Introduction
Acquired aplastic anemia (AA) is a rare
hematological disease characterized by bone marrow hypocellularity
and varying degrees of pancytopenia (1). Although etiological studies have
demonstrated that drugs (e.g. chloramphenicol), toxic chemicals
(e.g. benzene, pesticides), viruses (e.g. hepatitis virus),
autoimmune diseases (e.g. lupus erythematosus) and even pregnancy
are associated with AA, the pathogenesis of this disease remains
largely elusive (1). The final
common sign of bone marrow failure is fatty replacement and a
marked decrease in the number of marrow CD34+ cells
(1); however, the underlying
mechanism has remained elusive. In the majority of cases,
autoreactive cytotoxic T-lymphocytes cause this disease by
suppressing or destroying marrow CD34+ cells. However,
the shorter telomeres of patients with AA and the approximately
three-fold increase in the incidence of AA in certain parts of
China indicate that other factors may also contribute to the
pathogenesis of AA (1,2).
Although AA is non-malignant, it is a
life-threatening condition. Prior to the development of allogeneic
hematopoietic stem cell transplantation (allo-HSCT) and
immunosuppressive therapy (IST), half of the patients with AA did
not survive for >4-6 months (3).
Allo-HSCT is generally considered as the first-line treatment for
young patients who may have a human leukocyte antigen
(HLA)-identical sibling donor (1,4,5). Compared with allo-HSCT, IST is used
more frequently, particularly for older patients, and in countries
and areas where optimal donors may not be available. Unfortunately,
30-40% of patients with AA respond poorly to IST, although the IST
regimen has become the standard of care (6). To the best of our knowledge,
universally accepted predictors of unresponsiveness to IST have yet
to be identified. Therefore, the present meta-analysis was
performed to assess several potential predictors of IST
unresponsiveness in patients with AA. The results may be helpful in
establishing an appropriate therapeutic plan, improving IST
efficacy and even providing novel insight into the pathogenesis of
AA.
Materials and methods
Data sources and searches
The PubMed (January 1980 to December 2017), EMbase
(January 1980 to December 2017) and Cochrane (January 1980 to
December 2017) databases were searched by utilizing the following
search terms: (‘Immunosuppressive therapy’ or ‘immunosuppressive
treatment’) and (‘aplastic anemia’, ‘aplastic anemia’ or ‘bone
marrow failure’). The references of all studies and reviews
retrieved were manually scanned to identify additional studies. The
language was limited to English.
Study selection
The studies were first screened based on the title
and abstract. The full text was then retrieved for further
assessment by two authors (WJ and SP). Eligible studies were
independently selected by two authors (WJ and SP) according to the
following inclusion criteria: a) Clinical trials, prospective
studies or retrospective case-control studies; b) diagnostic
criteria for severity of AA as determined by the Camitta criteria
(Supplemental Table SII) (7); c) anti-thymocyte globulin (ATG) and
cyclosporine A (CsA)-based IST regimens; d) response to IST
determined using the Bacigalupo criteria (Supplemental Table SIII) (8) and evaluation after a six-month
treatment; e) available odds ratios (ORs) and 95% confidence
intervals (CIs) of factors affecting no response to IST; f)
reported in English.
Data extraction and quality
assessment
Two investigators (WJ and SP) independently
extracted the data from all eligible studies. Any disagreements
were resolved through discussion with senior investigators (JW and
XW). The author name, year of publication, journal and country, as
well as the number, age and severity of the cases were recorded.
Factors associated with unresponsiveness were retrieved from all
eligible studies (Table I). The
authors were contacted to request any missing information when
necessary.
 | Table ICharacteristics of the studies
included. |
Table I
Characteristics of the studies
included.
| First author
(year) | Journal | Cases (n) | Median age
(range) | Country | Severity of AA, n
(%) | Factors | QS | (Refs.) |
|---|
| Ihan, et al
(1997) | Int J Hematol | 35 | 23 (16~57y) | Turkey | Not provided | Age, sex,
HLA-DR2 | 6 | (10) |
| Maciejewski et
al (2001) | Blood | 281 | Not provided | USA | Not provided | HLA-DR2 | 7 | (11) |
| Saunthararajah
et al (2002) | Blood | 99 | 34y,
21±0.7ya | USA | SAA, 99(100) | HLA-DR2 | 7 | (12) |
| Oguz et al
(2002) | Haematologica | 17 | Pediatric
cases | Turkey | SAA, 17(100) | HLA-DR2 | 7 | (13) |
| Gupta et al
(2006) | Br J Haematol | 81 | 5-73y | UK | NSAA, 39 (48.1);
SAA, 28 (34.6); VSAA, 16 (19.8) | CAs | 7 | (14) |
| Saracco et
al (2008) | Br J Haematol | 42 | 15-239m | Italy | NSAA, 6 (14.5);
SAA, 19 (45.0); VSAA, 17 (40.5) | Age, sex, ANC,
platelet, HLA-DR2, severity, interval from diagnosis to
therapy | 7 | (15) |
| Scheinberg et
al (2009) | Br J Haematol | 316 | 31y (18-52y) | USA | SAA, 316(100) | Age, ANC, ARC, ALC,
PNH clone | 7 | (16) |
| Kim et al
(2010) | Genes Chromosomes
Cancer | 600 | 67y (17-87y) | Korea | NSAA, 301 (50.2);
SAA, 255 (42.5); VSAA, 44 (7.3) | Age, sex, severity,
PNH clone, CAs, HB(C)V | 7 | (17) |
| Song et al
(2010) | Hum Immunol | 37 | 35y (3-66y) | Korea | SAA, 37(100) | HLA-DR2 | 7 | (18) |
| Yoshida et
al (2011) | Haematologica | 312 | 8y (<18y) | Japan | NSAA, 49 (15.7);
SAA, 107 (34.3); VSAA, 156 (40.4) | sex, severity,
etiology, peripheral blood parameters at diagnosis, interval from
diagnosis to therapy | 7 | (19) |
All eligible studies were independently assessed by
two investigators (WJ and SP) according to the Newcastle-Ottawa
Scale (NOS) (9) (Supplemental
Table SI). The NOS scores ranged
between 0 (worst) and 9 (best). Scores of 6 to 9 were rated as
being of ‘high quality’, whereas scores of 0-5 were considered to
be of ‘poor quality’. Disagreements were resolved as described in
the data extraction section.
Definition of assessment factors
The factors were selected for assessment according
to the following criteria: a) Available ORs of factors affecting no
response to IST and 95% CI; b) at least two studies including those
data.
Data synthesis and analysis
Data were analyzed using Review Manager software
(version for Windows; the Cochrane Collaboration). The
heterogeneity of included studies was assessed using the
χ2-test (P<0.10) and calculation of the I2
statistic. The fixed-effects model was applied, but when
statistically significant heterogeneity was detected (P<0.10 or
I2>50%), the random-effects model was adopted. The
results were expressed as ORs with 95% CI and were presented in
forest plots. ORs were used to assess the extent of the association
of the factors with poor response of the patients with AA to IST.
Differences were considered statistically significant if P≤0.05
(two-tailed). Publication bias was assessed using Begg's funnel
plots.
Results
Studies and factors included
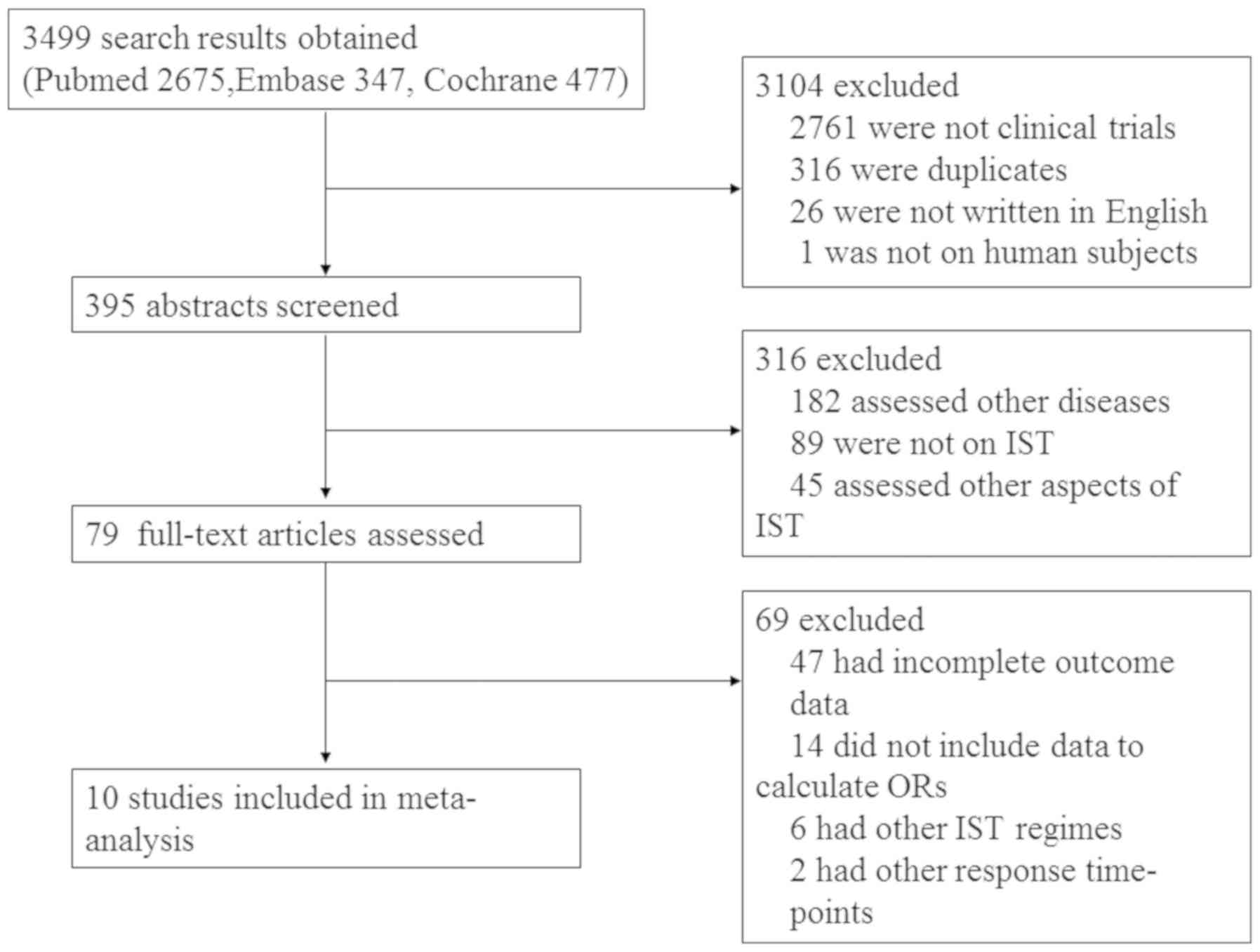
Following the search strategy, 3,499 studies were
identified, 10 of which were ultimately included in the present
meta-analysis (Fig. 1). The
characteristics of the studies (10-19)
included are summarized in Table I.
All of the studies were retrospective and their publication date
range was between 1997 and 2011; a total of 1,820 cases in various
countries and involving multiple ethnicities were reported, most of
which were idiopathic. The mean NOS score was 6.9 (range, 6-7, Table SI), indicating that the quality of
the meta-analysis was acceptable. Although the present
meta-analysis included the full spectrum of AA (Table I), 4 studies including 469 patients
only reported on severe AAb (12,13,16,18) and
2 including 316 patients did not include any data on the severity
distribution of the cases (10,11).
With regard to the age range of the patients in these studies
(10-19,
Table I), 5 studies including 795
patients focused on mixed-age groups (10,14,15,17,18), 1
including 316 patients on adult patients(≥18 years) (16), 2 including 329 patients on pediatric
patients(<18 years) (13,19), 1 of 99 patients on mixed-age or adult
cases (12) and 1 study of 281
patients did not provide the age of the subjects (11).
In the studies included, 7 factors were identified
for analysis: Age (≥60 years), sex, absolute neutrophil count
(ANC), severity of the disease, paroxysmal nocturnal hemoglobinuria
(PNH) clone, HLA-DR2 and cytogenetic abnormalities (CAs) (Table I). Although these factors were not
included in each study, it was possible to analyze them by
combining the data of at least 2 studies from which the ORs were
able to be calculated. As a higher age was indicated to be a factor
predicting unresponsiveness to IST in previous studies and there is
a peak in incidence between the ages of 65 and 69 years (16,20,21), age
(≥60 years) was included as a factor.
Meta-analysis results for factors
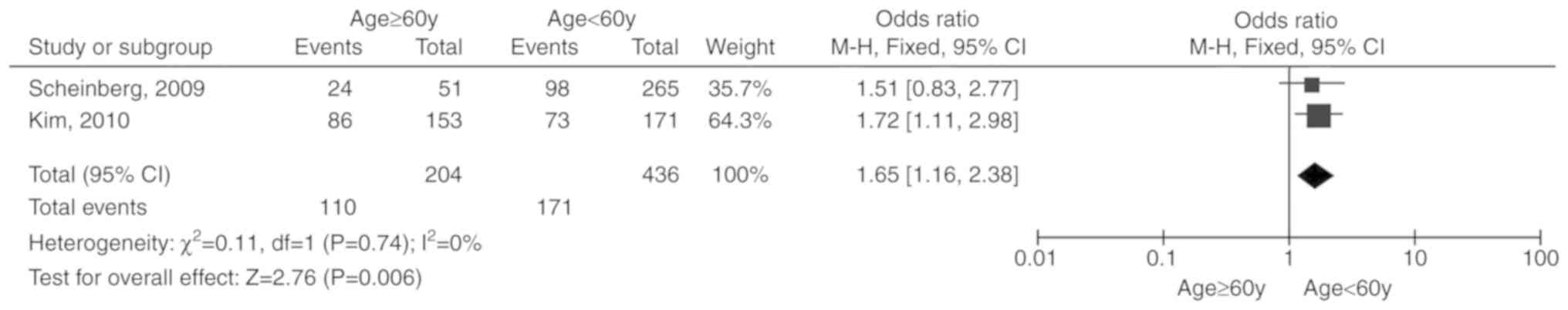
included Age (≥60 years)
Two studies (16,17)
including 640 patients were reported. Due to the lack of
significant heterogeneity among the included studies (P=0.74,
I2=0%), the fixed-effects model was selected. The total
OR (95% CI) was 1.65 (1.16, 2.38). The results of the meta-analysis
indicated a statistically significant association between age (≥60
years) and unresponsiveness to IST (P=0.006; Fig. 2).
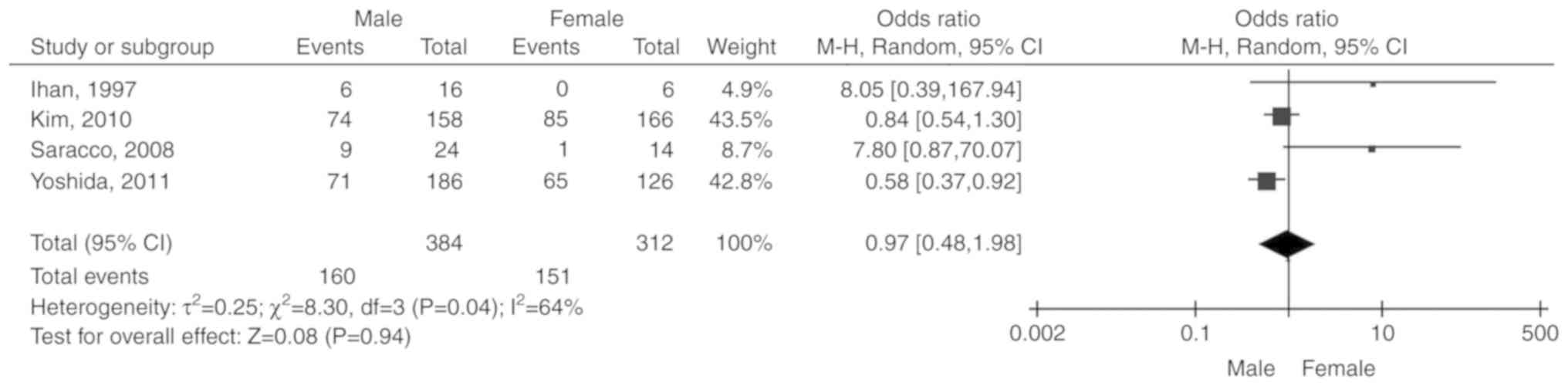
Sex
Four studies (10,15,17,19)
including 696 patients were reported. Due to the significant
heterogeneity among the included studies (P=0.04,
I2=64%), the random-effects model was selected. The
total OR (95% CI) was 0.97 (0.48, 1.98). The results of the
meta-analysis did not indicate any statistically significant
association between sex and unresponsiveness to IST (P=0.94;
Fig. 3).
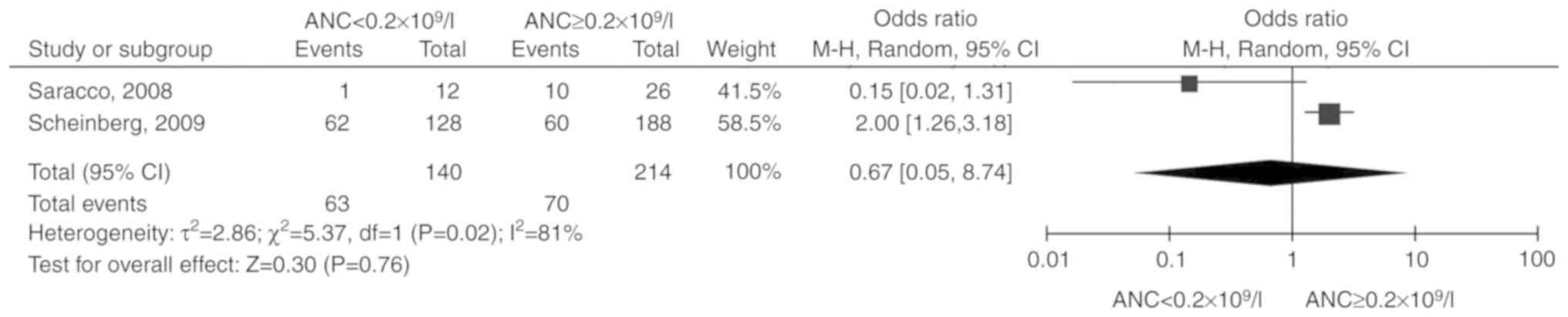
ANC
Two studies (15,16)
including 354 patients were reported. Due to the significant
heterogeneity among the included studies (P=0.02,
I2=81%), the random-effects model was selected. The
total OR (95% CI) was 0.67 (0.05, 8.74). The results of the
meta-analysis did not indicate a statistically significant
association between ANC and unresponsiveness to IST (P=0.76;
Fig. 4).
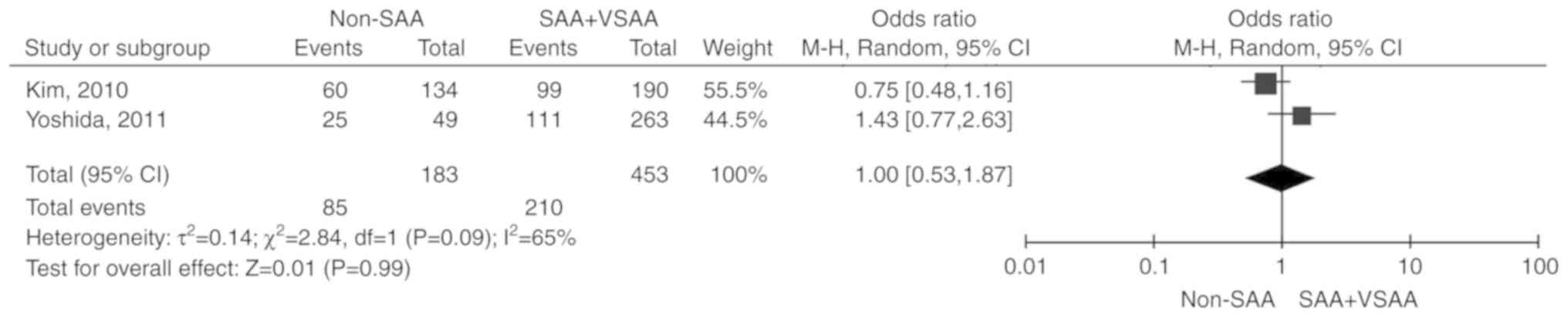
Severity of disease
Two studies (17,19)
including 636 patients were reported. Due to the significant
heterogeneity among the included studies (P=0.09,
I2=65%), the random-effects model was selected. The
total OR (95% CI) was 1.00 (0.53, 1.87). The results of the
meta-analysis did not indicate any statistically significant
association between the severity of the disease and
unresponsiveness to IST (P=0.99; Fig.
5).
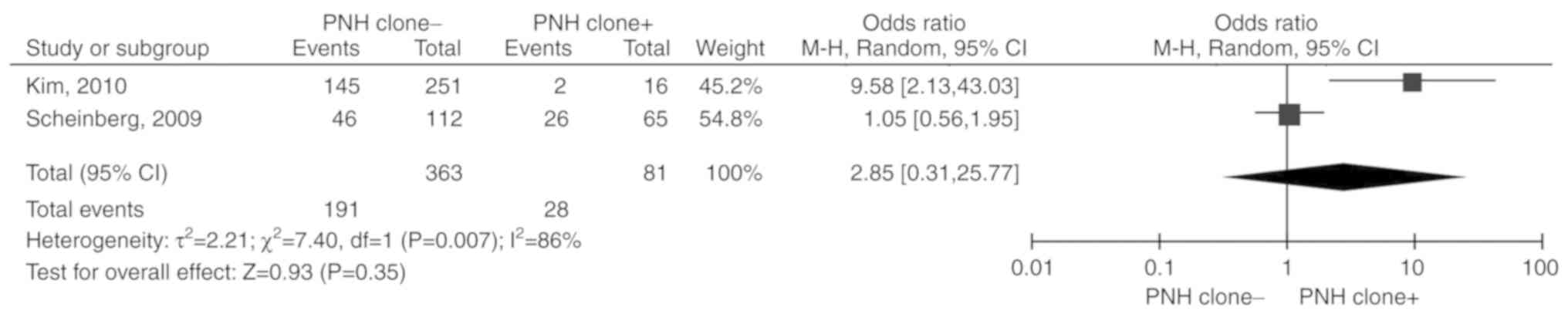
PNH clone
Two studies (16,17)
including 444 patients were reported. Due to the significant
heterogeneity among the included studies (P=0.007,
I2=86%), the random-effects model was selected. The
total OR (95% CI) was 2.85 (0.31, 25.77). The results of the
meta-analysis did not indicate any statistically significant
association between PNH clone and unresponsiveness to IST (P=0.35;
Fig. 6).
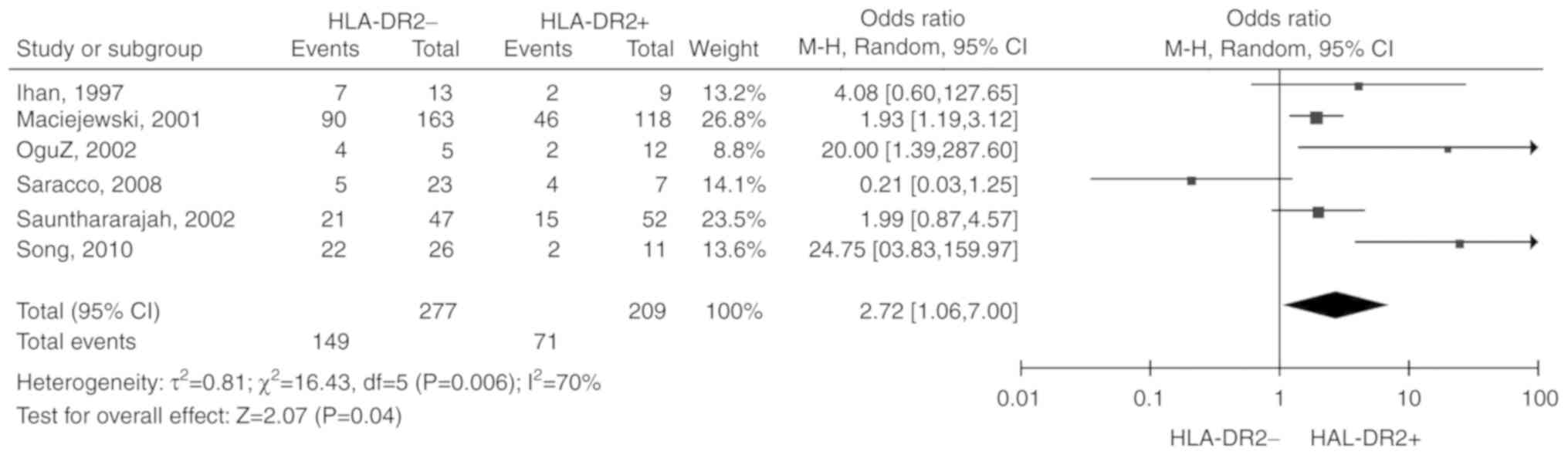
HLA-DR2
6 studies (10-13,15,18)
including 486 patients were reported. Due to the significant
heterogeneity among the included studies (P=0.006,
I2=70%), the random-effects model was selected. The
total OR (95% CI) was 2.72 (1.06, 7.00). The results of the
meta-analysis indicated a statistically significant association
between HLA-DR2 negative status and unresponsiveness to IST
(P=0.04; Fig. 7).
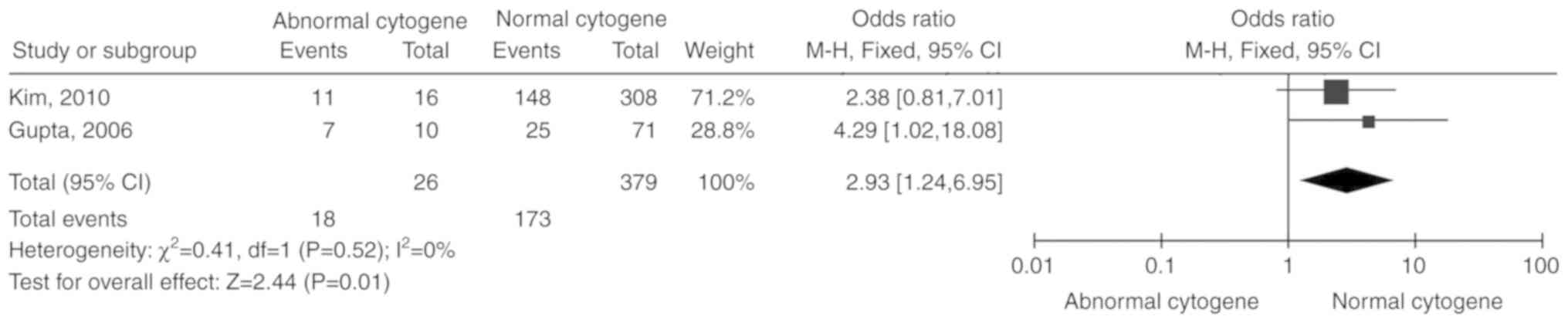
CAs
Two studies (14,17)
including 405 patients were reported. Due to the lack of
significant heterogeneity among the included studies (P=0.52,
I2=0%), the fixed-effects model was selected. The total
OR (95% CI) was 2.93 (1.24, 6.95). The results of the meta-analysis
indicated a statistically significant association between CAs and
unresponsiveness to IST (P=0.01; Fig.
8).
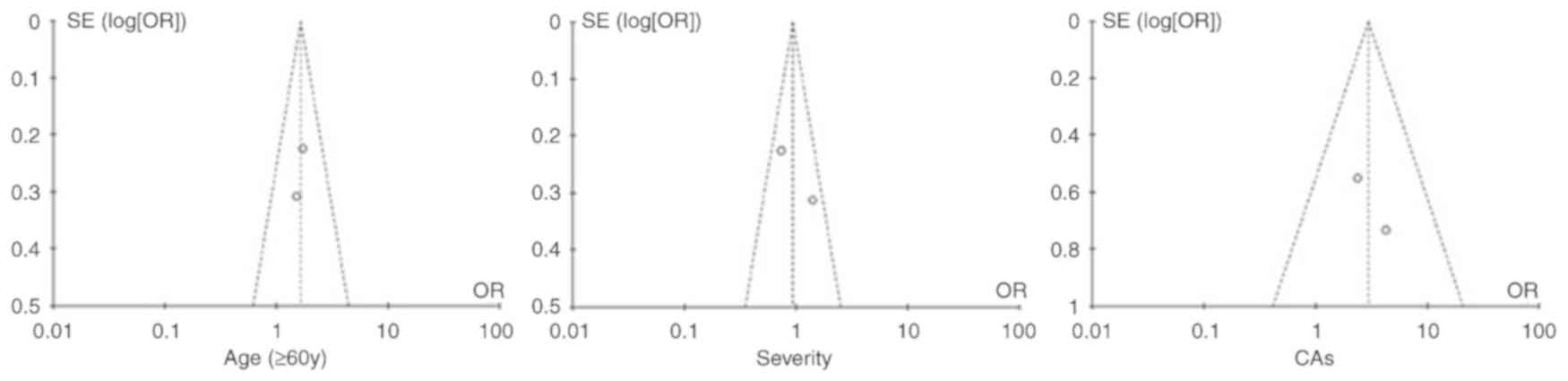
Publication bias
As indicated by the funnel plots (Fig. 9), the studies providing age (≥60
years), disease severity and CAs analysis were inside the 95% CIs,
with an even distribution around the vertical symmetry, which
indicates that there was no publication bias. However, the other
factors were associated with publication bias, with asymmetrical
funnel plots (Fig. S1).
Discussion
AA is a clinical manifestation of bone marrow
failure, wherein the hematopoietic stem and precursor cells are
nearly absent and are replaced by fat. Accordingly, the varying
degrees of decrease in the number of peripheral blood cells result
in anemia, infection and bleeding (1,22). HSCT
and IST have become the first-line treatments for AA and the 5-year
survival rates are similar for patients receiving those therapies
(1,22). However, allo-HSCT is limited by age,
requirement for a suitably matched sibling donor and
post-transplantation complications. Thus, IST is the modality most
widely used in AA patients in several countries and geographical
areas (1,22). Although IST is one of the most
effective treatments for AA, it is associated with several
problems, including unresponsiveness, relapse and malignant
transformation (1,22). Several common risk factors associated
with unresponsiveness have been reported by previous studies in
different populations and areas (1,10-19,22).
However, widely accepted predictors of AA have yet to be
identified. To the best of our knowledge, the present study was the
first meta-analysis of risk factors associated with
unresponsiveness to IST in AA. A total of 3 factors, namely age,
HLA-DR2 negativity and CAs, were revealed to be statistically
significantly associated with unresponsiveness of AA to IST,
whereas sex, ANC, disease severity and PNH clone did not exhibit
such an association.
Among the three risk factors, old age is most widely
recognized. Several studies from different countries and areas have
reported that old age is a major prognostic indicator of poor
response to IST in AA (20,23). Age is also associated with the
incidence of AA (1), with two
incidence peaks observed between 15 and 25 years, as well as
between 65 and 69 years (1).
However, old age is usually negatively associated with the
incidence of autoimmune diseases (21,24). In
addition, anemia in old patients has the unique characteristics of
clinical presentation and prognosis (25,26).
These results suggest that anemia in old patients may have a
distinct pathogenesis. Certain studies consider that the high
incidence and specific presentation of older patients with AA may
be linked to telomere length and hematopoietic stem cell number
(2,4). However, these previous studies lack
convincing evidence and even contradict one another. For instance,
numerous studies pointed out that short telomere length may favor
the development of AA (2,4,27,28), but
older patients with AA do not have a shorter telomere length
compared with healthy older subjects (26). Therefore, old patients with AA should
be more thoroughly investigated, particularly regarding the
pathogenesis of this disease, in order to improve its
treatment.
Similar to age, HLA-DR2 was indicated to be
associated with response to IST in previous studies (10,11,13,15,18). In
general, patients with HLA-DR2 exhibit a good response to CsA or
the combined protocol of CsA + ATG/ALG, but not to ATG/ALG alone.
The mechanism underlying their association remains elusive, but the
fact that HLA-DR2 is highly expressed in patients with AA and is
associated with a good response to IST suggests a key role for
HLA-DR2 in AA. It was previously hypothesized that HLA-DR2, which
is frequently over-represented in autoimmune diseases, including
anti-glomerular basement membrane disease (29) and multiple sclerosis (30), may be a predisposing factor for the
immune process of autoimmune diseases, partly as certain HLA
haplocytes are not only susceptible to present
foreign/self-antigen-derived pathogenic peptides, but are also
involved in the formation of autoantigens (13,29,30). In
addition, HLA-DR2 is associated with a decrease in the production
of tumor necrosis factor (TNF)-α (31,32).
However, TNF-α release increases following activation of interferon
(IFN)-γ (32). Furthermore, the
TNF-α and IFN-γ cytokines have an important role in the depletion
of bone marrow CD34+ cells in AA (1). Therefore, HLA-DR2 may be involved in
the formation of AA through an autoimmune mechanism to a certain
extent, whereas HLA-DR2-negative patients with AA may harbor
mechanisms other than autoimmunity. This condition is associated
with a poor response to IST.
Compared with the former factors, there are certain
issues regarding the association between CAs and response to IST in
AA. In certain institutions, AA with an abnormal karyotype at the
time of diagnosis is considered as myelodysplastic syndrome
(33); however, the incidence of CAs
at initial diagnosis is approximately 4-15% (17,19,34),
which is not accompanied by morphological changes (17,19,34-39).
The mechanism and function of CAs in AA have remained to be fully
elucidated due to the scarcity of uniform, systematic and
large-scale studies. Based on previous studies, trisomy 8, trisomy
6 and monosomy 7 are the most common CAs among patients with AA
(17,19,34-39).
Trisomy 8 and trisomy 6 are frequently present at the time of
diagnosis, whereas monosomy 7 almost always develops after
treatment (17,19,34-39).
Studies on various ethnic groups and geographical areas report that
trisomy 8 responds well to IST (17,19,33,39),
whereas the opposite was observed for trisomy 6 and monosomy 7
(17,19,33,36,39),
with trisomy 6 reported to be ‘the sole predictable marker for IST
unresponsiveness’ (34). The CAs of
AA are not constant over the course of the disease and are
transiently present in certain cases (38-40).
The different clinical courses of CAs may be associated with their
different biology after development, including Fas expression,
sensitivity to external stimuli and gene expression profiles,
although all of them are considered to initially result from genome
instability under immune pressure (41-46).
Although the CAs of AA occur at random and are secondary
cytogenetic events, the fact that an individual CA is always
associated with a specific clinical presentation, along with the
multifactorial pathogenesis of AA, cannot exclude the possible
involvement of the initial CAs in CD34+ cell depletion
(41,42). For instance, trisomy 6, one of the
most common CAs in AA and a predictor of poor response to IST, was
characterized by hypoplastic bone marrow in most studies and was
observed in early hematopoietic progenitor cells (10,46,47),
suggesting that trisomy 6 may be an intrinsic factor causing bone
marrow hypoplasia. These results suggest different molecular
mechanisms of action and different behavior of these CAs in AA;
therefore, further studies should assess individual CAs, rather
than CAs as a group.
There were several limitations to this
meta-analysis. First, the factors including sex, ANC, PNH clone and
HLA-DR2 are associated with publication bias and the quality of
included studies are only 6-7 out of 10 on NOS score although three
databases were searched. Furthermore, all of the studies included
are clinical trials, wherein the deficiencies may be magnified
through meta-analysis. In addition, in three of the selected
studies, although the source of the patients was different, they
were from the same area; thus, the same patients may have been
included in more than one study, which may overemphasize the
results. However, without more details, it is difficult to
determine whether this magnification exists and to what extent.
Furthermore, the quality of original data from the studies included
cannot be improved by the present meta-analysis. In addition, each
factor was analyzed using data from different studies. Using the
same studies would be ideal for the analysis of these factors;
however, due to the scarcity of published studies on response to
IST in AA, there were no more than two studies that shared more
than two factors. Finally, only univariate analysis was performed
in the present meta-analysis, which may compromise the accuracy of
the conclusions. This limitation is attributed to the fact that the
analysis results were obtained from different sources. However, the
intention was not to reach a definitive conclusion, but rather to
detect risk factors of AA unresponsiveness to IST for future
research.
In conclusion, the present study demonstrated that
age (≥60 years), HLA-DR2 negativity and CAs are risk factors of
unresponsiveness to IST. This result may be helpful for
establishing a treatment guideline for AA. Furthermore, this result
may even provide novel clues to the pathogenesis of AA. Some
studies suggest that in addition to an abnormal immune system,
other factors including telomere length, HLA or CAs, may trigger or
be involved in the development of this disease (48-50),
which may lead to diverse patterns of responsiveness to IST.
Important future research includes large, multicenter clinical
trials and laboratory research, in order to help fully elucidate
the pathogenesis of AA.
Supplementary Material
Funnel plots for the assessment of
publication bias for the factors of sex, ANC, PNH clone and
HLA.DR2. SE, standard error; OR, odds ratio; HLA, human leukocyte
antigen; PNH, paroxysmal nocturnal hemoglobinuria; ANC, absolute
neutrophil count.
Newcastle–Ottawa Scale quality score
of the studies included.
Definitions of the severity of
AA.
Definition of response.
Acknowledgements
Not applicable.
Funding
This study was supported by Xinhua Hospital,
Shanghai Jiao Tong University School of Medicine (grant no.
XH2049).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
All authors contributed to the scientific work and
therefore share collective responsibility and accountability for
the results. WJ and XW conceived and designed the study. JW and PS
collected and analyzed the data and interpreted the results; JW and
PS drafted the manuscript. All authors have read the manuscript and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaushansky KWW: Williams hematology. New
York: McGraw-Hill Medical xxiii: pp.2439, 2010.
|
|
2
|
Townsley DM, Dumitriu B and Young NS: Bone
marrow failure and the telomeropathies. Blood. 124:2775–2783.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Marsh JC: Results of immunosuppression in
aplastic anaemia. Acta Haematol. 103:26–32. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Risitano AM: Immunosuppressive therapies
in the management of immune-mediated marrow failures in adults:
Where we stand and where we are going. Br J Haematol. 152:127–140.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Scheinberg P: Aplastic anemia: Therapeutic
updates in immunosuppression and transplantation. Hematology Am Soc
Hematol Educ Program. 2012:292–300. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Scheinberg P and Young NS: How I treat
acquired aplastic anemia. Blood. 120:1185–1196. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Marsh JC, Ball SE, Cavenagh J, Darbyshire
P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca
J, et al: Guidelines for the diagnosis and management of aplastic
anaemia. Br J Haematol. 147:43–70. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bacigalupo A, Broccia G, Corda G, Arcese
W, Carotenuto M, Gallamini A, Locatelli F, Mori PG, Saracco P,
Todeschini G, et al: Antilymphocyte globulin, cyclosporin, and
granulocyte colony-stimulating factor in patients with acquired
severe aplastic anemia (SAA): A pilot study of the EBMT SAA Working
Party. Blood. 85:1348–1353. 1995.PubMed/NCBI
|
|
9
|
Margulis AV, Pladevall M, Riera-Guardia N,
Varas-Lorenzo C, Hazell L, Berkman ND, Viswanathan M and
Perez-Gutthann S: Quality assessment of observational studies in a
drug-safety systematic review, comparison of two tools: The
Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol.
6:359–368. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ihan O, Beksaç M, Arslan O, Ozcan M, Koç
H, Akan H, Gürman G, Konuk N and Uysal A: HLA DR2: A predictive
marker in response to cyclosporine therapy in aplastic anemia. Int
J Hematol. 66:291–295. 1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maciejewski JP, Follmann D, Nakamura R,
Saunthararajah Y, Rivera CE, Simonis T, Brown KE, Barrett JA and
Young NS: Increased frequency of HLA-DR2 in patients with
paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia
syndrome. Blood. 98:3513–3519. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saunthararajah Y, Nakamura R, Nam JM,
Robyn J, Loberiza F, Maciejewski JP, Simonis T, Molldrem J, Young
NS and Barrett AJ: HLA-DR15 (DR2) is overrepresented in
myelodysplastic syndrome and aplastic anemia and predicts a
response to immunosuppression in myelodysplastic syndrome. Blood.
100:1570–1574. 2002.PubMed/NCBI
|
|
13
|
Oguz FS, Yalman N, Diler AS, Oguz R, Anak
S and Dorak MT: HLA-DRB1*15 and pediatric aplastic
anemia. Haematologica. 87:772–774. 2002.PubMed/NCBI
|
|
14
|
Gupta V, Brooker C, Tooze JA, Yi QL, Sage
D, Turner D, Kangasabapathy P and Marsh JC: Clinical relevance of
cytogenetic abnormalities at diagnosis of acquired aplastic anaemia
in adults. Br J Haematol. 134:95–99. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Saracco P, Quarello P, Iori AP, Zecca M,
Longoni D, Svahn J, Varotto S, Del Vecchio GC, Dufour C, Ramenghi
U, et al: Cyclosporin A response and dependence in children with
acquired aplastic anaemia: A multicentre retrospective study with
long-term observation follow-up. Br J Haematol. 140:197–205.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Scheinberg P, Wu CO, Nunez O and Young NS:
Predicting response to immunosuppressive therapy and survival in
severe aplastic anaemia. Br J Haematol. 144:206–216.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim SY, Lee JW, Lee SE, Cho BS, Kim M, Eom
KS, Kim YJ, Kim HJ, Lee S, Min CK, et al: The characteristics and
clinical outcome of adult patients with aplastic anemia and
abnormal cytogenetics at diagnosis. Genes Chromosomes Cancer.
49:844–850. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song EY, Kang HJ, Shin HY, Ahn HS, Kim I,
Yoon SS, Park S, Kim BK and Park MH: Association of human leukocyte
antigen class II alleles with response to immunosuppressive therapy
in Korean aplastic anemia patients. Hum Immunol. 71:88–92.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yoshida N, Yagasaki H, Hama A, Takahashi
Y, Kosaka Y, Kobayashi R, Yabe H, Kaneko T, Tsuchida M, Ohara A, et
al: Predicting response to immunosuppressive therapy in childhood
aplastic anemia. Haematologica. 96:771–774. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Marsh JC and Kulasekararaj AG: Management
of the refractory aplastic anemia patient: What are the options?
Hematology Am Soc Hematol Educ Program. 2013:87–94. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reddy V, Khan S, Wingard JR and Mehta P:
Treatment results in aplastic anemia trials need to be analyzed
separately for pediatric and adult populations. Blood.
94:1833–1834. 1999.PubMed/NCBI
|
|
22
|
Shin SH and Lee JW: The optimal
immunosuppressive therapy for aplastic anemia. Int J Hematol.
97:564–572. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Locasciulli A, Oneto R, Bacigalupo A,
Socié G, Korthof E, Bekassy A, Schrezenmeier H, Passweg J and
Führer M: Severe Aplastic Anemia Working Party of the European
Blood and MarrowTransplant Group: Outcome of patients with acquired
aplastic anemia given first line bone marrow transplantation or
immunosuppressive treatment in the last decade: A report from the
European Group for Blood and Marrow Transplantation (EBMT).
Haematologica. 92:11–18. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vadasz Z, Haj T, Kessel A and Toubi E:
Age-related autoimmunity. BMC Med. 11(94)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Andro M, Le Squere P, Estivin S and
Gentric A: Anaemia and cognitive performances in the elderly: A
systematic review. Eur J Neurol. 20:1234–1240. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
den Elzen WP and Gussekloo J: Anaemia in
older persons. Neth J Med. 69:260–267. 2011.PubMed/NCBI
|
|
27
|
Young NS: Pathophysiologic mechanisms in
acquired aplastic anemia. Hematology Am Soc Hematol Educ Program.
72–77. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Young NS, Calado RT and Scheinberg P:
Current concepts in the pathophysiology and treatment of aplastic
anemia. Blood. 108:2509–2519. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nepom GT: Class II antigens and disease
susceptibility. Annu Rev Med. 46:17–25. 1995.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Haegert DG and Marrosu MG: Genetic
susceptibility to multiple sclerosis. Ann Neurol. 36 (Suppl
2):S204–S210. 1994.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Peces R, Urra JM and de la Torre M:
Influence of HLA-DR phenotype on tumor necrosis factor-alpha
production in renal-transplant recipients. Nephron. 71:180–183.
1995.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bendtzen K, Morling N, Fomsgaard A,
Svenson M, Jakobsen B, Odum N and Svejgaard A: Association between
HLA-DR2 and production of tumour necrosis factor alpha and
interleukin 1 by mononuclear cells activated by lipopolysaccharide.
Scand J Immunol. 28:599–606. 1988.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Maciejewski JP, Risitano A, Sloand EM,
Nunez O and Young NS: Distinct clinical outcomes for cytogenetic
abnormalities evolving from aplastic anemia. Blood. 99:3129–3135.
2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ohga S, Ohara A, Hibi S, Kojima S, Bessho
F, Tsuchiya S, Ohshima Y, Yoshida N, Kashii Y, Nishimura S, et al:
Treatment responses of childhood aplastic anaemia with chromosomal
aberrations at diagnosis. Br J Haematol. 118:313–319.
2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mikhailova N, Sessarego M, Fugazza G,
Caimo A, De Filippi S, van Lint MT, Bregante S, Valeriani A,
Mordini N, Lamparelli T, et al: Cytogenetic abnormalities in
patients with severe aplastic anemia. Haematologica. 81:418–422.
1996.PubMed/NCBI
|
|
36
|
Kearns WG, Sutton JF, Maciejewski JP,
Young NS and Liu JM: Genomic instability in bone marrow failure
syndromes. Am J Hematol. 76:220–224. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Keung YK, Pettenati MJ, Cruz JM, Powell
BL, Woodruff RD and Buss DH: Bone marrow cytogenetic abnormalities
of aplastic anemia. Am J Hematol. 66:167–171. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Piaggio G, Podestà M, Pitto A, Sessarego
M, Figari O, Fugazza G, Benvenuto F, Bruno B, Van Lint MT, Truini
M, et al: Coexistence of normal and clonal haemopoiesis in aplastic
anaemia patients treated with immunosuppressive therapy. Br J
Haematol. 107:505–511. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Geary CG, Harrison CJ, Philpott NJ, Hows
JM, Gordon-Smith EC and Marsh JC: Abnormal cytogenetic clones in
patients with aplastic anaemia: Response to immunosuppressive
therapy. Br J Haematol. 104:271–274. 1999.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Appelbaum FR, Barrall J, Storb R, Ramberg
R, Doney K, Sale GE and Thomas ED: Clonal cytogenetic abnormalities
in patients with otherwise typical aplastic anemia. Exp Hematol.
15:1134–1139. 1987.PubMed/NCBI
|
|
41
|
Afable MG II, Tiu RV and Maciejewski JP:
Clonal evolution in aplastic anemia. Hematology Am Soc Hematol Educ
Program. 2011:90–95. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sloand EM, Kim S, Fuhrer M, Risitano AM,
Nakamura R, Maciejewski JP, Barrett AJ and Young NS: Fas-mediated
apoptosis is important in regulating cell replication and death in
trisomy 8 hematopoietic cells but not in cells with other
cytogenetic abnormalities. Blood. 100:4427–4432. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen G, Zeng W, Miyazato A, Billings E,
Maciejewski JP, Kajigaya S, Sloand EM and Young NS: Distinctive
gene expression profiles of CD34 cells from patients with
myelodysplastic syndrome characterized by specific chromosomal
abnormalities. Blood. 104:4210–4218. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kojima S, Ohara A, Tsuchida M, Kudoh T,
Hanada R, Okimoto Y, Kaneko T, Takano T, Ikuta K and Tsukimoto I:
Japan Childhood Aplastic Anemia Study Group: Risk factors for
evolution of acquired aplastic anemia into myelodysplastic syndrome
and acute myeloid leukemia after immunosuppressive therapy in
children. Blood. 100:786–790. 2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sloand EM, Yong AS, Ramkissoon S, Solomou
E, Bruno TC, Kim S, Fuhrer M, Kajigaya S, Barrett AJ and Young NS:
Granulocyte colony-stimulating factor preferentially stimulates
proliferation of monosomy 7 cells bearing the isoform IV receptor.
Proc Natl Acad Sci USA. 103:14483–14488. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mohamed AN, Varterasian ML, Dobin SM,
McConnell TS, Wolman SR, Rankin C, Willman CL, Head DR and Slovak
ML: Trisomy 6 as a primary karyotypic aberration in hematologic
disorders. Cancer Genet Cytogenet. 106:152–155. 1998.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Argiropoulos B, Clifford B, Crocker S,
Sinclair-Bourque E, McCready E, McGowan-Jordan J, Johnston DL and
Padmore R: HLA-DR(negative), CD34(negative) hypergranular acute
myeloid leukemia with trisomy 6 and del(5)(q22q33): Case report and
review of the literature. J Pediat Hematol Oncol. 33:e289–e295.
2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Boddu PC and Kadia TM: Molecular
pathogenesis of acquired aplastic anemia. Eur J Haematol.
102:103–110. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shallis RM, Ahmad R and Zeidan AM:
Aplastic anemia: Etiology, molecular pathogenesis, and emerging
concepts. Eur J Haematol. 101:711–720. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wang L and Liu H: Pathogenesis of aplastic
anemia. Hematology. 24:559–566. 2019.PubMed/NCBI View Article : Google Scholar
|