Introduction
Osteoarthritis (OA), is the most prevalent joint
disease characterized by loss of cartilage, subchondral bone
sclerosis or cyst and osteophyte formation (1). The clinical manifestations of OA
include joint stiffness, chronic pain and limited movement
(2). Traditional treatment can only
temporarily relieve clinical symptoms, and cannot effectively
inhibit the pathological progress of OA (3). Therefore, it is important to understand
the pathogenesis of OA and investigate novel safe and effective
treatments. Degeneration of articular cartilage is one of the major
pathological changes in OA (3). Many
cytokines, growth factors and enzymes, such as interleukin (IL)-1β
and collagenase, are involved in articular cartilage degeneration
(4). IL-1β is secreted by synovial
cells and OA inflammatory cells, and stimulates the production of
proteolytic enzymes, such as matrix degrading enzymes and
collagenase, causing synovial inflammation and bone resorption
(4). Collagenase is upregulated in
OA cartilage, and intra-articular injection of collagenase has
successfully established an animal model of OA (4). In addition, matrix metalloproteinases
(MMPs) have been shown to play an important role in the
pathogenesis of OA (4). Inflammatory
cytokines increase the secretion of MMP-13, and promote the
degradation of collagen II (Col II) in chondrocytes, leading to the
occurrence of OA (5).
Irisin, a myokine produced by skeletal muscle in
response to physical exercise, promotes transdifferentiation of
white adipose tissue into brown adipose tissue (6). Previous studies have suggested that
irisin is also involved in the control of bone metabolism. Faienza
et al (7) identified that
irisin influences the treatment of pediatric patients with type 1
diabetes and promotes pediatric bone health. Previous studies have
shown that irisin can directly enhance osteogenic differentiation
of bone stromal cells and improve cortical quality (8). In addition, previous studies have
suggested that irisin can activate the Wnt/β-catenin signaling
pathway in MC3T3-E1 cells to promote osteoblast differentiation in
OA mice (9,10). A previous study reported that irisin
inhibits osteoclast differentiation by inhibiting the receptor of
nuclear factor C1 of T cells activated by NF-κB ligand in RAW264.7
cells (9). Irisin can effectively
enhance the osteogenesis process and reduce the occurrence of
osteoporosis and fracture (9).
Many signaling pathways regulating joint formation
and homeostasis are thought to be key factors in the pathogenesis
of OA (10). The Wnt/β-catenin
signaling pathway is considered to be one of the most important
pathways associated with postnatal metabolism of articular
cartilage matrix, differentiation and apoptosis of articular
chondrocytes (10,11). β-catenin is a key factor in the
Wnt/β-catenin signaling pathway and its expression level in the
nucleus directly reflects the activation level of this signaling
pathway (11). When the
Wnt/β-catenin signaling pathway is activated, β-catenin can
regulate the function of chondrocytes and change their
physiological state, resulting in OA and other related diseases
(10).
The SW1353 cell line was initiated in 1977, and was
later considered to be a valuable in vitro system for
investigating catabolic gene regulation with IL-1β, tumor necrosis
factor-α and fibroblast growth factors (12,13). At
present, previous studies have focused on the bone and subchondral
bone in OA joints. To the best of our knowledge, there are no
studies investigating whether irisin directly acts on cartilage and
plays a protective role in the process of OA. Furthermore, to the
best of our knowledge, there are no data showing the close
interaction between irisin and the Wnt/β-catenin and NF-кB
signaling pathways in SW1353 cells. The present results suggested
that irisin inhibited the Wnt/β-catenin and NF-кB signaling
pathways in SW1353 cells.
Materials and methods
Materials
Recombinant human full-length irisin protein (112
amino acids, FNDC5 sequence 32-143) was purchased from Phoenix
Pharmaceuticals, Inc. Recombinant human IL-1β was purchased from
Bio-Techne, and lithium chloride (LiCl; molecular weight, 42.39400)
was purchased from Shanghai Mintchem Development Co., Ltd.
Cell culture
The chondrosarcoma cell line SW1353, originating
from a 72-year-old woman, was purchased from Procell Life Science
& Technology, Co., Ltd. Cells were cultured with DMEM (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a 5%
CO2 incubator at 37˚C.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 kit (Gibco; Thermo Fisher Scientific, Inc.)
was used to evaluate the cytotoxicity of IL-1β and irisin. The
experiment was performed according to the manufacturer's
instructions. Cells (100 µl/well; ~5,000/well) were incubated for 4
h in 96-well plates in a humidified incubator (at 37˚C; 5%
CO2). Different concentrations of IL-1β (0, 5, 10, 20 or
50 ng/ml) or irisin (0, 10, 20, 50 or 100 mM) were added for 12,
24, 36 or 48 h. Then, 10 µl of CCK-8 solution was added to each
well of the plate using a repeating pipettor and incubated at 37˚C
for 4 h. The optical density was measured at a wavelength of 450 nm
using a microplate reader (Bio-Rad Model 550; Bio-Rad Laboratories,
Inc.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
SW1353 cells (5x105 in each dish) were
seeded in a 6 cm dish and treated with 10 ng/ml IL-1β and/or 20 mM
irisin at 37˚C for 24 h. The cells were harvested and then washed
with PBS. Total RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Then, 5 µl RNA was used for
electrophoresis with a 1% agarose gel to detect the integrity of
the RNA. The cDNA was synthesized from 1 µg total RNA using cDNA
synthesis (Tiangen Biotech Co., Ltd.) according to the
manufacturer's instructions. The expression levels of MMP-13, Col
II and Wnt-1 were analyzed using the primer sequences and β-catenin
as the reference gene as listed in Table
I.
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5'-3') | Length, bp |
|---|
| MMP-13 | F:
ACCCCAACCCTAAACATCC | 155 |
| | R:
CGTTAAAAACAGCTCCGCA | |
| Collagen II | F:
TGGTCTGAGGGGTCTTCC | 172 |
| | R:
CTGGTCACCTGGTTTTCC | |
| β-catenin | F:
CCAGTGGATTCTGTGTTGTT | 170 |
| | R:
ATTTGAAGGCAGTCTGTCGT | |
| Wnt-1 | F:
CACAAACCGCCCTCCCCC | 142 |
| | R:
GCAGCTCGCAGCCGTCCA | |
| β-actin | F:
CCAAGGCCAACCGCGAGAA | 187 |
| | R:
GCATGGGGGAGGGCATACC | |
The experimental operation of the reaction system
was carried out according to the manufacturer's instructions. The
20 µl reaction mixture consisted of 10 µl 2*SuperReal
PreMix Plus (SYBR Green; Tiangen Biotech Co., Ltd.) and each
primer. The StepOne System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used for qPCR using the TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems, Foster City, CA,
USA). The thermocycling conditions were as follows: Initial
denaturation at 95˚C for 1 min; 40 cycles of 95˚C for 10 sec, 58˚C
for 30 sec and 72˚C for 30 sec; and then the melt curve was
analyzed. Target gene levels were analyzed using the
2-ΔΔCq method (14).
Western blot analysis
SW1353 cells (5x105 in each dish) were
seeded in a 6 cm dish and treated with 10 ng/ml IL-1β and/or 20 mM
irisin at 37˚C for 24 h, then RIPA lysis buffer (Cell Signaling
Technology, Inc.) was used to extract protein. Protein
concentration was determined using a bicinchoninic acid kit
(Sigma-Aldrich; Merck KGaA). A total of 20 µg of protein was loaded
into each well of a 10% SDS-PAGE gel. After gel electrophoresis,
the protein bands were separated from the gel, transferred to PVDF
membranes after blocking with 5% non-fat milk at room temperature
for 1 h by transfer electrophoresis and then incubated at 37˚C for
1 h with the following antibodies: MMP-13 (1:10,000; cat. no.
DF6494; Affinity Biosciences), Col II (1:10,000 dilution; cat. no.
AF5456; Affinity Biosciences), phospho-NFκB (1:10,000; cat. no.
AF2006; Affinity Biosciences), inhibitor of NF-κB (IκB) α
(1:10,000; cat. no. AF002; Affinity Biosciences), Wnt-1 (1:10,000;
cat. no. DF514; Affinity Biosciences) and β-catenin (1:2,000; cat.
no. ab1008; Abcam). β-actin (cat. no. ab051, Abcam) was used as the
endogenous control. The membranes were then incubated with a goat
anti-rabbit secondary antibody (1:10,000; cat. no. ab97051; Abcam)
at 37˚C for 1 h. Protein bands were visualized with an ECL kit
(Abcam). The data of study groups were quantitatively analyzed
relative to the NC group using SPSS 19.0 (IBM Corp.).
Immunofluorescence analysis
SW1353 cells (6x104) were cultured in a
24-well plate and treated with 10 ng/ml IL-1β and/or 20 mM irisin
at 37˚C for 24 h. After the culture solution was aspirated, cells
were washed with PBS, and fixed with acetone at 4˚C for 10 min, and
blocked with 5% BSA in PBS at 37˚C for 60 min. After the treatment
with the primary antibody (rabbit LC3 antibody; 1:250; cat. no.
DF674; Affinity Biosciences) and the secondary antibody
(anti-rabbit IgG; 1:500; cat. no. AF313; Affinity Biosciences) at
37˚C for 1 h, the DAPI staining solution was added dropwise at 37˚C
for 10 min, and the images were taken using a fluorescence
microscope (magnification, 400x; Carl Zeiss AG).
Activation of the Wnt/β-catenin
signaling pathway
LiCl is a specific activator of the Wnt/β-catenin
signaling pathway. To investigate the effect of irisin on the
activated Wnt/β-catenin signaling pathway in a non-inflammatory
environment, the cells were pretreated with 10 mM LiCl at 37˚C for
24 h and treated with 20 nM irisin at 37˚C for a further 24 h.
Phosphorylated-p65 (p-p65) primary antibody (1:500; cat. no. BC336)
was purchased from Affinity Biosciences.
Statistical analysis
Data were analyzed using SPSS software version 12.0
(SPSS, Inc.) and are presented as the mean ± SD. Graphs were drawn
using GraphPad Prism 7.00 (GraphPad Software, Inc.). One-way ANOVA
with subsequent Tukey's test was used for multiple comparisons. The
experiment was repeated three times in each group. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of irisin and IL-1β on cell
viability of SW1353 cells
Effects of irisin and IL-1β on the cytotoxicity of
SW1353 cells were evaluated by CCK-8 assay. Irisin at
concentrations of 0, 10, 20, 50 and 100 mM did not have a
significant effect on cell viability, after 24 h (Fig. 1A) or 48 h (Fig. 1B) of incubation. The concentration of
20 mM was chosen for the following experiments as previously
described [12]. Furthermore, IL-1β at concentrations of
0, 5, 10, 20 and 50 ng/ml led to no difference in cell viability at
12 h (Fig. 1C) or 24 h (Fig. 1D). However, at 36 h (Fig. 1E) and 48 h (Fig. 1F) of incubation, IL-1β (10, 20 and 50
ng/ml) significantly decreased cell viability (P<0.05,
P<0.001, P<0.001), and the effects of 50 ng/ml IL-1β were
more significant (P<0.001). Therefore, 10 ng/ml IL-1β was used
to stimulate cells.
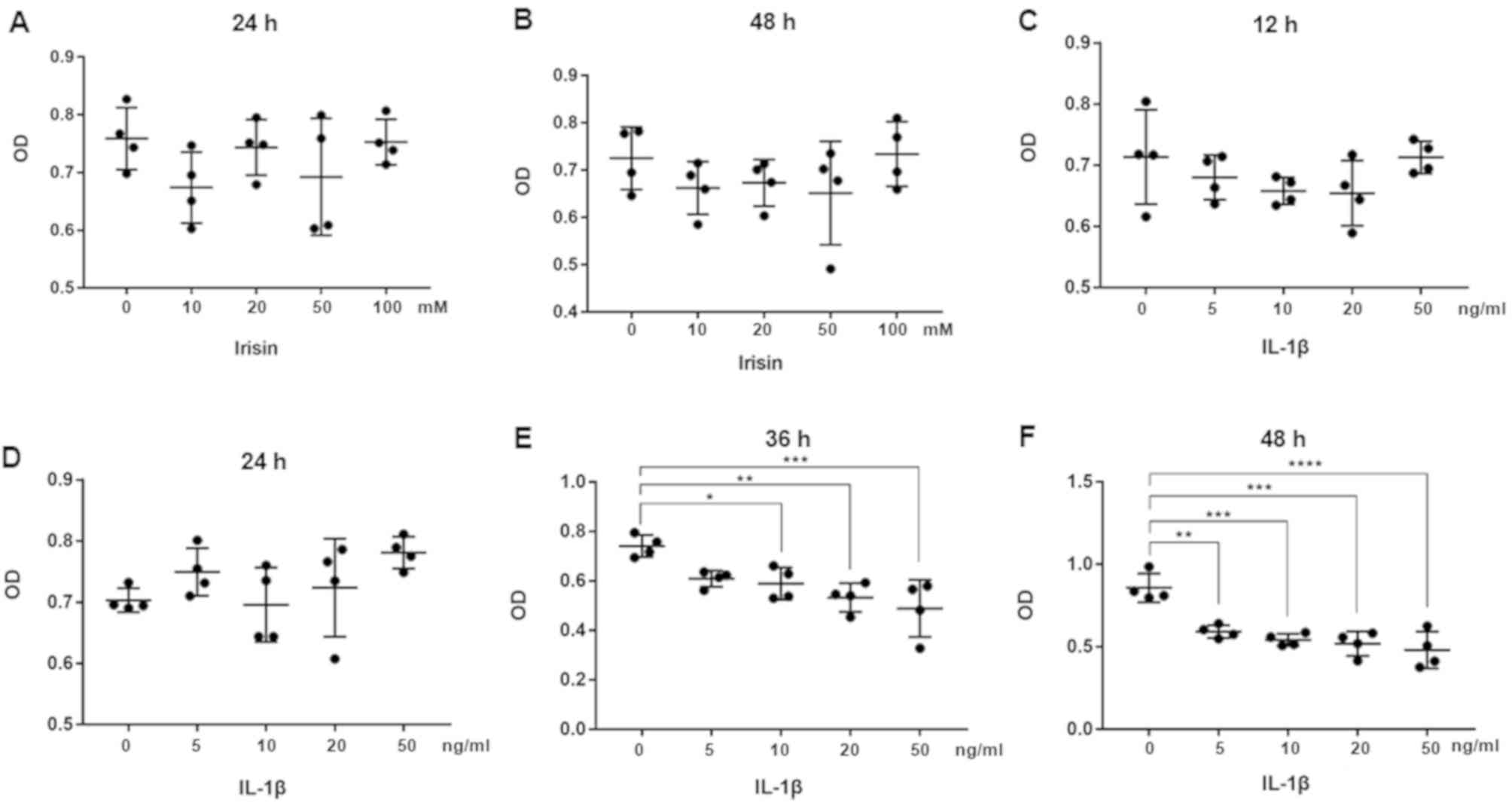 | Figure 1Effect of irisin on the viability of
SW1353 cells. SW1353 cells were seeded (100 µl/well; ~5,000/well)
in 96-well plates, treated with different concentrations of irisin,
and assessed by CCK-8 assay at (A) 24 h and (B) 48 h. n=5/group.
Cell viability in SW1353 cells treated with IL-1β was assessed by
CCK-8 assay at (C) 12, (D) 24, (E) 36 and (F) 48 h. n=5/group.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. IL-1β,
interleukin-1β; CCK-8, Cell Counting Kit-8; OD, optical
density. |
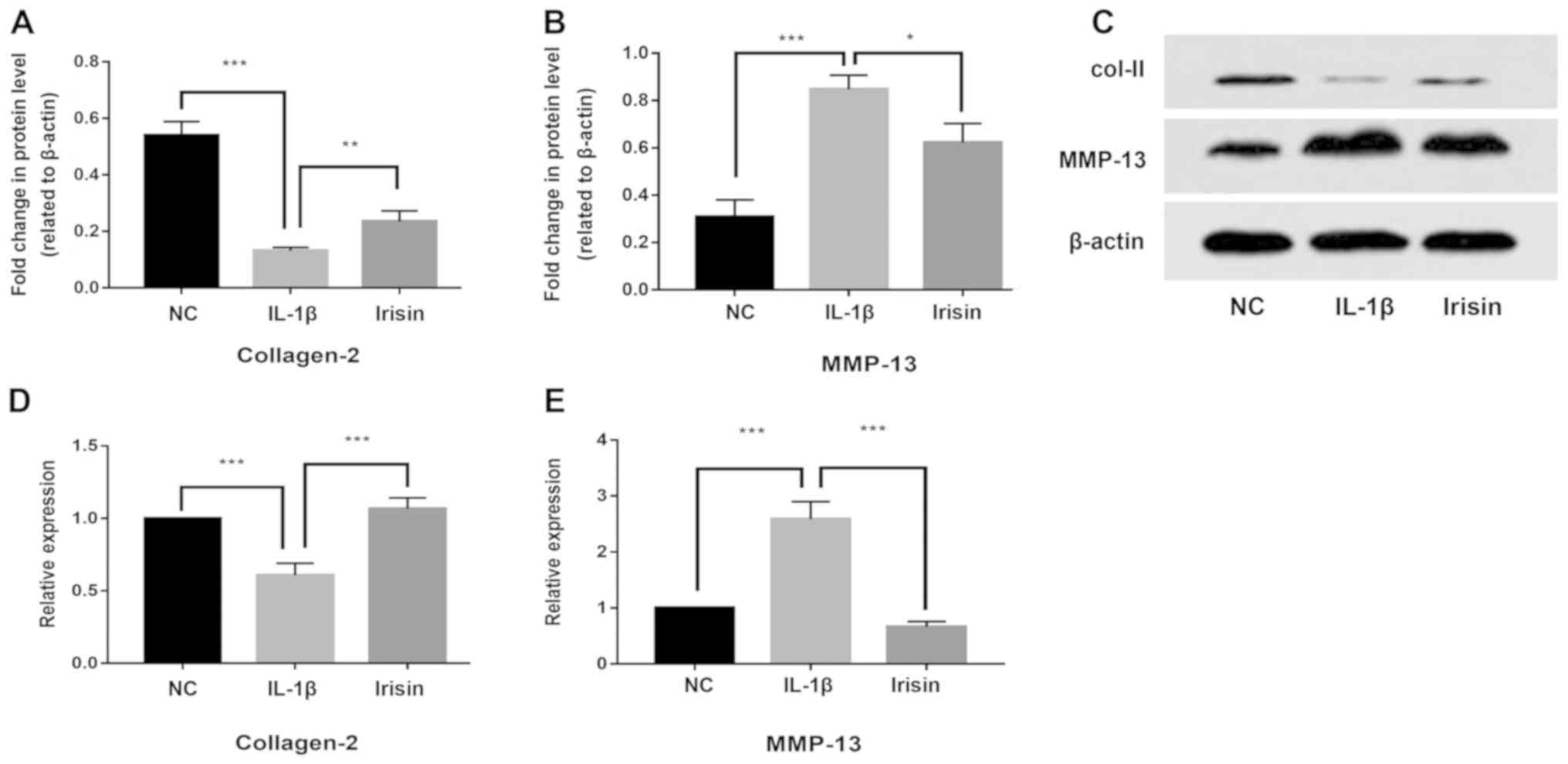
Effect of irisin on IL-1β-induced
expression of MMP-13 and Col II in SW1353 cells
The present study investigated the effect of irisin
on IL-1β-induced MMP-13 and Col II expression levels using RT-qPCR,
western blotting and immunofluorescence analysis. The present
results indicated that the expression of Col II at the protein
(Fig. 2A and C) and mRNA (Fig.
2D) levels was significantly decreased by IL-1β treatment,
whereas irisin treatment reversed the IL-1β induced-decrease of Col
II expression levels (P<0.05). Moreover, the expression of
MMP-13 at the protein (Fig. 2B and
C) and mRNA (Fig. 2E) levels in the SW1353 cells was
upregulated by IL-1β treatment, and the effect of IL-1β was
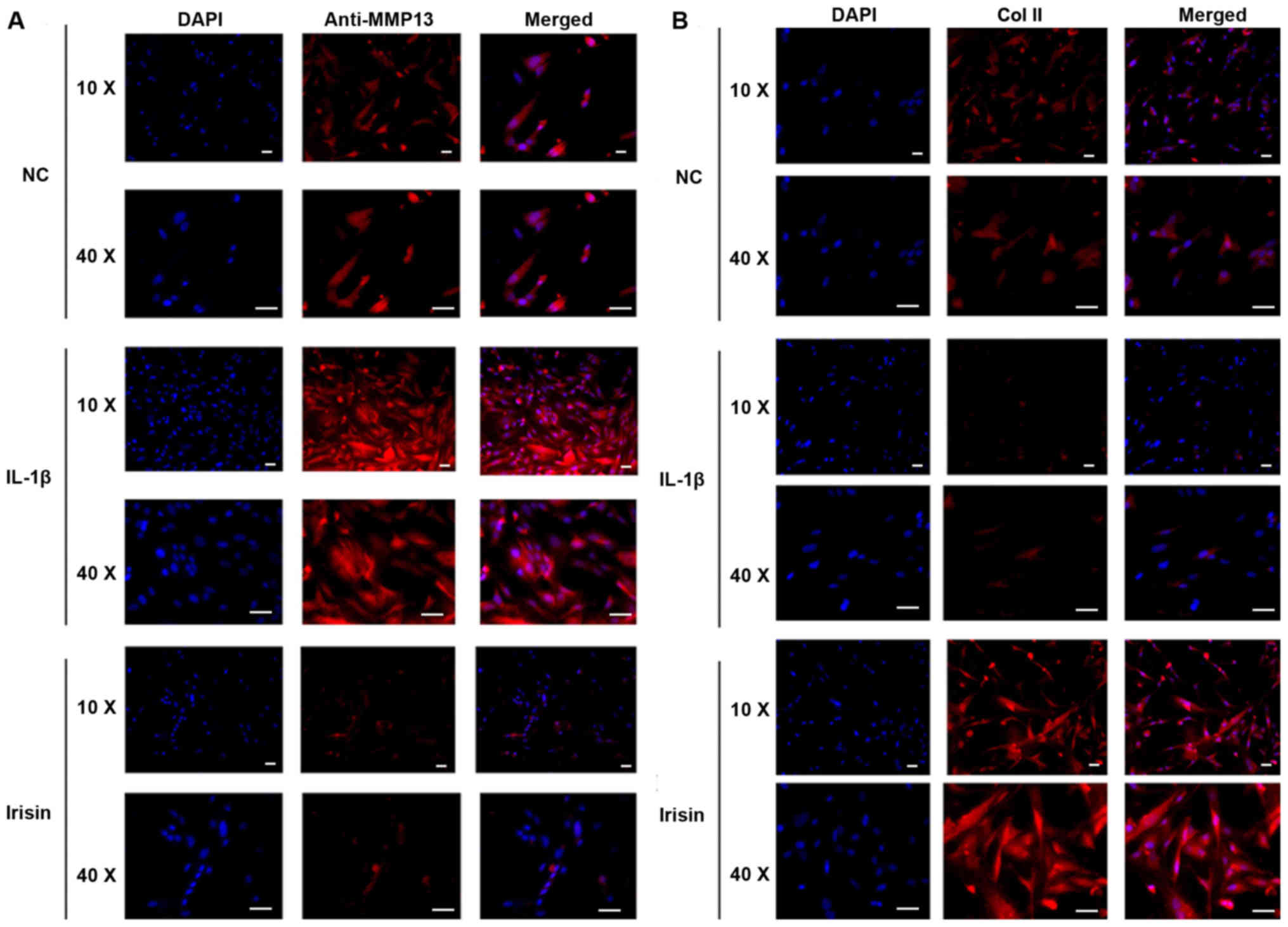
reversed by irisin treatment (P<0.05). Immunofluorescence
analysis supported the present RT-qPCR results. The expression
level of MMP-13 in SW1353 cells was downregulated after irisin
treatment, whereas IL-1β treatment upregulated the expression level
of MMP-13 in SW1353 cells compared with the control groups
(Fig. 3A). However, the expression
level of Col II in SW1353 cells was downregulated following IL-1β
intervention, and subsequently upregulated by the irisin treatment
compared with the control group (Fig.
3B).
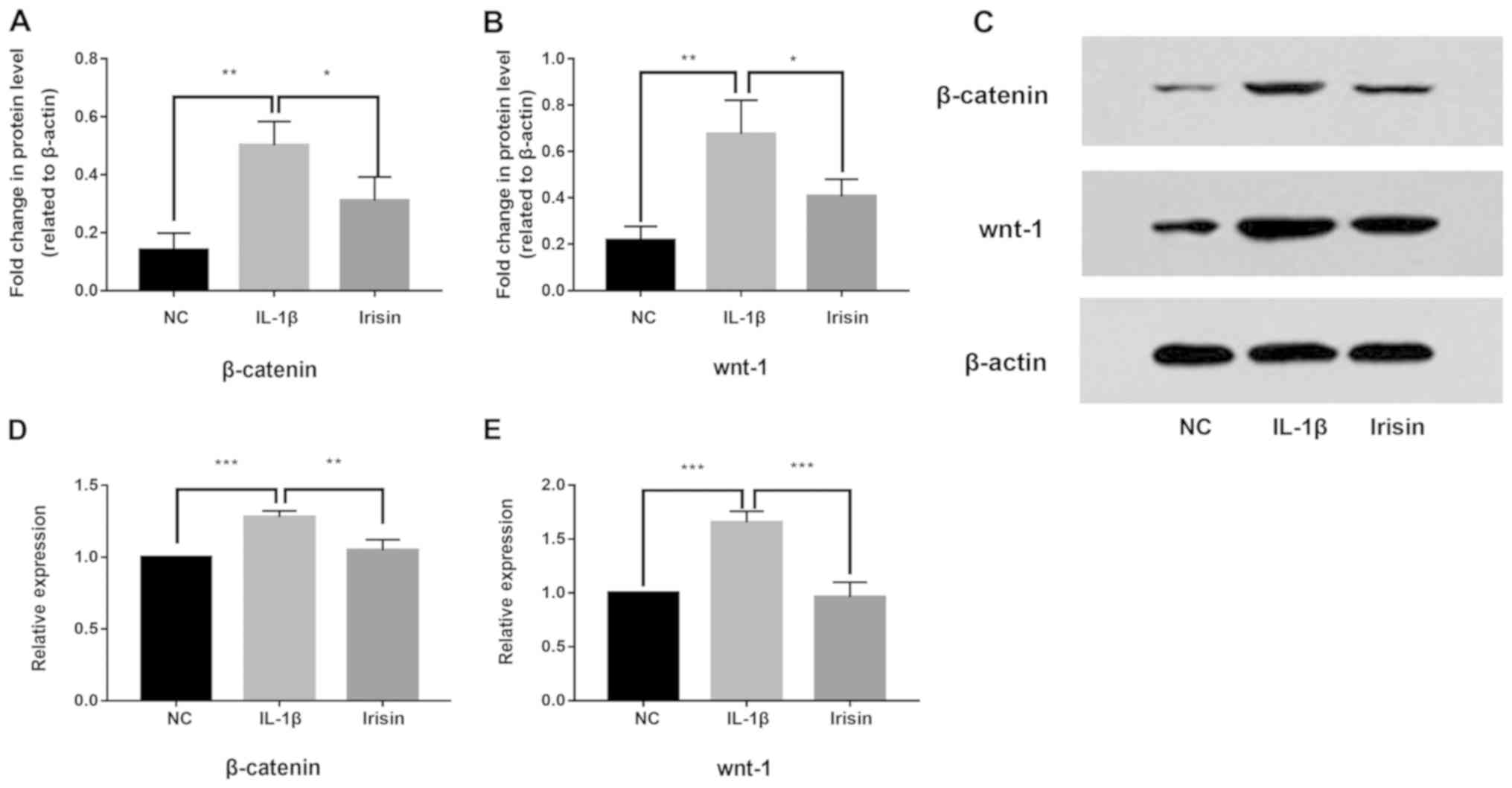
Effect of irisin on IL-1β-induced
activation of the Wnt/β-catenin signaling pathway in SW1353
cells
To study the anti-inflammatory mechanism of irisin,
western blotting and RT-qPCR were used to investigated its effects
on IL-1β-induced activation of the Wnt/β-catenin signaling pathway
in SW1353 cells. The present results suggested that, compared with
the negative control group, the protein and mRNA expression levels
of β-catenin (Fig. 4A, C and D) and
Wnt-1 (Fig. 4B, C and E) in
SW1353 cells were upregulated by IL-1β treatment (P<0.05),
indicating the activation of the Wnt/β-catenin signaling pathway.
However, following irisin treatment, IL-1β-induced activation was
suppressed (P<0.05). The present results suggested the
IL-1β-induced activity of the Wnt/β-catenin signaling pathway was
significantly decreased by irisin treatment.
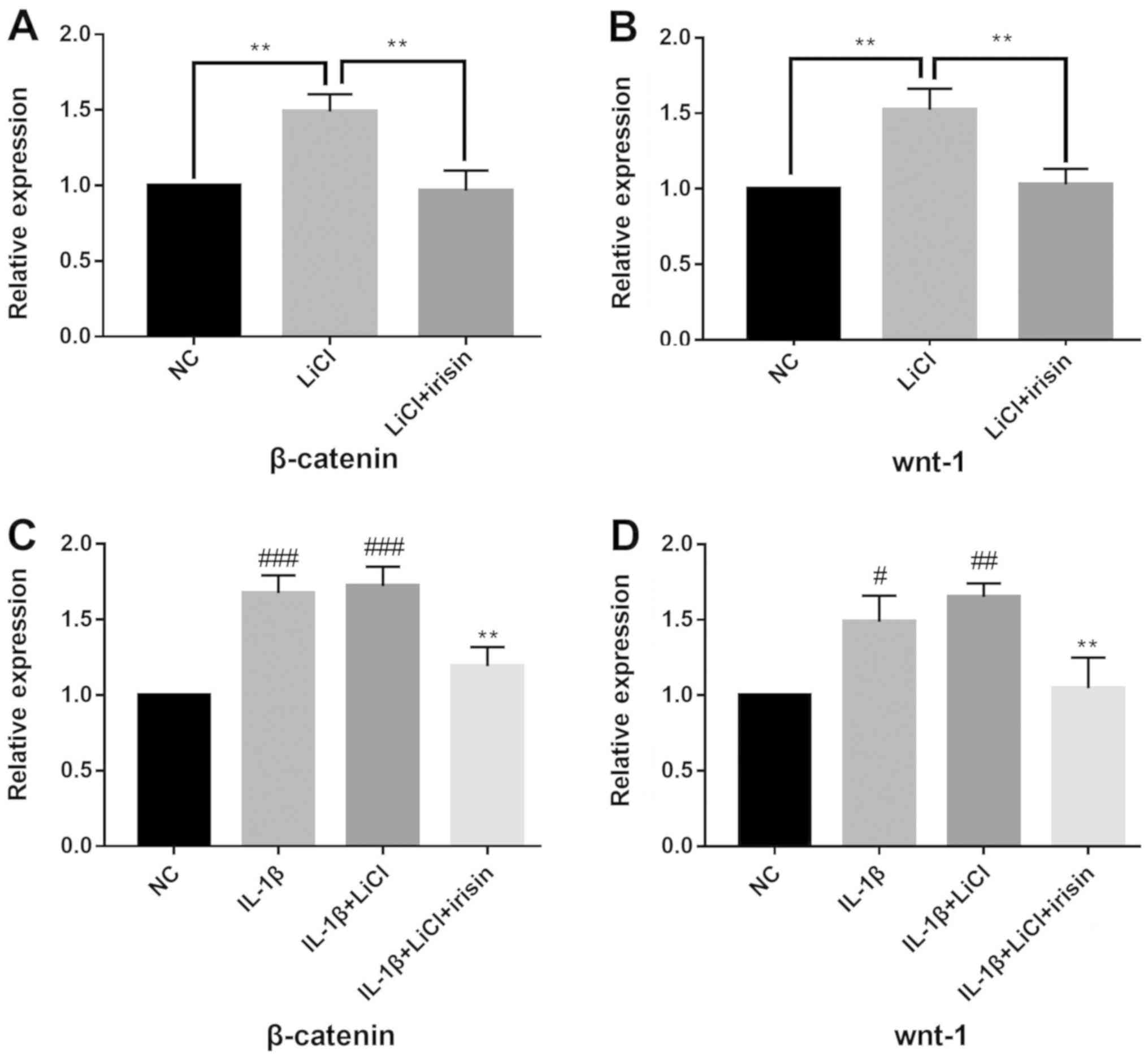
Effect of irisin on the Wnt/β-catenin
signaling pathway
LiCl significantly increased the mRNA expression
levels of β-catenin (Fig. 5A) and
Wnt-1 (Fig. 5B; P<0.05). Irisin
treatment significantly decreased the expression levels of Wnt-1
and β-catenin that were induced by LiCl (P<0.05). SW1353 cells
co-cultured with IL-1β and LiCl were also treated with irisin, and
the present results indicated that treatment with irisin decreased
the expression levels of β-catenin and Wnt-1 (Fig. 5C and D). The present results suggested that
irisin may exerted its functions in SW1353 cells by inhibiting the
Wnt/β-catenin signaling pathway (Fig.
5).
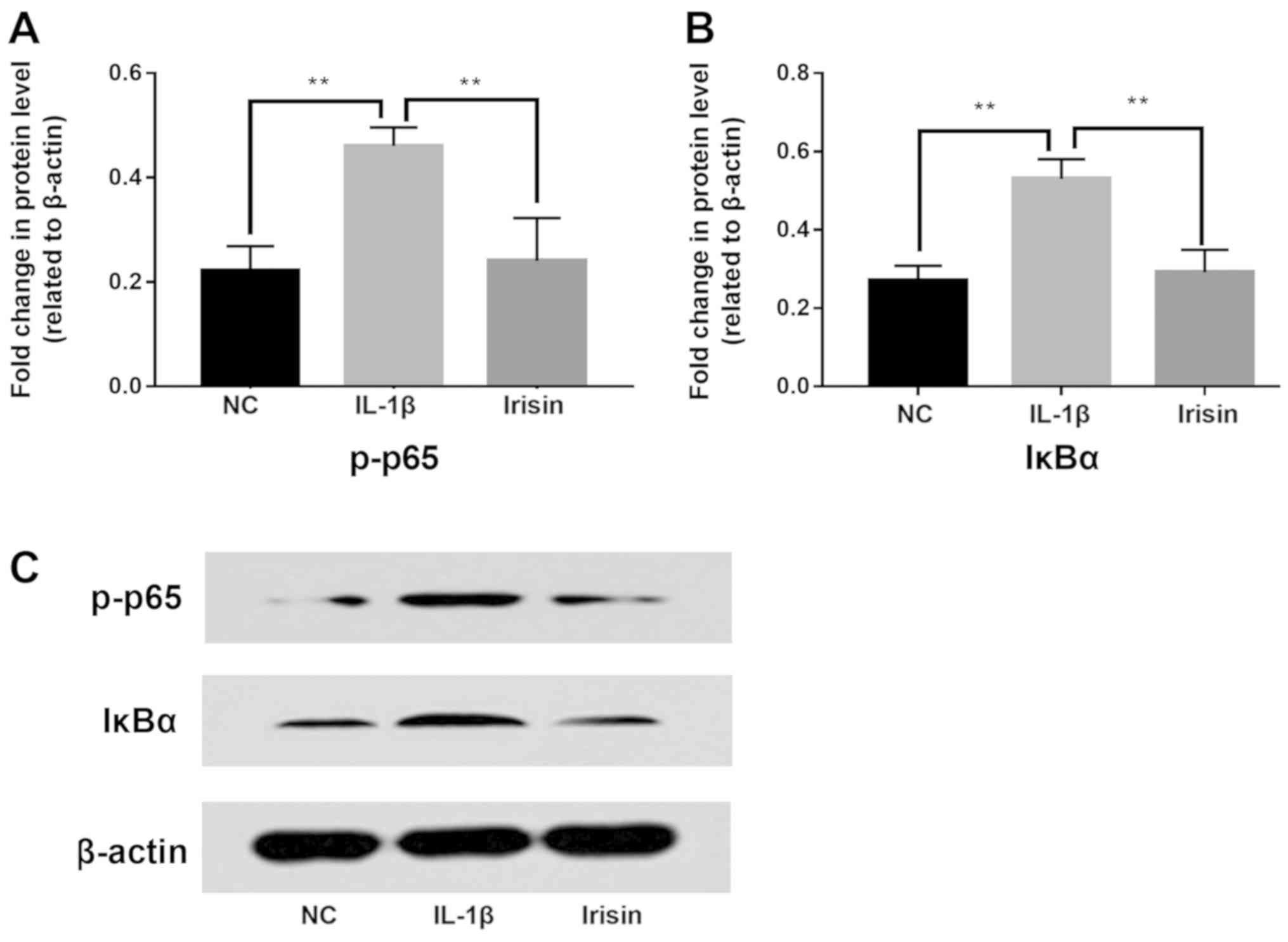
Effect of irisin on IL-1β-induced
activation of NF-κB signaling in SW1353 cells
To investigate the effect of irisin on the NF-κB
signaling pathway in IL-1β-induced SW1353 cells, the changes in
IκBα and phosphorylated-p65 (p-p65) were detected by western blot
analysis (Fig. 6C). Statistical
analysis showed that the expression levels of p-p65 (Fig. 6A) and IκBα (Fig. 6B) were significantly increased by
IL-1β, but were significantly decreased after irisin treatment
compared with the IL-1β group. Therefore, the present results
indicated that irisin could inhibit the level of cytoplasmic p-p65
and downregulate the activity of the NF-κB pathway.
Discussion
Different types of SW1353 cells have been applied in
cell models of OA. Huang et al (14) utilized and treated SW1353 cells with
IL-1β to imitate the microenvironment of OA for in vitro
experiment. Feng et al (15)
applied human SW1353 chondrocytes to evaluate the effect of salicin
in OA. Lu et al (16)
investigated the chondroprotective role of sesamol by
downregulating MMP expression via retention of the NF-κB signaling
pathway in activated SW1353 cells. In addition, Tetsunaga et
al (17) analyzed the effect of
runt-related transcription factor 2 on the mechanical
stress-induced MMP-13 and A disintegrin and metalloproteinase with
thrombospondin motifs 5 expression in SW1353 chondrocyte-like
cells. Cheng et al (18)
successfully established a cellular model of OA by stimulating
SW1353 cells with IL-1β. All these previous studies have shown that
SW1353 cells are a feasible cell line with which to establish OA
models.
The present study used RT-qPCR, western blotting and
immunofluorescence analysis to investigate whether irisin can
protect against OA induced by IL-1β in SW1353 cells. Previous
studies have confirmed that IL-1β plays an important role in the
development of OA and can stimulate chondrocytes to produce OA
(19,20). The present study performed CCK-8
analysis to identify the optimum concentration of IL-1β for
modeling OA. Cartilage degradation in OA is mediated by the MMP
family (21). MMP-13, as a member of
the MMP family, can degrade collagen and reduce cartilage
composition (22). High levels of
MMPs are considered to be one of the main characteristics of OA
(22,23), which can combine with low levels of
chondrocyte-specific proteins, such as Sox9 and collagen II
(24), leading to cartilage
degeneration. Therefore, it is important to regulate the expression
levels of MMPs to facilitate the prevention and treatment of OA.
The present results indicated that five different concentrations of
irisin did not affect the viability of SW1353 cells. However,
treatment with 20 mM irisin significantly reduced the expression
levels of MMP-13 in SW1353 cells. In addition, irisin treatment
increased the expression level of Col-II. The present results
suggested that irisin exerted its protective effects by decreasing
the expression level of MMP-13 and increasing the expression level
of Col-II in IL-1β-induced SW1353 cells.
Previous study has shown that exercise causes
production of skeletal muscle and release of irisin into the blood,
which has beneficial effects in increasing bone mass, lowering body
mass index, increasing insulin sensitivity and releasing total body
energy (25). Colaianni et al
(25) found that, compared with a
restricted exercise group, the expression of irisin in mice after 3
weeks of treadmill exercise was increased significantly at both the
mRNA and protein levels. A previous study showed that irisin can
directly act on bone stromal cells and enhance osteogenic
differentiation (25). A recent
study also indicated that irisin activates the Wnt/β-catenin
signaling pathway in pre-osteoblasts to promote osteoblast
differentiation (9). A clinical
investigation into OA showed that irisin expression levels in
circulating and synovial fluids, and CRP expression levels in
circulating fluid are closely related to the severity of OA
(26), suggesting that irisin is
associated with OA. Cartilage degeneration is an important part of
the pathogenesis of OA (26). To the
best of our knowledge, the present study is the first to
investigate the direct effects of irisin on IL-1β-induced SW1353
cells.
Studies have shown that several signaling pathways
regulating joint formation and homeostasis are key factors in the
pathogenesis of OA (26). The NF-кB
and Wnt/β-catenin signaling pathways are two major pathways
involved in OA development (26).
Wnt protein is a promoter of the Wnt/β-catenin signaling pathway
(26). β-catenin is a key factor in
the Wnt/β-catenin signaling pathway, which is encoded by the
catenin β-1 gene (26). The
expression level of β-catenin in the nucleus directly reflects the
activation level of this signaling pathway (26). Under normal conditions, β-catenin
remains stable in the cytoplasm. In the absence of Wnt protein
promoter stimulation, β-catenin forms a complex with axis
inhibitor, adenomatous polyposis coli and glycogen synthase kinase
3 (GSK3), among others. β-catenin is then phosphorylated by casein
kinase 1 and GSK3, and the free β-catenin in the cytoplasm is
reduced, and thereby the transcription of Wnt protein target genes
in the nucleus is inhibited (27).
Thus, the β-catenin expression level remains low due to continuous
degradation. When the Wnt/β-catenin signaling pathway is activated,
β-catenin becomes stable as proteasomal degradation is reduced, and
stabilized β-catenin is then translocated into the nucleus to
upregulate various inflammation-related genes, such as those
encoding MMPs (27). Previous
studies on OA showed the interaction of irisin and Wnt/β-catenin in
osteogenesis (9); therefore, the
present study investigated this interaction in IL-1β-induced SW1353
cells. The present RT-qPCR and western blotting results identified
that irisin downregulated IL-1β-induced upregulation of Wnt-1 and
β-catenin at both protein and mRNA expression levels in SW1353
cells. Therefore, the present results suggested that irisin could
inhibit the Wnt/β-catenin signaling pathway. LiCl acts as a
classical activator of the Wnt pathway, and can regulate GSK-3β
(27). The primary role of GSK-3β is
to phosphorylate free β-catenin (27). The present results suggested that
irisin inhibited the Wnt pathway activated by LiCl, and this effect
was also identified with irisin-treated cells co-treated with IL-1β
and LiCl. Therefore, the present results suggested that irisin
regulated the Wnt/β-catenin signaling pathway by inhibiting the
phosphorylation of β-catenin.
The NF-κB pathway is another important pathway in
the OA process, and could be a potential therapeutic target for OA
(28). When the NF-κB pathway is
activated by inflammatory mediators such as IL-1β, NF-κB-p65 in the
cytoplasm, it is then translocated into the nucleus where it
upregulates multiple inflammation-related genes, such as MMPs,
cyclooxygenase-2 and prostaglandin E2(28). Exposing chondrocytes to a variety of
inflammatory cytokines leads to the degradation of IκB and further
translocation of p65 into the nucleus (29). In the present study, western blot
analysis indicated that IL-1β induced a significant increase in
IκBα and p-p65 expression levels in SW1353 cells; however, this
effect was reversed by irisin treatment. The present results
suggested that irisin may reduce p-p65 in the cytoplasm to inhibit
the NF-κB pathway, activated by inflammatory factors. Previous
studies have shown that there is some crosstalk between the
Wnt/β-catenin and NF-κB pathways (28,29).
Activation of the Wnt/β-catenin pathway can enhance β-transducing
repeat-containing protein-mediated degradation of IκB, and IκB
kinase can also inhibit the degradation of β-catenin protein
(29). However, the exact target of
irisin in mitigating the IL-1β-induced inflammatory response is
still unclear, and future studies will need investigate this in
depth.
In conclusion, the present results suggested that
irisin suppressed the activation of the Wnt/β-catenin and NF-κB
signaling pathways, and may facilitate the treatment of OA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660373).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJL, YM and QHJ were responsible for the acquisition
and interpretation of data. YL, QL and SW conducted the conception
and design of the study, drafted the article. All authors read and
approved final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krasnokutsky S, Attur M, Palmer G, Samuels
J and Abramson SB: Current concepts in the pathogenesis of
osteoarthritis. Osteoarthritis Cartilage. 16 (Suppl 3):S1–S3.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Buckland J: Osteoarthritis: Targeting
cartilage erosion in OA. Nat Rev Rheumatol. 6(64)2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lahm A, Kasch R, Mrosek E, Spank H,
Erggelet C, Esser J and Merk H: Semiquantitative analysis of ECM
molecules in the different cartilage layers in early and advanced
osteoarthritis of the knee joint. Histol Histopathol. 27:609–615.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang JH, Shih KS, Wu YW, Wang AW and Yang
CR: Histone deacetylase inhibitors increase microRNA-146a
expression and enhance negative regulation of interleukin-1β
signaling in osteoarthritis fbroblast-like synoviocytes.
Osteoarthritis Cartilage. 21:1987–1996. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Klatt AR, Klinger G, Paul-Klausch B, Kühn
G, Renno JH, Wagener R, Paulsson M, Schmidt J, Malchau G and
Wielckens K: Matrilin-3 activates the expression of
osteoarthritis-associated genes in primary human chondrocytes. FEBS
Lett. 583:3611–3617. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Boström P, Wu J, Jedrychowski MP, Korde A,
Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al: A
PGC1-a-dependent myokine that drives brown-fat-like development of
white fat and thermogenesis. Nature. 481:463–468. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Faienza MF, Brunetti G, Sanesi L,
Colaianni G, Celi M, Piacente L, D'Amato G, Schipani E, Colucci S
and Grano M: High irisin levels are associated with better glycemic
control and bone health in children with type 1 diabetes. Diab Res
Clin Pract. 141:10–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Colaianni G, Cuscito C, Mongelli T,
Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M,
Mori G, et al: The myokine irisin increases cortical bone mass.
Proc Natl Acad Sci USA. 112:12157–12162. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang J, Valverde P, Zhu X, Murray D, Wu
Y, Yu L, Jiang H, Dard MM, Huang J, Xu Z, et al: Exercise-induced
irisin in bone and systemic irisin administration reveal new
regulatory mechanisms of bone metabolism. Bone Res.
5(16056)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weng X, Lin P, Liu F, et al: Achyranthes
bidentata polysaccharides activate the Wnt/β-catenin signaling
pathway to promote chondrocyte proliferation. Int J Mol Med.
3:1045–1050. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lotz MK and Carames B: Autophagy and
cartilage homeostasis mechanisms in joint health, aging and OA. Nat
Rev Rheumatol. 7:579–587. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liacini A, Sylvester J, Li WQ, Huang W,
Dehnade F, Ahmad M and Zafarullah M: Induction of matrix
metalloproteinase-13 gene expression by TNF-alpha is mediated by
MAP kinases, AP-1, and NF-kappaB transcription factors in articular
chondrocytes. Exp Cell Res. 288:208–217. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schaefer JF, Millham ML, de Crombrugghe B
and Buckbinder L: FGF signaling antagonizes cytokine-mediated
repression of Sox9 in SW1353 chondrosarcoma cells. Osteoarthritis
Cartilage. 11:233–241. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang Y, Wan G and Tao J: C1q/TNF-related
protein-3 exerts the chondroprotective effects in IL-1β-treated
SW1353 cells by regulating the FGFR1 signaling. Biomed
Pharmacother. 85:41–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Feng G and Zhang S: Salicin inhibits
AGE-induced degradation of type II collagen and aggrecan in human
SW1353 chondrocytes: Therapeutic potential in osteoarthritis. Artif
Cells Nanomed Biotechnol. 47:1043–1049. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lu YC, Jayakumar T, Duann YF, Chou YC,
Hsieh CY, Yu SY, Sheu JR and Hsiao G: Chondroprotective role of
sesamol by inhibiting MMPs expression via retaining NF-κB signaling
in activated SW1353 cells. J Agric Food Chem. 11:4969–4978.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tetsunaga T, Nishida K, Furumatsu T,
Naruse K, Hirohata S, Yoshida A, Saito T and Ozaki T: Regulation of
mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2
transcriptional factor in SW1353 chondrocyte-like cells.
Osteoarthritis Cartilage. 19:222–232. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng NT, Meng H, Ma LF, Zhang L, Yu HM,
Wang ZZ and Guo A: Role of autophagy in the progression of
osteoarthritis: The autophagy inhibitor, 3-methyladenine,
aggravates the severity of experimental osteoarthritis. Int J Mol
Med. 39:1224–1232. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen WP, Hu ZN, Jin LB and Wu LD:
Licochalcone A inhibits MMPs and ADAMTSs via the NF-κB and
Wnt/β-catenin signaling pathways in rat chondrocytes. Cell Physiol
Biochem. 43:937–944. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Troeberg L and Nagase H: Proteases
involved in cartilage matrix degradation in osteoarthritis. Biochim
Biophys Acta. 1824:133–145. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tchetverikov I, Lohmander LS, Verzijl N,
Huizinga TW, TeKoppele JM, Hanemaaijer R and DeGroot J: MMP protein
and activity levels in synovial fluid from patients with joint
injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis.
64:694–698. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Matyas JR, Huang D, Chung M and Adams ME:
Regional quantification of cartilage type II collagen and aggrecan
messenger RNA in joints with early experimental osteoarthritis.
Arthritis Rheum. 46:1536–1543. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Colaianni G, Cuscito C, Mongelli T,
Oranger A, Mori G, Brunetti G, Colucci S, Cinti S and Grano M:
Irisin enhances osteoblast differentiation in vitro. Int J
Endocrinol. 2014(902186)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Marcu KB, Otero M, Olivotto E, Borzi RM
and Goldring MB: NF-кB signaling: Multiple angles to target OA.
Curr Drug Targets. 11:599–613. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ma B and Hottiger MO: Crosstalk between
Wnt/β-catenin and NF-кB signaling pathway during inflammation.
Front Immunol. 7(378)2016.PubMed/NCBI View Article : Google Scholar
|