Introduction
Polycystic ovary syndrome (PCOS) is a common female
endocrine disorder, affecting 6-12% of women of reproductive age
globally (1). It is characterized by
symptoms such as menstrual irregularity, anovulatory infertility,
hyperandrogenism and obesity, as well as metabolic dysfunctions
including insulin resistance, type 2 diabetes and dyslipidemia
(2). These symptoms can have a
serious impact on the quality of life and health of patients.
However, due to the complexity of this disease, the pathogenesis
remains unclear, and an optimal treatment has not been determined
(3). There is a growing need to
develop innovative interventions to treat this disease.
Recently, a series of studies have demonstrated that
vitamin D deficiency (VDD) is common in patients with PCOS and that
VDD may be associated with metabolic and endocrine disorders in
PCOS (4-7).
Compared with the general population, the prevalence of VDD is
relatively higher in PCOS patients (6). Vitamin D is a steroid hormone that is
involved in the balance of calcium phosphate and bone
mineralization (8). Vitamin D
receptors are expressed at 2,776 genomic positions and modulate the
expression of 229 genes in more than 30 different tissues,
including the pancreas, liver, immune cells, brain and ovaries
(9). As a result, vitamin D
supplementation for PCOS therapy has attracted attention.
Although a growing number of intervention studies
have assessed the relationship between vitamin D supplementation
and PCOS, there is a lack of convincing evidence demonstrating the
effect of vitamin D supplementation on PCOS. Therefore, the
objective of this meta-analysis was to quantitatively summarize the
available evidence to assess the effects of vitamin D
supplementation on metabolic and hormonal functions in patients
with PCOS.
Materials and methods
Data sources and search strategy
In the present study, a systematic search was
performed, searching for studies between January 1965 and July 2019
in PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), Embase
(https://www.elsevier.com/solutions/embase-biomedical-research),
Web of Science (http://webofscience.com) and Ovid Medline (http://ovidsp.ovid.com/). The search was initially
conducted using Medical Subject Headings and the following free key
words: Polycystic ovary syndrome, polycystic ovary disease, PCOS,
calcitriol and vitamin D. Randomized controlled trials (RCTs)
investigating the effects of vitamin D supplementation for patients
with PCOS were included in the current meta-analysis. A full manual
search of the bibliographies of selected studies was performed to
identify additional studies.
Inclusion and exclusion criteria
Studies were included if they met the following
criteria: A randomized, controlled clinical trial comparing the
effects of vitamin D supplementation with placebo; studies that
enrolled women with a strict diagnosis of PCOS based on diagnostic
criteria produced by the Rotterdam European Society of Human
Reproduction and Embryology (ESHRE) and the American Society of
Reproductive Medicine (ASRM); the main outcomes included body mass
index (BMI), total testosterone, dehydroepiandrosterone sulfate
(DHEAs), homeostasis model assessment of insulin resistance
(HOMA-IR), homeostasis model assessment of β-cell function
(HOMA-B), triglycerides, total cholesterol, low density
lipoprotein-cholesterol (LDL-C) or low density
lipoprotein-cholesterol (HDL-C). For each trial, the most recent
data was used in the present analysis.
The exclusion criteria were as follows: Studies
reporting other diseases, such as diabetes, Cushing's syndrome,
hyperthyroidism or other hormone-related disorders; studies
reporting other treatments, such as calcium or metformin; and
studies not published in English.
Data extraction
Data extraction and quality assessment were
performed by two authors independently. The following information
was extracted and entered into a database: The first author's name,
year of publication, country, study design, date of accrual,
diagnostic criteria for PCOS, the basic characteristics of
participants (principal baseline characteristics such as number and
age), the control interventions and the main outcomes which
included BMI, total testosterone, DHEAs, HOMA-IR, HOMA-B,
triglycerides, total cholesterol, LDL-C and HDL-C.
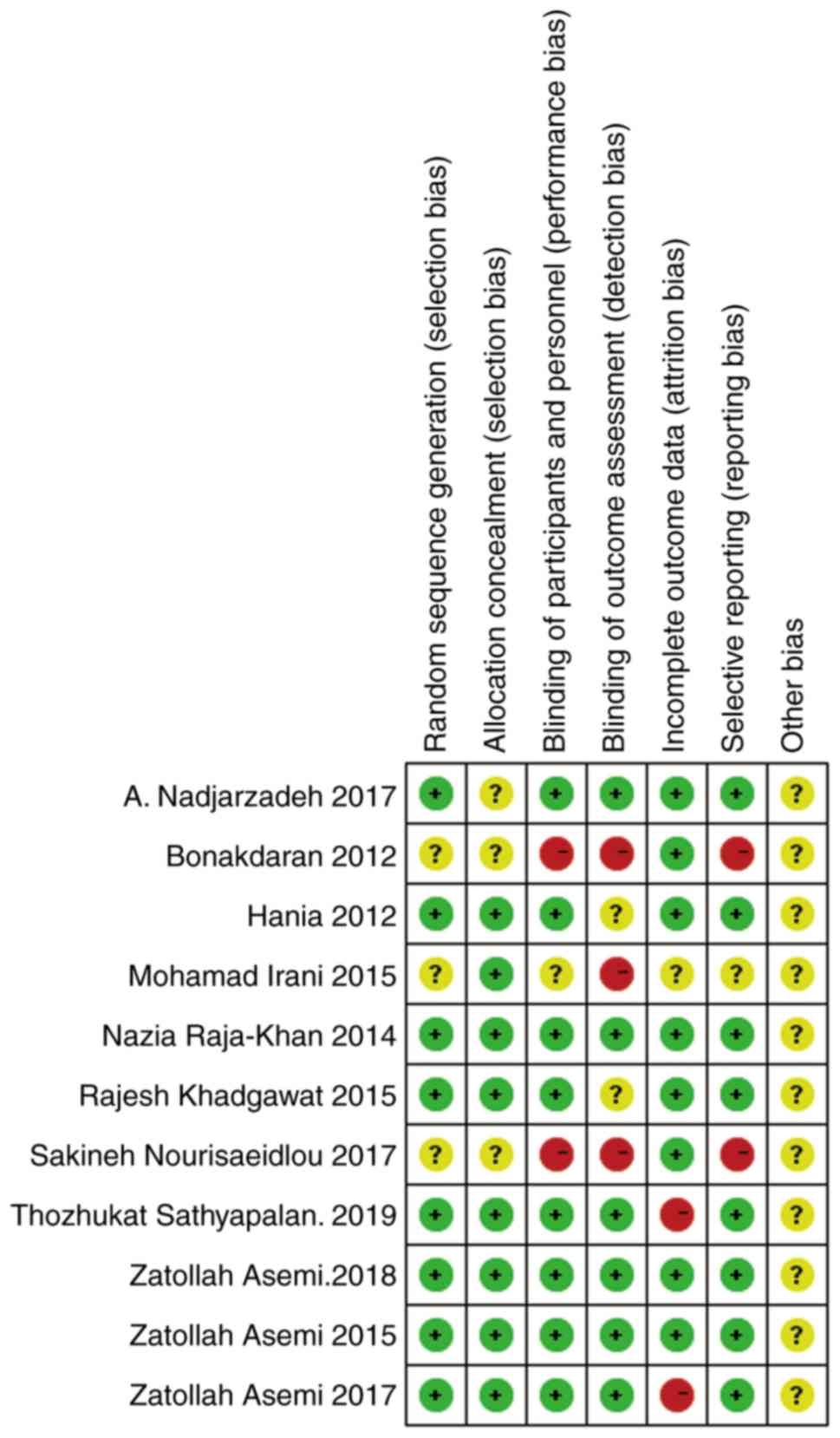
Study quality
The quality of each study was independently assessed
by two authors according to the following criteria: Studies
designed with case characteristics matched to controls; the studies
followed strict inclusion and exclusion criteria for patients; and
the methodological quality of the included studies described by
Jadad RCT guidelines (a randomized method, concealment allocation,
a blinding method and a follow-up) and the Cochrane risk bias
assessment tool (10).
Statistical analysis
Continuous data are presented as the mean and SD for
all included studies. Weighted mean differences (WMDs) with 95%
confidence intervals (CIs) were calculated. In order to measure the
heterogeneity among all included studies, Cochran's Q-tests
(χ2 test) were used. A Cochran Q-test with P>0.10 and
the I2 statistic <50% indicated statistical
homogeneity. If the results of the Cochran Q-test favored
homogeneity among the studies, the fixed-effect model was used to
calculate the total effect size. The random-effects model of
meta-analysis was used when unexplained statistical heterogeneity
was present. P≤0.05 and a 95% CI not containing 0 (WMD) were
considered to be statistically significant. Funnel plots were used
to assess publication bias. The current meta-analysis was performed
using Review Manager 5.3 software (https://community.cochrane.org/help/tools-and-software/revman-5/revman-5-download/installation)
provided by the Cochrane Collaboration.
Results
Study selection
A total of 2,123 relevant studies were obtained in
the preliminary examination. After reading the titles and
abstracts, 132 related studies were selected. After obtaining the
full texts and reviewing them one by one, 121 studies were further
excluded. Among them, 76 studies did not conform to the inclusion
criteria, 30 studies did not have complete data and 15 studies were
of low quality. A total of 11 studies were eventually included. The
literature screening process is shown in Fig. 1.
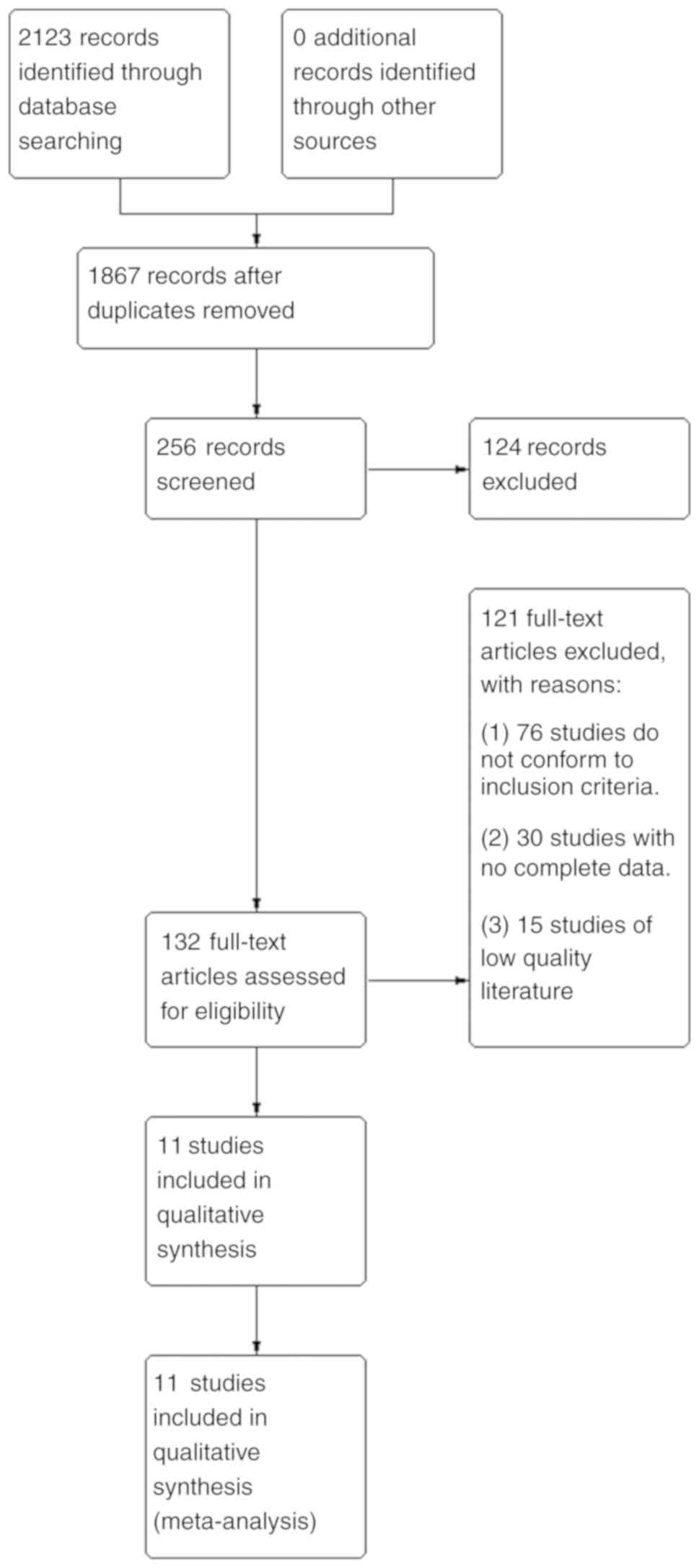 | Figure 1Literature retrieval process. Through
searches of the PubMed, Embase, Medline and Web of Science
databases, a total of 2,123 relevant studies were obtained. A total
of 1,867 studies were obtained after removing duplicates. Of these,
256 were obtained after limiting the research direction and
research type, and 124 of them were deleted after further reading
of the title and abstract of the article. The remaining 132 studies
were examined carefully. Among them, 76 studies did not meet the
inclusion criteria, 30 studies lacked complete data, and 15
low-quality studies were excluded. Finally, 11 studies were
selected for meta-analysis. |
Characteristics of included
studies
A total of 11 studies involving 483 participants
were included in the meta-analysis. Table I lists the basic characteristics of
all included studies. The diagnosis of PCOS in all 11 studies was
based on the guidelines of the ESHRE and ASRM. A total of seven
studies were conducted in Iran, two were in the United States, one
was in India and one was in the UK. A total of 11 studies compared
post-intervention differences in metabolic and endocrine parameters
between vitamin D supplements and placebo groups.
 | Table IMajor characteristics measured in the
current study. |
Table I
Major characteristics measured in the
current study.
| Authors, year | RCT | Date of
accrual | Region | No. of
patients | Age | Symptoms | Treatment | Course | Outcome | (Refs.) |
|---|
| Jafari-Sfidvajani
et al, 2018 | Y | March 2016-
February 2017 | Iran | 30 | 28.43±6.27 | PCOS, overweight,
VDD | Weight-loss
intervention + 50,000 IU/week oral vitamin D3 | 12 weeks | BMI, total
testosterone, DHEAS | (25) |
| | | | | 30 | 27.83±5.71 | | Weight
lossintervention + placebo | | | |
| Seyyed Abootorabi
et al, 2018 | Y | / | Iran | 19 | 26.21±4.62 | PCOS, VDD | 50,000 IU of oral
vitamin D3 | 8 weeks | HOMA-IR, HOMA-B,
QUICKI, FPG | (26) |
| | | | | 17 | 22.76±4.40 | | Placebo | | | |
| Maktabi et
al, 2017 | Y | April 2016 -June
2016 | Iran | 35 | 22.0±1.6 | Phenotype B of
PCOS, VDD | 50,000 IU vitamin D
every 2 weeks | 12 weeks | FPG, HOMA-IR,
HOMA-B, QUICKI, total testosterone, | (27) |
| | | | | 35 | 23.1±3.3 | | Placebo | | DHEAS,
triglycerides, total cholesterol, LDL-C, HDL-C | |
| Ardabili et
al, 2012 | Y | March 2010-June
2010 | Iran | 24 | 26.8±4.7 | PCOS, VDD | 50,000 IU of
vitamin D3 every 20 days | 8 weeks | BMI, HOMA, HOMA-IR,
QUICKI, FPG | (28) |
| | | | | 26 | 27.0±3.7 | | Placebo | | | |
| Raja-Khan et
al, 2014 | Y | July 2009-November
2010 | USA | 11 | 28.2±5.2 | PCOS | Vitamin D3 12,000
IU daily | 12 weeks | BMI, QUICKI,
HOMA-IR, FPG, LDL-C, HDL-C, triglyc | (29) |
| | | | | 11 | 28.7±5.6 | | Placebo | | erides, total
cholesterol, total testosterone | |
| Garg et al,
2015 | Y | / | India | 15 | 22.0±4.61 | PCOS | Metformin (1,500
mg/day) + vitamin D 4,000 IU/day (monthly dose of 120,000 IU) | 24 weeks | BMI, HOMA-IR, FPG,
DHEAs, triglycerides, total cholesterol, LDL-C, HDL-C, total
testosterone | (30) |
| | | | | 17 | 22.8±4.56 | | Metformin (1,500
mg/day) + placebo | | | |
| Dastorani et
al, 2018 | Y | December 2017-March
2018 | Iran | 20 | 29.9±4.4 | PCOS | 50,000 IU vitamin D
every other week | 8 weeks | FPG, Insulin,
HOMA-IR, QUICKI, Triglycerides, Total | (31) |
| | | | | 20 | 30.1±3.4 | | Placebo | | cholesterol, LDL-C,
HDL-C | |
| Javed et al,
2019 | Y | / | UK | 18 | 28.6±5.5 | VDD | Vitamin D 3200 IU
daily | 12 weeks | hs-CRP, BMI,
HOMA-IR, | (13) |
| | | | | 19 | 29.1±7.5 | PCOS | Placebo | | weight, lipid
profile, glucose levels, insulin levels, FAI, testosterone, TC,
LDL-C, HDL-C, TG | |
| Irani et al,
2015 | Y | October
2013-January 2015 | USA | 35 | 30.5±1.0 | VDD, PCOS | 50,000 IU of oral
vitamin D3 once weekly | 8 weeks | HOMA-IR, HOMA-B,,
triglycerides total cholesterol, | (32) |
| | | | | 18 | 29.6±1.7 | | Placebo once
weekly | | HDL-C, LDL-C,
DHEAS, total testosterone | |
| Foroozanfard et
al, 2015 | Y | June-August
2014 | Iran | 26 | / | VDD, PCOS | 50,000 IU vitamin D
weekly and calcium placebo daily Calcium placebo daily plus vitamin
D placebo weekly | 8 weeks | HOMA-B | (33) |
| Bonakdaran et
al, 2012 | Y | / | Iran | 15 | 24.7±3.3 | PCOS | Calcitriol 0.5
µg/day | 12 weeks | Total testosterone,
DHEAS, | (34) |
| | | | | 16 | 25.2±7.9 | | Placebo | | HOMA-IR | |
Assessment of bias risk
The quality assessment of eligible studies was
accomplished using the Jadad and Cochrane standards. The results
are shown in Table II, and Figs. 2 and 3.
 | Table IIQuality assessment of the included
studies using the Jadad Scale. |
Table II
Quality assessment of the included
studies using the Jadad Scale.
| Authors, year | Randomized
method | Concealment
allocation | Blinding
method | Follow-up | Total score | (Refs.) |
|---|
| Jafari-Sfidvajani
et al, 2018 | 2 | 1 | 2 | 1 | 6 | (25) |
| Seyyed Abootorabi
et al, 2018 | 1 | 1 | 0 | 1 | 3 | (26) |
| Maktabi et
al, 2017 | 2 | 2 | 2 | 0 | 6 | (27) |
| Foroozanfard et
al, 2015 | 2 | 2 | 2 | 1 | 7 | (33) |
| Irani et al,
2015 | 1 | 1 | 1 | 1 | 4 | (32) |
| Garg et al,
2015 | 2 | 2 | 1 | 1 | 6 | (30) |
| Raja-Khan et
al, 2014 | 2 | 2 | 2 | 1 | 7 | (29) |
| Ardabili et
al, 2012 | 2 | 2 | 1 | 1 | 6 | (28) |
| Bonakdaran et
al, 2012 | 1 | 1 | 0 | 1 | 3 | (34) |
| Javed et al,
2019 | 2 | 1 | 2 | 0 | 5 | (13) |
| Dastorani et
al, 2018 | 2 | 1 | 2 | 1 | 6 | (31) |
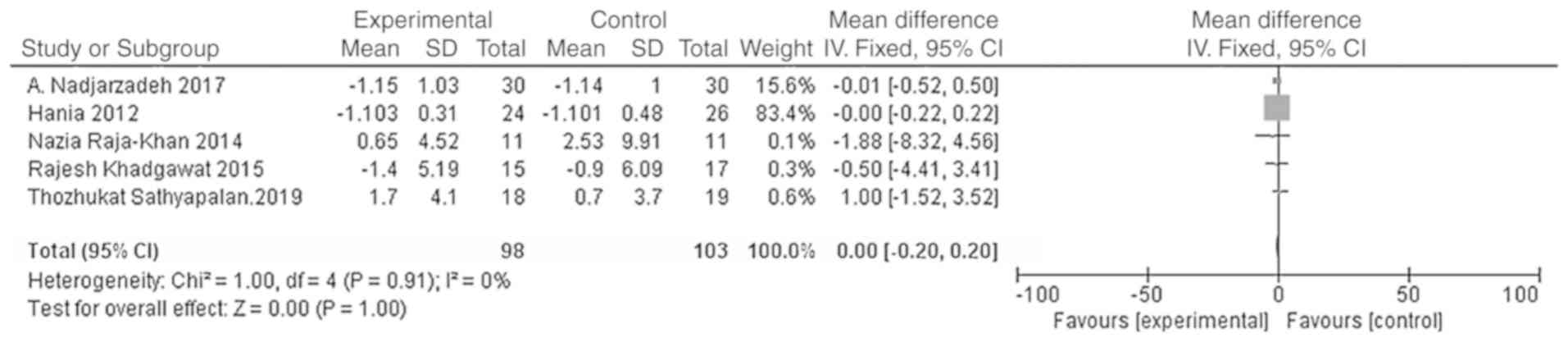
Meta-analysis
Effect of vitamin D supplementation on
the BMIs of patients with PCOS
A total of five studies were included with the main
outcome indicator being the BMI. Heterogeneity tests showed no
heterogeneity in each study (χ2=1.00; P=0.91;
I2=0%). The results of the meta-analysis using the fixed
effect model showed that the BMI of the vitamin D group was not
significantly different from that of the placebo group [WMD = 0.00,
95% CI (-0.20, 0.20), P=1.00; Fig.
4].
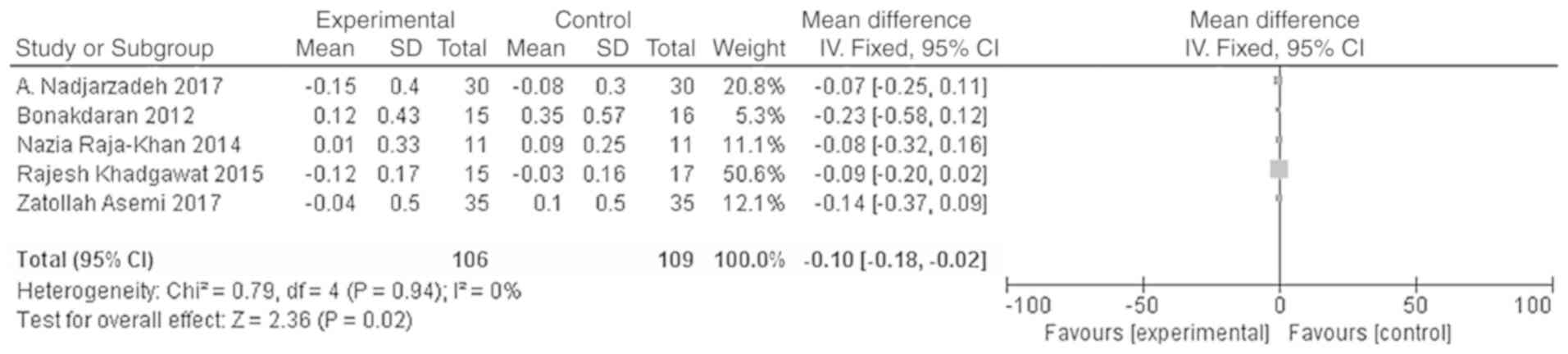
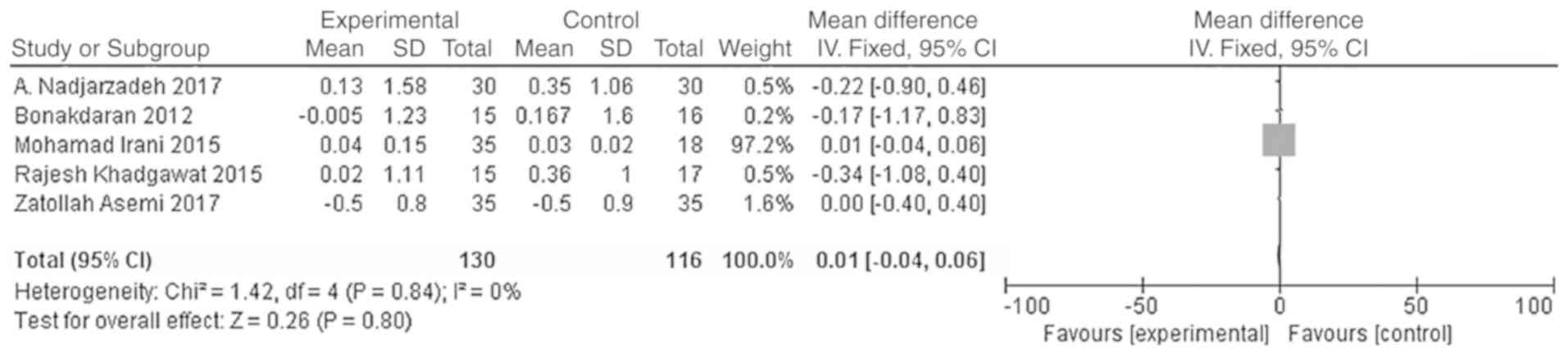
Effects of vitamin D supplementation
on androgen level in patients with PCOS
The main outcome indicators in the present study
were total testosterone and DHEA levels. Heterogeneity tests were
carried out according to the varying outcome indicators, and the
results showed that there was no heterogeneity in either study
(χ2=0.79, P=0.94, I2=0%; Fig. 5; χ2=1.42, P=0.84,
I2=0%; Fig. 6). The
meta-analysis was conducted using the fixed effect model. The
results showed that the total testosterone in the vitamin D group
was lower than that in the placebo group, and the difference was
statistically significant [WMD = -0.10, 95% CI (-0.18, -0.02),
P=0.02; Fig. 5]. However, the
difference in DHEA levels between the vitamin D group and the
placebo group was not statistically significant [WMD = 0.01, 95% CI
(-0.04, 0.06), P=0.80; Fig. 6].
Effects of vitamin D supplementation
on blood glucose metabolism in patients with PCOS
The outcome indicators that were used to evaluate
the effect of vitamin D treatment on the blood glucose metabolism
of patients were HOMA-IR and HOMA-B. The heterogeneity test was
carried out according to the different outcome indicators, and the
results showed that there was moderate heterogeneity among the
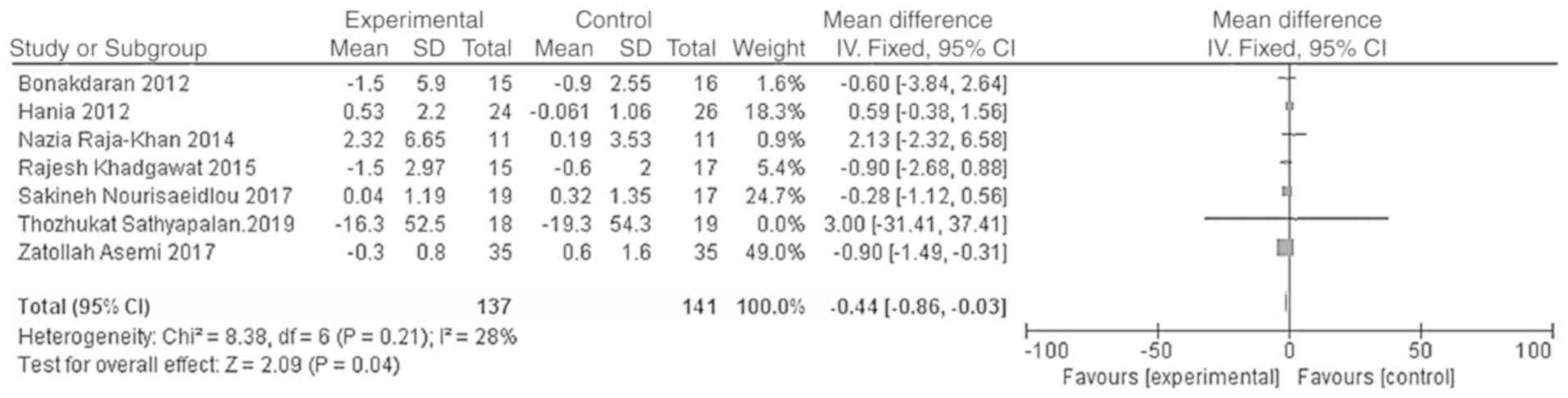
studies (HOMA-IR, χ2=8.38, P=0.21, I2=28%;
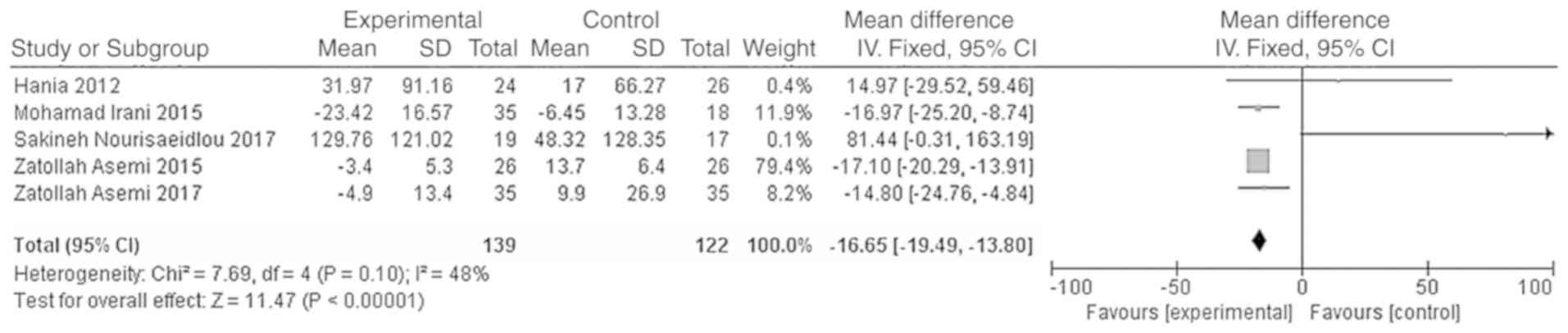
HOMA-B, χ2=7.69, P=0.10, I2=48%). The
indicators were analyzed using the fixed effect model.
Meta-analysis results showed that the HOMA-IR and HOMA-B levels in
the vitamin D group were significantly lower than those of the
placebo group, and the difference was statistically significant
[WMD = -0.44, 95% CI (-0.86, -0.03), P=0.04; Fig. 7; WMD = -16.65, 95% CI (-19.49,
-13.80), P<0.01; Fig. 8].
Effects of vitamin D supplementation
on lipid metabolism in patients with PCOS
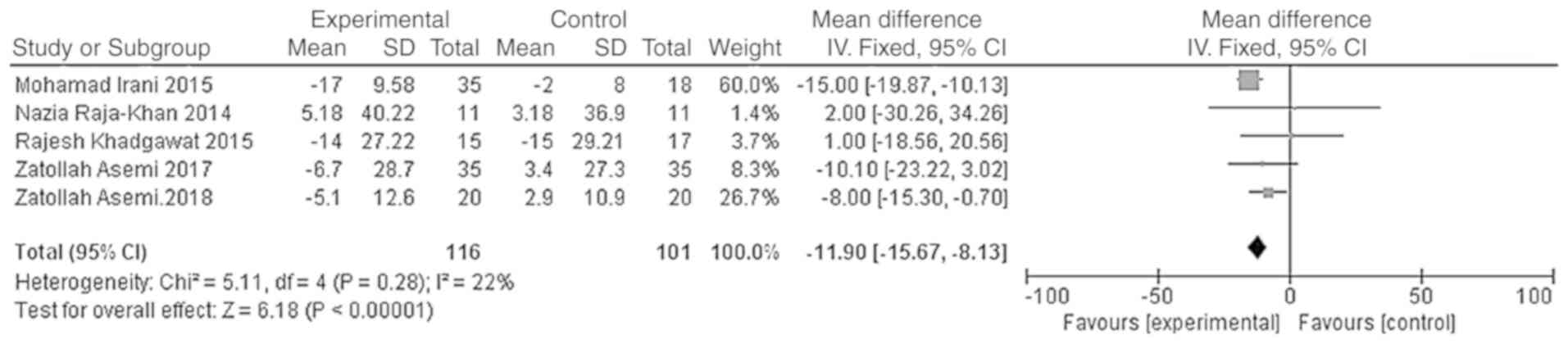
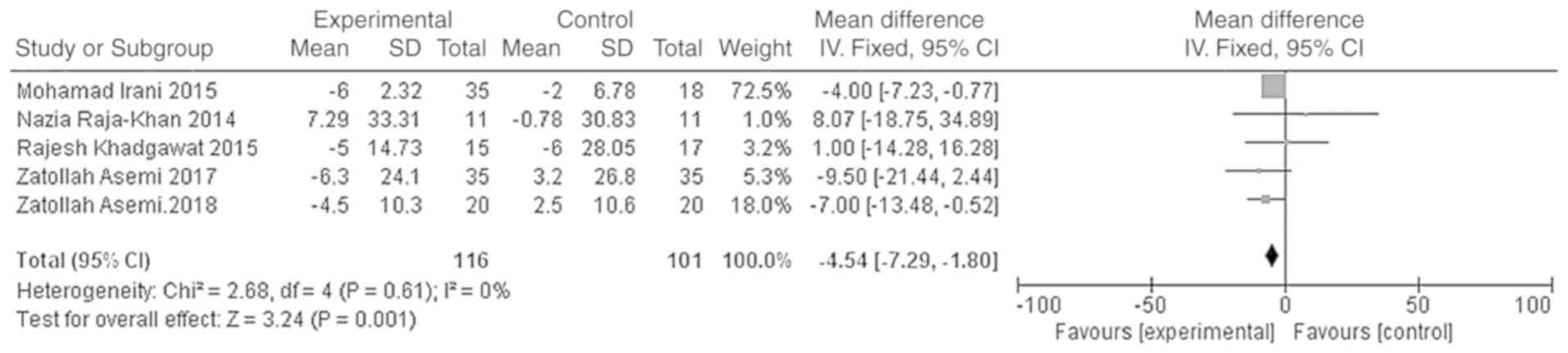
The outcome indicators that were used to evaluate
the effect of vitamin D on the lipid metabolism of patients were
triglycerides, total cholesterol, LDL-C and HDL-C. The
heterogeneity test was carried out according to different outcome
indicators, and the results showed that there was no heterogeneity
in each study (triglycerides, χ2=0.81, P=0.94,
I2=0%; total cholesterol, χ2=5.11, P=0.28,
I2=22%; LDL-C, χ2=2.68, P=0.61,
I2=0%; HDL-C, χ2=1.83, P=0.77,
I2=0%). The meta-analysis was conducted using the fixed
effect model. The results showed that the total cholesterol and
LDL-C levels of the vitamin D group were significantly lower than
those of the placebo group, and that the difference was
statistically significant [WMD =- 11.90, 95% CI (-15.67, -8.13),
P<0.01; Fig. 9]; WMD = -4.54, 95%
CI (-7.29, -1.80), P=0.001; Fig.
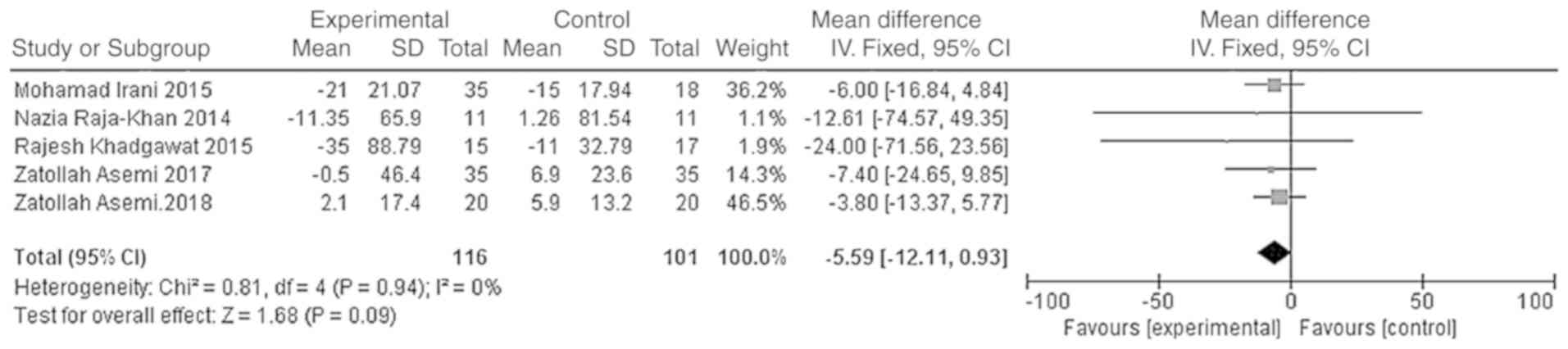
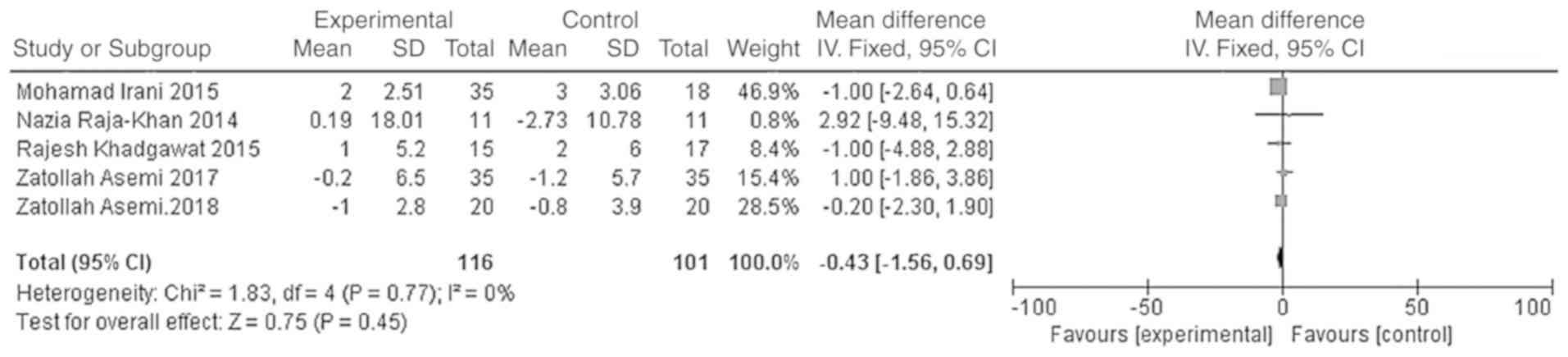
10]. However, the difference in the triglyceride and HDL-C
levels between the vitamin D group and the placebo group was not
statistically significant [WMD = -5.59, 95% CI (-12.11, 0.93),
P=0.09; Fig. 11; WMD = -0.43, 95%
CI (-1.56, 0.69), P=0.45; Fig.
12].
Sensitivity analyses and publication
bias
Sensitivity analyses were performed to evaluate the
stability of the results using the random-effect method. The
results showed that there was no significant change when any one of
the studies was excluded from the total analyses. Funnel plots were
used to evaluate the significance of publication bias. The results
of the funnel plots showed that there was no significant
publication bias for HDL-C (Fig.
S1), HOMA-IR (Fig. S3), LDL-C
(Fig. S4), total testosterone
(Fig. S6), BMI (Fig. S8) and DHEAs (Fig. S9). However, publication biases were
found in HOMA-B (Fig. S2), total
cholesterol (Fig. S5) and
triglycerides (Fig. S7).
Discussion
The current meta-analysis of RCTs was designed to
determine the efficacy of vitamin D supplementation in PCOS.
HOMA-IR, HOMA-B are both important factors in the development of
diabetes, and are also used as one of the main methods to measure
insulin sensitivity (11,12). In regards to lipid metabolism, LDL-C,
HDL-C, triglycerides and total cholesterol are common indicators of
the lipid profile of a patient, and LDL-C is an independent risk
factor for cardiovascular disease (13). Evidence from the current results
suggested that the use of vitamin D, as a treatment for patients
with PCOS, could improve insulin resistance, hyperandrogenism and a
number of the lipid metabolic parameters in PCOS patients in the
short-term follow-up intervention.
These outcomes are consistent with several previous
reports. Several similar studies (14-16)
have demonstrated that vitamin D supplementation therapy has a
beneficial effect on insulin resistance in women with PCOS. Wehr
et al (17) found that after
12 weeks of vitamin D treatment, total cholesterol and LDL-C levels
significantly increased, while there was no obvious change in HDL-C
and triglycerides levels. Following 24 weeks of intervention,
significant changes were observed in the levels of total
cholesterol, LDL-C and triglycerides. According to this study, some
of the non-significant results may be attributed to the short
duration of the intervention. A previous meta-analysis by
Azadi-Yazdi et al (18)
suggested that vitamin D supplementation may significantly affect
the serum total testosterone, whilst not being effective in
improving other markers of the androgenic profile. These results
are consistent with the results of the present study.
Insulin resistance is defined decreased sensitivity
of tissues to insulin, thereby resulting in reduced glucose
utilization. As glucose does not enter the target cells, serum
glucose concentrations increase, which stimulates pancreatic
β-cells to secrete more insulin into the blood. It is generally
considered that insulin resistance plays an pivotal role in the
pathogenesis of PCOS (19). Insulin
resistance is associated with the development of impaired glucose
tolerance and type 2 diabetes, as well as affecting lipid
metabolism (20). However, insulin
resistance also increases hyperandrogenism by affecting the
production of hepatic sex hormone-binding globulin (21). Vitamin D may contribute to the
development of insulin resistance by enhancing PPARγ expression,
which enhances insulin synthesis and release, and vitamin D may
promote insulin receptor expression or suppression of
proinflammatory cytokines (9,22,23).
However, a 2016 meta-analysis (24) reported that vitamin D supplementation
did not influence the glucose and lipid metabolic parameters in
PCOS patients. Due to the different interventions of the included
studies, the result of the present meta-analysis is different from
that of the 2016 meta-analysis, in which the intervention method
was vitamin D supplementation or metformin.
The current meta-analysis had a number of strengths,
including a comprehensive literature search, specified inclusion
and exclusion criteria, explicit methods for data extraction and
the inclusion of measures taken to properly reduce the influence of
bias and to assess heterogeneity. However, despite these strengths,
the current meta-analysis also had a number of limitations.
Firstly, the number of studies available for each meta-analysis was
less than seven, which may be too small for the assessment of
publication bias through funnel plots. Most studies examined had a
small sample size and short term follow-ups. There were also many
variations among studies such as doses and units of vitamin D that
were used, as well as the duration of interventions. The present
study was unable to investigate the curative effects of different
doses and treatment durations. Furthermore, varying levels of
exposure to sunlight may have had an impact on the analysis, which
may have affected the quality of the results. A number of the
studies also did not apply appropriate randomization and allocation
concealment, thus there is a risk of selection bias.
In conclusion, despite several limitations, the
present study found that vitamin D supplementation could affect
insulin resistance, lipid metabolism and, to an extent,
hyperandrogenism in patients with PCOS. Therefore, vitamin D should
be considered as a treatment for PCOS. There is a strong need for
further research to directly validate the results of the present
study. Further studies should include large sample sizes and
long-term interventions, with double-blind placebo controls. It
would also be of benefit to perform these studies worldwide,
especially in areas where women are exposed to abundant sunlight.
Additionally, it would be of interest to the effects of vitamin D
on the induction of ovulation or menstrual cycle regulation in
patients with PCOS. Further experiments should also be designed to
explore the pathogenesis between vitamin D and PCOS. Finally, an
optimal dose of vitamin D should be determined to allow for an
effective therapy to be developed.
Supplementary Material
Figure S1. Funnel plot for HDL-C. The
funnel plot shows the publication bias of HDL-C. The majority of
the studies were concentrated at the top of the funnel plot, and
each study was symmetrically distributed. There was no obvious
publication bias. HDL-C, high density lipoprotein-cholesterol; MD,
mean difference; SE, standard error.
Figure S2. Funnel plot for HOMA-B. The
funnel plot shows the publication bias of HOMA-B. Three of the
studies were distributed at the top of the funnel plot. Two of the
studies were scattered, showing the existence of publication bias.
HOMA-B, homeostasis model assessment of β-cell function; MD, mean
difference; SE, standard error.
Figure S3. Funnel plot for HOMA-IR.
The funnel plot shows the publication bias of HOMA-IR. Most of the
studies were concentrated at the top of the funnel plot, and each
study was symmetrically distributed. There was no obvious
publication bias. HOMA-IR, homeostasis model assessment of insulin
resistance; MD, mean difference; SE, standard error.
Figure S4. Funnel plot for LDL-C. The
funnel plot shows the publication bias of LDL-C. Most of the
studies were concentrated at the top of the funnel plot, and each
study was symmetrically distributed. There was no obvious
publication bias. LDL-C, low density lipoprotein-cholesterol; MD,
mean difference; SE, standard error.
Figure S5. Funnel plot for total
cholesterol. The funnel plot shows the publication bias of total
cholesterol. Most of the studies were concentrated on one side,
showing the existence of publication bias. MD, mean difference; SE,
standard error.
Figure S6. Funnel plot for total
testosterone: The funnel plot shows the publication bias of total
testosterone. Each study was symmetrically distributed. There was
no obvious publication bias. MD, mean difference; SE, standard
error.
Figure S7. Funnel plot for
triglycerides. The funnel plot shows the publication bias of
triglycerides. Three of the studies were distributed at the top of
the funnel plot. Two of the studies were concentrated on one side,
showing the existence of publication bias. MD, mean difference; SE,
standard error.
Figure S8. Funnel plot for BMI. The
funnel plot shows the publication bias of BMI. Each study was
symmetrically distributed. There was no obvious publication bias.
BMI, body mass index; MD, mean difference; SE, standard error.
Figure S9. Funnel plot for DHEAs. The
funnel plot shows the publication bias of DHEAs. Each study was
symmetrically distributed. There was no obvious publication bias.
DHEA, dehydroepiandrosterone sulfate; MD, mean difference; SE,
standard error.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CYM and XJF were involved in data management and
statistics, and drafted the manuscript. QZ and YC verifed the
extracted data following the literature search, monitored the study
and drafted the manuscript. QZ deisgned the current study. XJF and
YC conducted the searches and performed statistical anlaysis. QZ
and CYM performed extracted the data and contributed to quality
assessment. All authors contributed to drafting and revising the
manuscript and all authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bozdag G, Mumusoglu S, Zengin D, Karabulut
E and Yildiz BO: The prevalence and phenotypic features of
polycystic ovary syndrome: A systematic review and meta-analysis.
Hum Reprod. 31:2841–2855. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
Consensus Workshop Group. Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome (pcos). Hum Reprod 19: 41-47, 2004.
|
|
3
|
Jin P and Xie Y: Treatment strategies for
women with polycystic ovary syndrome. Gynecol Endocrinol.
34:272–277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wild RA, Rizzo M, Clifton S and Carmina E:
Lipid levels in polycystic ovary syndrome: Systematic review and
meta-analysis. Fertil Steril. 95:1073–1079. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thomson RL, Spedding S and Buckley JD:
Vitamin D in the aetiology and management of polycystic ovary
syndrome. Clin Endocrinol (Oxf). 77:343–350. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mousa A, Naderpoor N, Teede HJ, De Courten
MP, Scragg R and De Courten B: Vitamin D and cardiometabolic risk
factors and diseases. Minerva Endocrinol. 40:213–230.
2015.PubMed/NCBI
|
|
7
|
Trummer C, Pilz S, Schwetz V,
Obermayer-Pietsch B and Lerchbaum E: Vitamin D, PCOS and androgens
in men: A systematic review. Endocr Connect. 7:R95–R113.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gatti D, Idolazzi L and Fassio A: Vitamin
D: Not just bone, but also immunity. Minerva Med. 107:452–460.
2016.PubMed/NCBI
|
|
10
|
Jørgensen L, Paludan-Müller AS, Laursen
DR, Savović J, Boutron I, Sterne JA, Higgins JP and Hróbjartsson A:
Evaluation of the Cochrane tool for assessing risk of bias in
randomized clinical trials: Overview of published comments and
analysis of user practice in cochrane and non-cochrane reviews.
Syst Rev. 5(80)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Katz A, Nambi SS, Mather K, Baron AD,
Follmann DA, Sullivan G and Quon MJ: Quantitative insulin
sensitivity check index: A simple, accurate method for assessing
insulin sensitivity in humans. J Clin Endocrinol Metab.
85:2402–2410. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rössner SM, Neovius M, Mattsson A, Marcus
C and Norgren S: HOMA-IR and QUICKI Decide on a general standard
instead of making further comparisons. Acta Paediatr. 99:1735–1740.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Javed Z, Papageorgiou M, Deshmukh H,
Kilpatrick ES, Mann V, Corless L, Abouda G, Rigby AS, Atkin SL and
Sathyapalan T: A randomized, controlled trial of vitamin D
supplementation on cardiovascular risk factors, hormones, and liver
markers in women with polycystic ovary syndrome. Nutrients.
11(E188)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Selimoglu H, Duran C, Kiyici S, Ersoy C,
Guclu M, Ozkaya G, Tuncel E, Erturk E and Imamoglu S: The effect of
vitamin D replacement therapy on insulin resistance and androgen
levels in women with polycystic ovary syndrome. J Endocrinol
Invest. 33:234–238. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Łagowska K, Bajerska J and Jamka M: The
role of vitamin D oral supplementation in insulin resistance in
women with polycystic ovary syndrome: A systematic review and
meta-analysis of randomized controlled trials. Nutrients.
10(E1637)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hu Z, Chen J, Sun X, Wang L and Wang A:
Efficacy of vitamin D supplementation on glycemic control in type 2
diabetes patients: A meta-analysis of interventional studies.
Medicine (Baltimore). 98(e14970)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wehr E, Pieber TR and Obermayer-Pietsch B:
Effect of vitamin D3 treatment on glucose metabolism and menstrual
frequency in polycystic ovary syndrome women: A pilot study. J
Endocrinol Invest. 34:757–763. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Azadi-Yazdi M, Nadjarzadeh A,
Khosravi-Boroujeni H and Salehi-Abargouei A: The effect of vitamin
D supplementation on the androgenic profile in patients with
polycystic ovary syndrome: A systematic review and meta-analysis of
clinical trials. Horm Metab Res. 49:174–179. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cetkovic N, Pellicano R, Bjelica A and
Abenavoli L: Polycystic ovary syndrome and vitamin D serum levels.
Minerva Endocrinol. 44:82–84. 2019.
|
|
20
|
Dargham SR, Ahmed L, Kilpatrick ES and
Atkin SL: The prevalence and metabolic characteristics of
polycystic ovary syndrome in the Qatari population. PLoS One.
12(e0181467)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pugeat M, Crave J, Tourniaire J and Forest
M: Clinical utility of sex hormone-binding globulin measurement.
Horm Res. 45:148–155. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hoseini R, Damirchi A and Babaei P:
Vitamin D increases PPARγ expression and promotes beneficial
effects of physical activity in metabolic syndrome. Nutrition.
36:54–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pludowski P, Holick MF, Pilz S, Wagner CL,
Hollis BW, Grant WB, Shoenfeld Y, Lerchbaum E, Llewellyn DJ,
Kienreich K and Soni M: Vitamin D effects on musculoskeletal
health, immunity, autoimmunity, cardiovascular disease, cancer,
fertility, pregnancy, dementia and mortality-a review of recent
evidence. Autoimmun Rev. 12:976–989. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fang F, Ni K, Cai Y, Shang J, Zhang X and
Xiong C: Effect of vitamin D supplementation on polycystic ovary
syndrome: A systematic review and meta-analysis of randomized
controlled trials. Complement Ther Clin Pract. 26:53–60.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jafari-Sfidvajani S, Ahangari R, Hozoori
M, Mozaffari-Khosravi H, Fallahzadeh H and Nadjarzadeh A: The
effect of vitamin D supplementation in combination with low-calorie
diet on anthropometric indices and androgen hormones in women with
polycystic ovary syndrome: A double-blind, randomized,
placebo-controlled trial. J Endocrinol Invest. 41:597–607.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Seyyed Abootorabi M, Ayremlou P,
Behroozi-Lak T and Nourisaeidlou S: The effect of vitamin D
supplementation on insulin resistance, visceral fat and adiponectin
in vitamin D deficient women with polycystic ovary syndrome: A
randomized placebo-controlled trial. Gynecol Endocrinol.
34:489–494. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Maktabi M, Chamani M and Asemi Z: The
effects of vitamin D supplementation on metabolic status of
patients with polycystic ovary syndrome: A randomized,
double-blind, placebo-controlled trial. Horm Metab Res. 49:493–498.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ardabili HR, Gargari BP and Farzadi L:
Vitamin D supplementation has no effect on insulin resistance
assessment in women with polycystic ovary syndrome and vitamin D
deficiency. Nutr Res. 32:195–201. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Raja-Khan N, Shah J, Stetter CM, Lott ME,
Kunselman AR, Dodson WC and Legro RS: High-dose vitamin D
supplementation and measures of insulin sensitivity in polycystic
ovary syndrome: A randomized, controlled pilot trial. Fertil
Steril. 101:1740–1746. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Garg G, Kachhawa G, Ramot R, Khadgawat R,
Tandon N, Sreenivas V, Kriplani A and Gupta N: Effect of vitamin D
supplementation on insulin kinetics and cardiovascular risk factors
in polycystic ovarian syndrome: A pilot study. Endocr Connect.
4:108–116. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dastorani M, Aghadavod E, Mirhosseini N,
Foroozanfard F, Zadeh Modarres S, Amiri Siavashani M and Asemi Z:
The effects of vitamin D supplementation on metabolic profiles and
gene expression of insulin and lipid metabolism in infertile
polycystic ovary syndrome candidates for in vitro fertilization.
Reprod Biol Endocrinol. 16(94)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Irani M, Seifer DB, Grazi RV, Julka N,
Bhatt D, Kalgi B, Irani S, Tal O, Lambert-Messerlian G and Tal R:
Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS:
A randomized placebo-controlled trial. J Clin Endocrinol Metab.
100:4307–4314. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Foroozanfard F, Jamilian M, Bahmani F,
Talaee R, Talaee N, Hashemi T, Nasri K, Asemi Z and Esmaillzadeh A:
Calcium plus vitamin D supplementation influences biomarkers of
inflammation and oxidative stress in overweight and vitamin
D-deficient women with polycystic ovary syndrome: A randomized
double-blind placebo-controlled clinical trial. Clin Endocrinol
(Oxf). 83:888–894. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bonakdaran S, Mazloom Khorasani Z, Davachi
B and Mazloom Khorasani J: The effects of calcitriol on improvement
of insulin resistance, ovulation and comparison with metformin
therapy in PCOS patients: A randomized placebo-controlled clinical
trial. Iran J Reprod Med. 10:465–472. 2012.PubMed/NCBI
|