Introduction
Infertility is characterized by failure to establish
a clinical pregnancy after 12 months of regular and unprotected
sexual intercourse. In China, the frequency of infertility is
estimated to be 15-20% and male factors account for 50% among
infertile couples (1,2). Male infertility, occurs in a clinically
population and is influenced by factors including hormone status,
age, exercise, obesity, infectious disease and immunological or
psychological factors. In addition, various genetic impairments are
associated with problems of fertility, including azoospermia factor
deletion, mutations in the cystic fibrosis transmembrane
conductance regulator and numerical/structural chromosomal
abnormalities (3,4).
Small supernumerary marker chromosomes (sSMCs) are
defined as structurally abnormal chromosomes that cannot be clearly
characterized by conventional cytogenetic banding (5). While the incidence rate of sSMCs is
0.3-0.5/1,000 in the normal population, this occurrence is up to
0.125% in patients with fertility problems, with a male-to-female
ratio of 7.5:1(1). sSMCs may present as various forms, including
inverted duplicated (inv dup), complex rearranged, minute, ring or
neocentric chromosomes (6). Most
sSMCs result from short arms and pericentric regions of acrocentric
chromosomes, among which sSMC on chromosome 15 [sSMC(15)] is the
most common (7-9). To
date, the genotype-phenotype association between sSMCs and male
infertility has remained elusive. Therefore, more evidence is
required to determine this association.
The current study presents the case of a male
patient with oligoasthenoteratozoospermia (OAT) and sSMC(15). A
literature review on sSMC(15)-associated spermatozoa as a cause of
infertility in males was also performed.
Case report
A 38-year-old Chinese male was referred to the
Center for Reproductive Medicine and the Center for Prenatal
Diagnosis of the First Hospital of Jilin University (Changchun,
China) for consultation for infertility after 1 year of regular
unprotected sexual intercourse with no resulting pregnancy in
November 2017. No apparent abnormalities were observed in this
patient, except for infertility. A series of routine clinical
examinations were performed. Semen analysis indicated that the
patient had OAT according to the World Health Organization
guidelines (10). Reproductive
hormone levels were as follows: Luteinizing hormone, 4.65 mIU/ml
(normal range, 1.7-8.5 mIU/ml); follicle-stimulating hormone, 4.95
mIU/ml (normal range, 1.5-12.4 mIU/ml); estradiol, 14.3 pg/ml
(normal range, 28-248 pg/ml); testosterone, 6.46 nmol/l (normal
range, 9.9-27.8 nmol/l); and prolactin, 149.2 µIU/ml (normal range,
86-258 µIU/ml). All normal ranges were based on data provided by
the Center for Reproductive Medicine and Center for Prenatal
Diagnosis, The First Hospital, Jilin University. The present study
was approved by the Ethics Committee of the First Hospital of Jilin
University (Changchun, China; permit no. 2017-402). Informed
written consent was obtained from the patient for publication of
this case report and accompanying images.
G-banding analysis was performed according to
standard procedures on peripheral blood cells of the patient
(11). A total of 50 metaphase cells
were analyzed. The karyotype was described according to the
International System for Human Cytogenetic Nomenclature 2016
nomenclature (12). The result
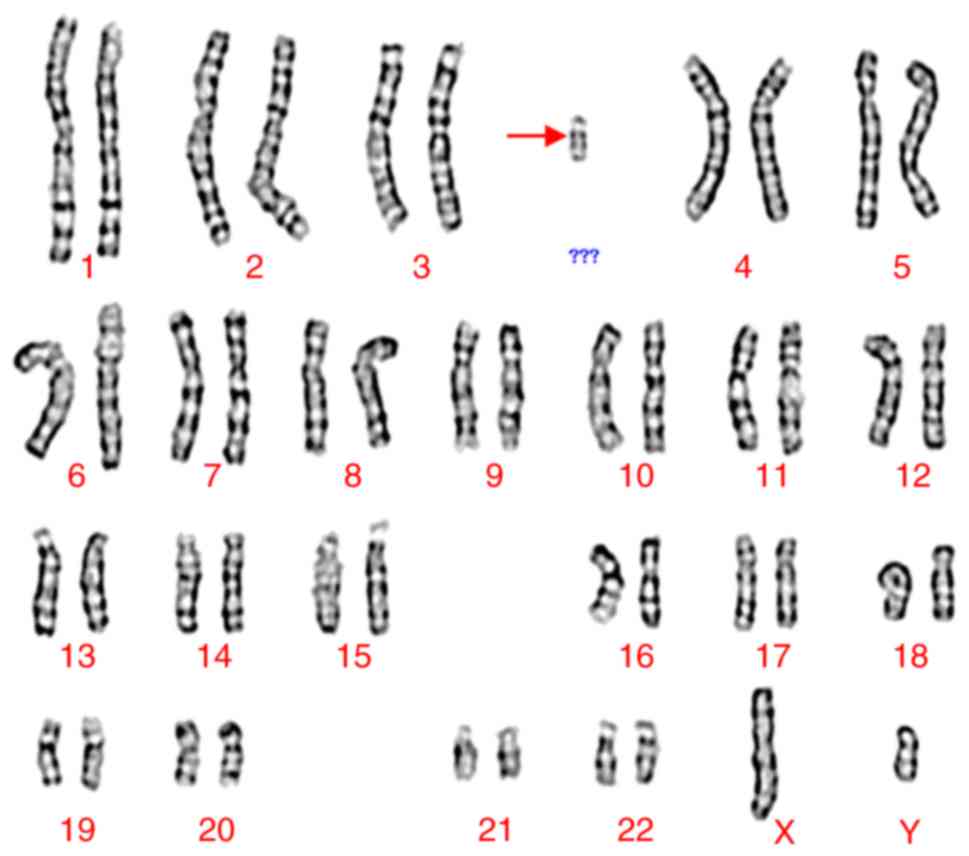
suggested that the patient had a non-mosaic abnormal karyotype of
47,XY,+mar (Fig. 1).
Chromosomal microarray analysis (CMA) was performed
on peripheral blood samples of the patient to analyze whole-genome
copy number variations and to detect heterozygous deletion by the
CytoScan 750 K array (Affymetrix; Themo Fisher Scientific, Inc.).
This method detects human genomic DNA copy number variations and
loss of heterozygosity with ≥50 probe labels and ≥200-kb
resolution, covering 22 pairs of autosomal and sex chromosomes.
Thresholds for genome-wide screening were set at ≥200 kb for gains,
≥100 kb for losses and ≥10 Mb for loss of heterozygosity. The
detected copy number variations were comprehensively evaluated
through the published literature and public databases, including
DECIPHER v9.28 (https://decipher.sanger.ac.uk/), the database of
genomic variants and Online Mendelian Inheritance in Man (OMIM;
https://www.ncbi.nlm.nih.gov/omim;
accessed June 1st 2019). The genomic coordinates were based on the
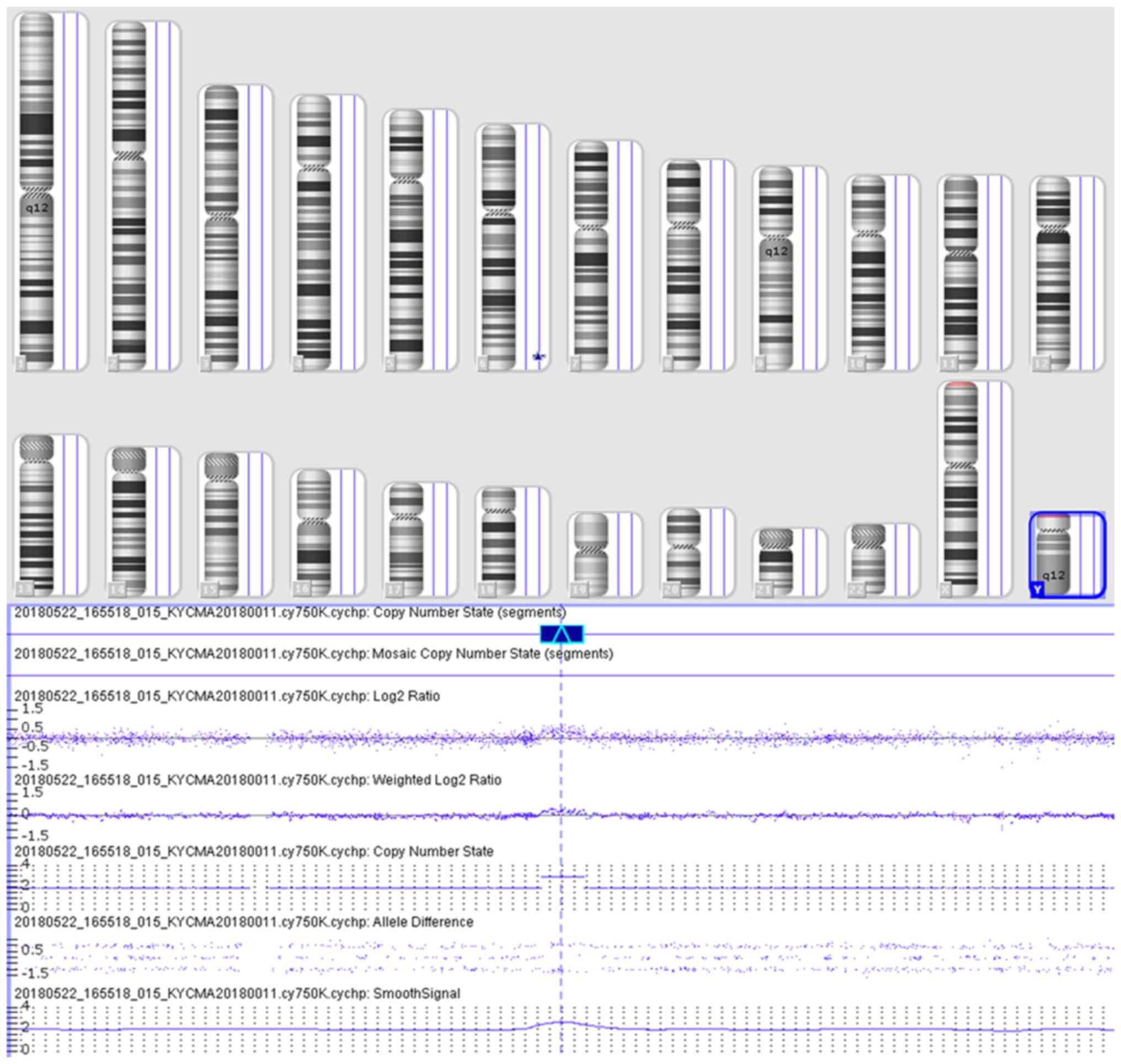
GRCh37/hg19 build of the human reference genome (13). A 0.44 Mb gain in 6q25.3q26 was
detected, which revealed
arr[hg19]6q25.3q26(160,569,492-161,010,647)x3 (Fig. 2).
Fluorescence in situ hybridization (FISH) was
used to further identify the origin of the sSMC. Specific probes
for chromosomes 13/21, 14/22 and 15 were used to investigate the
origin of the sSMC. The majority of the probes used in the present
study were commercial probes, including chromosomes 13/21, 14/22
centromere probes. The D13Z1 α-satellite probe was located at
13p11.1-q11.1 (cat no. LPE 013R/G-A; spectrum: Green), the
α-satellite D21Z1 probe was located at 21p11.1-q11.1 (cat no. LPE
013R/G-A; spectrum: Green), the α-satellite D14Z1 probe was located
at 14p11.1-q11.1 (cat no. LPE 014R/G-A; spectrum: Red), the
α-satellite D22Z1 probe was located at 22p11.1-q11.1 (cat no. LPE
014R/G-A; spectrum: Red). The satellite III D15Z1 probe was located
at 15p11.2 (spectrum: Green), the small nuclear ribonucleoprotein
polypeptide N Prader-Willi/Angelman probe (SNRPN) was located at
15q11-q13 (spectrum: Orange) and the PML probe was located at 15q24
(spectrum: Orange) (14). All probes
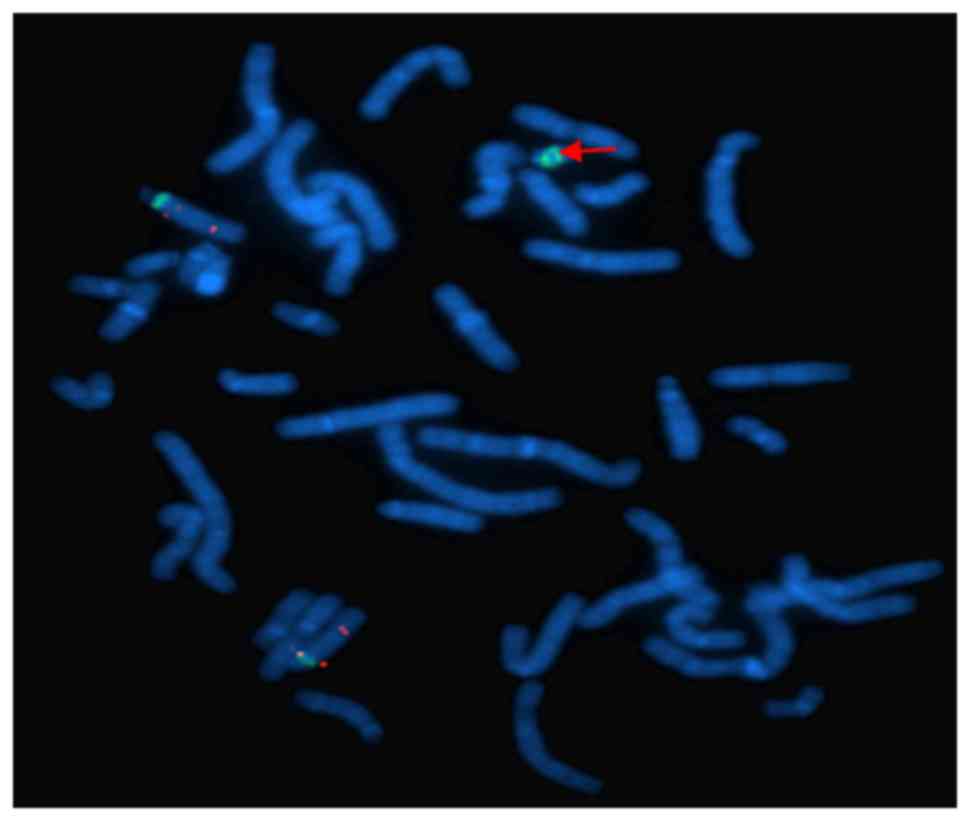
were supplied by Cytocell Technologies Ltd. FISH indicated that the
sSMC was positive twice for D15Z1 signals, which were located at
15p11.2, but negative for the probes SNRPN (15q11-13) and PML
(15q24) (Fig. 3).
These results indicated that the marker chromosome
was sSMC(15), which consisted of
two short arms, two centromeres and a pericentric region. The sSMC
was finally identified as inv dup(15)(q11.2). No chromosomal
analysis was performed in the proband's parents to determine
whether the sSMC of the proband was de novo or inherited.
According to the follow-up outcomes, the patient will pursue the
reproductive option of artificial insemination with donor
semen.
The present study focused on infertile patients with
sSMC(15), commonly presenting with impairment of spermatogenesis
and no apparent abnormalities. Based upon this selection criterion,
a systematic literature search was conducted by means of a Pubmed
literature search (http://www.ncbi.nlm.nih.gov/pubmed/; accessed May 16th
2019) using relevant terms and their combinations [sSMC(15) with
male infertility, marker chromosome 15 with male infertility and
sSMC(15) with spermatogenesis; Table
I] (15-19),
and by searching the sSMC database (http://ssmc-tl.com/sSMC.html; accessed May 16th 2019).
It was attempted to establish an association between non-mosaic
sSMC(15) and impairment of spermatogenesis in males. All cases are
listed in Table I and they were
divided into four groups as follows: i) OAT; ii) oligospermia; iii)
azoospermia and iv) cryptozoospermia, which are common
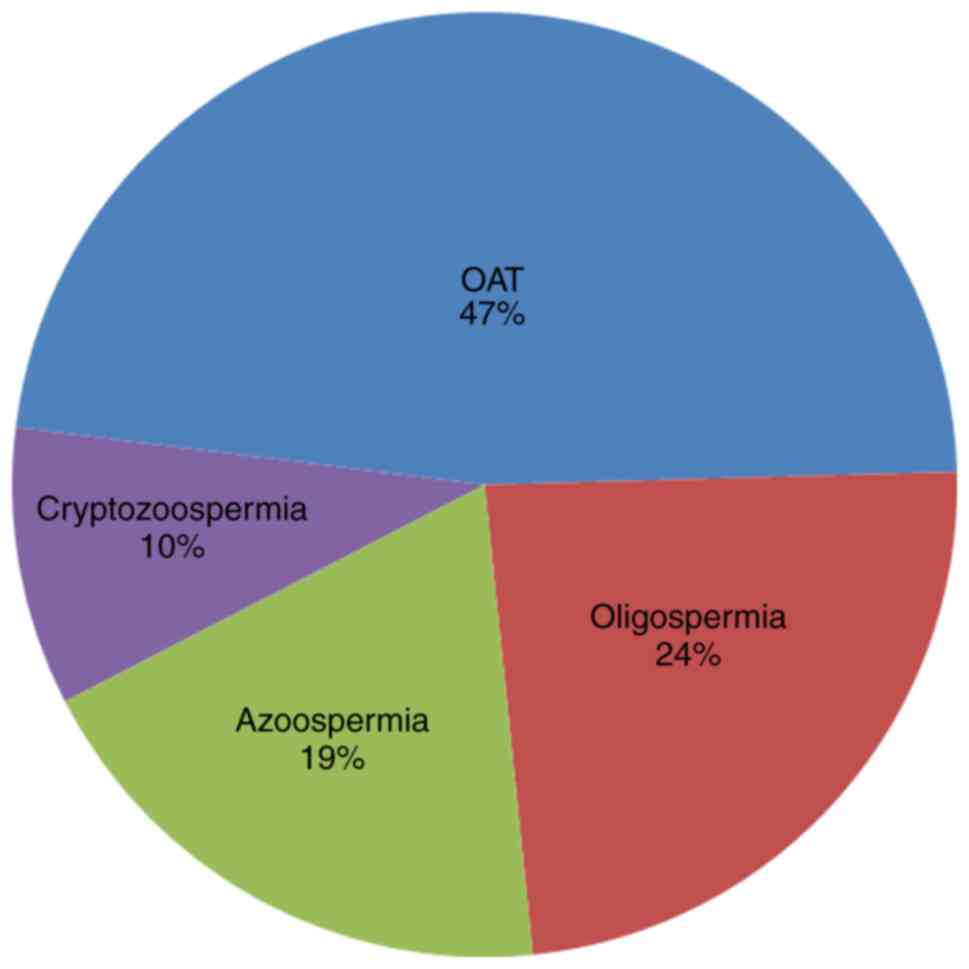
manifestations of spermatozoa in infertile males (20). It was revealed that in 90% (19/21) of
cases, sSMCs were described as inv dup(15) and involved the
centromere of chromosome 15. In addition, OAT and severe OAT
accounted for 47%, followed by oligospermia (24%), azoospermia
(19%) and cryptozoospermia (10%) (Fig.
4). In addition, no co-morbidities associated with sSMC(15)
were identified in any or all of these 4 groups. Furthermore,
multivariate statistical analyses were performed but no significant
associations were identified due to the lack of age specifications
in the azoospermia group. In conclusion, the effect of sSMC(15) on
abnormal spermatogenesis requires confirmation by further
studies.
 | Table ISummary of male sSMC(15) carriers with
spermatogenesis impairment and no apparent abnormalities based upon
the literature review and the sSMC database. |
Table I
Summary of male sSMC(15) carriers with
spermatogenesis impairment and no apparent abnormalities based upon
the literature review and the sSMC database.
| Case no. | Age at diagnosis
(years) | GTG result | Final result of the
sSMC | Diagnosis | (Refs.) |
|---|
| 1 | 38 | 47,XY,+mar[100%] | inv dup(15)
(q11.2) | OAT | Present case |
| 2 | Adult | 47,XY,+mar[100%] | inv dup(15)(q11) | Severe OAT | (15) |
| 3 | 39 | 47,XY,+mar[100%] | min(15)(p11→q11) | Oligospermia | (16) |
| 4 | 34 | 47,XY,+mar[100%] | inv
dup(15)(q11.1) | OAT | (17) |
| 5 | 41 | 47,XY,+mar[100%] | inv
dup(15)(q11.1) | OAT | (18) |
| 6 | 39 | 47,XY,+mar[100%] | inv
dup(15)(q11.1) | OAT | (17) |
| 7 | 37 |
47,XY,+mar[100%] | inv
dup(15)(q11.2) | Oligospermia | (17) |
| 8 | 30 |
47,XY,+mar[100%] | inv
dup(15)(q11.2) | OAT | (17) |
| 9 | 43 |
47,XY,+mar[100%] | inv
dup(15)(q11.2) |
Cryptozoospermia | (17) |
| 10 | Adult |
47,XY+mar[100%] | min or mar(15)
(pter→q11.2:) | Oligospermia | (17) |
| 11 | 33 |
47,XY,+mar[100%] | inv
dup(15)(q11.2~q13) | Oligospermia,
unilateral cryptorchidism | (19) |
| 12-14 | Postnatal |
47,XY,+mar[100%] | inv
dup(15)(q11.1) | Azoospermia | (17) |
| 15 | Postnatal |
47,XY,+mar[100%] | inv
dup(15)(q11.1) | OAT, seminoma | (17) |
| 16 | Postnatal |
47,XY,+mar[100%] | inv
dup(15)(q11.1) | Azoospermia | (17) |
| 17 | Postnatal |
47,XY,+mar[100%] | inv dup(15) | OAT | (17) |
| 18 | Postnatal |
47,XY,+mar[100%] | inv dup(15) | Oligospermia | (17) |
| 19 | Postnatal |
47,XY,+mar[100%] | inv dup(15) | Several OATs | (17) |
| 20 | Postnatal |
47,XY,+mar[100%] | inv dup(15) | Several OATs | (17) |
| 21 | Postnatal |
47,XY,+mar[100%] | inv dup(15) |
Cryptozoospermia | (17) |
Discussion
The present study reported on a male patient with
non-mosaic sSMC(15) who presented with OAT but had no other
apparent abnormalities. Cytogenetic analysis of the proband
indicated that the karyotype was 47,XY,+mar, while subsequent CMA
results further indicated a 0.44-Mb gain in 6q25.3q26. FISH results
indicated positive D15Z1 signals twice, which indicated that the
sSMC originated from chromosome 15. The sSMC was finally identified
as inv dup(15)(q11.2).
sSMCs are defined as structurally abnormal
chromosomes that may be detected in patients with developmental
and/or mental retardation and infertility, and in prenatal or
postnatal cases (21). The
genotype-phenotype correlation of sSMC is currently complex and
diverse due to its origin, size and constitution (14). Euchromatic sSMCs, encompassing gene
dosage-sensitive genes, may be harmful, while sSMCs only containing
heterochromatin are mostly harmless (22). The clinical phenotypes of sSMC(15)
are diverse due to the existence of chromosomal
euchromatin/heterochromatin. When sSMC(15) contains 15q euchromatin
and the Prader-Willi syndrome/Angelman syndrome critical region,
the relevant clinical manifestations include developmental
retardation, intellectual disability, epilepsy and autistic
behavior. By contrast, when sSMC(15) only contains heterochromatin,
it is considered harmless regarding clinical outcomes, although
exceptions have been recorded (23).
Of note, sSMCs may lead to only fertility problems without the
appearance of any additional clinical symptoms (1). Liehr and Hamid Al-Rikabi (4) pointed out that most sSMCs in infertile
males were derived from acrocentric chromosomes, particularly
sSMC(15), which accounted for up to 40% of all sSMCs. Patients with
sSMC(15) are generally clinically normal, while the risk of oligo-
or azoospermia is increased in infertile males, which may affect
spermatogenesis (24,25). Further research is required on
sSMC(15) due to the complex effect of the origin, size and
mosaicism of this sSMC on clinical phenotypes (25,26).
In the present study, the patient was diagnosed with
OAT with the karyotype 47,XY,+mar and the sSMC was further
identified as inv dup(15)(q11.2) by CMA and FISH analysis. Table I obtained by searching for relevant
cases showed that sSMC(15) was related to OAT, but the mechanism of
sSMC(15) on abnormal spermatogenesis needed further research.
Molecular cytogenetic techniques have critical roles in the
characterization of sSMCs and detection of genomic copy number
variations, chromosomal breakpoints and the genes involved
(4,14). In the present study, CMA analysis
detected a 0.44-Mb interstitial duplication in 6q25.3q26, which led
to arr[hg19]6q25.3q26(160,569,492-161,010,647)x3. This region
included solute carrier family 22 member 1 (SLC22A1; OMIM:602607),
SLC22A2 (OMIM:602608), SLC22A3 (OMIM:604842) and lipoprotein A
(LPA; OMIM:152200) genes. Mutation of LPA is associated with
susceptibility to coronary artery disease (27). SLC22A1, SLC22A2 and SLC22A3 are
members of cation transporter genes that are located in a cluster
on chromosome 6. Production of SLC22A1 is the major organic cation
uptake system in hepatocytes (28).
SLC22A2 may mediate the first step of tubular secretion of most
positively charged substances (29)
and SLC22A3 may have a significant role in the disposition of
cationic neurotoxins and neurotransmitters in the brain (30,31). The
duplications of these genes, likely benign variations, may not be
responsible for the spermatogenesis disorder in the infertile
patient of the present study.
Certain hypotheses may explain the reason for
infertility in the present case. First, there might be an
association between the nucleolar organizer region (NOR) and
meiotic abnormalities. The NOR is located on the short arms of
human acrocentric chromosomes and the chromosomal context of NOR
has a critical role in nuclear biology (32). Additional NOR activity beyond an
optimal threshold resulting from marker chromosomes may predispose
to meiotic disturbances (33).
Furthermore, an imbalance caused by increased heterochromatin may
have a negative effect on the maturation of germ cells during
meiosis (34). In addition, an
interchromosomal effect may increase the risk in chromosomal
non-disjunction of aneuploid sperm during meiosis, which may affect
the nuclear structure of sperm (35). An interchromosomal effect resulting
from the presence of sSMCs is likely lead to infertility to a
certain extent (1).
Although FISH analysis suggested that the breakpoint
of inv dup(15) was located between 15p11.2 and 15q11-13, the exact
breakpoint and the amount of extra euchromatin or heterochromatin
was not identified using the SNRPN probe in the present case.
Previous studies have indicated that males with maternal-inherited
sSMCs and females with paternal-inherited sSMCs were inclined to be
infertile (1,3,25).
However, the present study did not determine whether the presence
of sSMC(15) in the proband was parentally inherited or de
novo. Intracytoplasmic sperm injection may help infertile males
with an sSMC and spermatozoa obtain offspring. Pre-implantation
genetic diagnosis may detect sSMCs in pre-implantation embryos
through specific probes, and normal embryos may be selected for
transfer following in vitro fertilization (36,37).
Regardless of what choice has been made, prenatal diagnosis is
still necessary after the establishment of pregnancy. In addition,
explorative attempts have been made in pre-implantation human
embryos through targeted DNA excision technologies, including
clustered regularly interspaced short palindromic repeats. However,
off-target effects resulting from cutting non-targeted genes when
using DNA excision technologies and other adverse effects on the
developing embryo due to the techniques applied are major problems
in the process and the issues regarding medical ethics should not
be ignored (38).
In conclusion, the present case study reported on a
male patient with OAT and an sSMC derived from acrocentric
chromosome 15, which was identified by karyotype analysis, CMA and
FISH analysis. The present study not only underlines the
significance of the genotype-phenotype association of sSMC(15) and
male infertility, but also adds evidence to the diversity in the
quality of spermatozoa associated with sSMC(15). For infertile sSMC
carriers with spermatozoa, application of pre-implantation genetic
diagnosis may have a role in selecting normal embryos to a certain
extent, which may be beneficial for achieving offspring for such
patients. Furthermore, comprehensive evaluation of fertility and
genetic counseling is warranted in advance and prenatal diagnosis
after pregnancy should not be neglected.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Research
and Development Program of China (grant no. 2016YFC1000601).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
MS obtained the clinical information, collected data
from the literature and wrote the first draft of the manuscript. RW
and HZ collected patient data and participated in the analysis and
interpretation of data. YJ and JH participated in the analysis and
interpretation of data. SL and RL conceived and designed the study.
SL and RL reviewed the manuscript and were involved in its critical
revision prior to submission. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by Medical Ethics Committee
of The First Hospital of Jilin University (permit no. 2017-402).
The patient provided written informed consent to participate in
this study.
Patient consent for publication
The patient provided written informed consent for
the publication of the present case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Armanet N, Tosca L, Brisset S, Liehr T and
Tachdjian G: Small supernumerary marker chromosomes in human
infertility. Cytogenet Genome Res. 146:100–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Neto FT, Bach PV, Najari BB, Li PS and
Goldstein M: Genetics of Male Infertility. Curr Urol Rep.
17(70)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Manvelyan M, Riegel M, Santos M, Fuster C,
Pellestor F, Mazaurik ML, Schulze B, Polityko A, Tittelbach H,
Reising-Ackermann G, et al: Thirty-two new cases with small
supernumerary marker chromosomes detected in connection with
fertility problems: Detailed molecular cytogenetic characterization
and review of the literature. Int J Mol Med. 21:705–714.
2008.PubMed/NCBI
|
|
4
|
Liehr T and Hamid Al-Rikabi AB: Impaired
spermatogenesis due to small supernumerary marker chromosomes: The
reason for infertility is only reliably ascertainable by
cytogenetics. Sex Dev. 2018.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
5
|
Wang H, Wang T, Yang N, He Y, Chen L, Hong
L, Shao X and Li H, Zhu H and Li H: The clinical analysis of small
supernumerary marker chromosomes in 17 children with mos
45,X/46,X,+mar karyotype. Oncol Lett. 13:4385–4389. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Plaja A, Lloveras E, Martinez-Bouzas C,
Barreña B, Del Campo M, Fernández A, Herrero M, Barranco L, Palau
N, López-Aríztegui MA, et al: Trisomy 18p caused by a supernumerary
marker with a chromosome 13/21 centromere: A possible recurrent
chromosome aberration. Am J Med Genet A. 161A:2363–2368.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guediche N, Tosca L and Kara Terki A:
Array comparative genomic hybridization analysis of small
supernumerary marker chromosomes in human infertility. Reprod
Biomed Online. 24:72–82. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Quinonez SC, Gelehrter TD and Uhlmann WR:
A Marfan syndrome-like phenotype caused by a neocentromeric
supernumerary ring chromosome 15. Am J Med Genet A. 173:268–273.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Crolla JA, Youings SA, Ennis S and Jacobs
PA: Supernumerary marker chromosomes in man: Parental origin,
mosaicism and maternal age revisited. Eur J Hum Genet. 13:154–160.
2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
WHO Laboratory Manual for the Examination
and Processing of Human Semen. 5th edition. World Health
Organization, Geneva, 2010.
|
|
11
|
Zhang H, Wang R, Li L, Jiang Y, Zhang H
and Liu R: Clinical feature of infertile men carrying balanced
translocations involving chromosome 10: Case series and a review of
the literature. Medicine (Baltimore). 97(e0452)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
McGowan-Jordan J, Simons A and Schmid M:
ISCN (2016): An International System for Human Cytogenetic
Nomenclature. Basel, Switzerland: Karger. 2016.
|
|
13
|
Hochstenbach R, Nowakowska B, Volleth M,
Ummels A, Kutkowska-Kaźmierczak A, Obersztyn E, Ziemkiewicz K,
Gerloff C, Schanze D, Zenker M, et al: Multiple small supernumerary
marker chromosomes resulting from maternal meiosis i or ii errors.
Mol Syndromol. 6:210–221. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sun M, Zhang H, Li G, Guy CJ, Wang X, Lu
X, Gong F, Lee J, Hassed S and Li S: Molecular characterization of
20 small supernumerary marker chromosome cases using array
comparative genomic hybridization and fluorescence in situ
hybridization. Sci Rep. 7(10395)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Peschka B, Leygraaf J, Van der Ven K,
Montag M, Schartmann B, Schubert R, van der Ven H and Schwanitz G:
Type and frequency of chromosome aberrations in 781 couples
undergoing intracytoplasmic sperm injection. Hum Reprod.
14:2257–2263. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cetin Z, Berker Karaüzüm S, Yakut S, Mihçi
E, Baumer A, Wey E, Taçoy S, Bağci G and Lüleci G: M-FISH
applications in clinical genetics. Genet Couns. 16:257–268.
2005.PubMed/NCBI
|
|
17
|
Liehr T: Small supernumerary marker
chromosomes detected in connection with infertility. Zhonghua Nan
Ke Xue. 20:771–780. 2014.PubMed/NCBI
|
|
18
|
Paetzold U, Schwanitz G, Schubert R, van
der Ven K and Montag M: Sperm analyses, genetic counseling and
therapy in an infertile carrier of a supernumerary marker
chromosome 15. Adv Med Sci. 51:31–35. 2006.PubMed/NCBI
|
|
19
|
Vulcani-Freitas TM, Gil-da-Silva-Lopes VL,
Varella-Garcia M and Maciel-Guerra AT: Infertility and marker
chromosomes: Application of molecular cytogenetic techniques in a
case of inv dup(15). J Appl Genet. 47:89–91. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Magdi Y, Darwish E, Elbashir S, Majzoub A
and Agarwal A: Effect of modifiable lifestyle factors and
antioxidant treatment on semen parameters of men with severe
oligoasthenoteratozoospermia. Andrologia: Nov 10, 2017 (Epub ahead
of print). doi: 10.1111/and.12694.
|
|
21
|
Liehr T and Weise A: Frequency of small
supernumerary marker chromosomes in prenatal, newborn,
developmentally retarded and infertility diagnostics. Int J Mol
Med. 19:719–731. 2007.PubMed/NCBI
|
|
22
|
Liehr T and Kosyakova N: Small
supernumerary marker chromosomes (sSMC)-what about the
genotype-phenotype correlation? Tsitologiia. 55:165–166.
2013.PubMed/NCBI
|
|
23
|
Battaglia A: The inv dup (15) or idic (15)
syndrome (Tetrasomy 15q). Orphanet J Rare Dis. 3(30)2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cotter PD, Ko E, Larabell SK, Rademaker AW
and Martin RH: Segregation of a supernumerary del(15) marker
chromosome in sperm. Clin Genet. 58:488–492. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Koç A, Onur SO, Ergün MA and Perçin EF:
Supernumerary marker chromosome 15 in a male with azoospermia and
open bite deformity. Asian J Androl. 11:617–622. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liehr T: Small supernumerary marker
chromosomes-an update. Mol Cytogenet: Jan 21, 2014 (Epub ahead of
print). doi: 10.1186/1755-8166-7-S1-I11.
|
|
27
|
Clarke R, Peden JF, Hopewell JC, Kyriakou
T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, et
al: Genetic variants associated with Lp(a) lipoprotein level and
coronary disease. New Eng J Med. 361:2518–2528. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gründemann D, Gorboulev V, Gambaryan S,
Veyhl M and Koepsell H: Drug excretion mediated by a new prototype
of polyspecific transporter. Nature. 372:549–552. 1994.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Hoermann S, Gai Z, Kullak-Ublick GA and
Visentin M: Plasma membrane cholesterol regulates the allosteric
binding of 1-methyl-4-phenylpyridinium to organic cation
transporter 2 (SLC22A2). J Pharmacol Exp Ther. 372:46–53.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gründemann D, Schechinger B, Rappold GA
and Schömig E: Molecular identification of the
corticosterone-sensitive extraneuronal catecholamine transporter.
Nat Neurosci. 1:349–351. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Wu X, Huang W, Ganapathy ME, Wang H,
Kekuda R, Conway SJ, Leibach FH and Ganapathy V: Structure,
function, and regional distribution of the organic cation
transporter OCT3 in the kidney. Am J Physiol Renal Physiol.
279:F449–F458. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mangan H, Gailín MÓ and McStay B:
Integrating the genomic architecture of human nucleolar organizer
regions with the biophysical properties of nucleoli. FEBS J.
284:3977–3985. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Martín-Lucas MA, Pérez-Castillo A and
Abrisqueta JA: Infertility associated with two accessory
bisatellited chromosomes. Hum Genet. 73:133–136. 1986.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gentile M, Susca F, Resta N, Stella A,
Cascone A and Guanti G: Infertility in carriers of two bisatellited
marker chromosomes. Clin Genet. 44:71–75. 1993.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kirkpatrick G, Ren H, Liehr T, Chow V and
Ma S: Meiotic and sperm aneuploidy studies in three carriers of
Robertsonian translocations and small supernumerary marker
chromosomes. Fertil Steril. 103:1162–1169e7. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Perrin A, Nguyen MH, Delobel B, Guéganic
N, Basinko A, Le Bris MJ, Douet-Guilbert N, De Braekeleer M and
Morel F: Characterization and meiotic segregation of a
supernumerary marker chromosome in sperm of infertile males: Case
report and literature review. Eur J Med Genet. 55:743–746.
2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Oracova E, Musilova P, Kopecna O, Rybar R,
Vozdova M, Vesela K and Rubes J: Sperm and embryo analysis in a
carrier of supernumerary inv dup(15) marker chromosome. J Androl.
30:233–239. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ranisch R: Germline genome editing versus
preimplantation genetic diagnosis: Is there a case in favour of
germline interventions? Bioethics. 34:60–69. 2020.PubMed/NCBI View Article : Google Scholar
|