Introduction
Granulomatosis with polyangiitis (GPA) is a rare,
autoimmune-mediated systemic disease that is characterized by
necrotizing and granulomatous vasculitis of small blood vessels,
including arterioles, venules and capillaries (1). The incidence of GPA is ~1/100,000 in
the United Kingdom, Germany and Norway, where GPA usually occur in
older people, but are relatively rare in children and young people
(2). Although GPA mainly affects the
upper and lower respiratory tract, kidneys and eyes, neurological
manifestations and infectious diseases have been previously
associated with GPA (3-5).
The pathogenesis of GPA is considered to involve a
combination of environmental and infectious factors on the basis of
genetic susceptibility (6,7). This condition is closely associated
with the presence of anti-neutrophil cytoplasmic antibodies (ANCAs)
in blood, including perinuclear-ANCA (pANCA) and cytoplasmic-ANCA
(cANCA) (8). However, ≤10% patients
with GPA can test negative for ANCA (9). If the histopathological results and
highly suspected clinical features can be used to confirm the
diagnosis of GPA, positive ANCA serology is not a key element for
the diagnosis of GPA (10). GPA
involves the production of ANCA against proteinase 3 (PR3) in ~80%
of the GPA cases and against myeloperoxidase (MPO) in ~10% of the
GPA cases (6). The presence of
single nucleotide polymorphisms in the HLA-DPB1 locus, with
variants rs141530233 and rs1042169 being previously reported
examples, are at higher risk of vasculitis associated with ANCA
against PR3(11). Additionally, some
drugs have been reported to serve as triggers for ANCA-associated
GPA, including cefotaxime, anti-thyroid medication, anti-tumour
necrosis factor α agents; however, cases of ANCA-associated
vasculitis induced by pharmacological agent are normally resolved
following discontinuation of the drug in question (12).
At present, the diagnostic criteria of GPA are based
on the combination of clinical manifestation, ANCA serology,
radiology and histopathology, according to the ACR/EULAR
provisional 2017(10). The severity
of ANCA associated with GPA can be divided into mild, moderate and
severe based on the involvement of other organs (13). Although cyclophosphamide and
corticosteroid combination therapy have been applied for induction
therapy in GPA, cyclophosphamide has a potential side effects such
as fertility risks and teratogenicity, limiting the duration of
therapy (14). Although other
agents, including rituximab, methotrexate, azathioprine and
leflunomide, have demonstrated therapeutic effects of varying
degrees in patients with GPA (7), no
treatment option currently exists for patients with ANCA-negative
GPA. The present report documents a rare case of ANCA-negative GPA
involving main tract stenosis (MTS), where the patient with GPA
improved following treatment with oral prednisone only. The present
case study provides a prospective therapeutic option for
ANCA-negative GPA.
Case report
In January 2019, a 54-year-old woman presented with
a history of severe cough, wheezing, shortness of breath but no
fever. Immediately, she was admitted to the Jiangyou People's
hospital (Mianyang, Sichuan), where primary CT scans indicated
asymmetrical thickening of the tracheal wall and small calcified
nodules in the right upper and middle lobes. The patient was
diagnosed with an acute respiratory disease, who subsequently
confirmed by oral communication that cephalosporin antibiotics was
applied for ~half a month; however, the symptoms were not relieved.
On April 15, 2019, she was admitted to the Department of
Respiratory and Critical Care Medicine, Huashan Hospital (Shanghai,
China). A general physical examination suggested that the
expiratory and inspiratory breathing were restricted. Neurological
manifestations, skin lesions and superficial lymphadenopathy were
not visible or accessible.
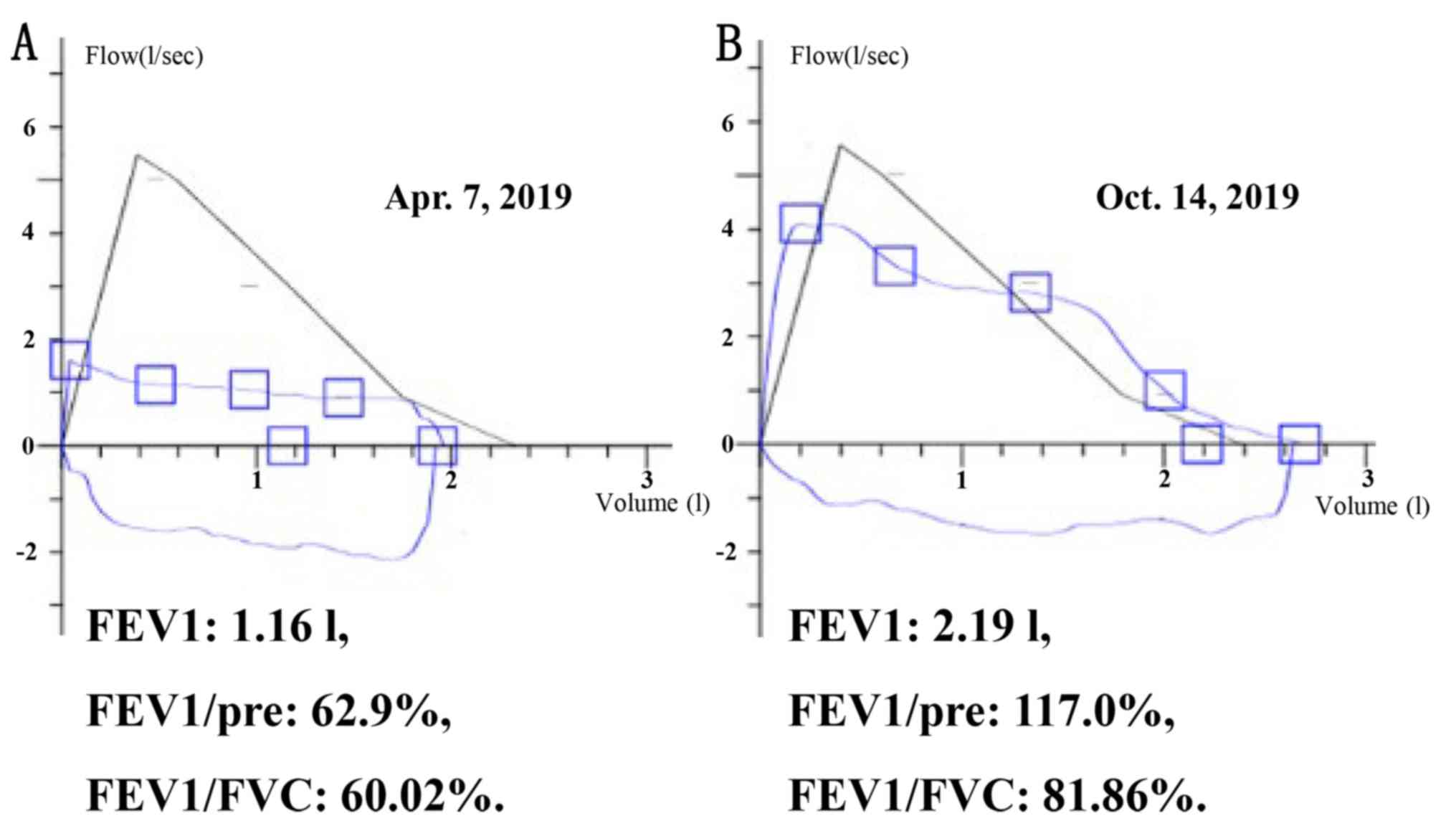
One week prior to admission to The Huashan hospital,
namely April 7, 2019, she was examined for pulmonary volume
capacity (PVC) and ventilation function (PVF) using the
MasterScreen™ PFT System (Vyaire Medical, Inc.) at the outpatient
department (Shanghai, China). PVC examination revealed the
following information: i) forced expiratory volume in 1 sec (FEV1),
1.16 l; ii) FEV1/pre-predicted value, 62.9%; iii) FEV1/forced vital
capacity (FVC), 60.02%; iv) peak expiratory flow, 1.60 l/sec; and
v) 75% of maximal expiratory flow, 1.14 l/sec (predicted value,
5.02 l/sec). PVF test showed that the top of the ascending line was
seriously low whereas the descending line plateaued above the
horizontal axis, indicating that the flow-volume loop was
insufficient. It suggested that the inhalation and exhalation of
the patient was obstructed (Fig.
1A).
On April 16, 2019, the results of blood tests were
as follows: i) Eosinophil count, 616x106 (normal range,
50-350x106 cells/l); ii) Erythrocyte sedimentation rate
(ESR), 29 mm/h (normal range: ≤20 mm/h); iii) anti-nuclear
antibodies, 1:1,000-fold (ANAs; normal range: <1:100-fold); iv)
anti-cytoplasmic reticular/mitochondrial antibodies-21, positive
(AC-21; normal range, negative); v) MPO-ANCA, 4.9 RU/ml (normal
range: 0~20 RU/ml); and vi) PR3-ANCA, 5.8 RU/ml (normal range, 0~20
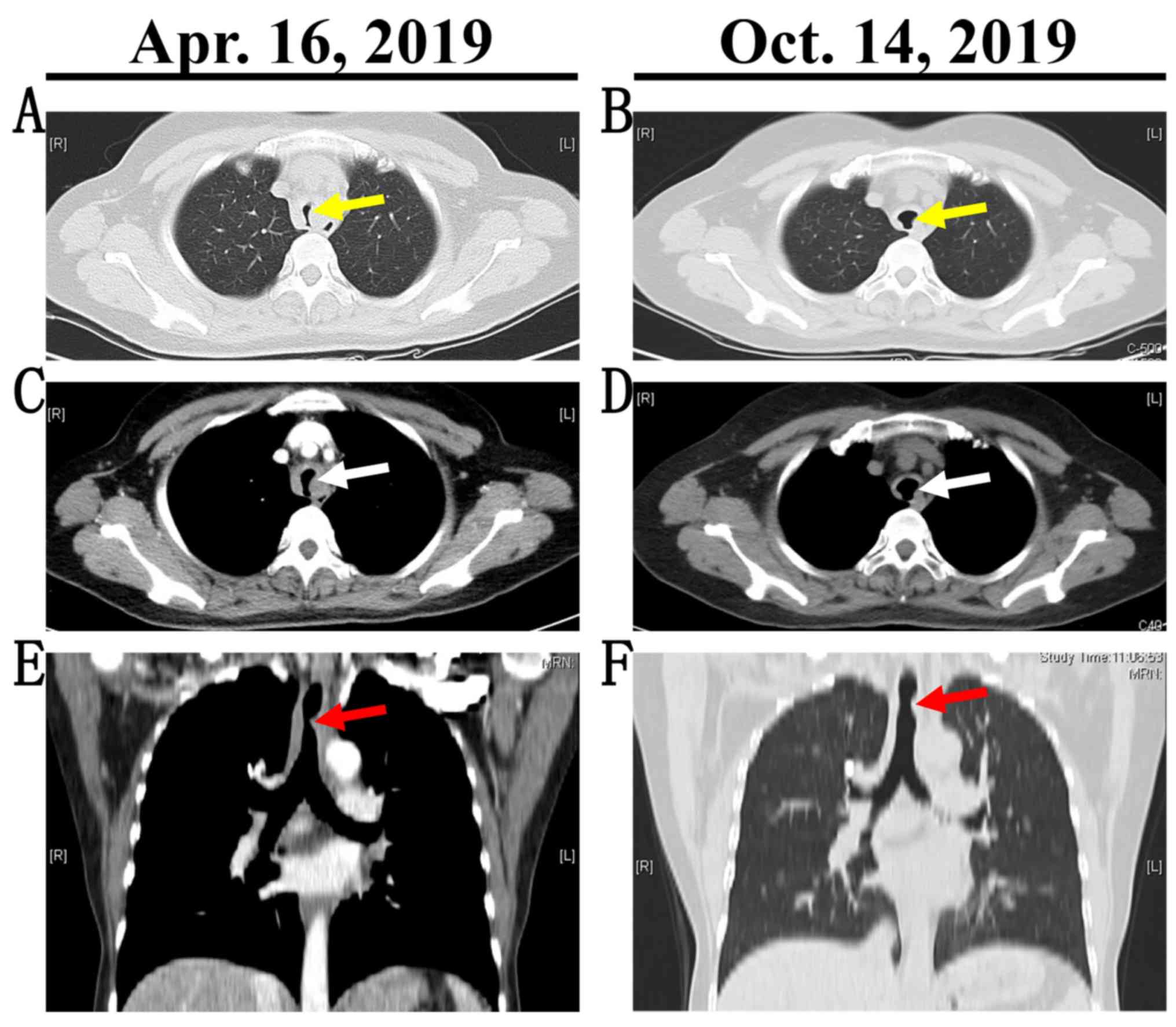
RU/ml). In addition to laboratory results, Chest CT enhanced and
three-dimensional reconstruction revealed stenosis of the main
trachea due to the uneven thickening of the tracheal wall (Fig. 2A, C
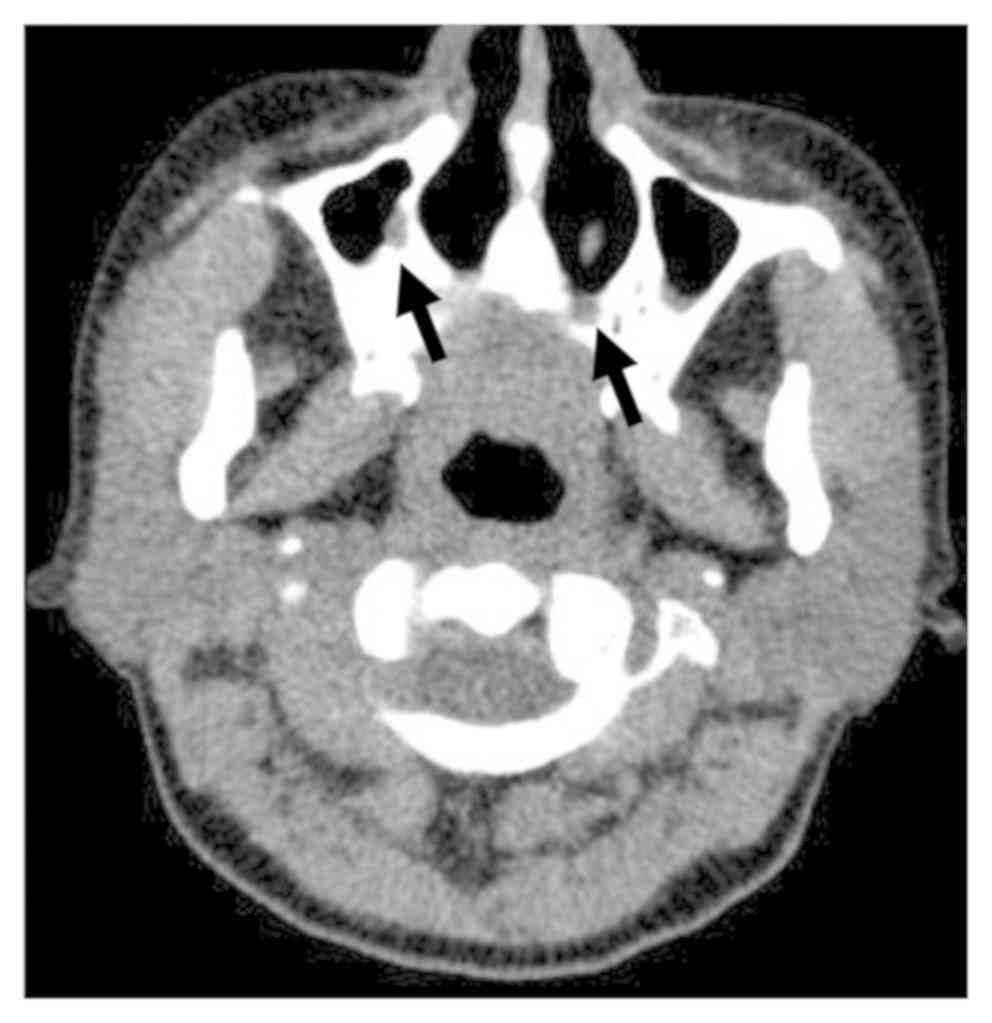
E). Sinus CT presented signs of
mucosal thickening in the maxillary sinusitis (Fig. 3).
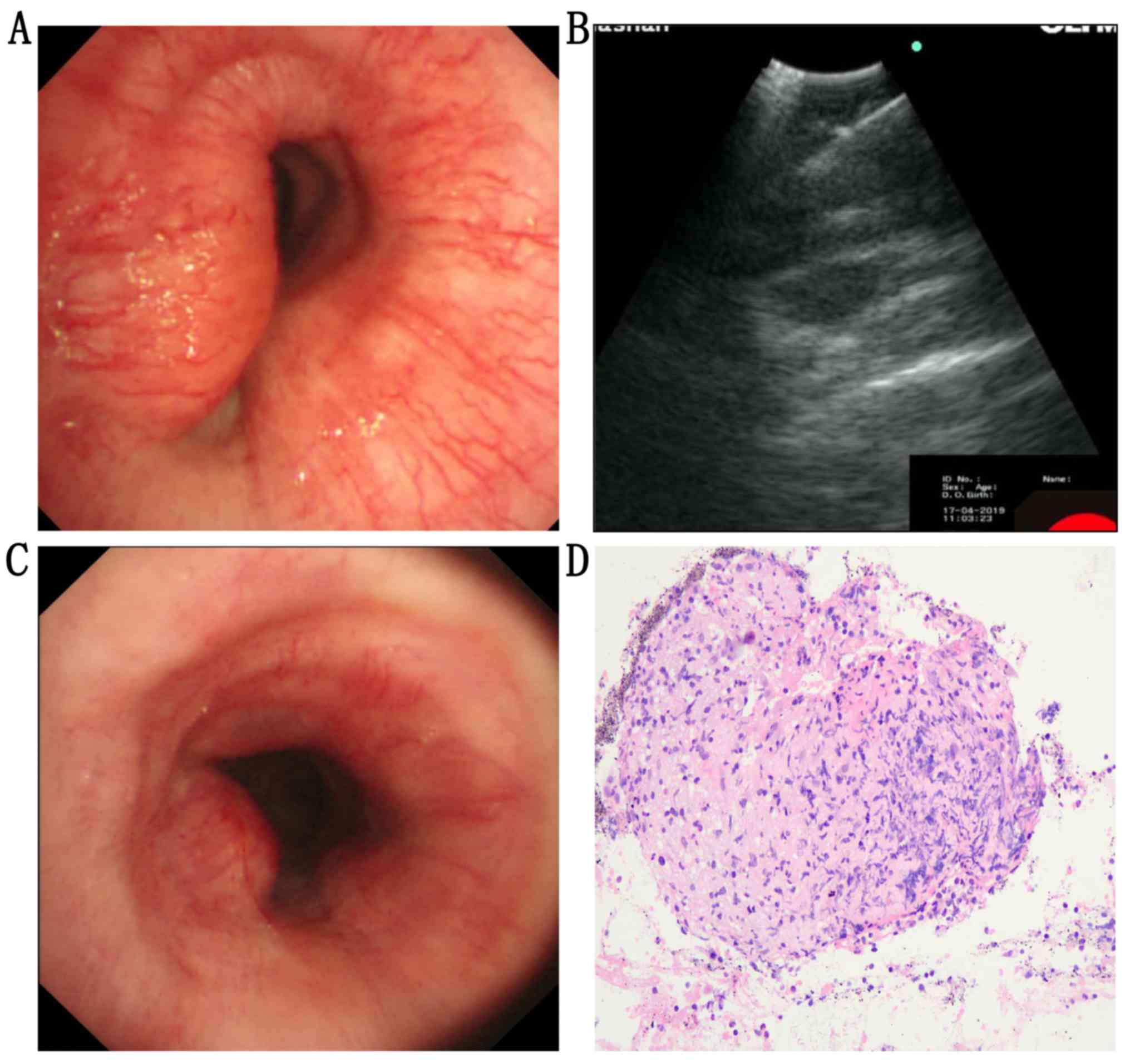
On April 17, 2019, endoscopic examination (EVIS
LUCERA CV-260SL; Olympus Corporation) indicated that the lumen of
the main trachea was significantly obstructed, with the absence of
bleeding on the surface (Fig. 4A).
An irregular low echo lesion was revealed by visualization using
endobronchial ultrasound (Fig. 4B).
We collected the lesion and performed immunohistochemistry (IHC).
The process was as follows: i) fixing with 10% neutral formalin for
>6 h at room temperature; ii) paraffin-embedded lesion was cut
into 3 mm slices; iii) blocking with 10% normal goat serum (Fuzhou
Maixin Biotech Co., Ltd.) for 10 min at room temperature; iv)
incubation with primary antibodies targeted against leukocyte
common antigen (LCA), vimentin (VIM), cytokeratin (CK),
chromogranin A (CgA), synaptophysin (Syn), thyroid transcription
factor-1 (TTF-1), Wilms tumor 1 (WT-1), tumor protein 63 (P63),
napsin A aspartic peptidase (NapsinA), tumor protein 40 (P40) at
37˚C for 1 h; v) After washing, incubating with horseradish
peroxidase (HRP) labeled secondary antibodies (Dako; Agilent
Technologies, Inc) at 37˚C for 30 min; vi) enhancing the signal
using a DAB kit (cat. no. dab-2032; Fuzhou Maixin Biotech Co.,
Ltd.); vii) counterstaining with hematoxylin for 2 min at room
temperature; viii) photographing under a light microscope
(magnification, x200; 55I/S-F1; Nikon Corporation). Sources and
instructions of antibodies above were listed in Table SI.
In addition, on April 17, 2019, the biopsy performed
using transbronchial needle aspiration (NA-201SX-4021-21 G; Olympus
Corporation) demonstrated chronic necrotizing granulomatous
inflammation (Fig. 4D). The results
of the IHC analysis were as follows: i) LCA, +; ii) VIM, +; iii)
CK, -; iv) CgA, -; v) Syn, -; vi) TTF-1, -; vii) WT-1, -; viii)
P63, -; ix) NapsinA, -; x) P40, -; xi) Ziehl-Neelsen staining, -;
and xii) periodic acid-Schiff staining, - (Fig. S1). According to the classification
criteria of ACR/EULAR provisional 2017(10), the patient scored 5 points, including
cartilaginous involvement (2 scores) and granuloma on biopsy (3
scores). The diagnosis of MTS caused by GPA was established.
Finally, the patient was treated with prednisone (5 mg/tablet). The
starting dose was 25 mg per day (0.5 mg/kg, orally), which was then
reduced to 20 mg per day after 2 months, followed by a reduction by
one tablet every two months.
After six months, the pulmonary function indicated
improved conditions: FEV1, 2.19 l; FEV1/pre, 117.0%; and FEV1/FVC,
81.86% (Fig. 1B). The chest CT scan
indicated that the narrow tract was relieved (Fig. 2B, D and F). The obstruction of main
trachea was significantly improved (Fig.
4C). The laboratory results were as follows: ESR, 13 mm/h
(normal range: ≤20 mm/h); ANAs, 1:320-fold (normal range,
<1:100-fold).
Discussion
The current case presents a patient of local
GPA-induced MTS with negative-ANCAs. The patient improved
significantly after the initial treatment of oral prednisone. It
has been reported that GPA involves the nasal, ears, oropharynx,
trachea and bronchi (15), where the
ANCA serology in patients with GPA are mostly positive (15,16).
However, if ANCAs is tested negative, the upper and lower
respiratory disease may be misdiagnosed due to the atypical
manifestation in GPA, (17).
Histopathological analysis serves an imperative role in the
diagnosis of GPA (8). The
involvement of the main airway could cause inspiratory and
expiratory changes. The flow-volume tracings from the current
patient indicated a fixed main airway obstruction. In addition, the
FEV1/FVC was decreased markedly. Positive correlation was observed
between the degree of airway obstruction and the platform change of
the respiratory phase (18).
Furthermore, since bronchoscopy visualized the main tract to be
compressed externally, the possibility of bronchogenic tumor could
not be ruled out (4). This was
initially suspected to be a neoplasm, but the appearance of the
lesion by endoscopic examination and biomarkers were contrary to
the expected diagnosis. A panel of tumor-related
immunohistochemical markers were also demonstrated to be negative.
The pathological results revealed chronic necrotizing granulomatous
inflammation (16).
GPA is an agnogenic, autoimmune-mediated systemic
condition, where airway ailments as a result of GPA is a benign
disease that is more prevalent in older adults (8). Currently, the standard treatment is a
combination of corticosteroids with cyclophosphamide in the
induction period, followed by lower doses of corticosteroids in
combination with azathioprine or methotrexate in the maintenance
period (14). However, a
population-based cohort study of 195 patients observed a persistent
increased risk for overall malignancies after the usage of
cyclophosphamide (19). Clinical
trials and observational studies have reached an agreement that
glucocorticoids are a fundamental drug for the standard induction
therapies in patients with GPA (20). A patient diagnosed with GPA
manifesting as bronchial stenosis with MPO-ANCA (+) demonstrated a
distinct reduction in a rash and improvement in arthralgia and
systemic malaise following treatment with prednisolone (21). Immunosuppressive therapies have
prolonged 5-year survival to 70-80%, but recurrence occur in ~50%
patients within 1.5 to 15 years (18,20).
Persistent positive-ANCAs (22), CD27-CD38hi B-cell frequency (23) and non-standardized glucocorticoid
therapy are key risk factors for a poor prognosis. Although
endobronchial involvement may improve symptoms, further studies are
required to investigate whether the incomplete excision of the
lesion can cause a relapse of the existing granulomata development
(24). Currently, targeting
molecules, including B-cell activating factor (25), C5a receptor (26) and interleukin-6(27), have been identified as potential
candidates for therapies in treating GPA and to reduce the
glucocorticoid-related drug reaction. However, further study is
required to optimize the treatment individually. In the present
case, although ANCAs serology was tested to be negative, GPA should
also be considered as a differential diagnosis.
Supplementary Material
Figure S1. Images of
immunohistochemistry. LCA and VIM were positive. Biomarkers
including CK, CgA, Syn, TTF.1, WT.1, P63, NapsinA and P40 were
negative. Both ZN and PAS staining were negative. Magnification,
x200. LCA, leukocyte common antigen; VIM, vimentin; CK,
cytokeratin; CgA, chromogranin A; Syn, synaptophysin; TTF.1,
thyroid transcription factor.1; WT.1, Wilms tumor 1; P63, tumor
protein 63; NapsinA, napsin A aspartic peptidase; P40, tumor
protein 40; ZN, Ziehl.Neelsen; PAS, periodic acid.Schiff.
Table SI. Antibody information.
Acknowledgements
Not applicable.
Funding
This study was supported by the Youth Project of
Shanghai Municipal Health Commission (grant no. 20164Y0139).
Availability of data and materials
The data collected and analyzed during this study
are included in this published article.
Authors' contributions
DBZ conceived and led the preparation of the
manuscript. PZ conceived and collected the data.; YYZ drafted the
manuscript. YZZ completed the bronchoscopy and collected biopsies,
as well as making the pathological sections. YYZ issued the lung
function report and drafted the manuscript. XJZ organized and
analyzed the figures and pathological sections.JL performed the
pathological photography, the literature search and discussed the
manuscript. ZWZ prepared the immunohistochemical analysis and
revised the manuscript. NZ designed the study, edited and revised
the manuscript. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Informed consent for all data and clinical history
was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lynch JP III, Derhovanessian A, Tazelaar H
and Belperio JA: Granulomatosis with polyangiitis (wegener's
granulomatosis): Evolving concepts in treatment. Semin Respir Crit
Care Med. 39:434–458. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lane SE, Watts R and Scott DG:
Epidemiology of systemic vasculitis. Curr Rheumatol Rep. 7:270–275.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Seo P and Stone JH: The antineutrophil
cytoplasmic antibody-associated vasculitides. Am J Med. 117:39–50.
2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Iijima Y, Kobayashi Y, Uchida Y, Tsutsui
T, Kakizaki Y, Naganuma T, Tsukamoto K, Oyama T and Miyashita Y: A
case report of granulomatous polyangiitis complicated by
tuberculous lymphadenitis. Medicine (Baltimore).
97(e12430)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Emamikhah M, Sina F, Mokhtari M, Shirani F
and Asadipanah M: Wegener's granulomatosis presenting as wallenberg
syndrome: A case report. J Stroke Cerebrovas Dis.
28(e107-e109)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cartin-Ceba R, Peikert T and Specks U:
Pathogenesis of ANCA-associated vasculitis. Curr Rheumatol Rep.
14:481–493. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lutalo PM and D'Cruz DP: Diagnosis and
classification of granulomatosis with polyangiitis (aka Wegener's
granulomatosis). J Autoimmun. 48-49:94–98. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rao JK, Allen NB, Feussner JR and
Weinberger M: A prospective study of antineutrophil cytoplasmic
antibody (c-ANCA) and clinical criteria in diagnosing Wegener's
granulomatosis. Lancet. 346:926–931. 1995.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bonaci-Nikolic B, Andrejevic S, Bukilica M
and Nikolic MM: Clinical and prognostic value of antineutrophil
cytoplasmic antibodies in Wegener's granulomatosis and microscopic
polyangiitis: Comment on the article by Russell et al.
Arthritis Rheum. 46:278–280. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sharma A, MB A, Naidu S, Rathi M, Pinto B,
Dhir V, Verma R, Sharma K, Nada R, Jain S, et al: Validation of the
ACR EULAR provisional 2017 classification criteria of
granulomatosis with polyangiitis (GPA) amongst patients with ANCA
associated vasculitis [abstract]. Arthritis Rheumato. 69
(Suppl):2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Merkel PA, Xie G, Monach PA, Ji X,
Ciavatta DJ, Byun J, Pinder BD, Zhao A, Zhang J, Tadesse Y, et al:
Identification of functional and expression polymorphisms
associated with risk for antineutrophil cytoplasmic
autoantibody-associated vasculitis. Arthritis Rheum. 69:1054–1066.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gao Y and Zhao MH: Review article:
Drug-induced anti-neutrophil cytoplasmic antibody-associated
vasculitis. Nephrol (Carlton). 14:33–41. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mahr AD, Neogi T, Lavalley MP, Davis JC,
Hoffman GS, McCune WJ, Specks U, Spiera RF, St Clair EW, Stone JH,
et al: Assessment of the item selection and weighting in the
Birmingham vasculitis activity score for Wegener's granulomatosis.
Arthritis Rheum. 59:884–891. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mukhtyar C, Guillevin L, Cid MC, Dasgupta
B, de Groot K, Gross W, Hauser T, Hellmich B, Jayne D, Kallenberg
CG, et al: EULAR recommendations for the management of primary
small and medium vessel vasculitis. Ann Rheum Dis. 68:310–317.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Polychronopoulos VS, Prakash UB, Golbin
JM, Edell ES and Specks U: Airway involvement in Wegener's
granulomatosis. Rheum Dis Clin North Am. 33:755–775.
2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Almouhawis HA, Leao JC, Fedele S and
Porter SR: Wegener's granulomatosis: A review of clinical features
and an update in diagnosis and treatment. J Oral Pathol Med.
42:507–516. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cadoni G, Prelajade D, Campobasso E, Calŏ
L, Agostino S, Manna R and Paludetti G: Wegener's granulomatosis: A
challenging disease for otorhinolaryngologists. Acta Otolaryngol.
125:1105–1110. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Semple D, Keogh J, Forni L and Venn R:
Clinical review: Vasculitis on the intensive care unit-part 2:
Treatment and prognosis. Crit Care. 9:193–197. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Heijl C, Westman K, Höglund P and Mohammad
AJ: Malignancies in patients with ANCA-associated vasculitis-A
population based cohort study. J Rheumatol.
pii(jrheum.181438)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nagasaka K, Harigai M, Hagino N, Hara A,
Horita T, Hayashi T, Itabashi M, Ito S, Katsumata Y, Kawashima S,
et al: Systematic review and meta-analysis for 2017 clinical
practice guidelines of the Japan research committee of the ministry
of health, labour, and welfare for intractable vasculitis for the
management of ANCA-associated vasculitis. Mod Rheumatol.
29:119–129. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peters JE, Salama AD and Ind PW: Wegener's
granulomatosis presenting as acute systemic vasculitis following 20
years of limited tracheobronchial disease. J Laryngol Otol.
123:1375–1377. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Heijl C, Mohammad AJ, Westman K and
Hoglund P: Long-term patient survival in a Swedish population-based
cohort of patients with ANCA-associated vasculitis. RMD Open.
3(e000435)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
von Borstel A, Land J, Abdulahad WH,
Rutgers A, Stegeman CA, Diepstra A, Heeringa P and Sanders JS:
CD27(+)CD38(hi) B cell frequency during remission predicts
relapsing disease in granulomatosis with polyangiitis patients.
Front Immunol. 10(2221)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cosano Povedano A, Munoz Cabrera L, Cosano
Povedano FJ, Rubio Sanchez J, Pascual Martinez N and Escribano
Duenas A: Endoscopic treatment of central airway stenosis: Five
years' experience. Arch Bronconeumol(Spanish). 41:322–327.
2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
McClure M, Gopaluni S, Jayne D and Jones
R: B cell therapy in ANCA-associated vasculitis: Current and
emerging treatment options. Nat Rev Rheumatol. 14:580–591.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bekker P, Dairaghi D, Seitz L, Leleti M,
Wang Y, Ertl L, Baumgart T, Shugarts S, Lohr L, Dang T, et al:
Correction: Characterization of pharmacologic and pharmacokinetic
properties of CCX168, a potent and selective orally administered
complement 5a receptor inhibitor, based on preclinical evaluation
and randomized phase 1 clinical study. PLoS One.
14(e0210593)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Berti A, Cavalli G, Campochiaro C,
Guglielmi B, Baldissera E, Cappio S, Sabbadini MG, Doglioni C and
Dagna L: Interleukin-6 in ANCA-associated vasculitis: Rationale for
successful treatment with tocilizumab. Semin Arthritis Rheum.
45:48–54. 2015.PubMed/NCBI View Article : Google Scholar
|