Introduction
Stroke, which is also known as cerebral infarction,
is the sudden deprivation of blood supply in the brain (1). In addition to having 6.1% mortality,
stroke is also associated with a high risk of disability and
recurrence around the world, accounting for 3% of the total health
expenditure (2-4).
This condition is mainly divided into two categories, ischemic
stroke and hemorrhagic stroke, of which the former is the most
prevalent, accounting for ~80% of total cases (5). Common risk factors for this disease
include high blood pressure, smoking, hyperlipidemia, obesity,
diabetes, coronary heart disease and a family history of stroke
(6). Among these factors, carotid
stenosis and occlusion as a result of atherosclerosis are main risk
factors for stroke (7).
In recent years, studies have demonstrated that
microRNAs (miRs/miRNAs) serve important roles in a number of
diseases, including vascular disease, metabolic disease and cancer
(8,9). Recent studies showed that miRNAs can
potentially serve as novel biomarkers and therapeutic targets for a
variety of pathological conditions (5). miRNAs have a wide range of biological
functions and remain stable in bodily fluids including blood, urine
and amniotic fluid (10,11). Previous reports found significant
differences in the expression of several miRNAs, including miR-30a,
miR-126 and Let-7b, in the peripheral blood of patients with stroke
at different time points (12,13). It
was previously reported that serum miR-21 and miR-221 levels in
addition to the peripheral blood levels of miR-210, miR-200c-3p,
miR-99b-5p and miR-150-5p could be used as biomarkers for stroke
(14,15).
The neuroprotective role of miR-135b in SH-SY5Y
cells and stress-enhanced memory has been previously demonstrated
(16,17). In Parkinson's disease (PD), the
protective role of miR-135b is achieved by targeting GSK-3β in
SH-SY5Y cells intoxicated with 1-methyl-4-phenyl-pyridinium
(16). Additionally, overexpression
of miR-135b-5p in the basolateral amygdala complex of
stress-resilient animals increased remote fear memory expression
and contributed to spontaneous renewal 14 days after death
(17). These observations suggested
the potentially important role of miR-135b in cerebral neurons.
However, the effect of miR-135b on acute ischemic stroke (AIS)
remains to be fully elucidated.
Therefore, the present study aimed to measure the
expression of miR-135b in the peripheral blood of patients with AIS
and to analyze the relationship between miR-135b expression and
disease etiology and severity to assess the occurrence of miR-135b
in AIS.
Materials and methods
General information
A total of 76 patients with AIS who were treated at
Southwest Hospital (Chongqing, China) between January 2018 and
April 2018 were selected as the case group (IS group) based on AIS
guidelines (18). The inclusion
criteria were as follows: i) Brain CT/MRI examination revealing
ischemic lesions; ii) numbness or weakness present in one limb or
face and speech difficulty; and iii) sudden onset of stroke, which
necessitated emergency admission to the Southwest Hospital. The
exclusion criteria were as follows: i) Hemorrhagic stroke, brain
trauma, hypertensive encephalopathy and encephalitis; ii) a
previous history of stroke and head trauma surgery; iii) other
diseases in the nervous and vascular system, such as meningitis,
myelitis, epilepsy and dementia; and iv) malignant brain tumors or
multiple sclerosis. According to the The Trial of Org 10172 in
Acute Stroke Treatment (TOAST) classification etiology, the 76
patients in the IS group were divided further into 33 cases of
aorta atheromatous plague, 19 cases of cardioembolism, 16 cases of
small arterial occlusion and 8 cases with unknown causes. In
addition, 60 healthy subjects in Southwest Hospital were selected
as the control group within the same time period. Informed and
signed consent was obtained from both groups of subjects and their
families. The present study was approved by the Ethics Committee of
Southwest Hospital (approval no. SH-20160986).
In the IS group, 5 ml peripheral blood was collected
on the day of admission and 14 days after admission, whilst 5 ml
peripheral blood was obtained from the control group (NC group) on
the day of physical examination. Subsequently, the serum samples
were kept at -80˚C until further use. Sex, age, blood glucose,
blood pressure and total biochemical indicators, including total
cholesterol, glycerol, platelet count and blood zymogen
time/international normalized ratio (INR), were routinely recorded
at 8:00 a.m. every day for 14 days. The severity of disease
affliction was evaluated according to the National Institutes of
Health Stroke Scale (NIHSS) (19) by
a professional physician. The score ranged from 0 to 42, with
higher scores positively associated with the severity of
neurological damage.
Serum total RNA extraction
Extraction of total RNA from serum was performed via
enrichment using an RNeasy Micro kit (cat. no. 74004; Qiagen GmbH)
according to the kit instructions. RNA concentration and purity
were determined using a BioPhotometer™ Plus nucleic acid protein
analyzer (Eppendorf) where samples with a total RNA absorbance of
1.8-2.0 were used for subsequent experiments. A total of 10 µl RNA
was used to assess RNA quality by denaturing agarose gel
electrophoresis. RNA samples were stored at -80˚C.
Reverse transcription
(RT)-quantitative PCR (RT-qPCR)
The RT reaction for mature miR-135b was performed
using a PrimeScript RT reagent kit (Takara Bio, Inc.) based on the
manufacturer's protocols. RT products were stored at -20˚C. The
subsequent qPCR mix was made up to 20 µl with the following:
fluorescent quantitative probe reaction mixture (10 µl) (2X
ProbeMixture), 1 µl template cDNA, 1 µl 20X miRNA probe, 1 µl
amplification primer premix and 7 µl RNase-free ddH2O.
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95˚C for 10 min, followed by 45 cycles of
95˚C for 15 sec and 60˚C for 1 min. U6 was used as external
reference for the standardization of extraction and reverse
transcription. Relative mRNA expression was normalized to U6 using
the 2-∆∆Cq method (20). The following primer pairs were used
for the PCR: U6, forward, 5'-GCTTCGGCAGCACATATACTAAAAT-3' and
reverse, 5'-CGCTTCACGAATTTGCGTGTCAT-3'; miR-135b-5p forward,
5'-GGTATGGCTTTTCATTCCT-3' and reverse,
5'-CAGTGCGTGTCGTGGAGT-3'.
Cell culture
PC12 cells were purchased from American Type Culture
Collection and cultured as described previously (21).
Transient transfection
miR-135b mimic (5'-UAUGGC UUUUCAUUCCUAUGUGA-3'),
mimic control (5'-UAU AUCG UGUUAUUAGCGUUCCU-3') (both from Shanghai
GenePharma Co., Ltd.), inhibitor (5'-UCACAUAGG AAUGAAAAGCCAUA-3')
or inhibitor control (5'-UUCAUC GUGUUAUUAGCGUUCCU-3') was
transfected into PC12 cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. In brief, PC12 cells were cultured at a
density of 1x106 cells/well and miR-135 mimic, mimic
control, inhibitor or inhibitor control were transfected after 24 h
at a final concentration of 20 nM. At 48 h after cell transfection,
transfection efficiency was detected using RT-qPCR.
Dual luciferase assay
TargetScan (http://www.targetscan.org/vert_72/) analysis
identified a conserved binding site for miR-135b in the
3'-untranslated region (3'-UTR) of transient receptor potential
cation channel subfamily C member 6 (TRPC6). The target open
reading frame from the genomic DNA of PC12 cells was then cloned
into a pmirGLO plasmid (Promega Corporation). PC12 cells were
cultured at a density of 1x106 cells/well in a six-well
plate. Subsequently, the miR-135b mimic + pmirGLO-TRPC6-3'UTR or NC
+ pmirGLO was transfected into PC12 cells at a final concentration
of 20 nM for 48 h using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. The cells were then collected, where the relative
luciferase units were determined using a
Dual-Luciferase® reporter assay kit (Promega
Corporation) according to the manufacturer's protocols. Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Western blot analysis
PC12 cells were collected and treated with RIPA
buffer (Beijing Solarbio Science & Technology Co., Ltd.)
containing 1% (v/v) phenylmethylsulfonyl fluoride (Beijing Solarbio
Science & Technology Co., Ltd.), 0.3% (v/v) protease inhibitor
(Sigma-Aldrich; Merck KGaA) and 0.1% (v/v) phosphorylated
proteinase inhibitor (Sigma-Aldrich; Merck KGaA). Western blots
were performed as previously described (22). Membranes were incubated with primary
antibodies against TRPC6 (cat. no. ab62461; 1:1,000; Abcam) and
anti-GAPDH (cat. no. 2118; 1:5,000; Cell Signaling Technology,
Inc.) overnight at 4˚C. Membranes were subsequently incubated with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(cat. no. ZB-2301; 1:5,000; Beijing Zhongshan Jinqiao Biotechnology
Co., Ltd.) for 2 h at room temperature, followed by three washes
with TBS-Tween-20. Enhanced chemiluminescence (ECL; EMD Millipore)
was used to determine protein concentrations according to the
manufacturer's protocol. Protein signal was detected using a Super
ECL Plus kit (Nanjing KeyGen Biotech Co., Ltd.). Relative protein
expression was normalized to GAPDH. All experiments were repeated
three times. ImageJ v1.43b software (National Institutes of Health)
was used for densitometric analysis.
Statistical analysis
Data were represented as the mean ± SD. Statistical
analysis was performed on SPSS 20.0 (IBM Corp.). Two-tailed
unpaired Student's t-test was used for comparisons between two
groups. One-way ANOVA multiple comparison test followed by Tukey's
post hoc test was used for comparisons between >2 groups. Chi
square test was performed for comparing categorical variables of
the clinical data. Receiver operating characteristic (ROC) curves
was used to assess miR-135b as a diagnostic biomarker, where the
area under the curve (AUC) was calculated. Correlation between
miR-135b expression and NIHSS scores was analyzed using the Pearson
correlation coefficient (r) method; logistic regression analysis
was used to explore the risk factors affecting AIS. Kaplan-Meier
analysis followed by log-rank test was used to analyze the overall
survival rate based the levels of miR-135b in peripheral blood.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical data
No significant differences in sex, mean age,
smoking, alcohol consumption, total cholesterol and triglyceride
levels were found between the IS and control groups (Table I). Systolic blood pressure, diastolic
blood pressure and fasting blood glucose in the IS group were found
to be significantly higher compared with those in the control group
(Table I). The platelet count in the
IS group was significantly lower compared with that in the control
group, whilst the prothrombin time/international normalized ratio
(INR) was significantly higher in the IS group compared with that
in the control group (Table I). The
NIHSS score was found to be 18.82±5.14 for the IS group (Table I).
 | Table IGeneral clinical data for patients
with AIS and healthy controls. |
Table I
General clinical data for patients
with AIS and healthy controls.
| Indicator | Healthy controls
(n=60) | AIS patients
(n=76) | P-value |
|---|
| Sex
(male/female) | 41/19 | 55/21 | 0.621 |
| Average age
(years) | 53.72±11.26 | 54.68±10.98 | 0.815 |
| Smoking [n
(%)] | 27 (45.00) | 39 (51.35) | 0.464 |
| Drinking [n
(%)] | 21 (35.00) | 33 (43.42) | 0.319 |
| Systolic blood
pressure (mmHg) | 121.15±10.28 | 152.62±10.75 | <0.001 |
| Diastolic blood
pressure (mmHg) | 79.84±8.63 | 98.52±6.85 | <0.001 |
| Previous
cerebrovascular event [n (%)] | 0 | 25 (32.9) | <0.001 |
| Family
cerebrovascular event [n (%)] | 0 (0.0) | 18 (23.7) | <0.001 |
| Homocysteine level
(μmol/l) | 11.46±1.85 | 22.56±6.35 | 0.043 |
| Uric acid
(μmol/l) | 235.56±73.11 | 359.03±96.71 | 0.031 |
| FBG (mol/l) | 5.38±1.32 | 7.25±1.96 | <0.001 |
| TC (mmol/l) | 5.12±1.38 | 5.24±1.76 | 0.589 |
| TG (mmol/l) | 1.46±0.35 | 1.44±0.48 | 0.762 |
| Platelet count
(x109/l3) | 215.82±33.56 | 175.78±26.84 | <0.001 |
| INR | 1.12±0.26 | 2.63±0.72 | <0.001 |
| NIHSS score | N/A | 18.82±5.14 | N/A |
| Intravenous
thrombolytic therapy [n (%)] | 0 (0.0) | 26 (34.2) | N/A |
Expression levels of peripheral blood
miR-135b in patients with IS and healthy controls
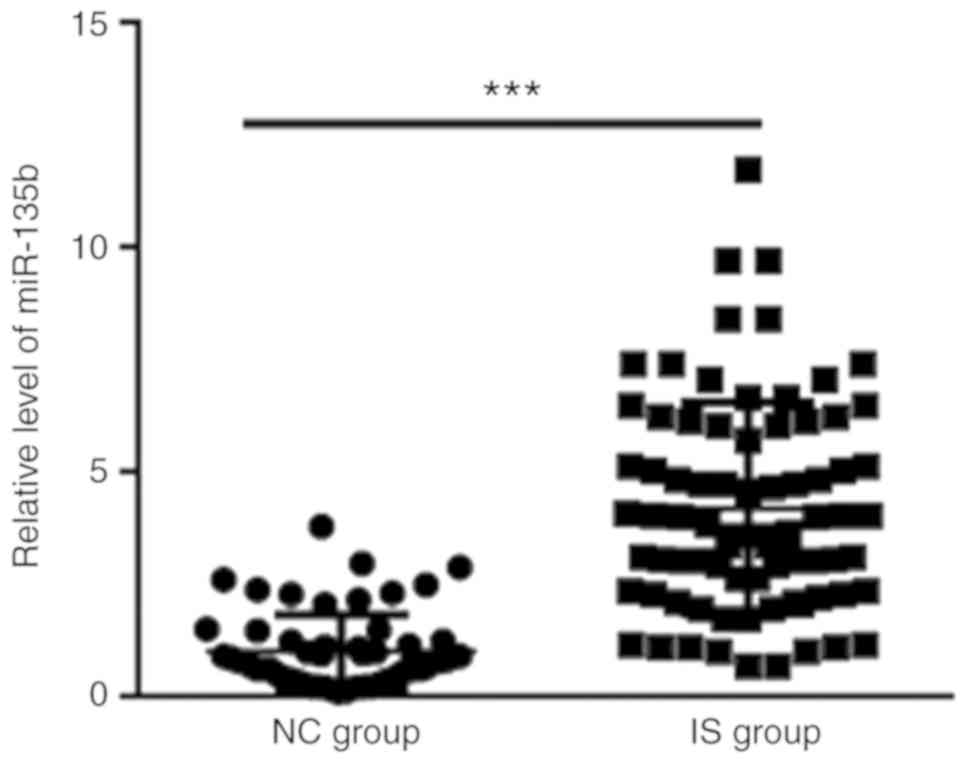
RT-qPCR was used to measure the expression levels of
miR-135b in the peripheral blood of IS and control groups. Serum
miR-135b expression levels was revealed to be significantly higher
in the IS group (4.20±2.35) compared with those in the NC group
(1.00±0.83) (Fig. 1).
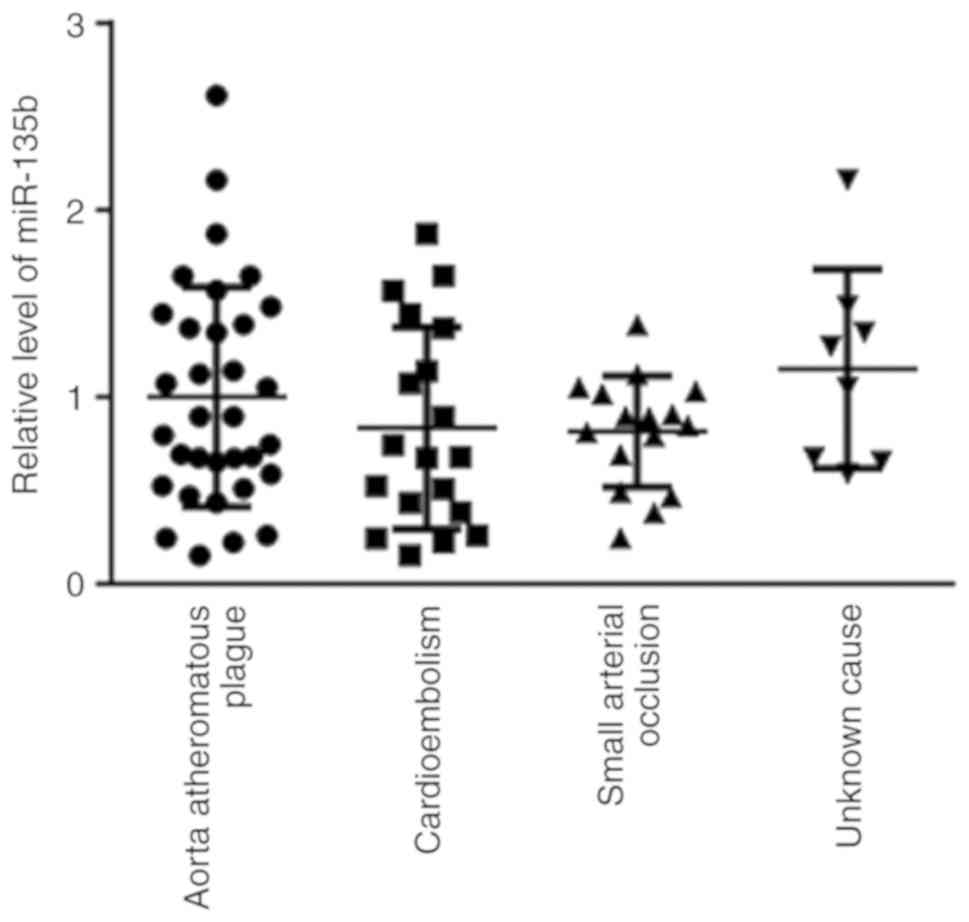
Peripheral blood miR-135b expression
levels among groups with different etiologies of AIS within the IS
group
No significant differences were found in the
expression levels of miR-135b among the 33 cases of aortic
atheromatous plague, 19 cases of cardioembolism, 16 cases of small
arterial occlusion and 8 cases with unknown causes (Fig. 2).
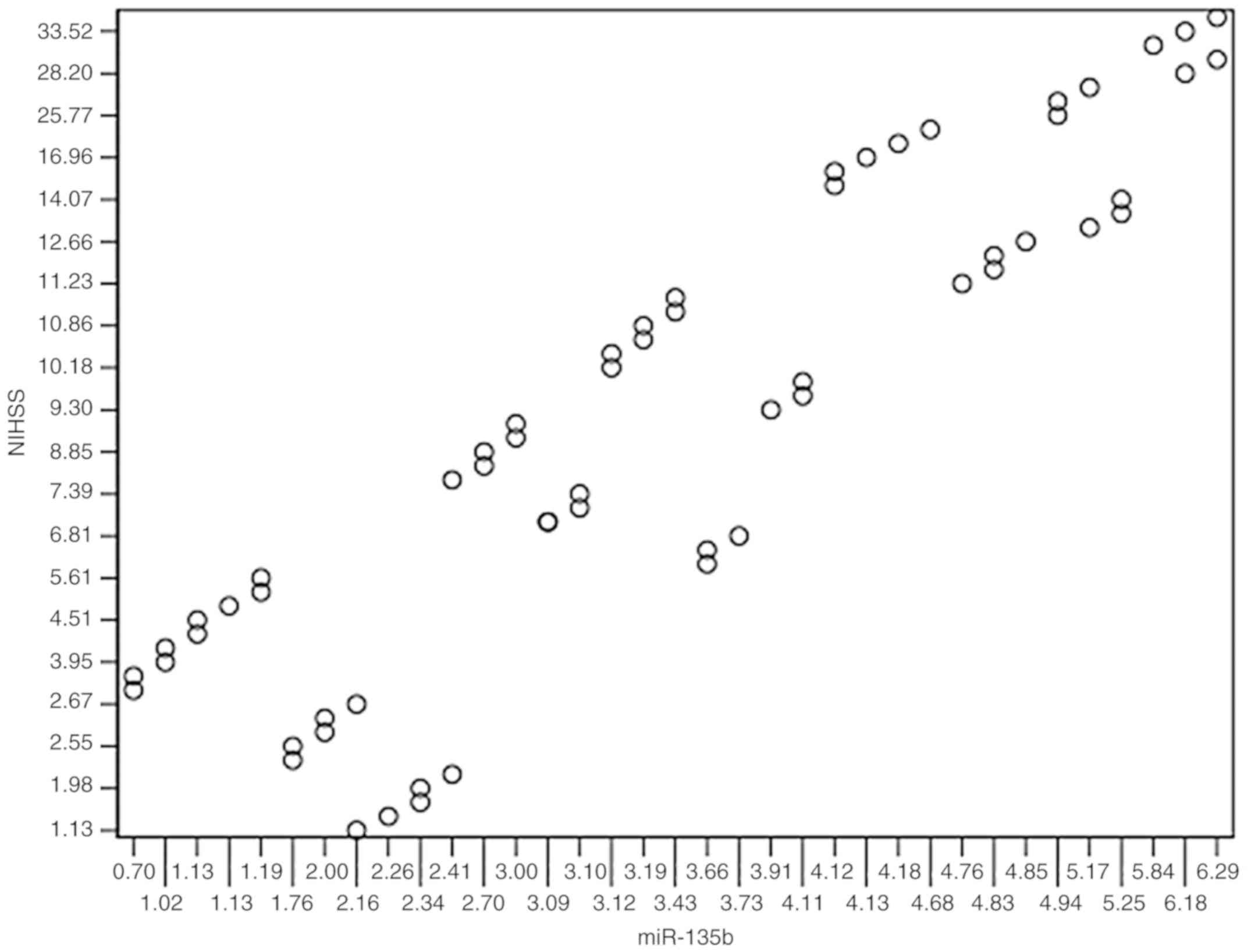
Relationship between peripheral blood
miR-135b expression levels and NIHSS score
A significant positive correlation was found between
miR-135b expression and the NIHSS scores in the peripheral blood of
patients in the IS group (r=-0.835; P<0.001; Fig. 3).
Multivariate logistic regression
analysis
Logistic multivariate regression analysis was
performed using AIS as the dependent variable, whilst hypertension,
hyperglycemia, platelet count, INR and miR-135b expression were
used as independent variables. Hypertension, hyperglycemia,
platelet count, INR and miR-135b expression were found to be risk
factors for AIS, as shown in Table
II.
 | Table IIMultivariate logistic regression
analysis results. |
Table II
Multivariate logistic regression
analysis results.
| Indicators | HR | 95% CI | P-value |
|---|
| Hypertension | 2.756 | 1.902-4.321 | <0.001 |
| Hyperglycemia | 1.862 | 1.543-2.356 | 0.014 |
| Platelet count | 2.094 | 1.678-2.635 | 0.001 |
| INR | 1.589 | 1.163-2.253 | 0.025 |
| miR-135b
expression | 2.356 | 1.825-3.287 | 0.002 |
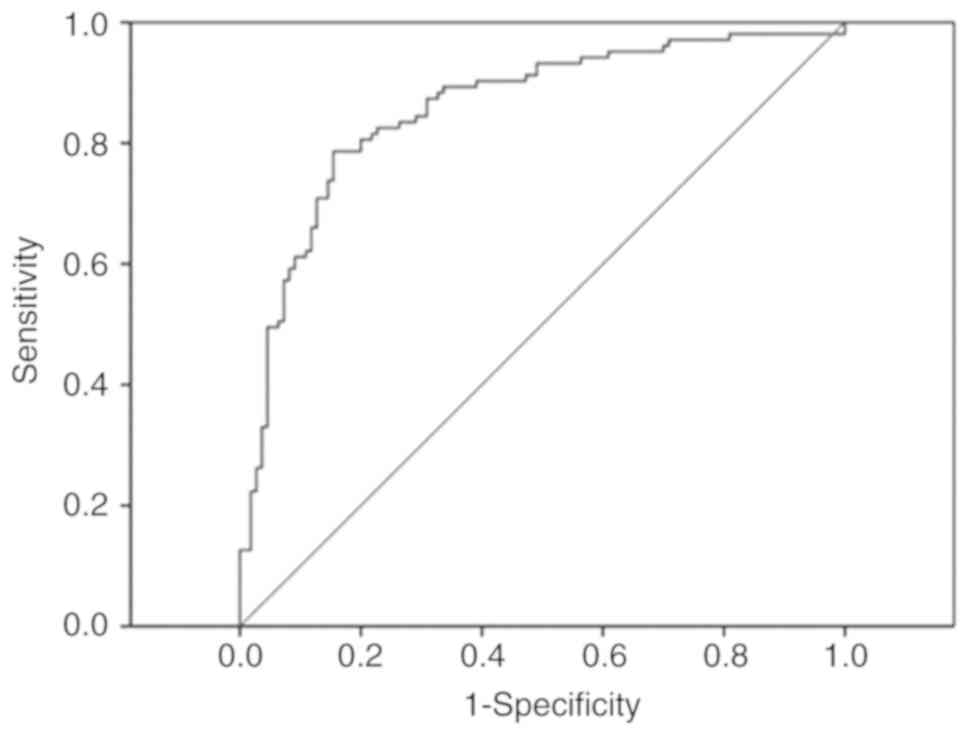
Sensitivity and specificity of
peripheral blood miR-135b levels for the diagnosis of IS
The sensitivity and specificity of peripheral blood
miR-135b in the diagnosis of IS were analyzed using ROC curve
analysis. The AUC of miR-135b was 0.78 (95% CI, 0.69-0.87; Fig. 4). When the cut-off value was set to
40 fmol/l, sensitivity and specificity of miR-135b for the
diagnosis of IS were calculated to be 79.25% and 64.71%,
respectively.
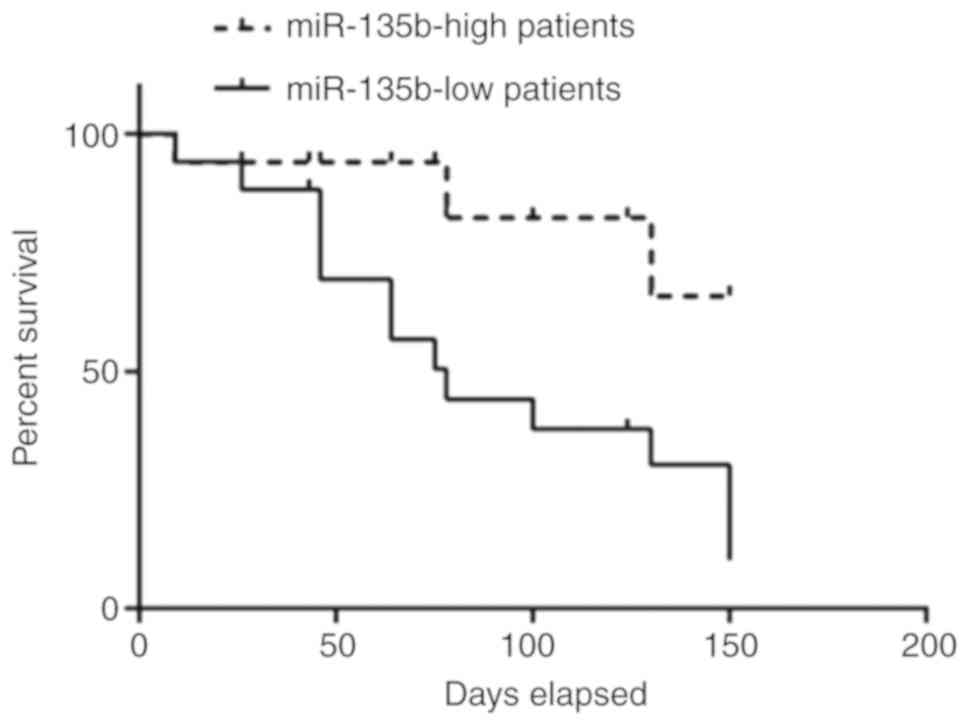
Lower miR-135b expression predicts
lower overall survival in patients with AIS
Association between serum miR-135b levels and
overall survival rates of patients with AIS was next evaluated. The
median value of 2.14 was applied for miR-135b expression in all 76
patients with AIS for separating those with high miR-135b
expression (n=27) from low miR-135b expression (n=49). Kaplan-Meier
curves indicated that patients with high miR-135b expression
exhibited significantly poorer survival compared with those with
low miR-135b expression (P=0.032; Fig.
5).
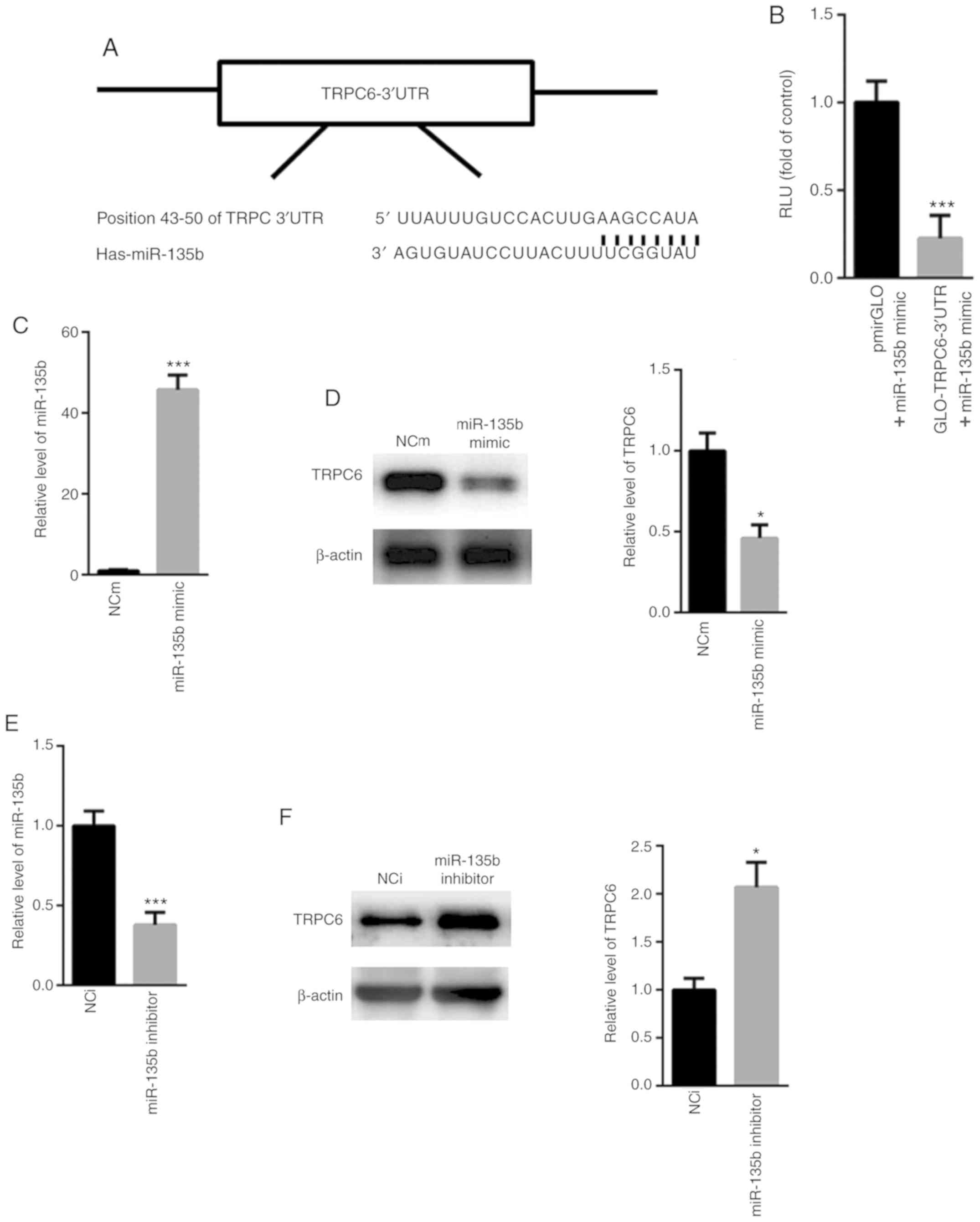
TRPC6 is a target gene of
miR-135b
Based on the aforementioned observations, the
potential target genes of miR-135b was subsequently analyzed.
Analysis using the TargetScan software identified a conserved
binding site in the 3'-UTR of TRPC6 (Fig. 6A). Dual luciferase reporter assay
showed that co-transfection with the miR-135b mimic significantly
reduced the relative luciferase activity of the pmirGLO-TRPC6-3'UTR
construct compared with cells co-transfected with the pmirGLO
construct (Fig. 6B). Further
analysis showed that transfection with the miR-135b mimic
significantly increased the levels of miR-135b expression whilst
significantly reducing the expression of TRPC6 in PC12 cells,
compared with corresponding NC mimic (NCm; Fig. 6C and D). By contrast, transfection with the
miR-135 inhibitor reduced the relative levels of miR-135b
expressions whilst enhancing the expression of TRPC6 compared with
NC inhibitor (NCi; Fig. 6E and
F). Taken together, these
observations indicate that TRPC6 is a target gene of miR-135b.
Discussion
At present, clinicians place heavy reliance on the
presentation of clinical symptoms and signs from patients combined
with head imaging to diagnose AIS (23). However, the early symptoms of AIS
onset lack specificity and imaging examination is expensive
(24), resulting in significant
economic burden on patients (25).
Therefore, there is a pressing need to explore novel circulating
markers for the diagnosis of AIS. Based on TOAST classification,
patients with AIS are classified into four categories: Aorta
atheromatous plague, cardioembolism, small arterial occlusion and
stroke of undetermined etiology (26). However, no significant difference in
serum miR-135b levels was identified among the four AIS groups in
the present study, indicating that miR-135b is a promising
biomarker for AIS but not for a specific subtype of AIS (23). A growing number of studies suggested
that changes in miRNA expression in the peripheral blood may be
closely associated with the development of AIS, which may be
exploited for the development of predictive, diagnostic and
prognostic markers for this disease (24,25).
miRNAs are single-stranded RNA molecules 18-25
nucleotides in length that regulate gene expression in many
cellular processes (2,27). Although the protective role of
miR-135b was previously reported in neurons (16,17), the
expression pattern and specific role of miR-135b in AIS remain
poorly understood. The present study demonstrated that miR-135b
expression in the peripheral blood samples obtained from the IS
group was higher compared with the healthy control group. At
present, NIHSS is the most commonly used scoring system for stroke
severity worldwide (28). The
present study found that miR-135b expression levels correlated
positively with the NIHSS score in a statistically significant
manner, suggesting that miR-135b may be a risk factor the
progression of AIS.
The diagnostic value of miR-135 in distinguishing
between patients with AIS and healthy controls was subsequently
evaluated. The present data showed that the sensitivity and
specificity of miR-135b for IS diagnosis revealed an AUC of 0.78
(95% CI, 0.69-0.87). When the cut-off value was set to 40 fmol/l,
the diagnostic sensitivity was 79.25% and the specificity was
64.71%. These findings suggested that serum miR-135b exhibited a
certain accuracy and feasibility as a biomarker of AIS. However, a
gap remains between the high specificity and high sensitivity of
this marker. Therefore, the combined detection of miRNAs with other
protein markers associated with ischemic stroke should be examined
in future research.
A number of reports have previously demonstrated
that circulating serum miRNAs may serve as potential non-invasive
biomarkers for patients with AIS (5,29). For
instance, Wang et al (29)
reported that serum miR-497 expression was significantly enhanced
in patients with AIS compared with healthy controls. Cheng et
al (5) suggested that
miR-148b-3p, miR-151b and miR-27b-3p may serve as potential
biomarkers for diagnosing AIS, with the specific combination of
miR-148b-3p and miR-27b-3p being more effective. In accordance with
these previous observations, the levels of serum miR-135 in
patients with AIS were analyzed based on etiology. Serum miR-135b
expression demonstrated positive correlation with the NIHSS scores.
However, correlation between serum miR-135b with inflammatory
factors, which has been reported to reflect the degree of brain
ischemic damage and stroke (30,31), was
not investigated in the present study.
The possible target genes of miR-135b were next
investigated. TRPC6, an important regulator of the neuronal
survival pathway (32), was
identified as a possible target gene of miR-135b by bioinformatics
analysis. Previous studies have demonstrated the beneficial effects
of TRPC6 in protecting against ischemic brain injury (33-35).
Guo et al (36) reported that
the enhanced expression of TRPC6 reduced neuronal cell death in
oxygen glucose-deprived cortical neurons, which serves an important
role in combined bone marrow stromal cells (BMSCs) and oxiracetam
treatments for cerebral ischemic infarction. A recent report showed
that the overexpression of TRPC6 via a CRISPR-based synergistic
activation mediator in BMSCs significantly reduced brain injury in
rats (33), whilst Li et al
(34) demonstrated that TRPC6
overexpression suppressed neurotoxicity and protected neurons from
ischemic brain damage. The present study hypothesize that miR-135b
may be involved in the process of AIS by targeting the TRPC6
neuronal survival pathway. Therefore, targeting miR-135b may be of
potential benefit for stroke prevention and therapy.
It should be noted a number of limitations remain
associated with the present study. A time profile of miR-135b would
be more practical, which will assist in elucidating the role of
miR-135b during disease onset and AIS prognosis. However, due to
the study design, the number of blood samples obtained remain
insufficient. In future studies, a larger cohort of samples should
be collected, where miR-135b expression will be studied over a
period of time. In addition, the present study mainly focused on
measuring the expression of miR-135b in the serum of patients with
AIS and to analyze the relationship between its expression levels
and the etiology and severity of the disease, thereby investigating
miR-135b occurrence in AIS. Investigations into the clinical values
of miR-135b in differentiating AIS, hemorrhagic stroke and other
brain diseases remain an attractive line of future research.
Specifically, samples obtained from patients with other brain
diseases could be investigated to elucidate the diagnostic value of
serum miR-135b levels further.
In conclusion, miR-135b was found to be closely
associated with the severity of patients with AIS in the present
study, where high levels of miR-135b in the peripheral blood can be
used to indicate the severity of neurological damage and are risk
factors for AIS. These findings may contribute to the future
clinical diagnosis and prognosis of AIS, however, the importance of
miR-135b intervention in the treatment of AIS warrants further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Southwest Hospital, Third Military Medical University (grant no.
TMMUSH-20150935).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY performed the experiments, analyzed the data,
wrote the manuscript, designed the experiments, analyzed the data
and gave final approval of the version to be published. XYZ, MH,
JJW and XMQ performed part of the RT-qPCR experiments. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Southwest Hospital, Third Military Medical
University (Chongqing, China). All patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Du K, Zhao C, Wang L, Wang Y, Zhang KZ,
Shen XY, Sun HX, Gao W and Lu X: MiR-191 inhibit angiogenesis after
acute ischemic stroke targeting VEZF1. Aging (Albany NY).
11:2762–2786. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen Z, Wang K, Huang J, Zheng G, Lv Y,
Luo N, Liang M and Huang L: Upregulated serum MiR-146b serves as a
biomarker for acute ischemic stroke. Cell Physiol Biochem.
45:397–405. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Marquez-Romero JM, Góngora-Rivera F,
Hernández-Curiel BC, Aburto-Murrieta Y, García-Cazares R,
Delgado-Garzón P, Murillo-Bonilla LM and Ochoa-Solórzano MA:
Endovascular treatment of ischemic stroke in a developing country.
Vasc Endovascular Surg: Feb 19, 2020 (Epub ahead of print).
|
|
4
|
Béjot Y, Daubail B and Giroud M:
Epidemiology of stroke and transient ischemic attacks: Current
knowledge and perspectives. Rev Neurol (Paris). 172:59–68.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cheng X, Kan P, Ma Z, Wang Y, Song W,
Huang C and Zhang B: Exploring the potential value of miR-148b-3p,
miR-151b and miR-27b-3p as biomarkers in acute ischemic stroke.
Biosci Rep. 38(38)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ji Q, Ji Y, Peng J, Zhou X, Chen X, Zhao
H, Xu T, Chen L and Xu Y: Increased brain-specific MiR-9 and
MiR-124 in the serum exosomes of acute ischemic stroke patients.
PLoS One. 11(e0163645)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jia L, Hao F, Wang W and Qu Y: Circulating
miR-145 is associated with plasma high-sensitivity C-reactive
protein in acute ischemic stroke patients. Cell Biochem Funct.
33:314–319. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Jiang M, Wang H, Jin M, Yang X, Ji H,
Jiang Y, Zhang H, Wu F, Wu G, Lai X, et al: Exosomes from
MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced,
autophagy-mediated brain injury by promoting M2
microglial/macrophage polarization. Cell Physiol Biochem.
47:864–878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jin F and Xing J: Circulating miR-126 and
miR-130a levels correlate with lower disease risk, disease
severity, and reduced inflammatory cytokine levels in acute
ischemic stroke patients. Neurol Sci. 39:1757–1765. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Khanna S, Rink C, Ghoorkhanian R, Gnyawali
S, Heigel M, Wijesinghe DS, Chalfant CE, Chan YC, Banerjee J, Huang
Y, et al: Loss of miR-29b following acute ischemic stroke
contributes to neural cell death and infarct size. J Cereb Blood
Flow Metab. 33:1197–1206. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li SH, Chen L, Pang XM, Su SY, Zhou X,
Chen CY, Huang LG, Li JP and Liu JL: Decreased miR-146a expression
in acute ischemic stroke directly targets the Fbxl10 mRNA and is
involved in modulating apoptosis. Neurochem Int. 107:156–167.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Su ZF, Sun ZW, Zhang Y, Wang S, Yu QG and
Wu ZB: Regulatory effects of miR-146a/b on the function of
endothelial progenitor cells in acute ischemic stroke in mice.
Kaohsiung J Med Sci. 33:369–378. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tiedt S, Prestel M, Malik R,
Schieferdecker N, Duering M, Kautzky V, Stoycheva I, Böck J,
Northoff BH, Klein M, et al: RNA-Seq identifies circulating
miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers
for acute ischemic stroke. Circ Res. 121:970–980. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Y, Zhang Y, Huang J, Chen X, Gu X,
Wang Y, Zeng L and Yang GY: Increase of circulating miR-223 and
insulin-like growth factor-1 is associated with the pathogenesis of
acute ischemic stroke in patients. BMC Neurol.
14(77)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu J, Du K and Lu X: Elevated expressions
of serum miR-15a, miR-16, and miR-17-5p are associated with acute
ischemic stroke. Int J Clin Exp Med. 8:21071–21079. 2015.PubMed/NCBI
|
|
16
|
Zhang J, Liu W, Wang Y, Zhao S and Chang
N: miR-135b plays a neuroprotective role by targeting GSK3β in
MPP+-intoxicated SH-SY5Y Cells. Dis Markers.
2017(5806146)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sillivan SE, Jamieson S, de Nijs L, Jones
M, Snidjers C, Klengel T, Joseph NF, Krauskopf J, Kleinjans J and
Vinkers CH: MicroRNA regulation of persistent stress-enhanced
memory. Mol Psychiatry: May 29, 2019 (Epub ahead of print).
|
|
18
|
Jiang B, Ball RL, Michel P, Jovin T, Desai
M, Eskandari A, Naqvi Z and Wintermark M: Prevalence of imaging
biomarkers to guide the planning of acute stroke reperfusion
trials. Stroke. 48:1675–1677. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kwah LK and Diong J: National Institutes
of Health Stroke Scale (NIHSS). J Physiother. 60(61)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT Method. Methods.
25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang X, Liu Y, Liu C, Xie W, Huang E,
Huang W, Wang J, Chen L, Wang H, Qiu P, et al: Inhibition of ROCK2
expression protects against methamphetamine-induced neurotoxicity
in PC12 cells. Brain Res. 1533:16–25. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rong F, Gao X, Liu K and Wu J:
Methotrexate remediates spinal cord injury in vivo and in
vitro via suppression of endoplasmic reticulum stress-induced
apoptosis. Exp Ther Med. 15:4191–4198. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiang W, Tian C, Lin J, Wu X, Pang G, Zhou
L, Pan S and Deng Z: Plasma let-7i and miR-15a expression are
associated with the effect of recombinant tissue plasminogen
activator treatment in acute ischemic stroke patients. Thromb Res.
158:121–125. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao B, Zhu Z, Hao J, Wan Z and Guo X:
Decreased plasma miR-335 expression in patients with acute ischemic
stroke and its association with calmodulin expression. J Int Med
Res. 44:1331–1338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou J, Chen L, Chen B, Huang S, Zeng C,
Wu H, Chen C and Long F: Increased serum exosomal miR-134
expression in the acute ischemic stroke patients. BMC Neurol.
18(198)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ko Y, Lee S, Chung JW, Han MK, Park JM,
Kang K, Park TH, Park SS, Cho YJ, Hong KS, et al: MRI-based
algorithm for acute ischemic stroke subtype classification. J
Stroke. 16:161–172. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shi FP, Wang XH, Zhang HX, Shang MM, Liu
XX, Sun HM and Song YP: MiR-103 regulates the angiogenesis of
ischemic stroke rats by targeting vascular endothelial growth
factor (VEGF). Iran J Basic Med Sci. 21:318–324. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yao S, Tang B, Li G, Fan R and Cao F:
miR-455 inhibits neuronal cell death by targeting TRAF3 in cerebral
ischemic stroke. Neuropsychiatr Dis Treat. 12:3083–3092.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang J, Lin M, Ren H, Yu Z, Guo T and Gu
B: Expression and clinical significance of serum miR-497 in
patients with acute cerebral infarction. Clin Lab.
65(65)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cojocaru IM, Cojocaru M, Tănăsescu R,
Iliescu I, Dumitrescu L and Silosi I: Expression of IL-6 activity
in patients with acute ischemic stroke. Rom J Intern Med.
47:393–396. 2009.PubMed/NCBI
|
|
31
|
Kwan J, Horsfield G, Bryant T, Gawne-Cain
M, Durward G, Byrne CD and Englyst NA: IL-6 is a predictive
biomarker for stroke associated infection and future mortality in
the elderly after an ischemic stroke. Exp Gerontol. 48:960–965.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Griesi-Oliveira K, Acab A, Gupta AR,
Sunaga DY, Chailangkarn T, Nicol X, Nunez Y, Walker MF, Murdoch JD,
Sanders SJ, et al: Modeling non-syndromic autism and the impact of
TRPC6 disruption in human neurons. Mol Psychiatry. 20:1350–1365.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li W, Yang F, Gao J, Tang Y, Wang J and
Pan Y: Over-expression of TRPC6 via CRISPR based synergistic
activation mediator in BMSCs ameliorates brain injury in a rat
model of cerebral ischemia/reperfusion. Neuroscience. 415:147–160.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li H, Huang J, Du W, Jia C, Yao H and Wang
Y: TRPC6 inhibited NMDA receptor activities and protected neurons
from ischemic excitotoxicity. J Neurochem. 123:1010–1018.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Du W, Huang J, Yao H, Zhou K, Duan B and
Wang Y: Inhibition of TRPC6 degradation suppresses ischemic brain
damage in rats. J Clin Invest. 120:3480–3492. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Guo C, Ma Y, Ma S, Mu F, Deng J, Duan J,
Xiong L, Yin Y, Wang Y, Xi M, et al: The role of TRPC6 in the
neuroprotection of calycosin against cerebral ischemic injury. Sci
Rep. 7(3039)2017.PubMed/NCBI View Article : Google Scholar
|