Introduction
Gestational diabetes mellitus (GDM) is defined as
impaired glucose tolerance in the second or third trimester of
pregnancy, with no apparent manifestation of diabetes during
pre-pregnancy (1). GDM is an
increasingly common pregnancy-associated complication (2), with an incidence as high as 18-36%
according to worldwide epidemiological surveys (3,4). GDM is
associated with various adverse maternal and fetal outcomes,
including pregnancy-induced hypertension, pre-eclampsia,
macrosomia, neonatal hypoglycemia and shoulder dystocia (5,6).
Concurrently, women with GDM have an increased risk of developing
type 2 diabetes mellitus (T2DM) post-pregnancy (7).
Previous studies have determined various risk
factors associated with GDM including maternal characteristics,
age, family history of diabetes and polycystic ovary syndrome
(PCOS) (8-10).
Currently, clinical management guidelines for
obstetrician-gynecologists produced by The American College of
Obstetricians and Gynecologists recommend early pregnancy glucose
screening for women at a high risk of increased blood glucose
levels (1), as well as for those
with risk factors such as hypertension, glycated hemoglobin levels
(HbA1c) ≥5.7%, impaired glucose tolerance or impaired fasting
glucose, a high-density lipoprotein cholesterol level ≤35 mg/dl and
triglyceride (TG) levels ≥250 mg/dl (7). Additionally, the 2 h 75 g oral glucose
tolerance test remains the gold standard for the clinical diagnosis
of GDM at 24 weeks of gestation (1).
A recent study has proposed a formula to predict the
probability of GDM between 8 and 20 weeks of gestation based on
maternal age, pre-pregnancy body mass index (BMI), fasting plasma
glucose and TG levels (11).
Additionally, numerous GDM predictive tools incorporate information
on maternal and biochemical factors (12). However, these traditional screening
methods are based on the mother's medical history, which can
demonstrate a high false-positive rating for predicting the disease
(13).
To the best of our knowledge, there are currently no
established criteria or models for predicting GDM that combine
sociodemographic characteristics, serological indicators and
ultrasound examinations. The early identification of risk factors
in pregnant women may help to predict the occurrence of GDM prior
to clinical diagnosis. Thus, there is an urgent requirement to
determine effective ways to predict the future development of GDM.
The present study aimed to develop a useful predictive tool for the
early identification of high-risk pregnant women, which could
provide a scientific basis for the early identification of
high-risk patients with GDM.
Materials and methods
Patient studies
The current study was approved by the Institutional
Review Board of Tangshan Gongren Hospital and written informed
consent was obtained from all patients. All procedures and methods
were performed in accordance with the approved guidelines.
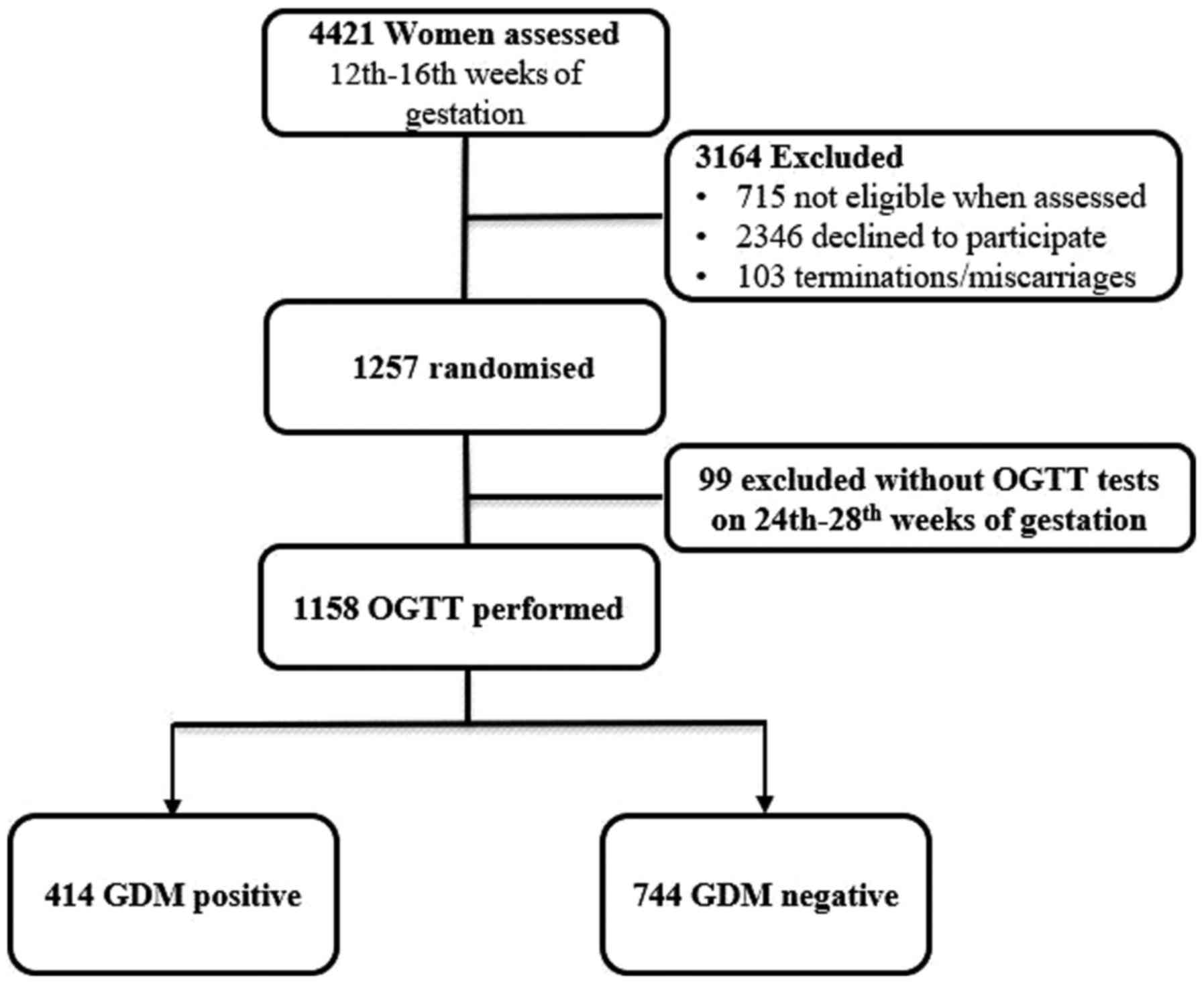
From January 2016 to May 2018, a total of 4,421
pregnant women (mean age 27.5±6.2 years, range 20-45) who received
routine pre-natal examinations at Tangshan Gongren Hospital and
eight other flagship hospitals (including First Hospital of
Tangshan Gongren Hospital group, Ninth Hospital of Tangshan Gongren
Hospital group, Tangshan Gongren Hospital group rehabilitation
hospital, Tangshan railway central hospital, Tangshan Gongren
Hospital group Fengnan hospital, Tangshan Gongren Hospital group
Qianan Yanshan hospital, Tangshan Gongren Hospital group Qianxi
Kangli hospital and Tangshan Gongren Hospital group Jidong
Sub-hospital) were observed.
The inclusion criteria included: i) Pregnancy at
12-16 weeks gestation; and ii) GDM diagnosis according to the 2017
GDM diagnostic criteria by the American Diabetes Association
(1). The exclusion criteria were: i)
Pregnancy with heart or cerebrovascular disease or vital organ
dysfunction; ii) prior antidiabetic medication; and/or iii)
pre-existing diabetes diagnosis [fasting blood glucose (BGL) levels
≥7.0 mmol/l, HbA1c levels ≥6.5% and/or random blood glucose levels
≥11.1 mmol/l]. During testing, 737 women were not eligible when
assessed. 2346 women declined to participate in the trial.103 women
quit the trial due to termination or miscarriages. For a variety of
reasons, 99 women didn't undergo a 75 g oral glucose tolerance test
(OGTT) at 24-28 weeks of gestation.
After further screening, selected pregnant women
underwent an OGTT between 24-28 weeks of gestation. Blood samples
were collected from patients for glucose and insulin measurement at
0, 0.5, 1 and 2 h after administration of OGTT to diagnose GDM. Of
these patients, 1,136 were enrolled in the present study and
included 406 women (35.75%) diagnosed with GDM and 730 women
(64.25%) that were negative for GDM (non-GDM; Fig. 1). Medical records of these enrolled
patients at 12-16 weeks of gestation were retrospectively
evaluated.
Data collections
Questionnaires were used to collect baseline
clinical data from pregnant women before 12 weeks of pregnancy,
including age, education, occupation, BMI, family history, parity,
a history of PCOS or of previous GDM, systolic blood pressure
(SBP), diastolic blood pressure and BGL levels. Right brachial
artery blood pressure was measured using a corrected mercury
sphygmomanometer between 7:00 and 9:00 a.m., with individuals
required to sit and rest for 15 min prior to measurement.
Occupations were divided into light work (clerks and civil
servants), medium work (teachers, students and self-employed) or
heavy work (farmers, medical staff and military personnel)
according to the China Physical Labor Intensity Grading Standard
(14).
Laboratory tests
HbA1c were measured at 12-16 weeks of gestation
using high-performance liquid chromatography (HPLC) with a cation
exchange chromatography column (Bio-Rex70, 200x6 mm, Bio-Rad,
Hercules, CA, USA). 15 µl of sample volume was injected into the
HPLC system. The HPLC system (, Waters, Milford, MA, USA) was
operated at a column temperature of 40°C and a flow rate of 1
ml/min. Mobile phase composition was as follows: mobile phase A,
PBS (pH6.6,40 mmol/l); mobile phase B, NaCl (pH 6.4, 300 mmol/l).
TG, total cholesterol (TC) and low-density lipoprotein cholesterol
(LDL-c) levels and liver function tests, including measuring
alanine transaminase (ALT), aspartate transaminase (AST) and
transglutaminase (GGT) plasma levels, were performed using an
automatic biochemistry analyzer (model AU600, Olympus Corporation;
7150, Hitachi, Ltd.) in the nine study center laboratories
(including central laboratory of Tangshan Gongren Hospital,
laboratory of First Hospital of Tangshan Gongren Hospital group,
laboratory of Ninth Hospital of Tangshan Gongren Hospital group,
laboratory of Tangshan Gongren Hospital group rehabilitation
hospital, laboratory of Tangshan railway central hospital,
laboratory of Tangshan Gongren Hospital group Fengnan hospital,
laboratory of Tangshan Gongren Hospital group Qianan Yanshan
hospital, laboratory of Tangshan Gongren Hospital group Qianxi
Kangli hospital and laboratory of Tangshan Gongren Hospital group
Jidong Sub-hospital). Serum hypersensitive C-reactive protein
(hs-CRP) was measured using immunoturbidimetry (Cias Latex CRP-H
kit; Kanto Chemical Co., Inc.). Serum visfatin and adiponectin
levels were analyzed using ELISA kits (Phoenix Pharmaceuticals,
Inc.; cat. no. EK-003-80 and EK-ADI-01, respectively).
Ultrasonography
A high-resolution Philips IU22 ultrasound system
(Philips Healthcare) equipped with a 3.5-10.0 MHz wide-band convex
sensor was used for ultrasonic measurements. To avoid any influence
caused by abdominal wall tension due to inhalation, abdominal
ultrasound examinations were performed in the supine position
during deep inhalation. Subcutaneous fat thickness (SFT) was
determined as the thickness of the fat between the liver surface
and the abdominal white line and visceral fat thickness (VFT) was
defined as the thickness of the fat between the skin-fat interface
and the white line (15).
Statistical analysis
Data were analyzed using SPSS software (version
13.0; SPSS, Inc.) and expressed as the mean±standard deviation or
the median and the interquartile range. Statistical differences
between non-GDM and GDM groups were determined using Student's
t-test or Wilcoxon test. Count data are expressed as numbers
and percentages and were evaluated using Fisher's exact probability
method. P<0.05 was considered to indicate a statistically
significant difference.
To assess the predictive variables of GDM, a
logistic regression model was established following adjustments for
age, pre-pregnancy BMI, a family history of diabetes, PCOS, a
history of GDM, SBP, and HbA1c, TG, TC, LDL-c, ALT, AST, GGT,
visfatin, hs-CRP, VFT and SFT levels. Logistic regression was used
to determine GDM risk predictors. According to previous reports
(16), the present study used the
stepwise forward selection method as the variable selection
procedure. Based on the results of logistic regression, the
predictability of the model was evaluated to give 95% confidence
intervals (CIs) and P-values. The logistic regression model was
evaluated using the area under the curve (AUC) of the receiver
operating characteristic curve and the Homer-Lemeshow
goodness-of-fit test was used to verify the efficiency of the
prediction model.
Results
Clinical data and biochemical
results
The sociodemographic and clinical characteristics of
patients in the non-GDM and GDM groups are summarized in Table I. The patients ranged in age from
20-45 years (GDM average; 28.69±4.73; non-GDM average; 26.40±3.64)
and the average pre-pregnancy BMI was 24.82±2.28 and 23.08±1.66
kg/m2 for the GDM and non-GDM groups, respectively. In
addition, there was no significant difference in liver function
between the two groups.
 | Table ISociodemographic and clinical
characteristics of patients in the non-GDM and GDM groups (mean ±
standard deviation). |
Table I
Sociodemographic and clinical
characteristics of patients in the non-GDM and GDM groups (mean ±
standard deviation).
| Variable | Non-GDM group
(n=730) | GDM group
(n=406) |
t/χ2 | P-value |
|---|
| Age (years) | 26.40±3.64 | 28.69±4.73 | -8.45 | <0.01 |
| BMI pregnancy
(kg/m2) | 23.08±1.66 | 24.82±2.28 | -13.52 | <0.01 |
| Family history of
diabetes mellitus (n, %) | 26 (3.6) | 80 (19.7) | 80.36 | <0.01 |
| History of GDM (n,
%) | 9 (1.2) | 25 (6.2) | 23.73 | <0.01 |
| Education (n,
%) | | | 3.54 | 0.17 |
|
High school
or below | 369 (50.5) | 203 (50.0) | | |
|
Diploma or
undergraduate | 301 (41.2) | 181 (44.6) | | |
|
Postgraduate
and above | 60 (8.2) | 22 (5.4) | | |
| Occupation (n,
%) | | | 4.42 | 0.11 |
|
Light
labor | 371 (50.8) | 204 (50.2) | | |
|
Medium
labor | 292 (40.0) | 178 (43.8) | | |
|
Heavy
labor | 67 (9.2) | 24 (5.9) | | |
| Parity (n, %) | | | 0.27 | 0.60 |
|
Primipara | 373 (51.1) | 214 (52.7) | | |
|
Multipara | 357 (48.9) | 192 (47.3) | | |
| PCOS (n, %) | 140 (19.2) | 142 (35.0) | 34.89 | <0.01 |
| HbA1c (%) | 5.66±0.35 | 6.11±1.43 | -6.28 | <0.01 |
| Fasting blood sugar
of pregnant women prior to 12 weeks of gestation (mmol/l) | 5.08±1.21 | 5.11±1.39 | -0.37 | 0.71 |
| SBP (mmHg) | 117.17±11.04 | 120.57±8.22 | -5.90 | <0.01 |
| DBP (mmHg) | 80.07±10.14 | 79.36±8.89 | 1.24 | 0.22 |
| Triglycerides
(mmol/l) | 1.81±0.70 | 1.94±0.84 | -2.75 | <0.01 |
| Total cholesterol
(mmol/l) | 5.20±0.72 | 6.29±1.59 | -13.23 | <0.01 |
| LDL cholesterol
(mmol/l) | 1.94±0.99 | 2.67±1.11 | -11.04 | <0.01 |
| ALT (mmol/l) | 17.60±3.14 | 17.43±3.34 | 0.83 | 0.41 |
| AST (mmol/l) | 17.10±3.18 | 17.28±2.82 | -0.99 | 0.32 |
| GGT (mmol/l) | 18.87±2.10 | 19.24±4.38 | -1.63 | 0.10 |
| hs-CRP (mg/l) | 1.69±0.17 | 2.16±0.60 | -15.63 | <0.01 |
| Visfatin
(ng/ml) | 8.98±1.09 | 10.28±1.43 | -16.01 | <0.01 |
| Adiponectin
(µg/ml) |
2,234.04±942.99 |
2,257.65±921.80 | -0.41 | 0.69 |
| VFT (mm) | 7.64±0.61 | 8.00±1.17 | -5.69 | <0.01 |
| SFT (mm) | 10.38±1.22 | 11.38±2.49 | -7.58 | <0.01 |
Compared with women in the non-GDM group, women in
the GDM group were older, demonstrated higher BMIs. were more prone
to PCOS, exhibited a family history of diabetes and were more
likely to present with a previous history of GDM. Additionally, the
GDM group demonstrated higher SBP, HbA1c, TGs, TC, LDL-c, visfatin,
hs-CRP, VFT and SFT (all P<0.01) compared with the non-GDM
group.
Results of logistic regression
analysis
Considering the strong association of numerous risk
factors and GDM, the forward stepwise variable-selection approach
was used to analyze independent predictors. The results revealed
that age, pre-pregnancy BMI, a family history of diabetes, a
history of GDM, PCOS, SBP and levels of TG, HbA1c, SBP, TC, LDL-c,
visfatin, hs-CRP, VFT and SFT should be included in the model as
these were all observed to be independent risk factors of GDM (all
P<0.01; Table II).
 | Table IILogistic regression analysis for the
clinical risk prediction model (mean ± standard deviation). |
Table II
Logistic regression analysis for the
clinical risk prediction model (mean ± standard deviation).
| Variable | β | S.E | Wald | OR (95% CI) | P-value |
|---|
| Age (years) | 0.154 | 0.027 | 33.445 | 1.166
(1.107-1.228) | <0.01 |
| BMI
pre-pregnancy | 0.580 | 0.063 | 84.959 | 1.787
(1.579-2.0212) | <0.01 |
| Family history of
diabetes mellitus | -1.071 | 0.424 | 6.377 | 0.343
(0.149-0.787) | 0.01 |
| History of GDM | -1.791 | 0.604 | 8.799 | 0.167
(0.051-0.545) | <0.01 |
| PCOS | -0.839 | 0.288 | 8.476 | 0.432
(0.246-0.7600) | <0.01 |
| HbA1c (%) | 0.833 | 0.207 | 16.134 | 2.299
(1.532-3.452) | <0.01 |
| SBP (mmHg) | 0.030 | 0.011 | 7.499 | 1.030
(1.009-1.053) | <0.01 |
| Triglycerides
(mmol/l) | 0.432 | 0.140 | 9.515 | 1.541
(1.171-2.028) | <0.01 |
| Total cholesterol
(mmol/l) | 1.046 | 0.163 | 41.017 | 2.846
(2.066-3.919) | <0.01 |
| LDL cholesterol
(mmol/l) | 0.579 | 0.103 | 31.887 | 1.784
(1.459-2.182) | <0.01 |
| hs-CRP (mg/l) | 1.427 | 0.296 | 23.212 | 4.165
(2.331-7.441) | <0.01 |
| Visfatin
(ng/ml) | 0.891 | 0.098 | 83.080 | 2.438
(2.013-2.952) | <0.01 |
| VFT (mm) | 0.760 | 0.133 | 32.574 | 2.139
(1.647-2.776) | <0.01 |
| SFT (mm) | 0.495 | 0.066 | 56.629 | 1.641
(1.443-1.867) | <0.01 |
Probability of basic and extended
prediction models
Model A retained 10 variables including age,
pre-pregnancy BMI, a family history of diabetes, GDM history, PCOS
history, and levels of HbA1C, SBP, TG, TC and LDL-c. Model B
included the above variables in addition to VFT and SFT and model C
contained all variables in model B plus visfatin and hs-CRP levels.
The AUC of model A was 0.846, whereas the addition of VFT and SFT
significantly increased the AUC of model B to 0.885 (Fig. 2). However, the addition of visfatin
and hs-CRP in model C (AUC, 0.911; 95% CI, 0.893-0.930) only
slightly improved the performance of model B (AUC, 0.885; 95% CI,
0.864-0.906). All three models were well-calibrated (Table III). Regression model C was the
best performing regression model with highly sensitive and strong
predictive power.
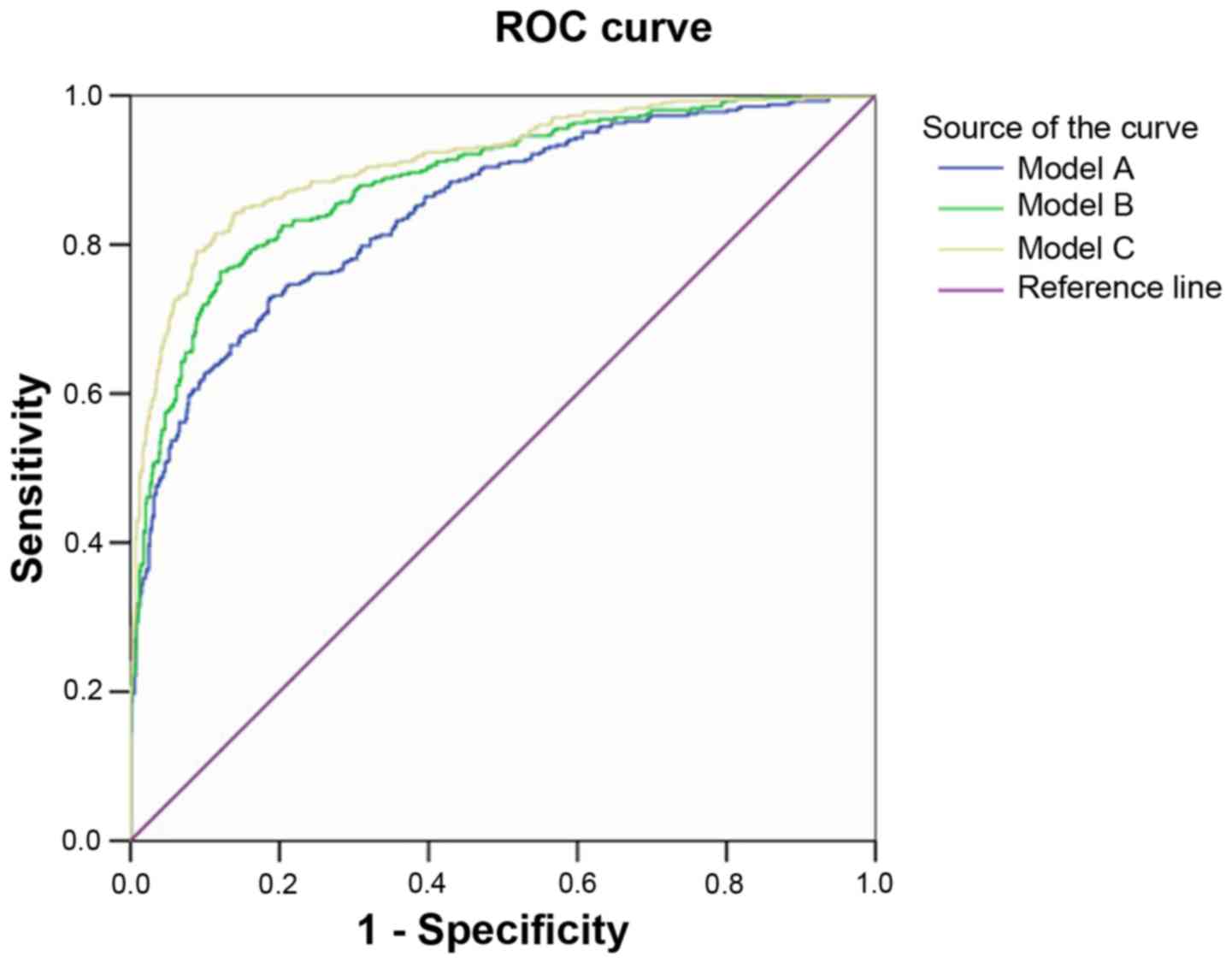 | Figure 2ROC curves of three different GDM
prediction models. Model A included the following variables: Age,
pre-pregnancy BMI, a family history of diabetes, GDM history, PCOS
history, and levels of HbA1C, SBP, TG, TC and LDL-c. Model B
included the following variables: Model A plus VFT and SFT. Model C
included the following variables: Model B plus visfatin and hs-CRP.
ROC, receiver operating characteristic; GDM, gestational diabetes
mellitus; BMI, body mass index; PCOS, polycystic ovary syndrome;
HBA1C, glycated hemoglobin; SBP, systolic blood pressure; TG,
triglyceride; TC, total cholesterol; LDL-c, low-density lipoprotein
cholesterol; VFT, visceral fat thickness; SFT, subcutaneous fat
thickness; hs-CRP, serum hypersensitive C-reactive protein. |
 | Table IIIPerformance of different models
predicting gestational diabetes mellitus. |
Table III
Performance of different models
predicting gestational diabetes mellitus.
| Model | Sensitivity
(%) | Specificity
(%) | Positive predictive
value (%) | Negative predictive
value (%) |
|---|
| A | 70.0 | 91.8 | 82.56 | 84.60 |
| B | 76.6 | 92.1 | 84.28 | 87.61 |
| C | 83.0 | 94.7 | 89.63 | 90.92 |
Discussion
In recent years, advanced etiological studies have
revealed GDM to be a multifactorial pregnancy disease which can be
induced by genetic factors, insulin resistance (IR), inflammation
and adipokine involvement (17). In
a case-controlled study reporting GDM incidence, the prevalence of
GDM in pregnant Chinese women was between 6.8-10.4%, with increased
prevalence in the Beijing area at 19.7% (18). Furthermore, it has been reported that
in woman >25 years old, the incidence of GDM increases with age
(19). This is due to the placenta
secreting insulin-like substances that induce IR, which occurs
alongside the age-associated decreased function of islet β-cells
(20).
Numerous studies have reported that increases in
pre-pregnancy BMI are independent risk factors for GDM (12,21).
During pregnancy, the secretion of insulin-antagonistic hormones
rapidly increases in obese women, which in turn increases the risk
of GDM (22). The present study
reported that women in the GDM group were older, had higher BMIs
and higher Hba1c levels compared with the non-GDM group.
Multivariate logistic regression analysis demonstrated that these
variables were significantly associated with an increased risk of
GDM.
Previous research has shown that pregnant women with
a history of diabetes hav a 3-fold higher risk of developing GDM
and this association is not affected by other confounding factors
(20,23). In addition, the fetuses of women with
GDM may be subjected to a hyperglycemic environment during
pregnancy that stimulates fetal islet β cell proliferation and
abnormal proliferation of adipocytes (24). These fetuses tend to develop obesity
in adulthood and are prone to abnormal glucose metabolism during
their own pregnancies. The results of the present study reported
that women with GDM were more likely to have a family history of
T2DM and a previous history of GDM, and were therefore included in
the final regression model. A previous study has suggested that
family history of T2DM influences GMD occurrence partially due to
overlapping genetic bases between the diseases (25).
GDM and PCOS are common endocrine diseases in women
of childbearing age, which are associated with central obesity and
IR (26,27). It has been suggested that PCOS may be
an early-stage manifestation of various IR-associated diseases,
including GDM, with the results of the present study confirming
that PCOS is independently associated with GDM (28). In addition, a second study
demonstrated that PCOS has a long-term effect on glucose metabolism
(29). Women with PCOS and GDM were
more likely to have impaired fasting blood glucose or impaired
glucose tolerance at 18 months post-delivery compared with women
that only exhibited GDM (29).
Furthermore, hyperandrogenism with accompanying anovulation is one
of the main features of PCOS and a previous study has demonstrated
that patients with PCOS had lower levels of free androgen and sex
hormone-binding globulin (SHBG) compared with patients without GDM
(30). A previous study also
demonstrated that obese women with PCOS and low SHBG levels were
more likely to develop GDM compared with those with high SHBG
levels (31).
During early pregnancy, estrogen and progesterone
stimulate maternal β cell proliferation and insulin secretion,
which promotes maternal fat and hepatic glycogen storage to support
fetal growth (32,33). In the first trimester of pregnancy,
insulin sensitivity may increase. However, as the pregnancy
progresses to mid-pregnancy, systemic insulin sensitivity declines
gradually (33). Previous studies
have reported that IR is more prevalent in patients with GDM,
leading to an increase in maternal blood glucose (17,18).
Additionally, there is evidence to suggest that IR is present in
women with gestational hypertension (34) and increased blood pressure in
pregnancy has been associated with the gradual impairment of
glucose tolerance (35). These
studies reinforce the results of the present study.
Patients with GDM have higher levels of TGs and
lower insulin sensitivity (36). In
addition, mRNA levels of genes involved in lipid and fatty acid
metabolism are often altered in women with GDM (37). Changes in maternal lipid metabolism
during pregnancy are characterized by a modest increase in lipids
during early pregnancy and a significant increase in lipids during
late pregnancy, particularly TGs and cholesterol (38). Lipid levels are closely associated
with the dysfunction of visceral abdominal adipose tissue (39). A previous study demonstrated that
central fat deposition is more closely associated with perinatal
diseases (such as GDM) compared with peripheral fat accumulation
(40). Ultrasounds are used to
measure visceral and subcutaneous fat thickness during pregnancy
due to their good practicability, objectivity and reproducibility,
with VFT and SFT generally considered as appropriate to measure the
distribution of visceral and subcutaneous fat. The present study
observed that pregnant women with GDM had more VFT and SFT and it
has been demonstrated that increased subcutaneous and visceral fat
may lead to increased IR in muscle and adipose tissue (41).
Hs-CRP is an acute-phase protein associated with an
increased risk of GDM (42). Given
the positive association between hs-CRP and the risk of GDM
development, the present study also observed that hs-CRP levels may
predict GDM risk. In addition, the current study demonstrated that
women with GDM exhibited higher concentrations of visfatin compared
with healthy pregnant controls, which is consistent with a previous
study (43). The study also reported
the association between serum visfatin, fasting blood glucose,
insulin and post-load insulin levels. Thus, altogether the
accumulating evidence suggests that dyslipidemia and inflammation
may serve an essential role in the progression of GDM.
However, as an important index of visceral fat
metabolism (particularly liver fat metabolism), there was no
significant difference in liver function between the two groups.
This may be due to the limited sample quantity the current study.
In future research, pregnant rats fed with a high fat diet could be
used to study the association between liver function and adipose
tissue.
In conclusion, age, pre-pregnancy BMI, a family
history of diabetes, PCOS and levels of TGs, HbA1c, TC, LDL-c,
visfatin, hs-CRP, VFT and SFT were all demonstrated to be
independent risk factors of GDM. By analyzing maternal
characteristics, sociodemographic characteristics, serological
indicators and ultrasound examinations, the results of the present
study suggested that this combined method adequately estimated the
risk factors for GDM in the second trimester. Following the
successful predictive ability of the model, it is hypothesized that
the prediction model developed in the present study may represent
an improved model capable of predicting GDM in women in the
Tangshan area. In addition, it is noteworthy that only Tangshan
Gongren Hospital and the other eight flagship hospitals in Tangshan
have been included in the present study to develop the GDM risk
prediction model. The results may not therefore be representative
of the total population. In future studies, large-scale
multi-center clinical trials are required before generalizing the
results to the population of China.
Acknowledgements
Not Applicable.
Funding
The present study was supported by Tangshan Science
and Technology Project (grant no. 17130224a).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, LZ and HF conceived and designed the study. LT,
XL, GZ, JQ and DZ performed data collection and ultrasonic
examination. LT and HF wrote the manuscript. DZ and HF reviewed and
edited the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
All research was conducted with the approval of the
Ethics Committee of Tangshan Gongren Hospital (clearance number
GRYY-LL-2016-03). All subjects signed written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Committee on Practice
Bulletins-Obstetrics. Practice bulletin no. 137: Gestational
diabetes mellitus. Obstet Gynecol. 122:406–416. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marathe PH Gao HX and Close KL: American
diabetes association standards of medical care in diabetes 2017. J
Diabetes. 9:320–324. 2017. View Article : Google Scholar
|
|
3
|
Duran A, Sáenz S, Torrejón MJ, Bordiú E,
Del Valle L, Galindo M, Perez N, Herraiz MA, Izquierdo N, Rubio MA,
et al: Introduction of IADPSG criteria for the screening and
diagnosis of gestational diabetes mellitus results in improved
pregnancy outcomes at a lower cost in a large cohort of pregnant
women: The St. carlos gestational diabetes study. Diabetes Care.
37:2442–2450. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Boyadzhieva MV, Atanasova I, Zacharieva S,
Tankova T and Dimitrova V: Comparative analysis of current
diagnostic criteria for gestational diabetes mellitus. Obstet Med.
5:71–77. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu X, Liu Y, Liu D, Li X, Rao Y, Sharma M
and Zhao Y: Prevalence and determinants of gestational diabetes
mellitus: A cross-sectional study in china. Int J Environ Res
Public Health. 14(pii: E1532)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hartling L, Dryden DM, Guthrie A, Muise M,
Vandermeer B and Donovan L: Benefits and harms of treating
gestational diabetes mellitus: A systematic review and
meta-analysis for the US preventive services task force and the
national institutes of health office of medical applications of
research. Ann Intern Med. 159:123–129. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shah BR, Retnakaran R and Booth GL:
Increased risk of cardiovascular disease in young women following
gestational diabetes mellitus. Diabetes Care. 31:1668–1669.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin PC, Hung CH, Chan TF, Lin KC, Hsu YY
and Ya-Ling Tzeng: The risk factors for gestational diabetes
mellitus: A retrospective study. Midwifery. 42:16–20.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Larrabure-Torrealva GT, Martinez S,
Luque-Fernandez MA, Sanchez SE, Mascaro PA, Ingar H, Castillo W,
Zumaeta R, Grande M, Motta V, et al: Prevalence and risk factors of
gestational diabetes mellitus: Findings from a universal screening
feasibility program in Lima, Peru. BMC Pregnancy Childbirth.
18(303)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Natamba BK, Namara AA and Nyirenda MJ:
Burden, risk factors and maternal and offspring outcomes of
gestational diabetes mellitus (GDM) in sub-Saharan Africa (SSA): A
systematic review and meta-analysis. BMC Pregnancy Childbirth.
19(450)2019. View Article : Google Scholar
|
|
11
|
Zheng T, Ye W, Wang X, Li X, Zhang J,
Little J, Zhou L and Zhang L: A simple model to predict risk of
gestational diabetes mellitus from 8 to 20 weeks of gestation in
chinese women. BMC Pregnancy Childbirth. 19(252)2019. View Article : Google Scholar
|
|
12
|
Sweeting AN, Wong J, Appelblom H, Ross GP,
Kouru H, Williams PF, Sairanen M and Hyett JA: A novel early
pregnancy risk prediction model for gestational diabetes mellitus.
Fetal Diagn Ther. 45:76–84. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Waugh N, Scotland G, McNamee P, Gillett M,
Brennan A, Goyder E, Williams R and John A: Screening for type 2
diabetes: Literature review and economic modelling. Health Technol
Assess. 11(iii-iv, ix-xi, 1-125)2007.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
State Bureau of Technical Supervision.
Classification on intensity of physical work (GB 3869-1997). China
Standard Press, Beijing. 1997.
|
|
15
|
Kaess BM, Pedley A, Massaro JM, Murabito
J, Hoffmann U and Fox CS: The ratio of visceral to subcutaneous
fat, a metric of body fat distribution, is a unique correlate of
cardiometabolic risk. Diabetologia. 55:2622–2630. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dowdy S, Weardon S and Chilko D: Multiple
Regression and Correlation. In: Statistics for Research 3rd
edition. Wiley & Sons, Inc., Hoboken NJ. 2005.
|
|
17
|
Carroll X, Liang X, Zhang W, Zhang W, Liu
G, Turner N and Leeper-Woodford S: Socioeconomic, environmental and
lifestyle factors associated with gestational diabetes mellitus: A
matched case-control study in Beijing, China. Sci Rep.
8(8103)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
McCabe CF and Perng W: Metabolomics of
diabetes in pregnancy. Curr Diab Rep. 17(57)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sajani TT, Rahman MT and Karim MR:
Maternal and fetal outcome of mothers with gestational diabetes
mellitus attending BIRDEM hospital. Mymensingh Med J. 23:290–298.
2014.PubMed/NCBI
|
|
20
|
Szoke E, Shrayyef MZ, Messing S, Woerle
HJ, van Haeften TW, Meyer C, Mitrakou A, Pimenta W and Gerich JE:
Effect of aging on glucose homeostasis: Accelerated deterioration
of beta-cell function in individuals with impaired glucose
tolerance. Diabetes Care. 31:539–543. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lauenborg J, Hansen T, Jensen DM,
Vestergaard H, Mølsted-Pedersen L, Hornnes P, Locht H, Pedersen O
and Damm P: Increasing incidence of diabetes after gestational
diabetes: A long-term follow-up in a Danish population. Diabetes
Care. 27:1194–1199. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Adane AA, Tooth LR and Mishra GD:
Pre-pregnancy weight change and incidence of gestational diabetes
mellitus: A finding from a prospective cohort study. Diabetes Res
Clin Pract. 124:72–80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhu WW, Yang HX, Wang C, Su RN, Feng H and
Kapur A: High prevalence of gestational diabetes mellitus in
Beijing: Effect of maternal birth weight and other risk factors.
Chin Med J (Engl). 130:1019–1025. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kubo A, Ferrara A, Windham GC, Greenspan
LC, Deardorff J, Hiatt RA, Quesenberry CP Jr, Laurent C, Mirabedi
AS and Kushi LH: Maternal hyperglycemia during pregnancy predicts
adiposity of the offspring. Diabetes Care. 37:2996–3002.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang C, Bao W, Rong Y, Yang H, Bowers K,
Yeung E and Kiely M: Genetic variants and the risk of gestational
diabetes mellitus: A systematic review. Hum Reprod Update.
19:376–390. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hildén K, Hanson U, Persson M and Fadl H:
Overweight and obesity: A remaining problem in women treated for
severe gestational diabetes. Diabet Med. 33:1045–1051.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Macut D, Bjekić-Macut J, Rahelić D and
Doknić M: Insulin and the polycystic ovary syndrome. Diabetes Res
Clin Pract. 130:163–170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Boomsma CM, Eijkemans MJ, Hughes EG,
Visser GH, Fauser BC and Macklon NS: A meta-analysis of pregnancy
outcomes in women with polycystic ovary syndrome. Hum Reprod
Update. 12:673–683. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Palomba S, Falbo A, Russo T, Rivoli L,
Orio M, Cosco AG, Vero R, Capula C, Tolino A, Zullo F, et al: The
risk of a persistent glucose metabolism impairment after
gestational diabetes mellitus is increased in patients with
polycystic ovary syndrome. Diabetes Care. 35:861–867.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li G, Huang W, Zhang L, Tian Z, Zheng W,
Wang T, Zhang T and Zhang W: A prospective cohort study of
early-pregnancy risk factors for gestational diabetes in women with
polycystic ovarian syndrome. Diabetes Metab Res Rev.
34(e3003)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
de Wilde MA, Veltman-Verhulst SM, Goverde
AJ, Lambalk CB, Laven JS, Franx A, Koster MP, Eijkemans MJ and
Fauser BC: Preconception predictors of gestational diabetes: A
multicentre prospective cohort study on the predominant
complication of pregnancy in polycystic ovary syndrome. Hum Reprod.
29:1327–1336. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Costrini NV and Kalkhoff RK: Relative
effects of pregnancy, estradiol and progesterone on plasma insulin
and pancreatic islet insulin secretion. J Clin Invest. 50:992–999.
1971.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lain KY and Catalano PM: Metabolic changes
in pregnancy. Clin Obstet Gynecol. 50:938–948. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bartha JL, Romero-Carmona R,
Torrejon-Cardoso R and Comino-Delgado R: Insulin, insulin-like
growth factor-1 and insulin resistance in women with
pregnancy-induced hypertension. Am J Obstet Gynecol. 187:735–740.
2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ma RM and Lao TT: Maternal mean arterial
pressure and oral glucose tolerance test results. Relationship in
normotensive women. J Reprod Med. 46:747–751. 2001.PubMed/NCBI
|
|
36
|
Scholtens DM, Muehlbauer MJ, Daya NR,
Stevens RD, Dyer AR, Lowe LP, Metzger BE, Newgard CB, Bain JR, Lowe
WL Jr, et al: Metabolomics reveals broad-scale metabolic
perturbations in hyperglycemic mothers during pregnancy. Diabetes
Care. 37:158–166. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Segura MT, Demmelmair H, Krauss-Etschmann
S, Nathan P, Dehmel S, Padilla MC, Rueda R, Koletzko B and Campoy
C: Maternal BMI and gestational diabetes alter placental lipid
transporters and fatty acid composition. Placenta. 57:144–151.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Herrera E and Ortega-Senovilla H:
Disturbances in lipid metabolism in diabetic pregnancy-Are these
the cause of the problem? Best Pract Res Clin Endocrinol Metab.
24:515–525. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xia MF, Chen Y, Lin HD, Ma H, Li XM,
Aleteng Q, Li Q, Wang D, Hu Y, Pan BS, et al: A indicator of
visceral adipose dysfunction to evaluate metabolic health in adult
Chinese. Sci Rep. 6(38214)2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Butte NF: Carbohydrate and lipid
metabolism in pregnancy: Normal compared with gestational diabetes
mellitus. Am J Clin Nutr. 71 (5 Suppl)(1256S-1261S)2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Catalano PM, McIntyre HD, Cruickshank JK,
McCance DR, Dyer AR, Metzger BE, Lowe LP, Trimble ER, Coustan DR,
Hadden DR, et al: The hyperglycemia and adverse pregnancy outcome
study: Associations of GDM and obesity with pregnancy outcomes.
Diabetes Care. 35:780–786. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Qiu C, Sorensen TK, Luthy DA and Williams
MA: A prospective study of maternal serum C-reactive protein (CRP)
concentrations and risk of gestational diabetes mellitus. Paediatr
Perinat Epidemiol. 18:377–384. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lewandowski KC, Stojanovic N, Press M,
Tuck SM, Szosland K, Bienkiewicz M, Vatish M, Lewinski A, Prelevic
GM and Randeva HS: Elevated serum levels of visfatin in gestational
diabetes: A comparative study across various degrees of glucose
tolerance. Diabetologia. 50:1033–1037. 2007.PubMed/NCBI View Article : Google Scholar
|