Introduction
The prevalence of skin cancer is increasing at an
alarming rate, with ~1 million new cases discovered annually
worldwide (1). Malignant cutaneous
melanoma occurs as a result of dysregulated proliferation of
melanocytes in the epidermis and is one of the most aggressive
forms of cutaneous neoplasms (2,3).
Malignant melanoma has a high metastatic rate, thus it is
considered to be the most severe skin cancer type (3–5). The
mortality rate of melanoma accounts for 65% of skin cancer deaths,
despite the incidence rate only representing approximately 3% of
all skin cancer types (6). Skin
cancer prognosis is poor and usually leads to mortality due to
metastasis (1). The median survival
time is 8 months, and the 5-year survival among patients with stage
IV metastatic melanoma is only approximately 10% (7,8).
Although there are many antimelanoma treatments, in many cases
melanoma is treatment-resistant (1).
Thus, malignant melanoma is difficult to successfully treat using
conventional surgery and chemotherapeutics (1).
Plant compounds are being increasingly used to
prevent and treat many diseases including tumors (9–11). A
cross-sectional survey in the West Midlands in the UK found that a
substantial number of patients with cancer are likely to be taking
herbal medicine (12). Currently,
over 60 herbal complexes are being studied as anticancer medicine
(11). The clinically used
plant-derived anticancer drugs can be divided into four important
groups: Vinca, alkaloids, taxanes and podophyllotoxins (13). Therefore, it is feasible to find new
Chinese herbal medicines that may inhibit melanoma metastasis
(11).
Plantamajoside (PMS) is a major ingredient isolated
from Plantago asiatica (14).
PMS has long been applied in food and medicine (14). PMS exhibits anti-inflammatory and
antioxidant properties (15,16). PMS has been applied to the treatment
of many diseases due to its protective effects against
cadmium-induced renal injury (17),
and its anti-inflammatory (15) and
antifibrotic effects (18).
Currently, the antitumor effects of PMS in esophageal squamous cell
carcinoma have been studied (19).
However, to the best of our knowledge, the effect of PMS on
malignant melanoma remains unknown.
Therefore, the aims of the present study were to
investigate the effect of PMS on malignant melanoma in
vitro, and to examine the underlying mechanism by which PMS may
affect malignant melanoma.
Materials and methods
Cell culture and treatment
The malignant melanoma cell line A2058 was obtained
from the Institute of Basic Medical Sciences, Chinese Academy of
Medical Sciences. Human primary epidermal melanocytes were obtained
from the American Type Culture Collection (cat. no. ATCC
PCS-200-013). A2058 cells were cultured in RPMI-1640 media (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (HyClone; GE
Healthcare Life Sciences) at 37°C with 5% CO2 for 24 h.
Normal melanocytes were grown in RPMI-1640 (HyClone; GE Healthcare
Life Sciences) containing 10% FSB (HyClone; GE Healthcare Life
Sciences), 100 U/ml penicillin and 100 µg/ml streptomycin (Beyotime
Institute of Biotechnology) in a humidified incubator with 5%
CO2.
PMS was purchased from Sigma-Aldrich (purity
>99%; Merck KGaA), and DMSO (Sigma-Aldrich; Merck KGaA) was used
to dissolve PMS. For PMS treatment, A2058 cells and normal
melanocytes were treated with different concentrations of PMS (0,
20, 80 and 160 µg/ml) at 37°C for 0, 24, 48 and 72 h respectively
following which the cells were subjected to further
experimentation.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 method was used to detect cell viability.
The cell concentration of normal melanocytes or A2058 cells was
adjusted to 1×104/ml, and 100 µl cell suspension was
added to each well of the 96-well plates. Then, A2058 cells were
treated with different concentrations of PMS (0, 20, 80 and 160
µg/ml) at 37°C for 0, 24, 48 and 72 h. The 96-well plates were
cultured at 37°C with 5% CO2 for 24 h. Subsequently, 10
µl CCK-8 reagent (Sigma-Aldrich; Merck KGaA) was added to each well
according to the manufacturer's protocol. The absorbance at the
wavelength of 450 nm was measured after 2 h of incubation at 37°C
using an automatic enzyme-linked immune detector. The experiment
was repeated three times, and data were normalized to the control
group, which was cells without any PMS treatment.
Flow cytometry
After treatment with different concentrations of PMS
(0, 20, 80 and 160 µg/ml) at 37°C for 48 h, 0.2% trypsin was used
to digest the normal melanocytes or A2058 cells. Cells were then
washed with PBS and fixed using the 70% ethanol overnight at 4°C.
The apoptotic rate of the cells was detected using the Annexin
V-FITC/PI kit (cat. no. 70-AP101-100; Hangzhou Multi Sciences
Biotech Co., Ltd.) according to the manufacturer's instructions.
The apoptotic rate of normal melanocytes was also measured using an
AnncxinV-FITC/PI Apoptosis Detection kit (cat no. A211-01; Vazyme
Biotech Co., Ltd.) as per the manufacturer's instructions. BD
FACSCalibur™ flow cytometer (Beckman Coulter, Inc.) coupled with
the CellQuest™ software (version 5.1; BD Biosciences) were used to
measure the apoptotic rate of cells. The experiments were performed
in triplicate.
Transwell assay
Polycarbonate filters (pore size, 8 µm; Corning,
Inc.) were used for the Transwell assay. Chamber inserts pre-coated
with 200 mg/ml of Matrigel at 37°C for 30 min were used for cell
invasion detection, whilst chamber inserts without Matrigel were
used for cell migration determination. The upper chamber was plated
with 200 µl RPMI-1640 media (Gibco; Thermo Fisher Scientific, Inc.)
containing 0.1% FBS and 1×105 A2058 cells treated with
different concentrations of PMS (0, 20, 80 and 160 µg/ml) at 37°C
for 48 h. The lower chamber was plated with 600 µl RPMI-1640 media
(Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS. After 48 h
incubation at 37°C, cells in the lower chamber were fixed with 4%
paraformaldehyde (cat. no. P0099; Beyotime Institute of
Biotechnology) at room temperature for 30 min and then stained with
0.5 ml 0.1% crystal violet (cat. no. C0121; Beyotime Institute of
Biotechnology) at room temperature for 15 min. migrated or invaded
cells were then counted using a light microscope (five fields per
chamber; magnification ×200). Each Transwell assay was repeated in
five independent experiments.
Reverse transcription-quantitative
PCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the total RNA from A2058
cells. Total RNA was reverse transcribed into cDNAs using the
temperature protocol of 70°C for 5 min, 37°C for 5 min and 42°C for
1 h. PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.)
was used according to the manufacturer's instructions. qPCR was
performed to analyze cDNAs using a TaqMan Universal PCR Master Mix
kit (Thermo Fisher Scientific, Inc.) following the manufacturer's
instructions. Primer sequences used for PCR were as follows:
GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′; Bcl-2 forward,
5′-TTCTTTGAGTTCGGTGGGGTC-3′ and reverse,
5′-TGCATATTTGTTTGGGGCAGG-3′; Bax forward,
5′-TCCACCAAGAAGCTGAGCGAG-3′ and reverse,
5′-GTCCAGCCCATGATGGTTCT-3′; and caspase-3 forward,
5′-TGTGGCATTGAGACAGAC-3′ and reverse, 5′-CACTTGCCATACAAACTA-3′. The
thermocycling conditions used for the qPCR were as following:
Initial denaturation at 95°C for 10 min; followed by 35 cycles of
95°C for 15 sec and 55°C for 40 sec. GAPDH was used as the
standardized control. ΔCq=Cqgene-Cqreference is the relative level
of gene expression, and the fold change in gene expression was
calculated by the 2−ΔΔCq method (20). The experiments were repeated in
triplicate.
Western blotting
Total proteins were collected using RIPA lysis
buffer (cat no. P0013E; Beyotime Institute of Biotechnology)
following the manufacturer's instructions. A bicinchoninic acid
assay kit (Pierce; Thermo Fisher Scientific, Inc.) was used to
quantify the protein samples. Protein samples (40 µg per lane) were
separated on 10% SDS-PAGE and electrophoretically transferred onto
a PVDF membrane (EMD Millipore). After blocking with 5% skim milk
for 2 h at room temperature, the membrane was incubated with
primary antibodies: Anti-AKT (1:1,000; cat. no. 4691; Cell
Signaling Technology, Inc.), anti-phosphorylated (p)-AKT (1:1,000;
cat. no. 4060; Cell Signaling Technology, Inc.), anti-Bcl-2
(1:1,000; cat. no. 4223; Cell Signaling Technology, Inc.), anti-Bax
(1:1,000; cat. no. 5023; Cell Signaling Technology, Inc.),
anti-total caspase-3 (1:1,000; cat. no. 29629; Cell Signaling
Technology, Inc.) and anti-β-actin (1:1,000; cat. no. 4970; Cell
Signaling Technology, Inc.), overnight at 4°C. Then, the membranes
were incubated with horseradish peroxidase-conjugated secondary
antibody (1:1,000; cat. no. 7074; Cell Signaling Technology, Inc.)
at room temperature for 2 h. Protein bands were visualized using
chemiluminescent ECL reagent (EMD Millipore) and quantified by
densitometry (QuantityOne 4.5.0 software; Bio-Rad Laboratories,
Inc.).
Statistical analysis
Data are presented as the mean ± SD of ≥3
independent experiments. One-way ANOVA followed by Tukey's post hoc
test was used for comparison between groups. All data analyses were
performed with SPSS 17.0 software (SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant different.
Results
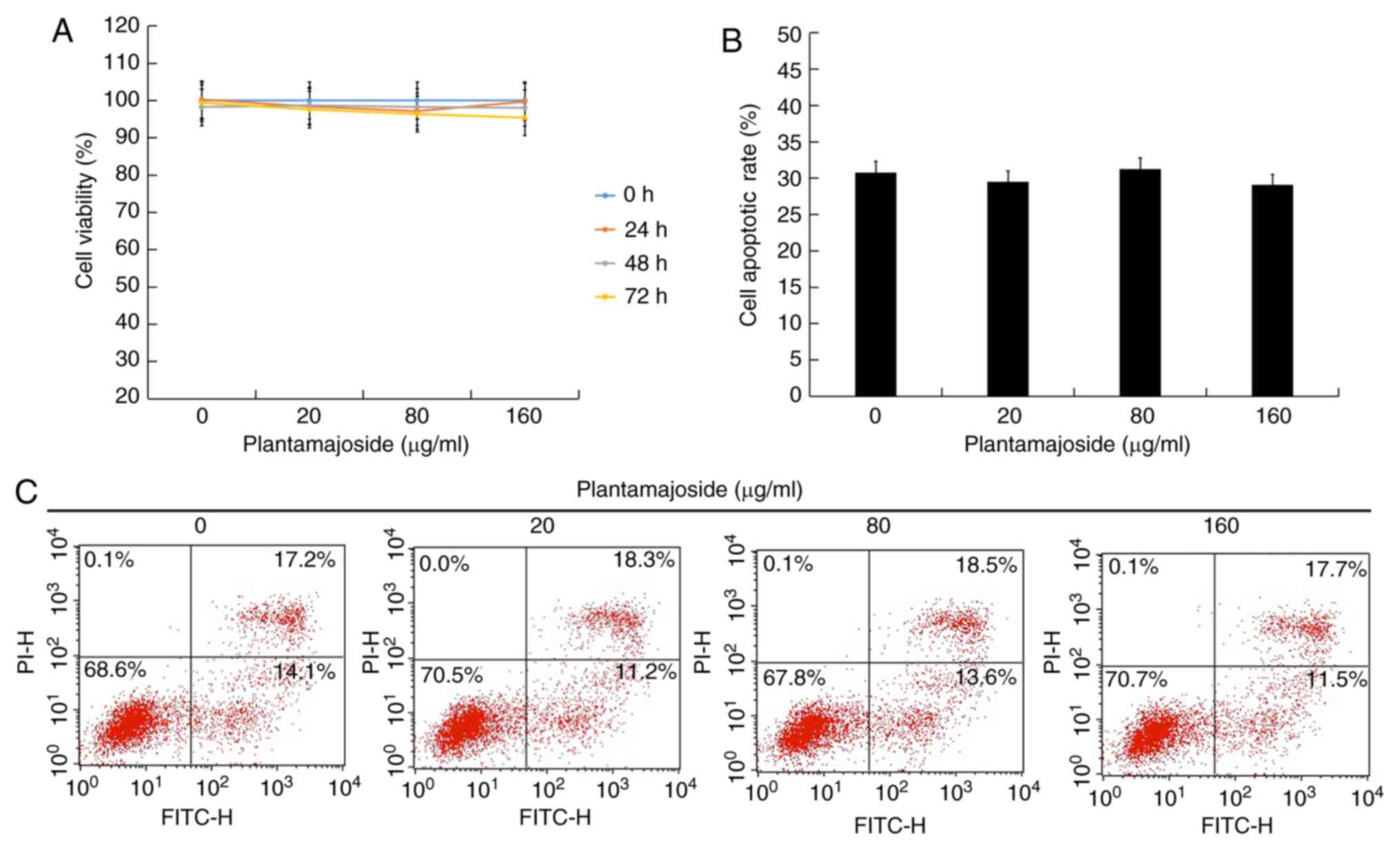
No significant effects of PMS on
normal melanocytes
To investigate the effect of PMS on malignant
melanoma cells, the present study examined the effect of PMS on
normal melanocytes. After treatment with different concentrations
of PMS (0, 20, 80 and 160 µg/ml) for 0, 24, 48 and 72 h, cell
viability was determined. The present results suggested that there
were no significant effects of PMS on the viability of normal
melanocytes (Fig. 1A). In addition,
after treatment with different concentrations of PMS (0, 20, 80 and
160 µg/ml) for 48 h, the apoptotic rate of normal melanocytes was
also investigated. The present results suggested that PMS had no
effect on normal melanocyte apoptosis (Fig. 1B and C).
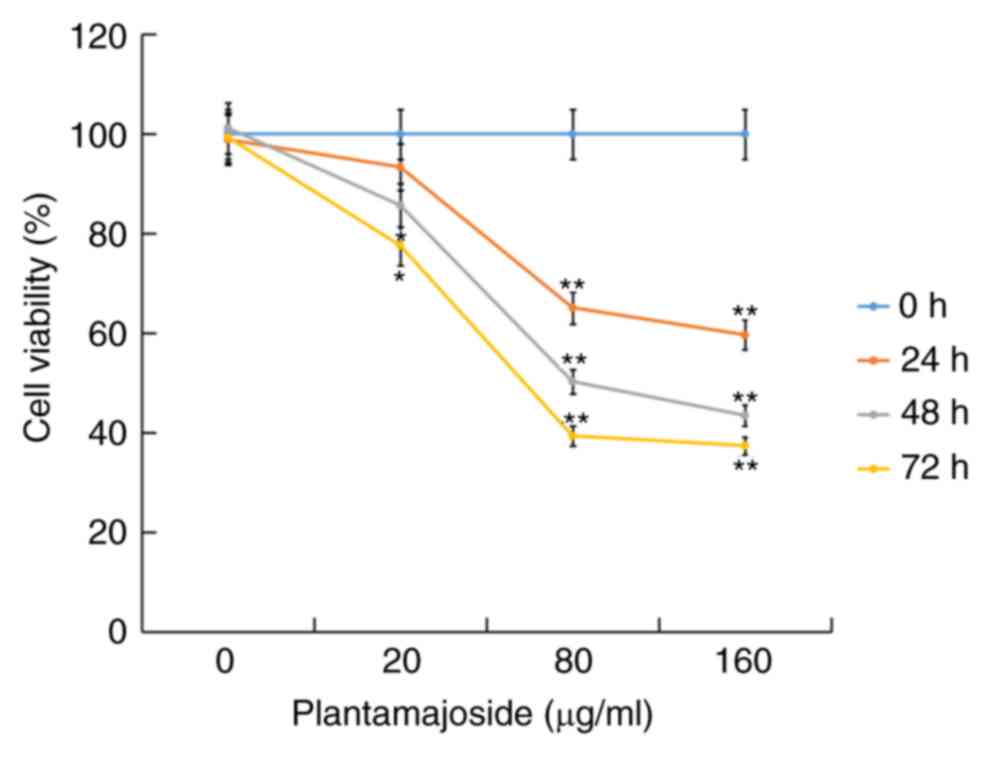
PMS inhibits cell viability in a
dose-dependent manner
The present study investigated the effect of PMS on
the malignant melanoma cells. A2058 cells were treated with
different concentrations of PMS (0, 20, 80 and 160 µg/ml) for 0,
24, 48 and 72 h, then cell viability was measured using a CCK-8
assay. The CCK-8 assay results suggested that PMS inhibited A2058
cell viability in a dose-dependent manner (Fig. 2).
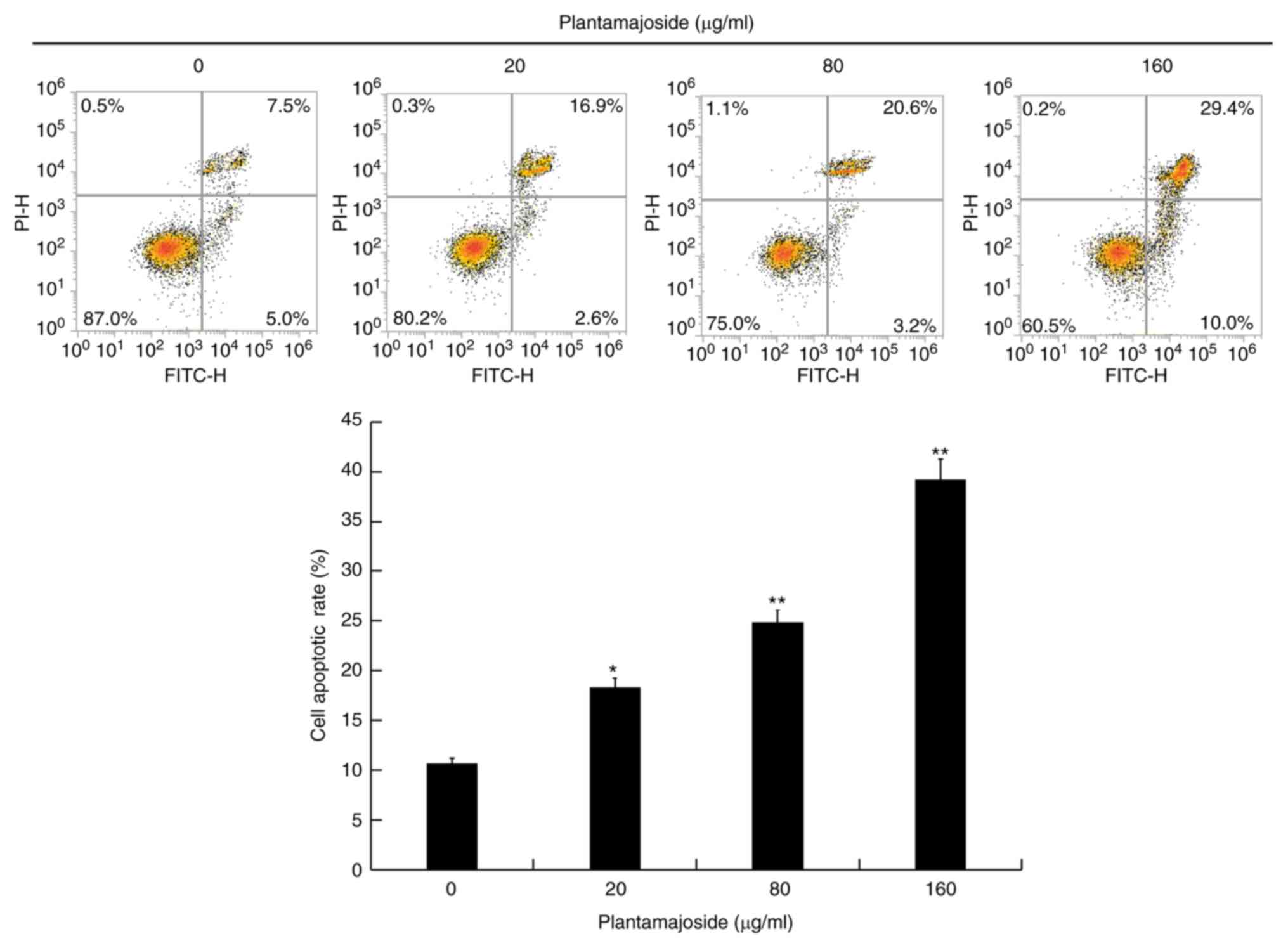
PMS induces cell apoptosis in a
dose-dependent manner
After treatment with different concentrations of PMS
(0, 20, 80 and 160 µg/ml) for 48 h, the apoptotic rate of malignant
melanoma cells was measured using Annexing V-FITC/PI staining. The
present results suggested that the apoptotic rate of A2058 cells
significantly increased after 48 h of PMS treatment, and that PMS
induced apoptosis in a dose-dependent manner (Fig. 3).
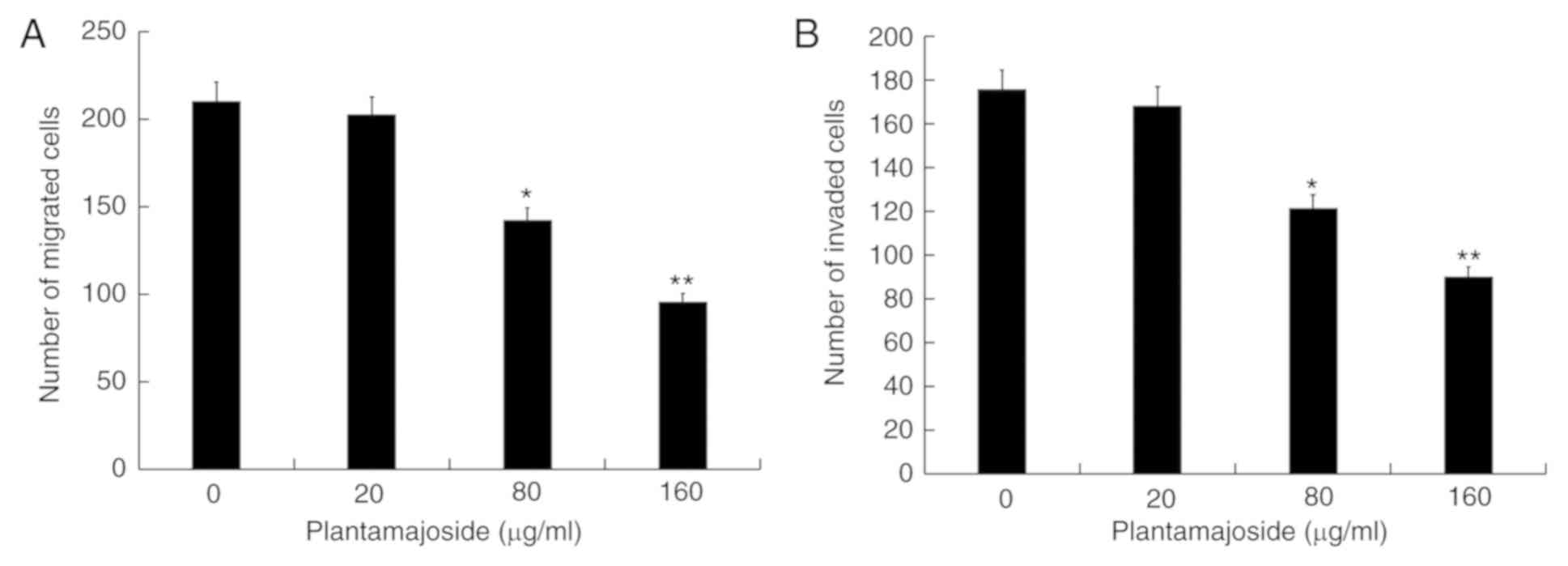
PMS inhibits cell migration and
invasion in a dose-dependent manner
Subsequently, after treatment with different
concentrations of PMS (0, 20, 80 and 160 µg/ml) for 48 h, a
Transwell assay was used to examine the effects of PMS on the
migratory and invasive abilities of malignant melanoma cells. The
Transwell assay results indicated that PMS inhibited cell migration
(Fig. 4A) and invasion (Fig. 4B) in a dose-dependent manner.
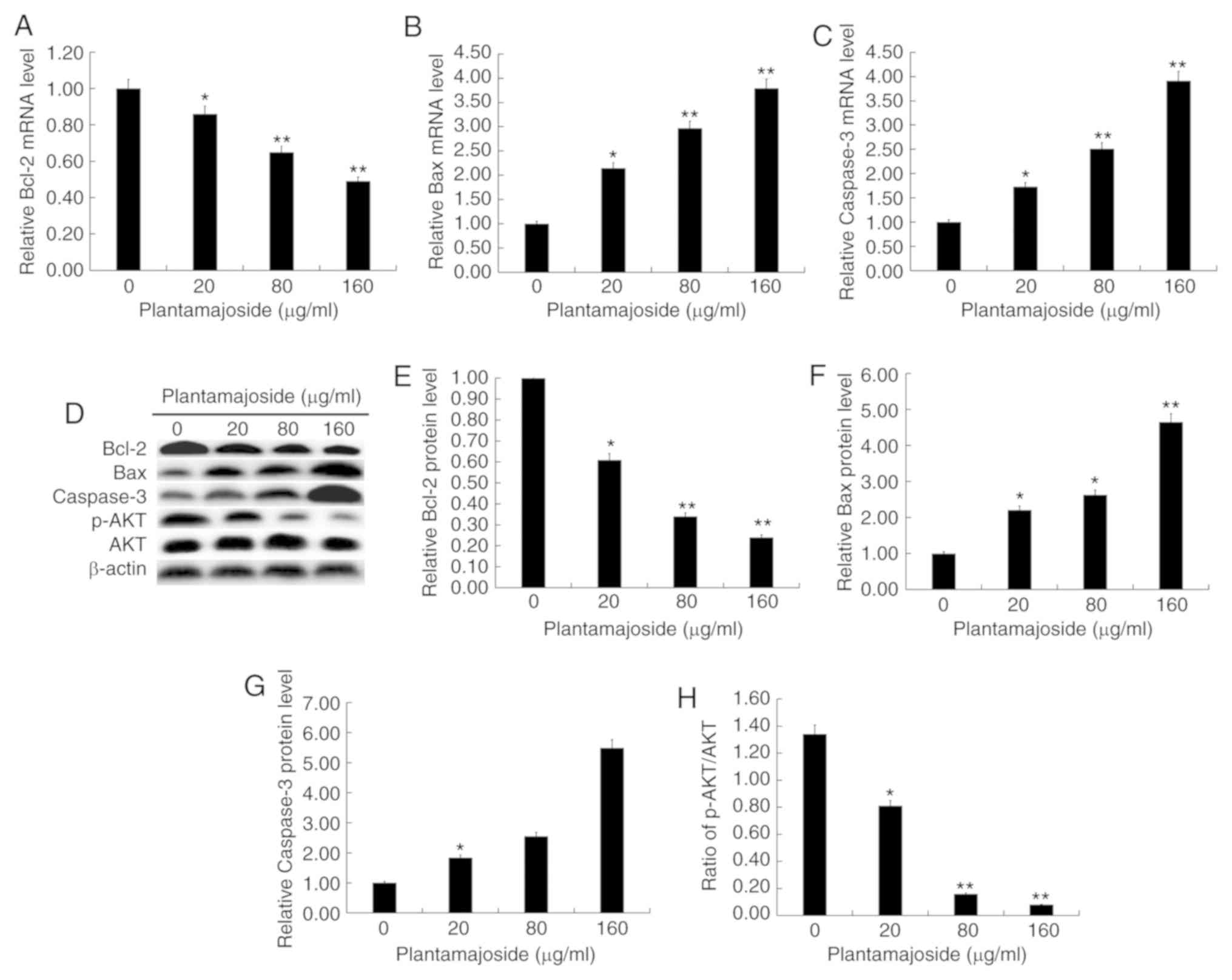
Effect of PMS on the expression levels
of apoptotic related genes and the PI3K/AKT signaling pathway
In order to study the mechanism by which PMS may
affect A2058 cells, the cells were treated with different
concentrations of PMS for 48 h. Then, the expression levels of the
apoptotic-related genes Bcl-2, Bax and caspase-3, and
the relative proteins in the PI3K/AKT signaling pathway including
AKT and p-AKT, were detected. The present results suggested that
PMS inhibited the mRNA expression level of Bcl-2, and
promoted the mRNA expression levels of Bax and
caspase-3 in a dose-dependent manner (Fig. 5A-C). Western blot analysis revealed
that PMS inhibited the protein expression level of Bcl-2, and
promoted the protein expression levels of Bax and caspase-3 in a
dose-dependent manner (Fig. 5D-G).
In addition, the protein expression level of p-AKT was inhibited by
PMS treatment in a dose-dependent manner. (Fig. 5D and H). Collectively, the present
results suggested that PMS may affect A2058 cells by impacting
apoptotic related genes expression levels and the PI3K/AKT
signaling pathway.
Discussion
Although the diagnosis and treatment of malignant
melanoma is improving, the median survival time of patients
diagnosed with malignant melanoma during metastasis is 6–9 months,
the 5-year survival rate is <15% and the prognosis is still poor
(21). Surgical removal of malignant
melanoma is the preferred method of therapy, but treatment with
radiation and/or chemotherapy is also used (6,21).
However, in the case of metastasis the cure rate is almost zero and
only palliative treatment is available (22). In addition, immunotherapy of
cytokines, as well as antibodies and BRAF inhibitors, are used in
the treatment of malignant melanoma (23,24).
However, these therapies can cause serious side effects (25,26).
Therefore, due to the aggressiveness of melanoma and the
complications associated with treatment, it is important to find
alternatives to improve melanoma treatment.
PMS has been previous found to exhibit antitumor
properties (19). Therefore, the
aims of the present study were to investigate the effect of PMS on
malignant melanoma and its underlying mechanisms in
vitro.
The present study examined the effect of PMS on
normal melanocytes. After treatment with different concentrations
of PMS (0, 20, 80 and 160 µg/ml) for 48 h, the present results
suggested that there were no significant effects of PMS on
viability and apoptosis of normal melanocytes. The present study
also investigated the effect of PMS on malignant melanoma cells.
A2058 cells were treated with different concentrations of PMS (0,
20, 80 and 160 µg/ml) for 0, 24, 48 and 72 h, then cell viability
was measured using a CCK-8 assay. In line with a previous study
(19), the present results suggested
that the viability of A2058 cells was significantly inhibited by
PMS treatment in a dose-dependent manner. Furthermore, the present
results indicated that PMS induced cell apoptosis in a
dose-dependent manner. Moreover, Pei et al (11) reported that PMS inhibited the growth
and metastasis of breast cancer by inhibiting the activity of
matrix metallopeptidase (MMP)-9 and MMP-2. The present study
investigated the effect of PMS on the migration and invasion of
malignant melanoma cells, and in line with results from a previous
study (11), the present results
indicated that PMS inhibited the migration and invasion of A2058
cells in a dose-dependent manner. Collectively, the present results
suggested that PMS could effectively inhibit malignant melanoma
cell growth and metastasis. Therefore, alternative treatments using
PMS alone or in combination with other methods may be beneficial in
the treatment of malignant melanoma.
A number of specific inhibitors targeting different
signaling pathways have been introduced into preclinical trials
such as the RAF inhibitors (RAF/MEK/ERK pathway) (27). A major research direction in the
therapy of advanced melanoma is individualized molecular-targeted
therapy, which specifically targets the key enzymes involved in the
cell cancerous signaling pathway for targeted inhibition and
different types of genetic mutations in patients (28). Among them, two survival signaling
pathways RAS/mitogen-activated protein kinase and PI3K/AKT have
been investigated (29–31). The PI3K/AKT signaling pathway is
continuously activated in cancer cells and is closely related to
cancer cell survival, proliferation, angiogenesis and invasion
(32). Inhibition of the PI3K/AKT
signaling pathway is expected to be an effective treatment for
cancer (33). However, to the best
of our knowledge, there is no research on the inhibition of the
PI3K/AKT signaling pathway by PMS in melanoma cells. Therefore, the
present study investigated the effect of PMS on the PI3K/AKT
signaling pathway by examining the protein expression level of
p-AKT by western blotting. The present results indicated that PMS
may inhibit malignant melanoma cell viability, invasion and
migration. In addition, PMS may be able to induce apoptosis by
regulating the expression levels of apoptotic-related genes and the
activation of the PI3K/AKT signaling pathway, thereby exerting
antimalignant effects on melanoma.
In conclusion, the present results suggested that
PMS could effectively inhibit cell viability, migration and
invasion, and induce apoptosis in malignant melanoma cells. The
present results suggested that these effects were mediated by the
regulation of apoptotic-related gene expression levels and
activation of the PI3K/AKT signaling pathway. Therefore, PMS may be
a promising new type of small molecule chemotherapy for treatment
of malignant melanoma. However, the present study is only a
preliminary study of the effect of PMS on melanoma and the effect
of PMS on melanoma in vivo will need to be investigated in
future studies. Follow-up experiments will need to continue to
examine the effects of PMS in vivo in different species, as
the effect of PMS on human malignant melanoma may be different to
in vitro results. Therefore, the role of PMS in human
malignant melanoma requires further experimental research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data sets used and/or generated during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YW contributed to study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. MZL and SGC contributed to data collection and
statistical analysis. QW contributed to data collection,
statistical analysis and manuscript preparation. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Sandhra MC, Alexandra APM, Nádia SVC,
Isadora CC, Poliane C, de Oliveira LCA and Herman S: Synthesis and
in vitro assessment of anticancer hydrogels composed by
carboxymethylcellulose-doxorubicin as potential transdermal
delivery systems for treatment of skin cancer. J Mol Liq.
266:425–440. 2018. View Article : Google Scholar
|
|
2
|
Haass NK, Smalley KSM, Li L and Herlyn M:
Adhesion, migration and communication in melanocytes and melanoma.
Pigment Cell Res. 18:150–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferreira LM, Cervi VF, Sari MHM, Barbieri
AV, Ramos AP, Copetti PM, de Brum GF, Nascimento K, Nadal JM,
Farago PV, et al: Diphenyl diselenide loaded poly(ε-caprolactone)
nanocapsules with selective antimelanoma activity: Development and
cytotoxic evaluation. Mater Sci Eng C Mater Biol Appl. 91:1–9.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coricovac D, Dehelean C, Moaca EA, Pinzaru
I, Bratu T, Navolan D and Boruga O: Cutaneous melanoma-A long road
from experimental models to clinical outcome: A review. Int J Mol
Sci. 19(pii): E15662018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mou K, Ding M, Han D, Zhou Y, Mu X, Liu W
and Wang L: miR-590-5p inhibits tumor growth in malignant melanoma
by suppressing YAP1 expression. Oncol Rep. 40:2056–2066.
2018.PubMed/NCBI
|
|
6
|
Dzwierzynski WW: Managing malignant
melanoma. Plast Reconstr Surg. 132:446e–460e. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Z, Lan B, Peng B, Wang X, Zhang G, Li
X and Guo F: Combination therapy with BH3 mimetic and hyperthermia
tends to be more effective on anti-melanoma treatment. Biochem
Biophys Res Commun. 503:249–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garbe C, Eigentler TK, Keilholz U,
Hauschild A and Kirkwood JM: Systematic review of medical treatment
in melanoma: Current status and future prospects. Oncologist.
16:5–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tariq A, Sadia S, Pan K, Ullah I, Mussarat
S, Sun F, Abiodun OO, Batbaatar A, Li Z, Song D, et al: A
systematic review on ethnomedicines of anti-cancer plants.
Phytother Res. 31:202–264. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chao J, Dai Y, Verpoorte R, Lam W, Cheng
YC, Pao LH, Zhang W and Chen S: Major achievements of
evidence-based traditional Chinese medicine in treating major
diseases. Biochem Pharmacol. 139:94–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pei S, Yang X, Wang H, Zhang H, Zhou B,
Zhang D and Lin D: Plantamajoside, a potential anti-tumor herbal
medicine inhibits breast cancer growth and pulmonary metastasis by
decreasing the activity of matrix metalloproteinase-9 and −2. BMC
Cancer. 15:9652015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Damery S, Gratus C, Grieve R, Warmington
S, Jones J, Routledge P, Greenfield S, Dowswell G, Sherriff J and
Wilson S: The use of herbal medicines by people with cancer: A
cross-sectional survey. Br J Cancer. 104:927–933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan T and Gurav P: PhytoNanotechnology:
Enhancing delivery of plant based Anti-cancer drugs. Front
Pharmacol. 8:10022018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samuelsen AB: The traditional uses,
chemical constituents and biological activities of plantago
major L. A review. J Ethnopharmacol. 71:1–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu F, Huang X, He JJ, Song C, Peng L,
Chen T and Wu BL: Plantamajoside attenuates inflammatory response
in LPS-stimulated human gingival fibroblasts by inhibiting PI3K/AKT
signaling pathway. Microb Pathog. 127:208–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han AR, Nam MH and Lee KW: Plantamajoside
Inhibits UVB and Advanced Glycation End products-induced MMP-1
expression by suppressing the MAPK and NF-κB pathways in HaCaT
cells. Photochem Photobiol. 92:708–719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung HY, Seo DW, Hong CO, Kim JY, Yang SY
and Lee KW: Nephroprotection of plantamajoside in rats treated with
cadmium. Environ Toxicol Pharmacol. 39:125–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y and Yan D: Plantamajoside exerts
antifibrosis effects in the liver by inhibiting hepatic stellate
cell activation. Exp Ther Med. 18:2421–2428. 2019.PubMed/NCBI
|
|
19
|
Li X, Chen D, Li M, Gao X, Shi G and Zhao
H: Plantamajoside inhibits lipopolysaccharide-induced
epithelial-mesenchymal transition through suppressing the
NF-κB/IL-6 signaling in esophageal squamous cell carcinoma cells.
Biomed Pharmacother. 102:1045–1051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozovska Z, Gabrisova V and Kucerova L:
Malignant melanoma: Diagnosis, treatment and cancer stem cells.
Neoplasma. 63:510–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Markovic SN, Erickson LA, Flotte TJ,
Kottschade LA, McWilliams RR, Jakub JW, Farley DR, Tran NV, Schild
SE, Olivier KR, et al: Metastatic malignant melanoma. G Ital
Dermatol Venereol. 144:1–26. 2009.PubMed/NCBI
|
|
23
|
Franklin C, Livingstone E, Roesch A,
Schilling B and Schadendorf D: Immunotherapy in melanoma: Recent
advances and future directions. Eur J Surg Oncol. 43:604–611. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Greco A, Safi D, Swami U, Ginader T,
Milhem M and Zakharia Y: Efficacy and adverse events in metastatic
melanoma patients treated with combination BRAF plus MEK inhibitors
versus BRAF inhibitors: A systematic review. Cancers (Basel).
11(pii): E19502019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stahl T and Loquai C: Treatment side
effects and follow-up of malignant melanoma. Radiologe. 55:136–143.
2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cancedda S, Rohrer Bley C, Aresu L,
Dacasto M, Leone VF, Pizzoni S, Gracis M and Marconato L: Efficacy
and side effects of radiation therapy in comparison with radiation
therapy and temozolomide in the treatment of measurable canine
malignant melanoma. Vet Comp Oncol. 14:e146–e157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bollag G, Hirth P, Tsai J, Zhang J,
Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, et al:
Clinical efficacy of a RAF inhibitor needs broad target blockade in
BRAF-mutant melanoma. Nature. 467:596–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hauschild A, Agarwala SS, Trefzer U, Hogg
D, Robert C, Hersey P, Eggermont A, Grabbe S, Gonzalez R, Gille J,
et al: Results of a Phase III, Randomized, Placebo-controlled study
of Sorafenib in combination with carboplatin and paclitaxel as
second-line treatment in patients with Unresectable stage III or
stage IV melanoma. J Clin Oncol. 27:2823–2830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nazarian R, Shi H, Wang Q, Kong X, Koya
RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, et al: Melanomas
acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS
upregulation. Nature. 468:973–977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davies MA: The role of the PI3K-AKT
pathway in melanoma. Cancer J. 18:142–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yajima I, Kumasaka MY, Thang ND, Goto Y,
Takeda K, Yamanoshita O, Iida M, Ohgami N, Tamura H, Kawamoto Y and
Kato M: RAS/RAF/MEK/ERK and PI3K/PTEN/AKT signaling in malignant
melanoma progression and therapy. Dermatol Res Pract.
2012:3541912012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|