Introduction
Osteosarcoma is a malignant bone tumor originating
in the skeletal system, which often occurs in adolescents (1,2), and is
characterized by a high metastasis rate, poor prognosis and a high
recurrence rate (3,4). Although adjuvant and neoadjuvant
chemotherapeutic strategies have been widely applied in the
treatment of osteosarcoma and improve the rate of long-term
survival (5,6), the 5-year overall survival rate of
patients with distant metastasis and a high degree of malignancy
remains low (approximately 30%) (7).
It is therefore of great importance to search for novel agents to
treat this disease.
The nuclear factor-κB (NF-κB) transcription factor
family includes NF-κB1, NF-κB2, RelA, RelB and c-Rel, which have
diverse biological activities (8).
The mechanisms underlying NF-κB-driven regulation of apoptosis
remain controversial and are poorly understood. Growing evidence
suggests that NF-κB can promote apoptosis by increasing the
expression of pro-apoptotic proteins, such as p53, Fas, Fas ligand
and death receptors (9-11).
As a downstream target of p53 and part of the BH3-only subset of
Bcl-2 family proteins, p53 upregulated modulator of apoptosis
(PUMA), a potent apoptosis inducer in various cancer cells, is
induced by p53 following exposure to DNA-damaging agents, such as
chemotherapeutic drugs (12,13). It has been demonstrated that NF-κB is
induced early after tumor necrosis factor-α (TNF-α) exposure and
upregulates PUMA expression (14).
Mutations in the NF-κB binding site of the PUMA promoter have been
suggested to abolish the response of the luciferase reporter
containing the NF-κB binding site to cytokines, indicating that
this NF-κB binding site may be crucial for PUMA gene transcription
(15).
Natural products may have biological and
pharmaceutical activities. A novel compound named
15-hydroxy-6α,12-epoxy-7β,10αH,11βH-spiroax-4-ene-12-one (HESEO),
isolated from the endophytic fungus Penicillium sp. FJ-1 of
the mangrove plant Avicennia marina, has been suggested to
inhibit the tumor growth of human xenograft osteosarcoma in nude
mice (16). In the present study,
the effects of HESEO on osteosarcoma were further investigated and
the results suggested that HESEO may have anti-tumor activities, as
evidenced by inhibition of cell proliferation through the induction
of apoptosis and an increased survival time, as well as a decreased
tumor burden in osteosarcoma tumor-bearing mice when compared with
control treatments. These results suggest a scientific rationale to
develop HESEO as a novel potential agent against osteosarcoma.
Materials and methods
Cell lines, reagents and
chemicals
MG-63, SaOS2, HOS and U2OS human bone osteosarcoma
and K7M2-WT murine osteosarcoma cell lines were purchased from the
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. RPMI 1640 medium and fetal bovine serum (FBS) were
purchased from Gibco; Thermo Fisher Scientific, Inc. Caspase
activity assay kits (cat. no. K106-100 for caspase-3; cat. no.
K119-110 for caspase-9) were purchased from R&D Systems, Inc.
JC-1 was obtained from Molecular Probes; Thermo Fisher Scientific,
Inc. Cytochrome c immunoassay kit (cat. no. DCTC0) was
purchased from R&D Systems, Inc. The Annexin V-FITC/PI
apoptosis detection kit and PCR reagents were obtained from Thermo
Fisher Scientific, Inc. Methotrexate (MTX) was purchased from
Shanxi Pude Pharmaceutical Co., Ltd. All solvents used in this
study were purchased from Sinopharm Chemical Reagent Co., Ltd. as
analytical grade. HESEO was provided by Dr. Xiao-Ming Shi from
Linyi People's Hospital, Shandong, China. For the in vitro
studies, HESEO was dissolved in DMSO. For the in vivo
experiment, HESEO was prepared in 0.5% carboxymethylated cellulose
freshly before use. MTX was dissolved in saline.
Laboratory animals
Female Balb/c mice (age, 6-weeks; weight, 18-20 g)
were purchased from Charles River Laboratories, Inc. and housed in
an animal facility at 23±2˚C and 40-70% humidity under a 12-h
light/dark cycle. Mice had free access to food and water throughout
the experimental period. All animal experimental procedures were
performed in strict accordance with the Chinese legislation on the
use and care of laboratory animals, and were approved by the
Ethical Committee on Animal Care and Use of Guizhou Medical
University. Animal health and behavior were monitored three times
every day. Mice were euthanized immediately when they showed signs
of distress, including palpable hypothermia, hunching, lethargy or
body weight loss of >20%, or when they reached the humane
endpoints of tumor volume reaching 1,500 mm3 or the
maximum diameter exhibited by a single subcutaneous tumor reaching
20 mm, in order to minimize their suffering. Mice were euthanized
by inhalation of CO2 at a flow rate of 1.6 l/min in an 8
l chamber for 5 min; death was confirmed by cervical
dislocation.
Cell proliferation
Cells were cultured in RPMI 1640 medium supplemented
with 10% FBS and 100 µg/ml streptomycin/penicillin at 37˚C and 5%
CO2. Cells were treated with HESEO at 1, 3 and 10 nM for
72 h and cell number was measured using a cell counter (Vi-Cell;
Beckman Coulter, Inc.).
Mitochondrial membrane potential (MMP)
measurement
MMP was measured using the staining reagent JC-1.
After treatment with HESEO at 1, 3 and 10 nM or DMSO as a negative
control for 48 h at 37˚C, MG-63 cells were suspended at
5x105 cells/ml and incubated with JC-1 at a final
concentration of 2 µM for 15 min at 37˚C in the dark. After washing
twice, the red/green fluorescence intensity was measured using a
fluorescence microplate reader at an excitation of 490 nm and
emission of 530/590 nm.
Cytochrome c assay
After treatment, MG-63 cells were collected and
fractionated with a subcellular protein fractionation kit for
cultured cells (cat. no. 78840; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The cytoplasmic fraction
was used to measure cytochrome c levels with the quantikine
ELISA kit (cat. no. DCTC0; R&D Systems, Inc.). Briefly, 100 µl
calibrator diluent was added to each well of a 96-well microplate
before 100 µl standard, control or sample was added to each well.
The wells were covered with a plate seal and incubated at room
temperature for 2 h. Each well was then aspirated and washed three
times before 200 µl conjugate was added to each well. The wells
were, once again, covered with a plate seal and incubated at room
temperature for 2 h. Wells were aspirated and washed four times
prior to the addition of 200 µl substrate solution to each well.
The wells were incubated at room temperature for 30 min before 50
µl stop solution was added to each well. The absorbance was read at
450 nm within 30 min.
Caspase activity measurement
After treatment, MG-63 cells were collected and
lysed with cell lysis buffer (cat. no. #9803, Cell Signaling
Technology, Inc.). Caspase activity was measured using assay kits
(cat. no. K106-100 for caspase-3; cat. no. K119-110 for caspase-9;
R&D Systems, Inc.), according to the manufacturer's
instructions.
Apoptosis analysis
After treatment, MG-63 cells were harvested and
washed with pre-chilled PBS, and then resuspended in binding buffer
to adjust the cell concentration to 5x105 cells/ml.
Subsequently, 5 µl Annexin V-FITC was added to 195 µl cell
suspension and incubated for 10 min at room temperature. Cells were
then washed and resuspended in 190 µl binding buffer, and 10 µl PI
was added to each sample for 1 min in the dark at room temperature.
Analysis was performed using a FACScan flow cytometer (BD
Biosciences). The data were analysed with FlowJo version 10 (FlowJo
LLC).
Reverse transcription-quantitative
PCR
After treatment, total RNA was extracted from MG-63
cells using a TRIzol® extraction kit (Invitrogen; Thermo
Fisher, Scientific, Inc.). The RNA concentration was measured using
a spectrophotometer. mRNA was transcribed into cDNA (25˚C for 5
min, 46˚C for 20 min, 95˚C for 1 min) using SuperScript master mix
(Bio-Rad Laboratories, Inc.). RT-qPCR was run on a StepOne system
using SYBR green Supermix (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were: 95˚C for 10 min, and 40 cycles of
95˚C for 15 sec and 60˚C for 60 sec. Subsequently, relative
expression levels were quantified using the
2-ΔΔCq method and were normalized
to β-actin (17). The gene-specific
primer sequences used were the following. Caspase-3:
Forward-5'-ATTGTGGAATTGATGCGTGA-3',
Reverse-3'-GGCAGGCCTGAATAATGAAA-5' (GenBank reference: AJ413269.1);
Caspase-9: Forward-5'-agggaagagggaatggaaga-3',
Reverse:-3'-GAGTCGTCACACTTCCAGCA-5' (GenBank reference:
AY214168.1); β-actin: Forward-5'-GCTCTTTTCCAGCCTTCCTT-3',
Reverse-3'-AGTACTTGCGCTCAGGAGGA-5' (GenBank reference:
HQ154074.1).
Western blot analysis
After treatment, MG-63 cells or mouse tumor tissues
were lysed with radioimmunoprecipitation assay lysis buffer (Thermo
Fisher Scientific, Inc.) containing a cocktail of protease
inhibitors. The lysates were centrifuged at 6,000 x g for 10 min at
4˚C, and protein concentrations were determined using the BCA
method. After boiling for 5 min, proteins (40 µg/lane) were
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on 4-12% gels and were then transferred to
polyvinylidene fluoride membranes. After blocking with 1% BSA
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature, proteins
were incubated overnight at 4˚C with the appropriate rabbit primary
antibodies: Anti-cleaved caspase-3 (cat. no. ab2302; 1:1,000),
anti-cleaved caspase-9 (cat. no. ab2324; 1:1,000), anti-Bcl-2
(cat.no. ab196495; 1:1,000), anti-Bax (cat. no. ab104156; 1:500),
anti-NF-κB phosphorylated (p)-p65 S536 (cat. no. ab86299; 1:3,000),
anti-NF-κB p65 (cat. no. ab16502; 1:1,000); anti-PUMA (cat. no.
ab9643; 1:500) and anti-β-actin (cat. no. ab227387; 1:3,000) and
then further incubated with the secondary horseradish
peroxidase-conjugated antibody (cat. no. ab205718; 1:3,000) for 2 h
at room temperature. All antibodies were supplied by Abcam. The
bands were detected using an ECL system (EMD Millipore).
Syngeneic tumor models
K7M2 cells (6x105 in 100 µl PBS) were
injected into Balb/c mice through the tail vein. Mice were divided
randomly into three groups (5 mice/group) and treatment was
initiated 14 days after cell injection. Mice were treated with
HESEO (1 or 2 mg/kg) by intragastric administration 5 days per week
or were treated with 0.5% carboxymethylated cellulose (vehicle; 50
mg/kg) as a negative control. For the combination study,
6x105 K7M2 cells in 100 µl PBS were injected into Balb/c
mice through the tail vein. Mice were divided randomly into four
groups (5 mice/group) and treatment was initiated 14 days after
cell injection. Mice were treated with HESEO (1 mg/kg) by
intragastric administration 5 days per week, with MTX (87.5 mg/kg)
by intraperitoneal injection weekly, or with the combination
treatment. Mice treated with vehicle served as the negative
control.
K7M2 cells (5x105) were subcutaneously
injected into Balb/c mice. When tumor volume reached 50-100
mm3, tumor-bearing mice were equally divided into three
different groups by tumor volume (5 mice/group). Mice were treated
with HESEO (1 or 2 mg/kg) by intragastric administration 5 days per
week or were treated with vehicle as a negative control. Once tumor
volume reached 1,500 mm3 or the maximum diameter
exhibited by a single subcutaneous tumor reached 20 mm, all
tumor-bearing mice were sacrificed to collect tumors for
analysis.
Statistical analysis
Data are presented as the mean ± SD and were
analyzed using SPSS version 12.0 (SPSS, Inc.). Data were analyzed
by one-way analysis of variance followed by Dunnett's post-hoc
test. For the subcutaneous tumor model, one-way analysis of
variance followed by Dunnett's post-hoc test was used to compare
differences among the groups. For the tail vein injection tumor
model, Kaplan-Meier survival analysis was used to compare
differences among the groups and P-values were calculated using the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference. Samples were measured in triplicate and
experiments were repeated three times.
Results
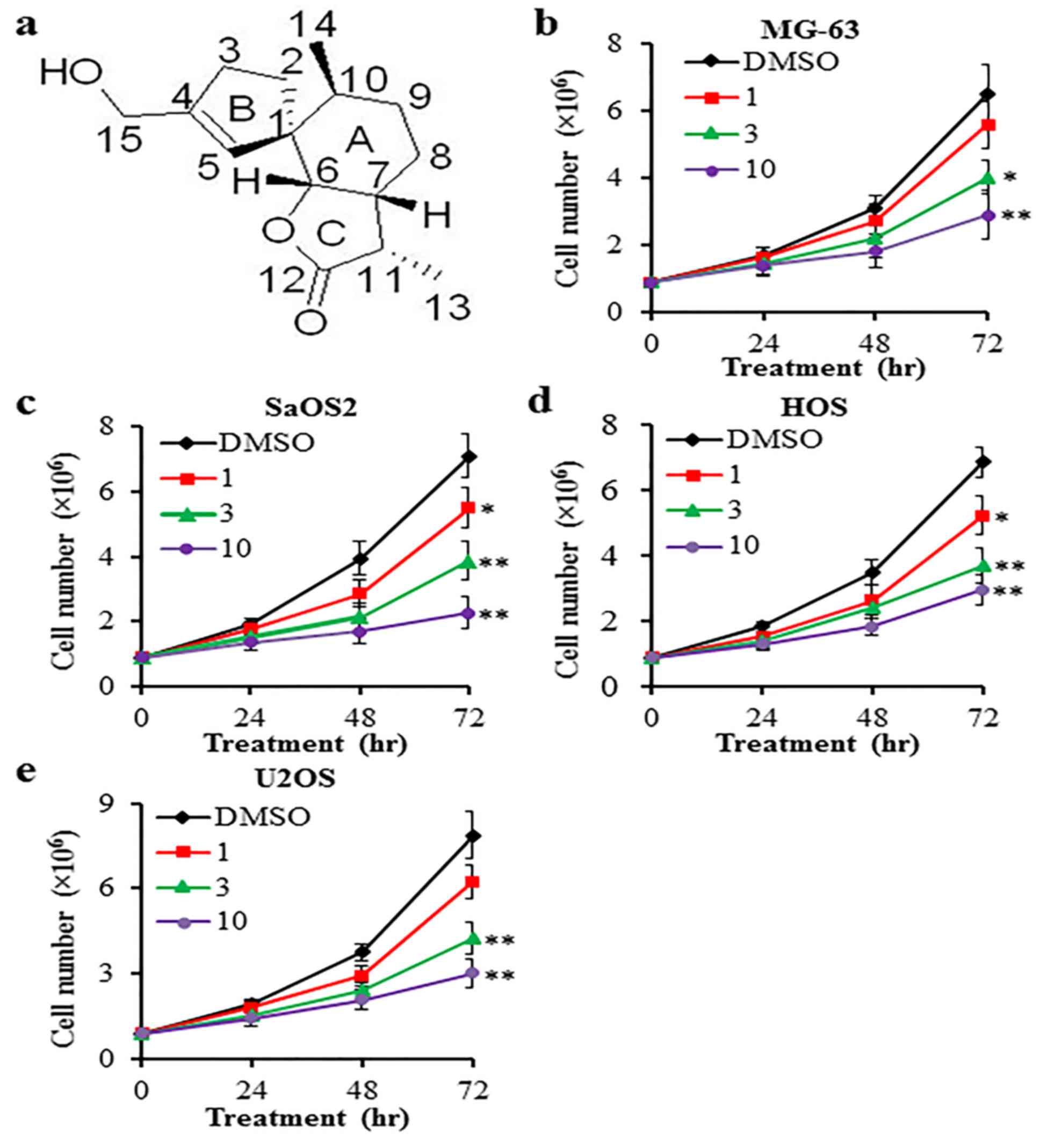
HESEO inhibits the proliferation of
osteosarcoma cells
Cell proliferation was measured by counting the cell
number after 72 h. HESEO demonstrated anti-proliferative activities
on MG-63, SaOS2, HOS and U2OS cells when compared with vehicle
control (DMSO; Fig. 1).
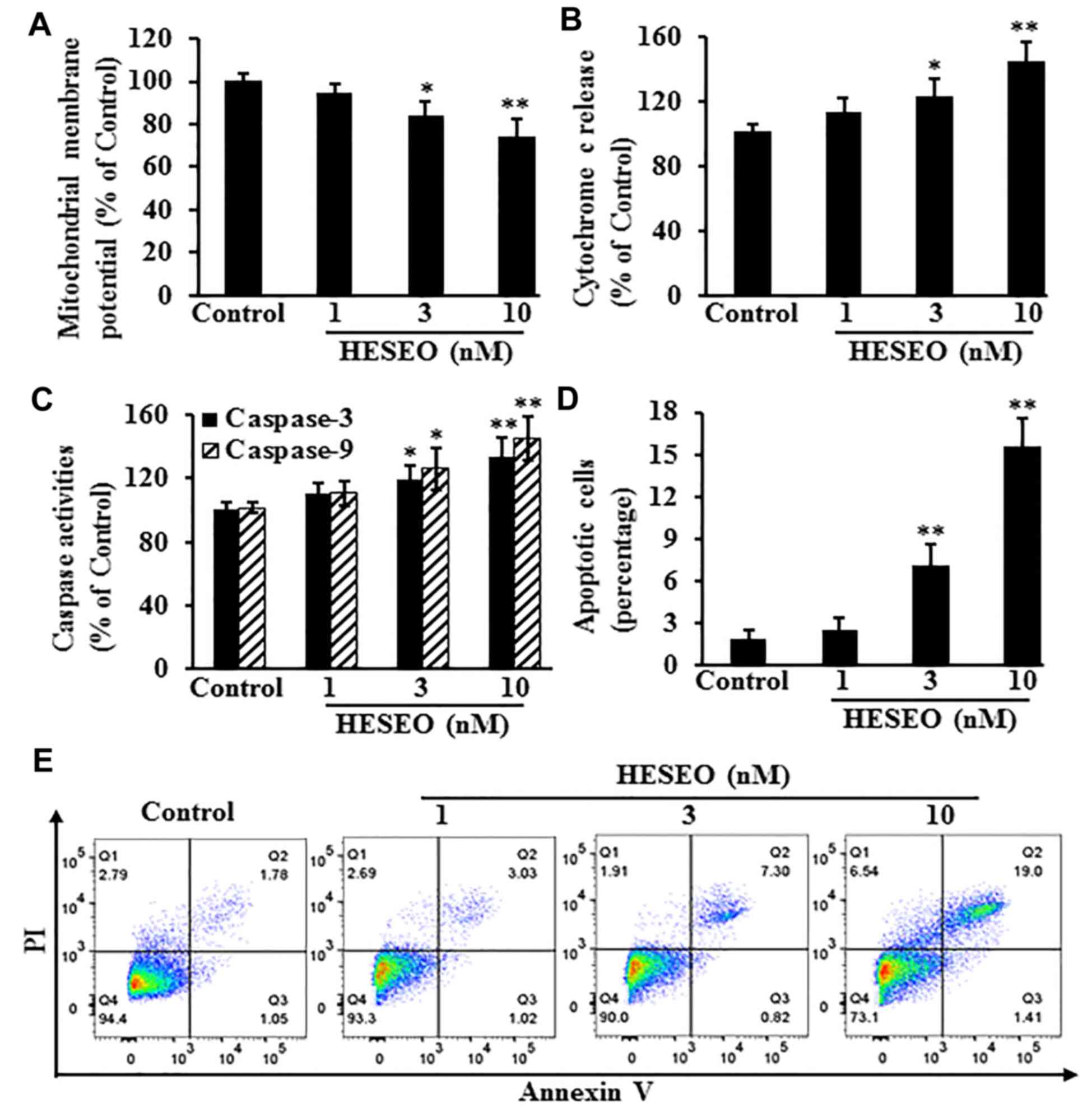
HESEO induces apoptosis of MG-63
cells
After treatment, levels of apoptosis-related markers
were detected. Compared with the DMSO control, HESEO reduced the
MMP (Fig. 2A), and increased the
release of cytochrome c (Fig.
2B), caspase-3/9 activities (Fig.
2C) and the percentage of apoptotic cells (Fig. 2D and E).
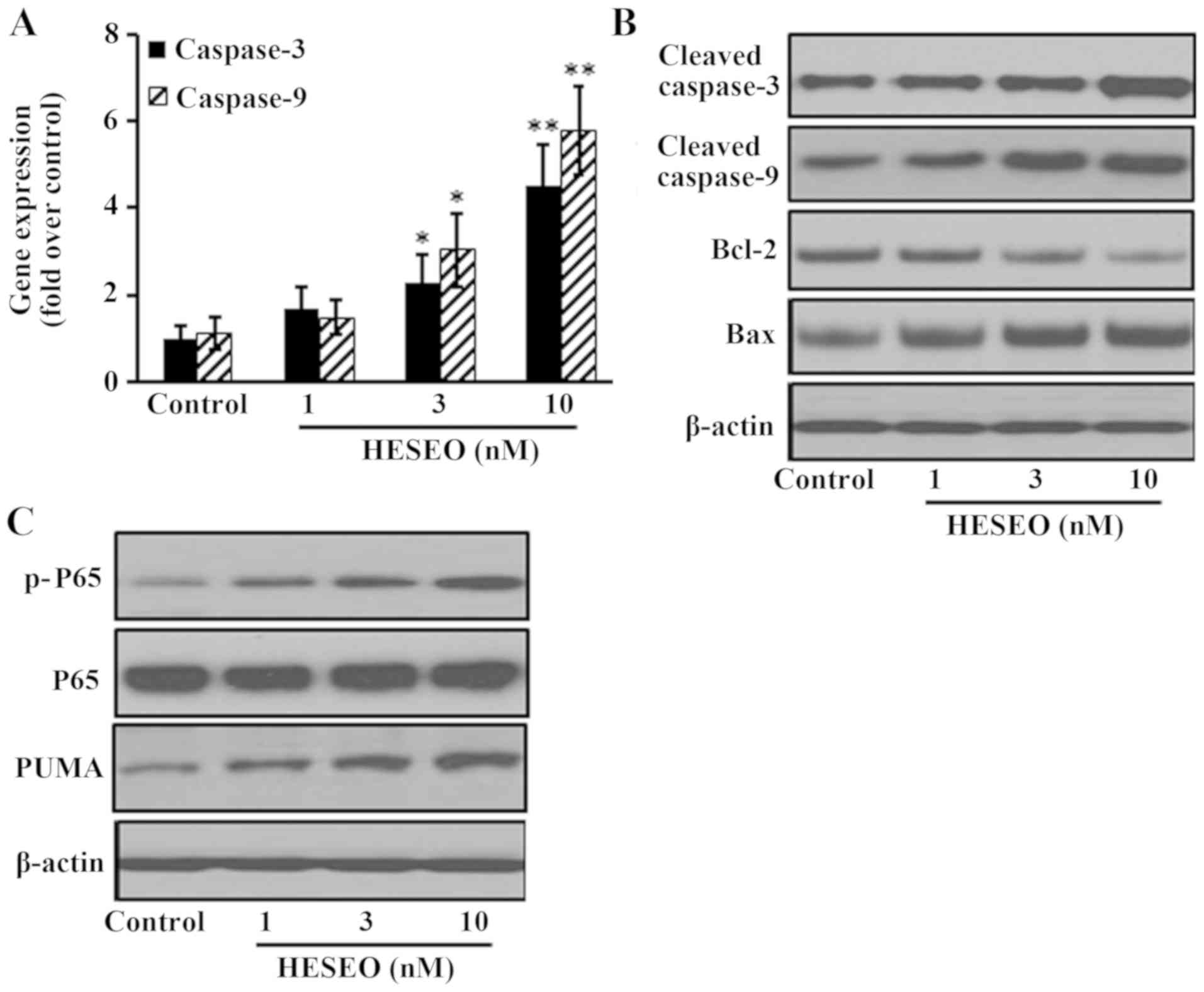
HESEO induces apoptosis through
increasing the expression of NF-κB p-P65 and PUMA
After HESEO treatment, the expression of
pro-apoptotic genes was significantly increased when compared with
control treatment (Fig. 3A). The
expression of pro-apoptotic proteins (cleaved caspase-3, cleaved
caspase-9, Bax) was also increased, whereas the expression levels
of anti-apoptotic proteins (Bcl-2) were decreased with HESEO
treatment compared with in the control group (Fig. 3B). HESEO treatment also increased the
expression levels of NF-κB p-P65 and PUMA compared with in the
control group (Fig. 3C).
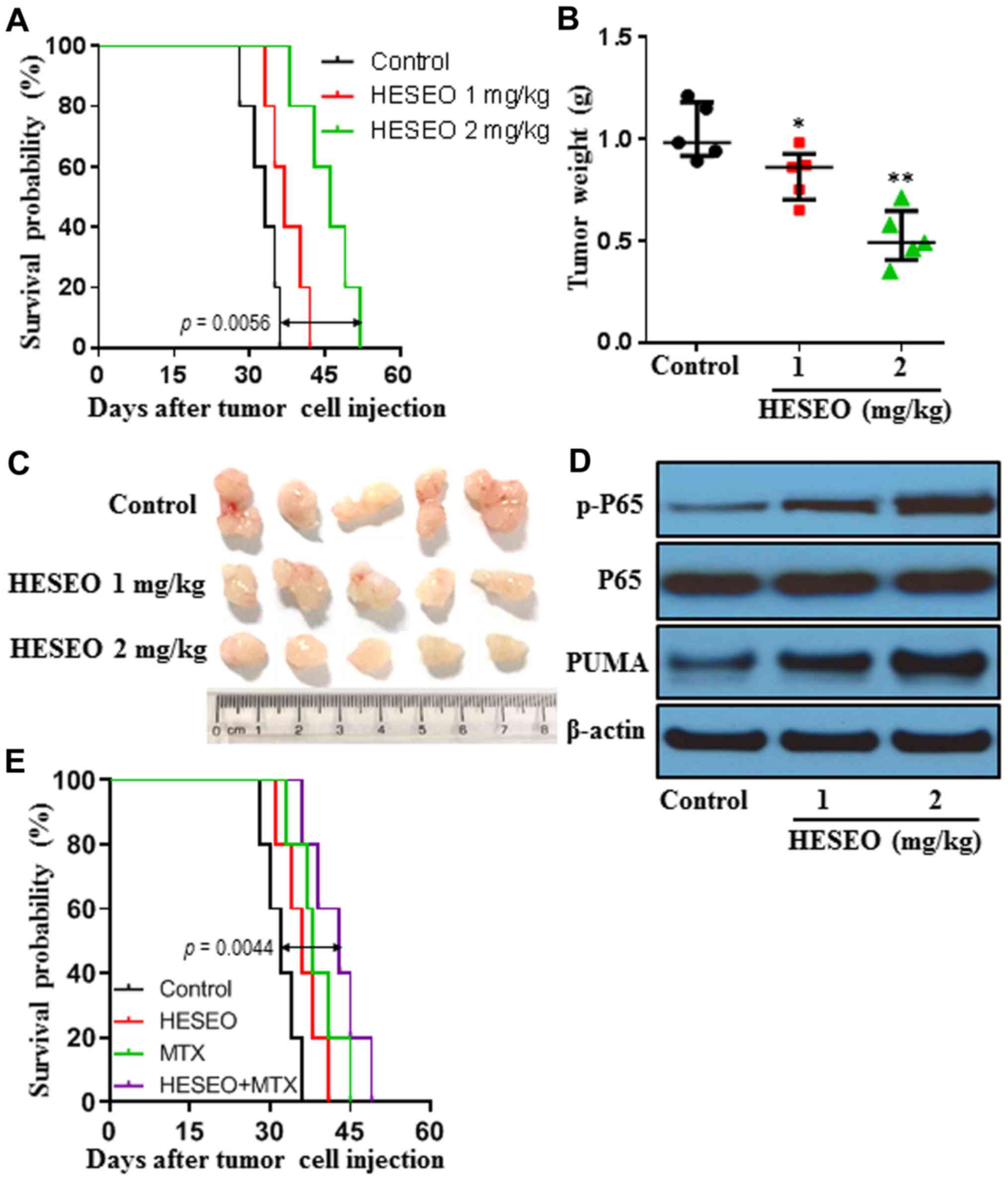
HESEO increases the survival time and
decreases the tumor burden of K7M2 tumor-bearing mice
The anti-tumor effects of HESEO were further
explored in syngeneic tumor models. K7M2 tumor-bearing mice were
treated with 1 or 2 mg/kg HESEO. HESEO treatment at 2 mg/kg
significantly increased the survival time of tumor-bearing mice
with lung metastasis (Fig. 4A) and
decreased the tumor burden of tumor-bearing mice in a subcutaneous
tumor model (Fig. 4B and C) when compared with control treatment.
Furthermore, HESEO treatment increased NF-κB p-P65 levels and PUMA
expression in tumor tissues of mice subcutaneously injected with
cells (Fig. 4D). In addition,
combination treatment with HESEO and the chemotherapeutic agent MTX
significantly increased the survival time of tumor-bearing mice
with lung metastasis over either treatment alone (Fig. 4E).
Discussion
As a genetically unstable and highly malignant
mesenchymal bone tumor, osteosarcoma is characterized by structural
chromosomal alterations (18-20).
Although advances in multimodality treatment, consisting of
radiation and chemotherapy, have led to a relatively good
prognosis, the 5-year survival rate for patients with osteosarcoma
has reduced in the past two decades, due to the presence of
pulmonary metastasis in 40-50% of patients (21,22).
Therefore, to improve survival levels, it is imperative to search
for effective treatment strategies. In the present study, HESEO, a
natural compound extracted from the endophytic fungus
Penicillium sp. FJ-1 isolated from Avicennia
marina, was found not only to inhibit the proliferation of
MG-63 cells, but also to inhibit the proliferation of other human
osteosarcoma cells when compared with the control group.
Furthermore, HESEO induced apoptosis of MG-63 cells, which may be
due to the increased expression of NF-κB p-P65 and PUMA, and
increased the survival time and decreased the tumor burden of
osteosarcoma tumor-bearing mice when compared with control
treatment. Other pathways, such as PI3K-AKT-mTOR, RAS-RAF-MAF and
cell cycle regulation, will be explored in future studies.
Mitochondrial dysfunction has been shown to induce
apoptosis and is suggested to be central to the apoptotic pathway
(23,24). As a group of proteolytic enzymes at
the end of the apoptotic signaling pathway, the caspase family
plays an essential role in apoptosis. Initiator caspases initiate
the apoptosis signal while the executioner caspases carry out the
mass proteolysis that leads to apoptosis (25). Notably, the Bcl-2 family exerts its
pro-apoptotic (through Bax and Bak) or anti-apoptotic (through
Bcl-2 and Bcl-XL) activities mainly through the
mitochondrial pathway. When cells are stimulated, intracellular Bax
translocates to the mitochondrial outer membrane and forms a
Bax/Bcl-2 heterodimer with Bcl-2 to induce the release of
cytochrome c from the mitochondria to the cytoplasm, thereby
inducing the downstream apoptosis cascade pathway (26,27). The
present study indicated that HESEO treatment caused MG-63 cell
damage, characterized by reduced MMP expression, increased
cytochrome c release, increased caspase activity, marked
downregulation of anti-apoptotic protein Bcl-2 expression, and
upregulation of the expression of pro-apoptotic proteins Bax and
caspase-3/caspase-9.
When inactive, NF-κB remains in the cytoplasm bound
to specific inhibitory proteins. Upon activation, NF-κB
translocates to the nucleus and binds to κB consensus sequences to
modulate numerous target genes (28). As a mediator of cell survival, NF-κB
signaling acts downstream of the TNF-α receptor. Notably, the TNF-α
signal bifurcates downstream of the TNF-α receptor into at least
two axes, one of which activates NF-κB to trigger cell death
(29). As a target of NF-κB and a
critical mediator of TNF-α-induced apoptosis, PUMA has been
demonstrated to be induced in response to DNA-damaging agents, such
as commonly used chemotherapeutic drugs and irradiation (30,31). The
pro-apoptotic function of PUMA is mediated by its interactions with
anti-apoptotic Bcl-2 family members, such as Bcl-2 and
Bcl-XL, leading to Bax/Bak-dependent mitochondrial
dysfunction, cytochrome c release and caspase activation.
The role of PUMA in DNA damage-induced apoptosis has been confirmed
using PUMA-knockout cells and mice (32-34).
MG-63 cells are p53-deficient, but anti-tumor drugs (such as
doxorubicin) can increase PUMA protein expression after 48 h
(35). In the present study, MG-63
cells were treated with HESEO for 48 h before PUMA expression was
determined. Similarly to doxorubicin, HESEO could increase PUMA
expression. Furthermore, the present study reported that HESEO
treatment increased NF-κB p-P65 levels, which may induce apoptosis
of MG-63 cells.
In conclusion, the present study demonstrated that
HESEO, isolated from the endophytic fungus Penicillium sp.
FJ-1 of Avicennia marina, may inhibit osteosarcoma
cell proliferation, and increase survival time and decrease tumor
burden in tumor-bearing mice, suggesting the scientific rationale
to develop HESEO as a therapeutic agent against osteosarcoma.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from The National
Natural Science Foundation of China (grant no. 81501563) and The
Suzhou Science and Technology Plan (grant no. SYS201611).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CFN and SZ designed and conceived the current study.
TZA, ZL, CY, XQH, PCL and JS performed the experiments and analyzed
the data. TZA, ZL, CFN and SZ prepared the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in compliance
with the Chinese legislation on the use and care of laboratory
animals, and were approved by The Ethical Committee on Animal Care
and Use of Guizhou Medical University (protocol no. 2017-0163).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Duong LM and Richardson LC: Descriptive
epidemiology of malignant primary osteosarcoma using
population-based registries, United States, 1999-2008. J Registry
Manag. 40:59–64. 2013.PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang S, Ren T, Huang Y, Bao X, Sun K, Shen
D and Guo W: BMPR2 and HIF1-α overexpression in resected
osteosarcoma correlates with distant metastasis and patient
survival. Chin J Cancer Res. 29:447–454. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Angelini A, Ceci F, Castellucci P,
Graziani T, Polverari G, Trovarelli G, Palmerini E, Ferrari S,
Fanti S and Ruggieri P: The role of 18F-FDG PET/CT in
the detection of osteosarcoma recurrence. Eur J Nucl Med Mol
Imaging. 44:1712–1720. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
O'Kane GM, Cadoo KA, Walsh EM, Emerson R,
Dervan P, O'Keane C, Hurson B, O'Toole G, Dudeney S, Kavanagh E, et
al: Perioperative chemotherapy in the treatment of osteosarcoma: A
26-year single institution review. Clin Sarcoma Res.
5(17)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hansen AR, Hughes BG, Paul S, Steadman P,
Sommerville S, Dickinson IC, Walpole ET, Thomson DB, Mar Fan HG and
Joubert WL: Single institution retrospective review of
perioperative chemotherapy in adult and adolescent patients with
operable osteosarcoma. Asia Pac J Clin Oncol. 12:e222–e228.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guenther LM, Rowe RG, Acharya PT, Swenson
DW, Meyer SC, Clinton CM, Guo D, Sridharan M, London WB, Grier HE,
et al: Response Evaluation Criteria in Solid Tumors (RECIST)
following neoadjuvant chemotherapy in osteosarcoma. Pediatr Blood
Cancer. 65(e26896)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hayden MS and Ghosh S: NF-κB in
immunobiology. Cell Res. 21:223–244. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Burstein E and Duckett CS: Dying for
NF-kappaB? Control of cell death by transcriptional regulation of
the apoptotic machinery. Curr Opin Cell Biol. 15:732–737.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dutta J, Fan Y, Gupta N, Fan G and Gelinas
C: Current insights into the regulation of programmed cell death by
NF-kappaB. Oncogene. 25:6800–6816. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu J, Zhang L, Hwang PM, Kinzler KW and
Vogelstein B: PUMA induces the rapid apoptosis of colorectal cancer
cells. Mol Cell. 7:673–682. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nakano K and Vousden KH: PUMA, a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gurzov EN, Ortis F, Cunha DA, Gosset G, Li
M, Cardozo AK and Eizirik DL: Signaling by IL-1beta+IFN-gamma and
ER stress converge on DP5/Hrk activation: A novel mechanism for
pancreatic beta-cell apoptosis. Cell Death Differ. 16:1539–1550.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gurzov EN, Germano CM, Cunha DA, Ortis F,
Vanderwinden JM, Marchetti P, Zhang L and Eizirik DL: p53
up-regulated modulator of apoptosis (PUMA) activation contributes
to pancreatic beta-cell apoptosis induced by proinflammatory
cytokines and endoplasmic reticulum stress. J Biol Chem.
285:19910–19920. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zheng C, Chen Y, Jiang LL and Shi XM:
Antiproliferative metabolites from the endophytic fungus
Penicillium sp. FJ-1 isolated from a mangrove Avicennia
marina. Phytochem Lett. 10:272–275. 2014. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li Y, Du W, Han J and Ge JB: LAMP3
promotes the invasion of osteosarcoma cells via SPP1 signaling. Mol
Med Rep. 16:5947–5953. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lussier DM, Johnson JL, Hingorani P and
Blattman JN: Combination immunotherapy with α-CTLA-4 and α-PD-L1
antibody blockade prevents immune escape and leads to complete
control of metastatic osteosarcoma. J Immunother Cancer.
3(21)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Strauss SJ, Ng T, Mendoza-Naranjo A,
Whelan J and Sorensen PH: Understanding micrometastatic disease and
Anoikis resistance in ewing family of tumors and osteosarcoma.
Oncologist. 15:627–635. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Carrle D and Bielack S: Osteosarcoma lung
metastases detection and principles of multimodal therapy. Cancer
Treat Res. 152:165–184. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Harting MT and Blakely ML: Management of
osteosarcoma pulmonary metastases. Semin Pediatr Surg. 15:25–29.
2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Z, Lu W, Li Y and Tang B: Alpinetin
promotes Bax translocation, induces apoptosis through the
mitochondrial pathway and arrests human gastric cancer cells at the
G2/M phase. Mol Med Rep. 7:915–920. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xue S, Chen YX, Qin SK, Yang AZ, Wang L,
Xu HJ and Geng HY: Raltitrexed induces mitochondrial-mediated
apoptosis in SGC7901 human gastric cancer cells. Mol Med Rep.
10:1927–1934. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xin BR, Liu JF, Kang J and Chan WP: (2R,
3S)-pinobanksin-3-cinnamate, a new flavonone from seeds of
Alpinia galanga willd., presents in vitro neuroprotective
effects. Mol Cell Toxicol. 10:165–172. 2014. View Article : Google Scholar
|
|
26
|
Zhao X, Kong F, Wang L and Zhang H: c-FLIP
and the NOXA/Mcl-1 axis participate in the synergistic effect of
pemetrexed plus cisplatin in human choroidal melanoma cells. PLoS
One. 12(e0184135)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao X, Liu X and Su L: Parthenolide
induces apoptosis via TNFRSF10B and PMAIP1 pathways in human lung
cancer cells. J Exp Clin Cancer Res. 33(3)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227.
2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen G and Goeddel DV: TNF-R1 signaling: A
beautiful pathway. Science. 296:1634–1635. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang P, Yu J and Zhang L: The nuclear
function of p53 is required for PUMA-mediated apoptosis induced by
DNA damage. Proc Natl Acad Sci USA. 104:4054–4059. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang P, Qiu W, Dudgeon C, Liu H, Huang C,
Zambetti GP, Yu J and Zhang L: PUMA is directly activated by
NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell
Death Differ. 16:1192–1202. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ming L, Wang P, Bank A, Yu J and Zhang L:
PUMA dissociates Bax and BCL-X(L) to induce apoptosis in colon
cancer cells. J Biol Chem. 281:16034–16042. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jeffers JR, Parganas E, Lee Y, Yang C,
Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, et al:
Puma is an essential mediator of p53-dependent and -independent
apoptotic pathways. Cancer Cell. 4:321–328. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Villunger A, Michalak EM, Coultas L,
Mullauer F, Bock G, Ausserlechner MJ, Adams JM and Strasser A: p53-
and drug-induced apoptotic responses mediated by BH3-only proteins
Puma and Noxa. Science. 302:1036–1038. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun YF, Xia P, Zhang HP, Liu B and Shi Y:
P53 is required for doxorubicin-induced apoptosis via the TGF-beta
signaling pathway in osteosarcoma-derived cell. Am J Cancer Res.
6:114–125. 2016.PubMed/NCBI
|