epithelial-mesenchymal transition (EMT)
(9,10) in breast and prostate cancers. A
number of genes, for example, those encoding BMI1 proto-oncogene,
polycomb ring finger (BMI1) (11),
E2F transcription factor 3 (11),
fibronectin 1 (FN1) (12), insulin
receptor substrate 1 (IRS1) (8),
PTEN (13), fascin actin-bundling
protein 1 (FSCN1) (14) and
platelet-derived growth factor receptor (PDGFR) (15), have been identified as distinct
miR200c target genes, which are involved in diverse biological
processes, including tumor cell proliferation, metabolism,
metastasis and angiogenesis. Downregulation of miR200c has been
shown to promote cell proliferation and metastasis, as well as to
induce stem-cell like phenotype, in various types of cancer
(16,17). Tang et al (18) reported reduced miR200c level in
prostate cancer cells by microarray analysis. A previous study
published by the authors' research group also demonstrated that
miR200c is downregulated in prostate cancer and that it was
associated with the growth and progression of prostate cancer cells
(8).
α-methylacyl-coenzyme A racemase (AMACR/P504S), an
enzyme involved in fatty acid metabolism, was shown to be
overexpressed in prostate cancer, which led to the promotion of
cell proliferation (18). The same
study also revealed that artificial suppression of AMACR with RNA
interference significantly inhibited the proliferation of prostate
cancer cells (19). It has also been
reported that AMACR is overexpressed in other tumor types,
including gastric (20) and colon
(21) adenocarcinoma, breast cancer
(1), gastrointestinal stromal tumors
(22), and myxofibrosarcomas
(23). Potential mechanisms of AMACR
overexpression in prostate cancer and other tumors include
transcriptional activation, for example, by CCAAT-enhancer-binding
protein (C/EBP, and gene amplification (21–26). On
the basis of the reduced expression of miR200c in prostate cancer
cells and the authors' pilot bioinformatics analysis, the present
study proposed the hypothesis that miR200c may be a negative
regulator of AMACR.
The aim of the present study was to investigate the
regulatory effect of miR200c on AMACR and to explore the biological
functions of the miR200c-AMACR axis. In addition, experimental
evidence is presented to show how miR200c directly targets AMACR by
binding to the 90–97 nucleotide (nt) seed sequence of the AMACR
mRNA 3′-UTR and that artificial upregulation of miR200c and/or
downregulation of AMACR suppresses prostate cancer cell
proliferation, migration and invasiveness.
Materials and methods
Cell lines and tissue samples
The human prostate cancer cell lines PC-3, DU145,
CL1, LNCaP and C4-2B were obtained from the American Type Culture
Collection. The cell lines were maintained in RPMI-1640 medium
(Life Technologies; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal calf serum (FCS; Life Technologies; Thermo Fisher
Scientific, Inc.) and 100 µg/ml penicillin and 100 µg/ml
streptomycin. The 293 cell line was obtained from the American Type
Culture Collection and maintained in DMEM (Life Technologies;
Thermo Fisher Scientific, Inc.) with 10% FCS. Cells were cultured
at 37°C with 5% CO2. Benign prostatic hyperplasia (BPH)
tissue (n=3; patient age 65–77) and prostate cancer tissue samples
(n=3; patient age 72–81; Gleason score 7–9) were collected from
March to May 2018 by transurethral resection of the prostate. All
tissue samples were collected in the West China Hospital according
to the ethical guidelines and procedures approved by the
institutional supervisory committee. Informed consent was obtained
according to the institutional guidelines. Fresh tissue samples
were stored immediately at −80°C.
RNA isolation, stem-loop reverse
transcription (RT) and conventional RT-PCR
Total RNA was isolated using TRIzol®
reagent (Life Technologies; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. SMART® MMLV
reverse transcriptase (Takara Biotechnology Co., Ltd.) was used for
RT (temperature protocol: 16°C for 30 min, 42°C for 30 min, 85°C
for 5 min and 10°C for 10 min). Stem-loop RT-PCR was employed to
examine the mature miR200c, using the primer for miR200c designed
with the sequence:
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCCATC-3′. The
random RT primer, 5′-(dN)9−3′ (Takara Biotechnology Co.,
Ltd.), was used for other genes. The PCR primers sequences were as
follows: miR200c (forward, 5′-GCATAGCCCGTAATACTGCCGGGTA-3′;
reverse, 5′-GTGCAGGGTCCGAGGT-3′, 67 bp); U6 (forward,
5′-TGGAACGATACAGAGAAGATTAGCA-3′; reverse,
5′-AACGCTTCACGAATTTGCGT-3′, 66 bp); AMACR (forward,
5′-gcttatttatgccaggctgag-3′; reverse, 5′-cttcccacagactcaatttctg-3′,
314 bp); and β-actin (forward, 5′-CTGGCACCACACCTTCTACAATG-3′;
reverse, 5′-CCTCGTAGATGGGCACAGTGTG-3′, 248 bp). Thermocycling
conditions were as follows: Initial denaturation at 95°C for 5 min,
30 cycles of denaturation at 95°C for 30 sec, annealing at 56°C for
30 sec and extension at 72°C before a final extension at 72°C for
10 min.
The amplification products were resolved by 2%
agarose gel electrophoresis and visualized by staining with the
fluorescent dye, Goldview™ (Beijing Solarbio Science &
Technology Co., Ltd.).
RT-quantitative (q)PCR
RT-qPCR was performed on a Light Cycler 2.0 RT-qPCR
instrument (Roche Diagnostics GmbH) using SYBRGreen as fluorescence
(Bio-Rad Laboratories, Inc.) and data were analyzed with Light
Cycler software, version 4.05 (Roche Diagnostics GmbH) as
previously described (27). The
β-actin gene was used as control. The copy number of target genes
(relative to β-actin) was determined using the 2−ΔΔCq
method (28), where
ΔΔCq=ΔCqexp-ΔCqcon=(Cqexp-target-Cqexp-actin)-(Cqcon-target-Cqcon-actin),
in which ‘exp’ represents the experimental group, ‘con’ the control
group and ‘target’ represents the gene of interest.
Western blot analysis
The primary antibodies used were as follows: AMACR
(rabbit monoclonal, cat. no. ab175280; 1:1,000; Abcam) and GAPDH
(mouse monoclonal, 1:5,000; cat. no. KC-5G4; Kangchen Bio-tech,
Inc.). Horseradish peroxidase-labeled secondary antibodies (Both
used at 1:10,000; goat anti-mouse IgG secondary antibody, cat. no.
31430 and goat anti-rabbit IgG secondary antibody, cat. no. 31460)
were from Zymed; Thermo Fisher Scientific, Inc. Western blotting
was performed as previously described (29). Briefly, after incubation, cells were
trypsinized and lysed with RIPA cell lysis buffer containing PMSF
(Beyotime Institute of Biotechnology) at 4°C for 10 min. The total
protein was obtained by centrifugation at 12,000 g at 4°C for 10
min and quantified with a Bicinchoninic acid protein assay kit
(Tiangen Biotech Co., Ltd.). Then total protein (40 µg/sample) was
separated on a 10% SDS-PAGE gel and transferred to polyvinylidene
difluoride membrane. After blocking with 5% nonfat milk at 37°C for
2 h, the membrane was incubated with antibodies against AMACR at
4°C overnight. After washing, the membrane was incubated with
HRP-secondary antibody at 37°C for 1 h. Then the membrane was
washed and analyzed with ECL detection system (EMD Millipore). The
expression of GAPDH was used as internal reference. Images were
captured using the ChemiDox XRS system (Bio-Rad Laboratories,
Inc.). Quantification was performed using ImageJ software (version
1.47; National Institutes of Health).
Recombinant adenoviral vectors for
overexpression of miR200c
The recombinant adenoviral vector for overexpression
of miR200c, Ad-miR200c, was constructed according to the protocol
previously reported (30). Briefly,
the pri-miR200c sequence was amplified from 293 cell genomic DNA.
PCR products were cloned into plasmid pMD19-T (Takara Biotechnology
Co., Ltd.), verified by sequencing and subcloned into shuttle
plasmid pAdTrack-CMV to construct pAdTrack-miR200c.
pAdTrack-miR200c, linearized with PmeI, was used to
transform BJ5183-AD-1 cells harboring the adenoviral pAdeasy-1
vector (Stratagene; Agilent Technologies, Inc.) for homologous
recombination. Colonies were screened by plasmid miniprep and
PacI restriction analysis to obtain clones with recombinant
miR200c (designated ‘pAdeasy-miR200c’). PacI-linearized
pAdeasy-miR200c was used to transfect 293 cells to obtain packaged
recombinant miR200c adenovirus (designated Ad-miR200c). Ad-miR200c
was amplified by repeated infection and verified by PCR. The
pAdTrack-CMV empty vector was used as control (designated
‘Ad-control’). The titers and the multiplicity of infection were
determined according to the manufacturer's protocols. After
transfection for 48 h, green fluorescence was observed using an
inverted fluorescence microscope (1X71; Olympus Corporation).
Luciferase reporter constructs and
site-directed mutagenesis
The seed sequences of AMACR 3′-UTR (90–97 nt) with
flanking sequences were amplified from the genomic DNA of 293
cells. The 3′-UTR of the AMACR mRNA was analyzed using TargetScan
6.2 (http://www.targetscan.org/). PCR
products were cloned into plasmid pMD19-T (Takara Biotechnology
Co., Ltd.) and subsequently subcloned into the vector pGL3-promoter
(Promega Corporation) and inserted into the 3′-UTR downstream of
the luciferase coding sequence. Overlapping PCR was used for
site-directed mutagenesis of the seed sequences (CAGTATTA mutated
to GGGGTTT) to construct pGL3-AMACR-UTRmutant (mut), the PCR
primers for which were 5′-ATGGAGGAAGGGGTTTCAGT-3′ (forward) and
5′-ACTGAAACCCCTTCCTCCAT-3′ (reverse).
Dual reporter gene assays
Cells were cultured in 24-well plates
(1×105 cells/well) and transfected with the reporter
constructs (0.8 µg) using Novagen® NanoJuice™
transfection reagent (EMD Millipore). The pRL-CMV vector (0.02 µg;
Promega Corporation) was co-transfected as the internal control.
The luciferase expression of pGL3-promoter plasmid was used as
baseline. Cells were infected with Ad-miR200c and subsequently
trypsinized with 0.25% trypsin at 48 h post-transfection. The
firefly and Renilla luciferase activities were determined
using a Luminometer TD-20/20 (Turner BioSystems) with the Dual
Luciferase® Reporter Assay system (Promega Corporation).
The relative luciferase activity was determined by calculating the
ratios of firefly/Renilla luciferase activities.
RNA interference
Double-stranded small-interfering RNAs (siRNAs) and
controls (si-CON, 5′- GCGCGCTTTGTAGGATTCG-3′) were designed,
synthesized, and purified by Guangzhou RiboBio Co., Ltd. The three
siRNAs targeting AMACR were designed as follows:
5′-GCCACGATATCAACTATTT-3′ (si-AMACR1), 5′-GCACCTTTCTATACGACTT-3′
(si-AMACR2) and 5′-GGAGGTTGTTCATCATGAT-3′ (si-AMACR3). Cells were
transfected with 0.1 nmol siRNA or si-CON using Novagen®
NanoJuice™ transfection reagent (EMD Millipore) and collected 24 h
later.
Artificial overexpression of AMACR in
PC-3 and DU145 cells infected by Ad-miR200c
The full-length cDNA of the AMACR coding sequence
was cloned into the thymine and adenine clone vector (pMD19-T) and
subcloned into the pcDNA3.1 vector (Clontech Laboratories, Inc.).
The primers used for cloning were as follows:
5′-AGATCTcatggcactgcagggca-3′ (forward) and
5′-GGTACCtagagactagcttttacctt-3′ (reverse). PC-3 and DU145 cells
infected by Ad-miR200c were transfected with AMACR expression
plasmid (pcDNA3.1-AMACR) or pcDNA3.1 blank vector (pcDNA3.1-blank)
using Novagen® NanoJuice™ transfection reagent (EMD
Millipore). After incubation for 4 h in serum-free medium, the
medium was replaced with DMEM medium containing 10% FCS and cells
were incubated for a further 44 h before analysis.
Cell Counting Kit-8 (CCK-8) assay
The cell suspension was seeded in 96-well plates
(3,000 cells/well) and pre-incubated for 24 h with RPMI-1640 media
containing 10% FCS, penicillin and streptomycin. Cells were
subsequently treated with si-AMACR or si-CON for 0, 24, 48, or 72
h, respectively. Cells were also treated with Ad-CON, Ad-miR200c,
or Ad-miR200c + pcDNA3.1-AMACR for 0, 24 or 48 h, respectively.
Subsequently, 10 µl CCK-8 (Dojindo Molecular Technologies, Inc.)
was added to each well and incubated for 2 h at 37°C. The
absorbance was examined at 450 nm using a scanning multi-well
spectrophotometer (Thermo Fisher Scientific, Inc.). The
proliferation index (PI) was calculated as
PI=[(Asample-Ablank)/(Acontrol-Ablank)]
×100%.
Wound-healing assay
DU145 and PC-3 cells were seeded in 12-well plates
(3×105 cells/well) and incubated with PRMI-1640 media at
37°C with 5% CO2 until the cells reached ~95–100%
confluence. A wound was created using a 200 µl pipette tip to
scratch a vertical wound on the cell monolayer of each well, which
was subsequently washed with PBS twice to remove the floating
cells. The widths of the wounds were measured under an inverted
light microscope (DM IL; Leica Microsystems GmbH) at 0 h and at 24
h after incubation in serum free medium.
Transwell assays
Transwell cell migration and invasion assays were
performed using a Transwell insert (8-µm pore size) with or without
BD Matrigel™ (Becton, Dickinson and Company). A total of 100 µl
Matrigel was added to the upper chamber surface of the membrane for
the invasion assay. A total of 1×105 cells in 200 µl
serum free medium were plated in the upper chambers and 800 µl
medium containing 10% serum was added to the lower chambers. After
48 h incubation, the membrane was washed with PBS, fixed in 4%
paraformaldehyde at room temperature for 30 min and stained with
0.1% crystal violet at room temperature for 10 min. Cells on the
upper side of the membrane were removed using cotton swabs and
cells that had migrated to the lower side were counted using an
inverted light microscope at 100× magnification.
Statistical analysis
All experiments were performed at least in
triplicate, unless otherwise stated. Data are presented as the mean
± standard deviation. SPSS version 17 software (SPSS, Inc.) was
used for general analysis of the statistical data. All quantitative
data were analyzed using Student's t-test or one-way analysis of
variance with Tukey's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Low expression of miR200c and
overexpression of AMACR in prostate cancer cells and prostate
cancer tissue
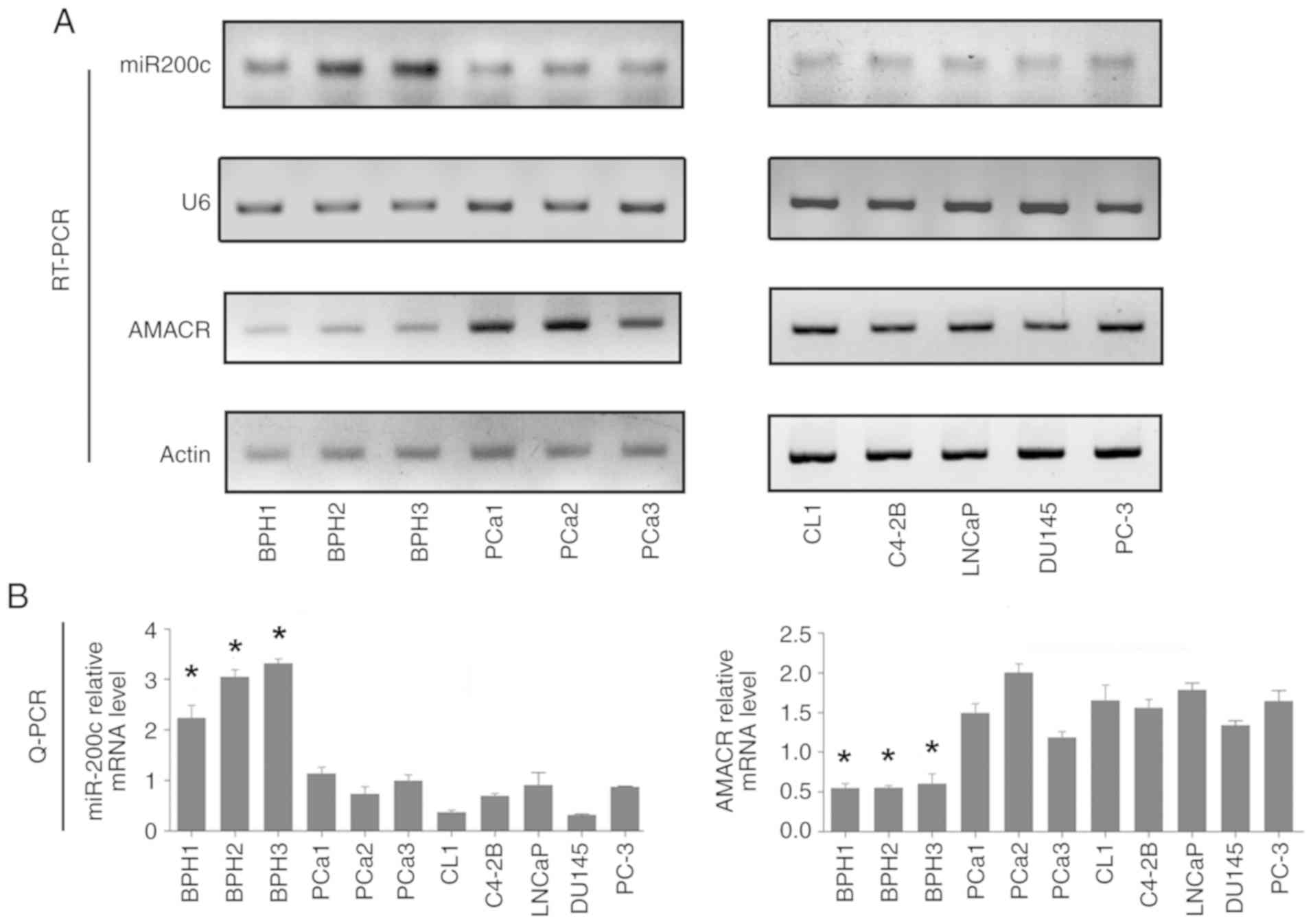
The expression of mature miR200c and AMACR mRNA was
investigated by stem loop RT-PCR and conventional RT-PCR. The
results revealed high expression levels of miR200c and low levels
of AMACR, in BPH tissue samples (BPH-1, BPH-2 and BPH-3; Fig. 1A). In contrast, in prostate cancer
tissue samples and prostate cancer cell lines (CL1, C4-2B, LNCaP,
PC-3 and DU145 cell lines), decreased levels of miR200c and
increased levels of AMACR were observed compared with (Fig. 1A). The RT-qPCR results showed that
the expression levels of miR200c in the prostate cancer tissue
samples and cell lines were ~25% compared with that in the BPH
samples, whereas the AMACR level was ~3-4-fold higher in the
prostate cancer tissue samples and cell lines compared with that of
BPH (Fig. 1B).
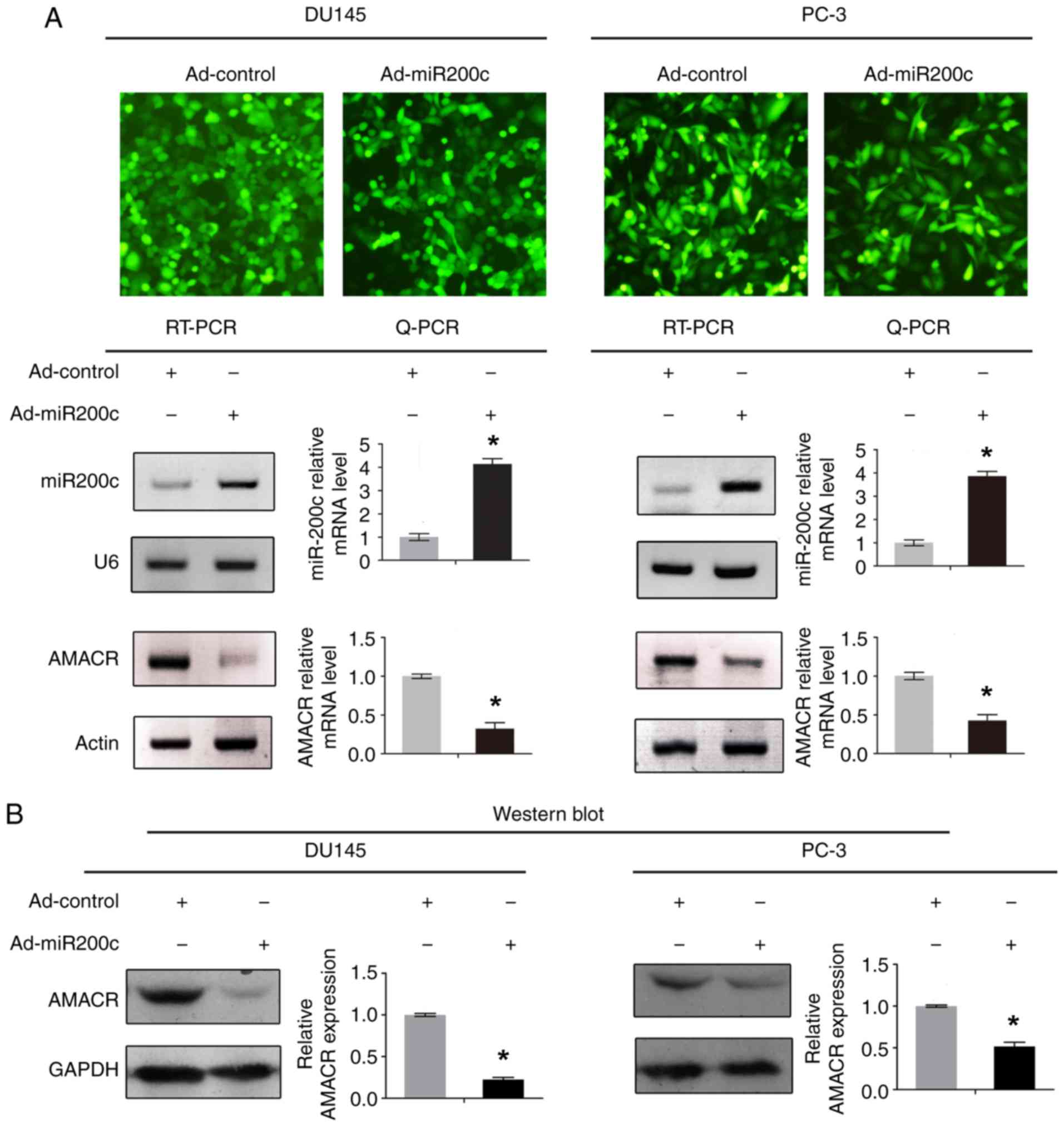
Artificial overexpression of miR200c
by adenoviral vectors leads to downregulation of AMACR
The effect of miR200c on AMACR expression level is
shown in Fig. 2. Concomitantly with
the artificial overexpression of mature miR200c, the mRNA and
protein levels of AMACR were both significantly downregulated in
DU145 and PC-3 cells compared with the control (P<0.05; Fig. 2A and B).
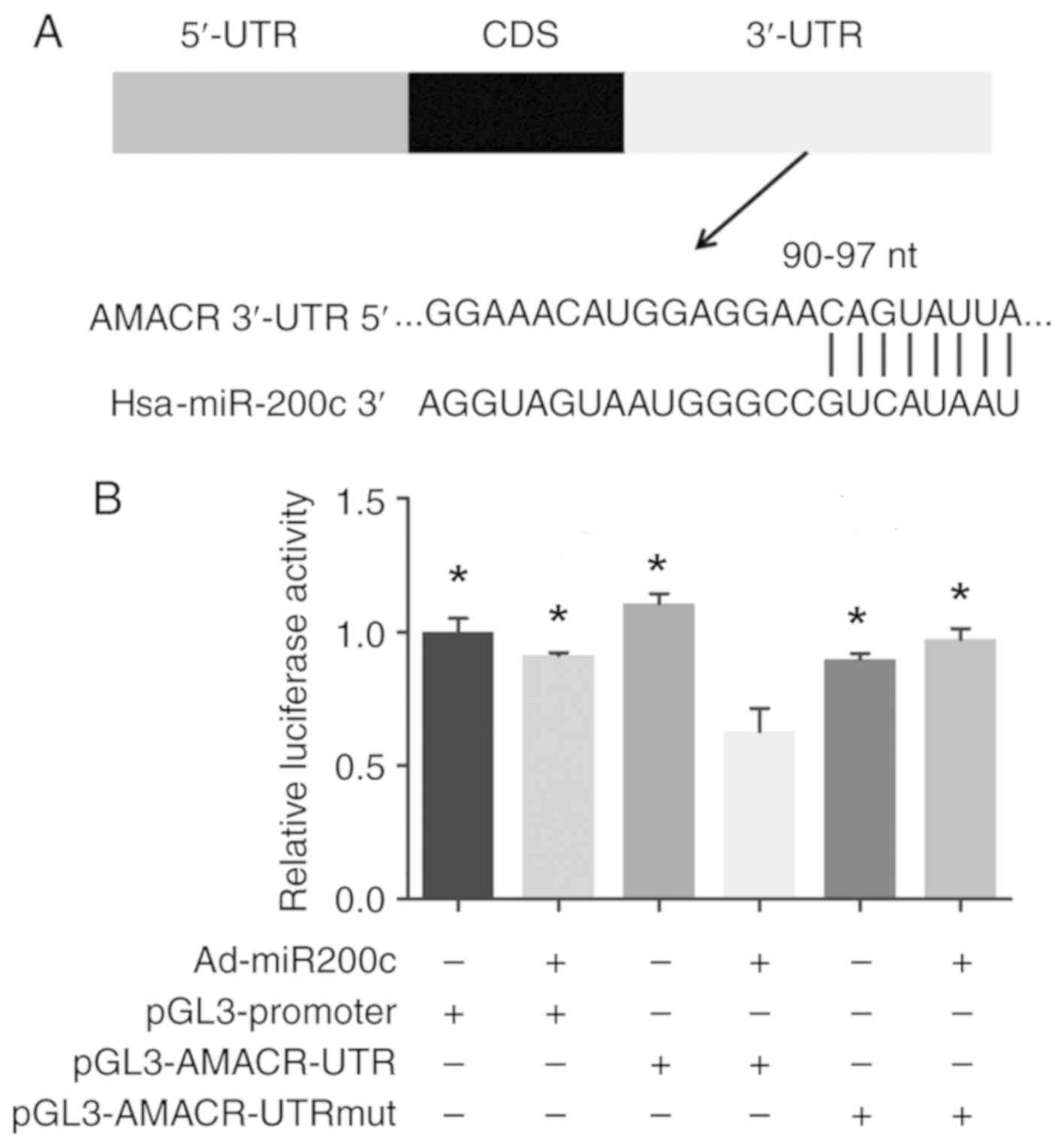
Identification of potential miR200c
seed sequences in the AMACR 3′-UTR
The 2,107-nt 3′-UTR of the AMACR mRNA was analyzed,
which identified 90–97 nts of the 3′-UTR to be the potential seed
sequence targeted by miR200c. Sequence analysis of the genomic DNA
revealed no deletions or mutations in the AMACR 3′-UTR in DU145 and
PC-3 cells (Fig. 3A).
miR200c targets the 3′-UTR of AMACR,
as revealed by dual-reporter gene assay
Luciferase reporter gene constructs (pGL3-AMACR-UTR)
were prepared, in which the potential seed sequence for miR200c in
the AMACR 3′-UTR was inserted downstream of the luciferase coding
sequence, as were constructs in which the seed sequence was mutated
(pGL3-AMACR-UTRmut). Following artificial overexpression of miR200c
by Ad-miR200c, dual-reporter assay revealed a significant decrease
in luciferase reporter gene activity in cells treated with the
pGL3-AMACR-UTR construct compared with controls, whereas mutations
of the seed sequence significantly restored the luciferase gene
activity in constructs bearing mutated 3′-UTR (P<0.05; Fig. 3B).
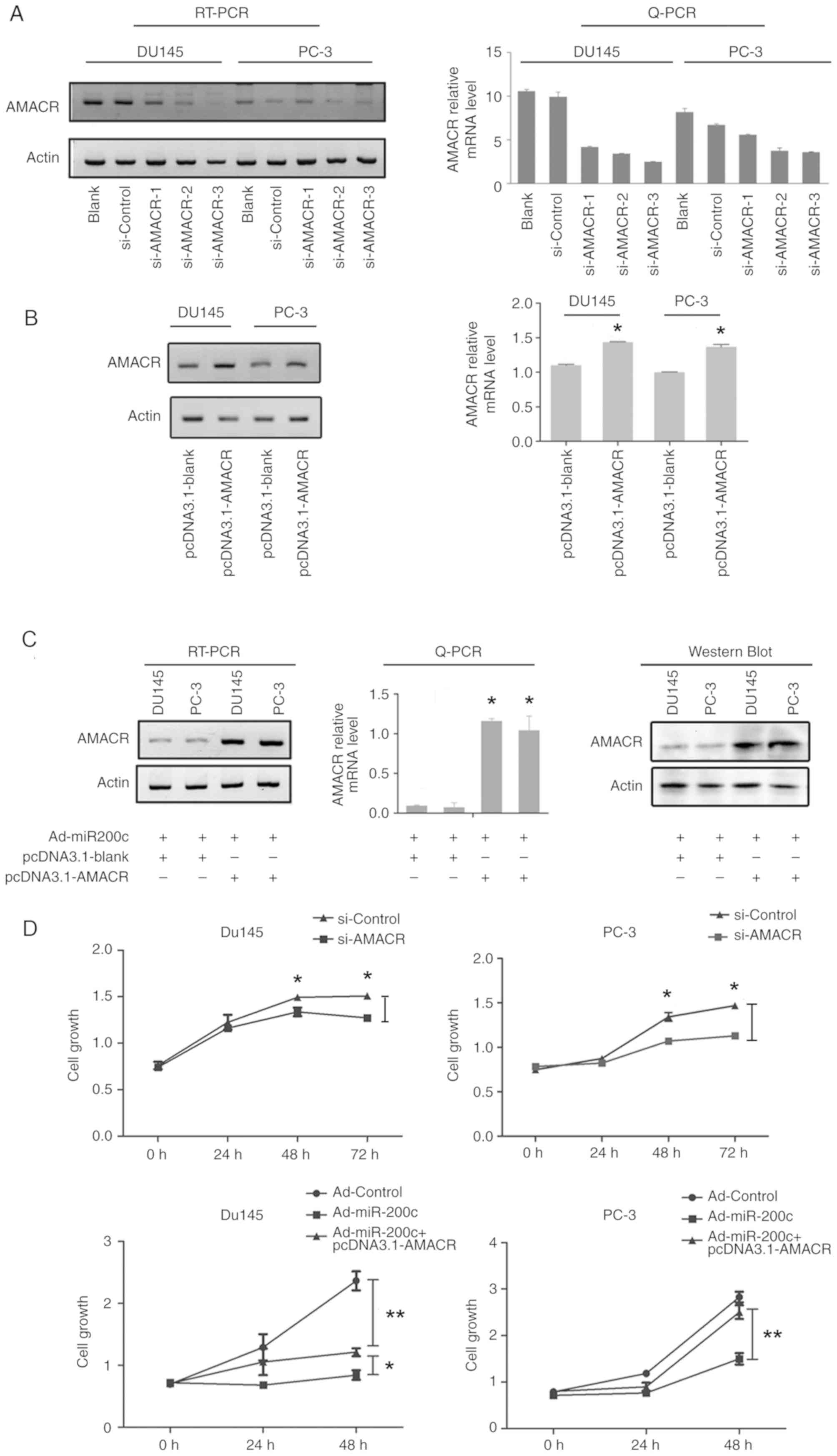
Knockdown of AMACR by RNAi or
artificial overexpression of miR200c suppresses PC-3 cell
proliferation, migration and invasiveness
siRNAs for AMACR were designed, which knocked down
the AMACR levels in DU145 and PC-3 cells. Additionally,
transfection of pcDNA3.1-AMACR also led to significant knockdown in
both cell lines (P<0.05; Fig. 4A and
B). The decrease in the mRNA expression level of AMACR in DU145
and PC-3 cells infected with Ad-miR200c could be reversed by
artificial overexpression transfection of the pcDNA3.1-AMACR vector
(P<0.05; Fig. 4C). The CCK-8 cell
proliferation assay revealed that DU145 and PC-3 cell proliferation
was inhibited by si-AMACR or by artificial overexpression of
miR200c, whereas artificial AMACR overexpression by pcDNA3.1-AMACR
transfection reversed the effects elicited by Ad-miR200c (P<0.01
or P<0.05; Fig. 4D).
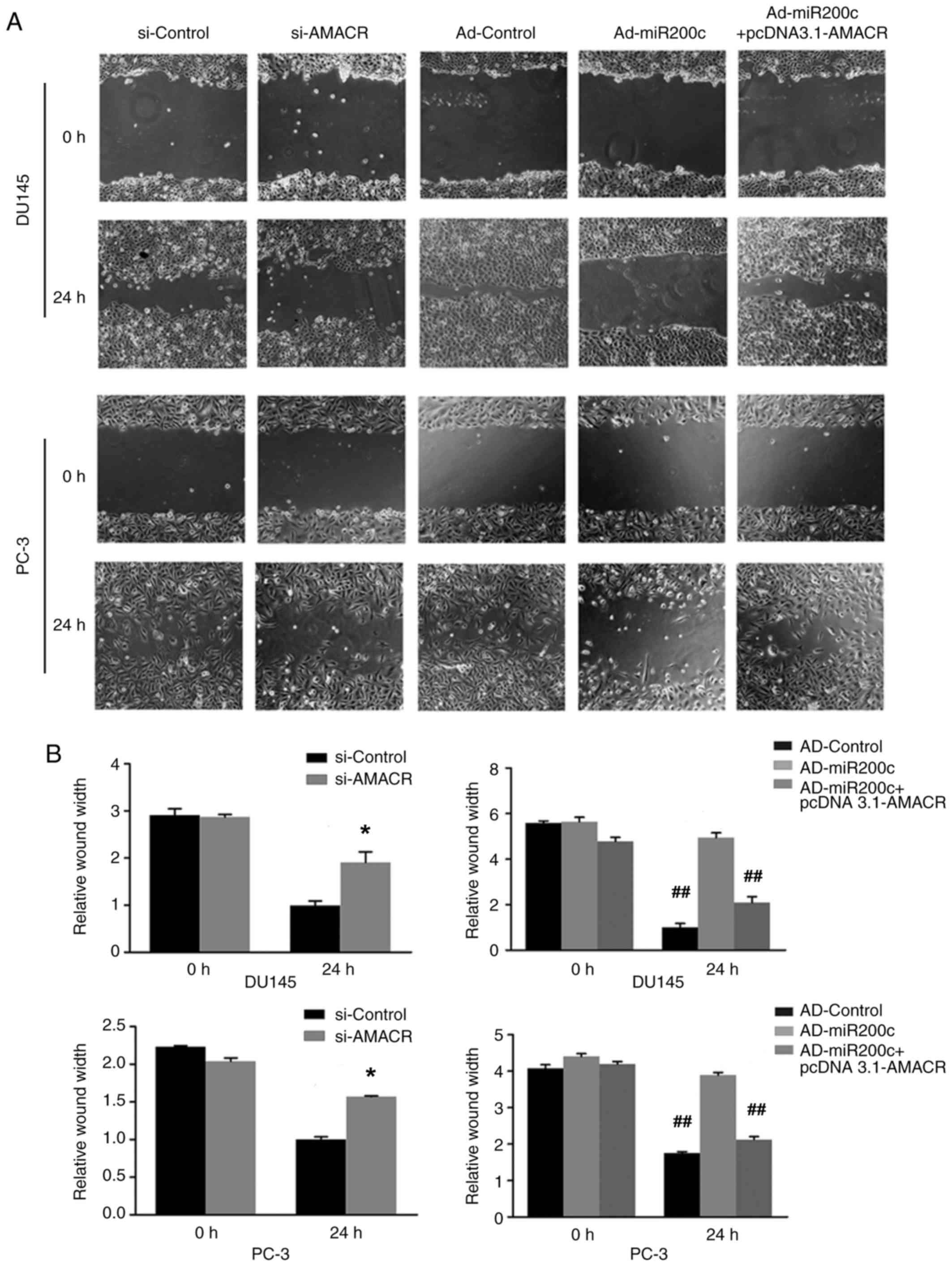
The wound-healing assays demonstrated decreased
levels of cell migration following treatment of the prostate cancer
cells with si-AMACR or artificial overexpression of miR200c
compared with si-Control, a result that was reversed by artificial
overexpression of AMACR using pcDNA3.1-AMACR (P<0.05; Fig. 5A and B). Similarly, Transwell assays
revealed that si-AMACR or artificial overexpression of miR200c
inhibited prostate cancer cell migration and invasiveness compared
with controls, whereas artificial AMACR overexpression by
pcDNA3.1-AMACR reversed the effects of Ad-miR200c (P<0.05;
Fig. 5C and D).
Discussion
The present study aimed to investigate the
regulatory effects of miR200c on AMACR and to explore the
biological effects of miR200c-AMACR deregulation on prostate cancer
cell proliferation, migration and invasion. The authors' pilot
studies and bioinformatics analysis (data not shown) indicated that
miR200c may regulate AMACR at post-transcriptional level. The
present study showed that artificial overexpression of miR200c
downregulated AMACR by interacting with the 3′-UTR of AMACR.
Previous studies have shown that miR-200c serves a
critical role in inhibiting EMT, cell motility and tumor metastasis
of various cancer types (9,10). The present results showed that
knockdown of AMACR and artificial overexpression of miR200c led to
marked inhibition of proliferation, migration and invasion of
prostate cancer cells (9).
AMACR is an enzyme associated with lipid metabolism;
specifically, the β-oxidation of dietary branched-chain fatty acids
and bile acid synthesis (30). AMACR
may be upregulated via different mechanisms in tumors, including an
increase in the amount of branched-chain fatty acids in the diet
(31), transcriptional activation by
C/EBP (which may function as an enhancer) (26), Sp1 (21) or Brachyury (24), or by downregulation of inhibitory
miRNAs (32). The branched-chain
fatty acid, phytanic acid, predominantly exists in red meat and
dairy products, an excessive intake of which is associated with
both an increased risk of prostate cancer and an increase in the
expression level of AMACR in prostate cancer cells (31). To the best of the authors' knowledge,
the present study is the first to identify AMACR as a novel target
of post-transcriptional regulation by miR200c.
The expression of AMACR is regulated by several
transcription factors. The C/EBP family member, C/EBP, activates
the expression of AMACR transcription (23), whereas the promoter activity of AMACR
was shown to be downregulated by C/EBP, p53 and the p50 subunit of
nuclear factor-κB, which may interact with the basal transcription
machinery or other transcription factors (25). Recently, Brachyury, a T-box
transcription factor, was shown to directly bind to and promote the
promoter activity of AMACR in prostate cancer (24). Sp1 may lead to overexpression of
AMACR by binding to the CpG island of the promoter region, whereas
ZNF202 may inhibit AMACR expression (21). It has also been reported that, in
gastrointestinal stromal tumors (22) and myxofibrosarcomas (23), two distinct soft tissue tumors, AMACR
gene amplification resulted in its overexpression, which promoted
tumor cell proliferation and cell cycle progression.
MiR200c, a major member of the miR200 family, has
been shown to promote expression of the cell-adhesion molecule
E-cadherin by suppressing the oncogenic transcription factor ZEB1,
thus inhibiting EMT and metastatic potential of tumor cells
(9,10). A variety of genes have been
identified as distinct miR200c target genes, which are involved in
diverse biological processes. For example, miR200c was shown to
suppress renal cancer cell growth and metastasis by targeting BMI1
and E2F3, which are well recognized as oncogenes involved in cell
cycle regulation and cell proliferation (11). miR200c was also shown to bind to FN1,
the gene encoding fibronectin-1, a cell-adhesion molecule involved
in multiple cellular processes, including embryogenesis, blood
coagulation, wound healing and tumor metastasis, which also
suppresses the proliferation, migration and invasion of gastric
cancer cells (12). The present
research group previously identified FSCN1 as a target inhibited by
miR200c, the loss of which promoted expression of the actin-binding
protein, fascin-1 and urothelial carcinoma invasiveness (14). PDGFR-β has also been shown to be an
miR200c target, which mediates endothelial differentiation in
triple-negative breast cancer (15).
The present authors have previously reported that the gene for IRS1
is a target of miR200c, the downregulation of which, in prostate
cancer, results in overexpression of IRS1, which promotes the
proliferation of prostate cancer cells (8). The multi-targeting function of miR200c
as a tumor-suppressor miRNA indicates that it has a pivotal role in
inhibiting cell growth and migration.
The biological effects of AMACR overexpression may
be manifold. As discussed above, AMACR overexpression has been
associated with tumor cell proliferation in various tumor types,
including prostate cancer (21–24).
Interference of AMACR was shown to reduce cell proliferation and
in vivo tumor growth in prostate cancer xenografts (19,33). In
the authors' future research, they will study other regulatory
mechanisms related with miR200c or AMACR more deeply and
thoroughly. Taken together, the experiments in the present study
have indicated that knockdown of AMACR expression, or artificial
overexpression of miR200c may suppress prostate cancer cell
proliferation, migration and invasiveness. The miR200c-AMACR
regulatory mechanism may be involved in prostate cancer
carcinogenesis and progression and could be exploited as a putative
therapeutic target for prostate cancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was support by grants from the
Natural Science Foundation of China (grant nos. NSFC81572540,
81572541, 81272820, 81272848 and 81302225).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HX performed experiments and analyzed data, and
drafted the manuscript. LN contributed to design of study, miR200C
experiments, and data analysis. MZ contributed to data analysis and
interpretation. JG contributed to CCK-8 assays and analyzed data.
XC interpreted data and revised the manuscript. MX contributed to
design of study and analysis of data. ZS contributed to the wound
healing and transwell assays and analyzed data. NC and QZ designed
the study, analyzed data, revised and finalized the manuscript. All
authors have given approval of the final version of the
mauscript.
Ethics approval and consent to
participate
The present study followed institutional ethical
guidelines and was supported by grants approved by ethics committee
of the authors' institution. All tissue samples were collected in
the West China Hospital according to the ethical guidelines and
procedures approved by the institutional supervisory committee.
Informed written consent was obtained according to the
institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Knezevic J, Pfefferle AD, Petrovic I,
Greene SB, Perou CM and Rosen JM: Expression of miR-200c in
claudin-low breast cancer alters stem cell functionality, enhances
chemosensitivity and reduces metastatic potential. Oncogene.
34:5997–6006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jurmeister S, Baumann M, Balwierz A,
Keklikoglou I, Ward A, Uhlmann S, Zhang JD, Wiemann S and Sahin Ö:
MicroRNA-200c represses migration and invasion of breast cancer
cells by targeting actin-regulatory proteins FHOD1 and PPM1F. Mol
Cell Biol. 32:633–651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou X, Wang Y, Shan B, Han J, Zhu H, Lv
Y, Fan X, Sang M, Liu XD and Liu W: The downregulation of
miR-200c/141 promotes ZEB1/2 expression and gastric cancer
progression. Med Oncol. 32:4282015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valladares-Ayerbes M, Reboredo M,
Medina-Villaamil V, Iglesias-Díaz P, Lorenzo-Patiño MJ, Haz M,
Santamarina I, Blanco M, Fernández-Tajes J, Quindós M, et al:
Circulating miR-200c as a diagnostic and prognostic biomarker for
gastric cancer. J Transl Med. 10:1862012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toiyama Y, Hur K, Tanaka K, Inoue Y,
Kusunoki M, Boland CR and Goel A: Serum miR-200c is a novel
prognostic and metastasis-predictive biomarker in patients with
colorectal cancer. Ann Surg. 259:735–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao YC and Wu J: MicroRNA-200c and
microRNA-141 as potential diagnostic and prognostic biomarkers for
ovarian cancer. Tumour Biol. 36:4843–4850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Butz H, Szabó PM, Khella HW, Nofech-Mozes
R, Patocs A and Yousef GM: miRNA-target network reveals miR-124as a
key miRNA contributing to clear cell renal cell carcinoma
aggressive behaviour by targeting CAV1 and FLOT1. Oncotarget.
6:12543–12557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su W, Xu M, Chen X, Nie L, Chen N, Gong J,
Zhang M, Su Z, Huang L and Zhou Q: MiR200c targets IRS1 and
suppresses prostate cancer cell growth. Prostate. 75:855–862. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puhr M, Hoefer J, Schäfer G, Erb HH, Oh
SJ, Klocker H, Heidegger I, Neuwirt H and Culig Z:
Epithelial-to-mesenchymal transition leads to docetaxel resistance
in prostate cancer and is mediated by reduced expression of
miR-200c and miR-205. Am J Pathol. 181:2188–2201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Radisky DC: miR-200c at the nexus of
epithelial-mesenchymal transition, resistance to apoptosis, and the
breast cancer stem cell phenotype. Breast Cancer Res. 13:1102011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu M, Liang Z, Chen L, Tan G, Liu L, Wang
K, Chen H and Liu J: MicroRNA-200c suppresses cell growth and
metastasis by targeting Bmi-1 and E2F3 in renal cancer cells. Exp
Ther Med. 13:1329–1336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Sun Z, Li Y, Fan D and Jiang H:
MicroRNA-200c binding to FN1 suppresses the proliferation,
migration and invasion of gastric cancer cells. Biomed
Pharmacother. 88:285–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen P, Guo X, Zhang L, Zhang W, Zhou Q,
Tian Z, Zheng Y, Liao Q, Wang H, Li G, et al: MiR-200c is a
cMyc-activated miRNA that promotes nasopharyngeal carcinoma by
downregulating PTEN. Oncotarget. 8:5206–5218. 2017.PubMed/NCBI
|
|
14
|
Zhang M, Nie L, Su Z, Xu M, Chen N, Gong
J, Xie H, Zhong J, Tan J, Xu Y, et al: MicroRNA200c suppresses
urothelial carcinoma invasiveness by targeting FSCN1. Int J Clin
Exp Pathol. 10:5665–5674. 2017.
|
|
15
|
D'Ippolito E, Plantamura I, Bongiovanni L,
Casalini P, Baroni S, Piovan C, Orlandi R, Gualeni AV, Gloghini A,
Rossini A, et al: miR-9 and miR-200 regulate PDGFRβ-mediated
endothelial differentiation of tumor cells in triple-negative
breast cancer. Cancer Res. 76:5562–5572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimono Y, Zabala M, Cho RW, Lobo N,
Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al:
Downregulation of miRNA-200c links breast cancer stem cells with
normal stem cells. Cell. 138:592–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang X, Tang X, Gal J, Kyprianou N, Zhu H
and Tang G: Detection of microRNAs in prostate cancer cells by
microRNA array. Methods Mol Biol. 732:69–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zha S, Ferdinandusse S, Denis S, Wanders
RJ, Ewing CM, Luo J, De Marzo AM and Isaacs WB:
Alpha-methylacyl-CoA racemase as an androgen-independent growth
modifier in prostate cancer. Cancer Res. 63:7365–7376.
2003.PubMed/NCBI
|
|
20
|
Jindal Y, Singh A, Kumar R, Varma K, Misra
V, Misra SP and Dwivedi M: Expression of alpha methylacyl CoA
racemase (AMACR) in gastric adenocarcinoma and its correlation with
helicobacter pylori infection. J Clin Diagn Res. 10:EC10–EC12.
2016.PubMed/NCBI
|
|
21
|
Zhang X, Leav I, Revelo MP, Deka R,
Medvedovic M, Jiang Z and Ho SM: Deletion hotspots in AMACR
promoter CpG island are cis-regulatory elements controlling the
gene expression in the colon. PLoS Genet. 5:e10003342009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li CF, Chen LT, Lan J, Chou FF, Lin CY,
Chen YY, Chen TJ, Li SH, Yu SC, Fang FM, et al: AMACR amplification
and overexpression in primary imatinib-naïve gastrointestinal
stromal tumors: A driver of cell proliferation indicating adverse
prognosis. Oncotarget. 5:11588–11603. 2014.PubMed/NCBI
|
|
23
|
Li CF, Fang FM, Lan J, Wang JW, Kung HJ,
Chen LT, Chen TJ, Li SH, Wang YH, Tai HC, et al: AMACR
amplification in myxofibrosarcomas: A mechanism of overexpression
that promotes cell proliferation with therapeutic relevance. Clin
Cancer Res. 20:6141–6152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinto F, Pértega-Gomes N, Vizcaíno JR,
Andrade RP, Cárcano FM and Reis RM: Brachyury as a potential
modulator of androgen receptor activity and a key player in therapy
resistance in prostate cancer. Oncotarget. 7:28891–28902. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen W, Wu W, Zhao J, Yu C, Liu W, Jiang A
and Zhang J: Molecular cloning and preliminary analysis of the
human alpha-methylacyl-CoA racemase promoter. Mol Biol Rep.
36:423–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zha S and Isaacs WB: A nonclassic CCAAT
enhancer element binding protein binding site contributes to
alpha-methylacyl-CoA racemase expression in prostate cancer. Mol
Cancer Res. 3:110–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen N, Chen X, Huang R, Zeng H, Gong J,
Meng W, Lu Y, Zhao F, Wang L and Zhou Q: BCL-xL is a target gene
regulated by hypoxia-inducible factor-1{alpha}. J Biol Chem.
284:10004–10012. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Gong J, Zeng H, Chen N, Huang R,
Huang Y, Nie L, Xu M, Xia J, Zhao F, et al: MicroRNA145 targets
BNIP3 and suppresses prostate cancer progression. Cancer Res.
70:2728–2738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferdinandusse S, Denis S, IJlst L,
Dacremont G, Waterham HR and Wanders RJ: Subcellular localization
and physiological role of alpha-methylacyl-CoA racemase. J Lipid
Res. 41:1890–1896. 2000.PubMed/NCBI
|
|
31
|
Mobley JA, Leav I, Zielie P, Wotkowitz C,
Evans J, Lam YW, L'Esperance BS, Jiang Z and Ho SM: Branched fatty
acids in dairy and beef products markedly enhance
alpha-methylacyl-CoA racemase expression in prostate cancer cells
in vitro. Cancer Epidemiol Biomarkers Prev. 12:775–783.
2003.PubMed/NCBI
|
|
32
|
Erdmann K, Kaulke K, Thomae C, Huebner D,
Sergon M, Froehner M, Wirth MP and Fuessel S: Elevated expression
of prostate cancer-associated genes is linked to down-regulation of
microRNAs. BMC Cancer. 14:822014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Festuccia C, Gravina GL, Mancini A, Muzi
P, Cesare ED, Kirk R, Smith M, Hughes S, Gibson R, Lian LY, et al:
Trifluoroibuprofen inhibits α-methylacyl coenzyme A racemase
(AMACR/P504S), reduces cancer cell proliferation and inhibits in
vivo tumor growth in aggressive prostate cancer models. Anticancer
Agents Med Chem. 14:1031–1041. 2014. View Article : Google Scholar : PubMed/NCBI
|