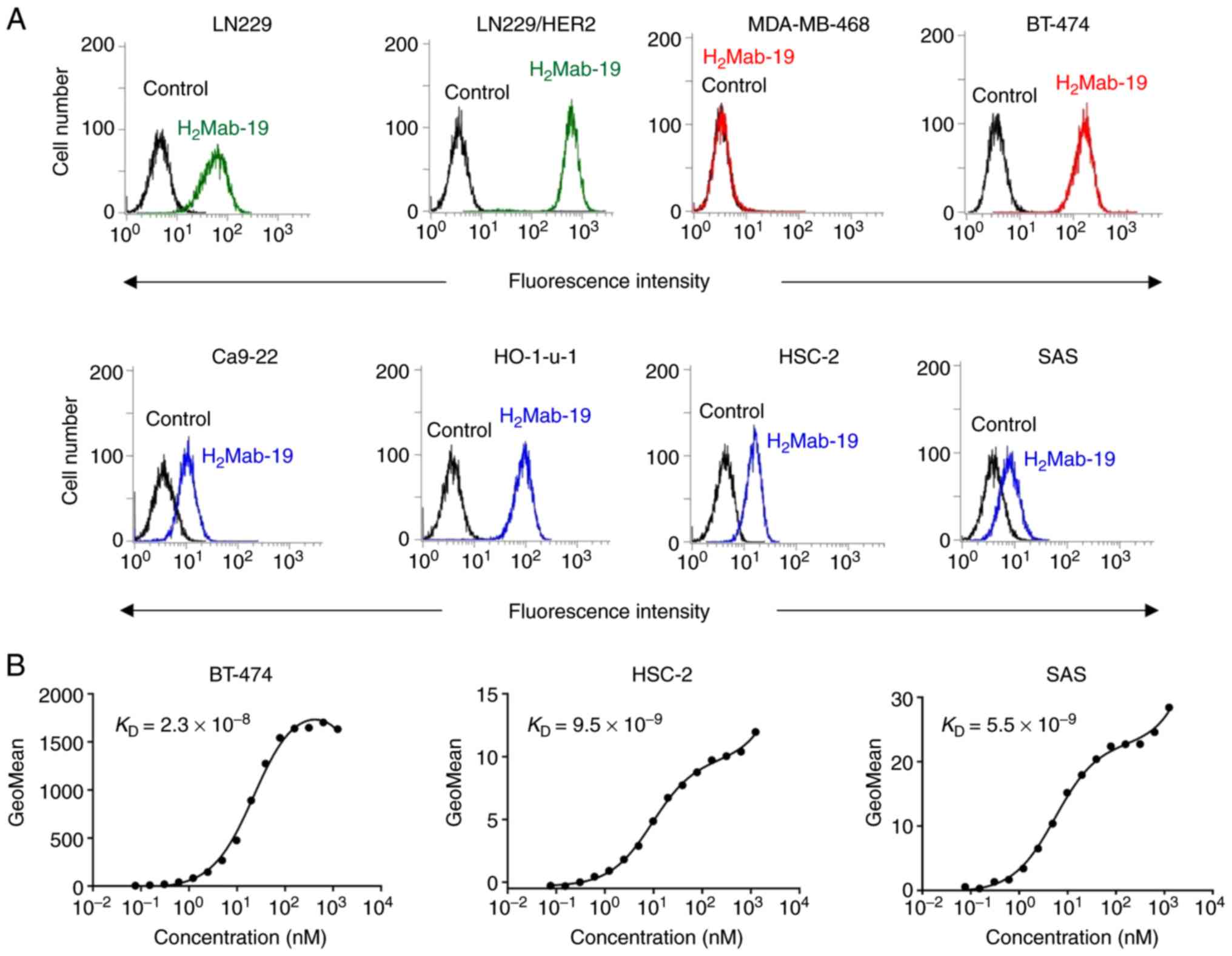
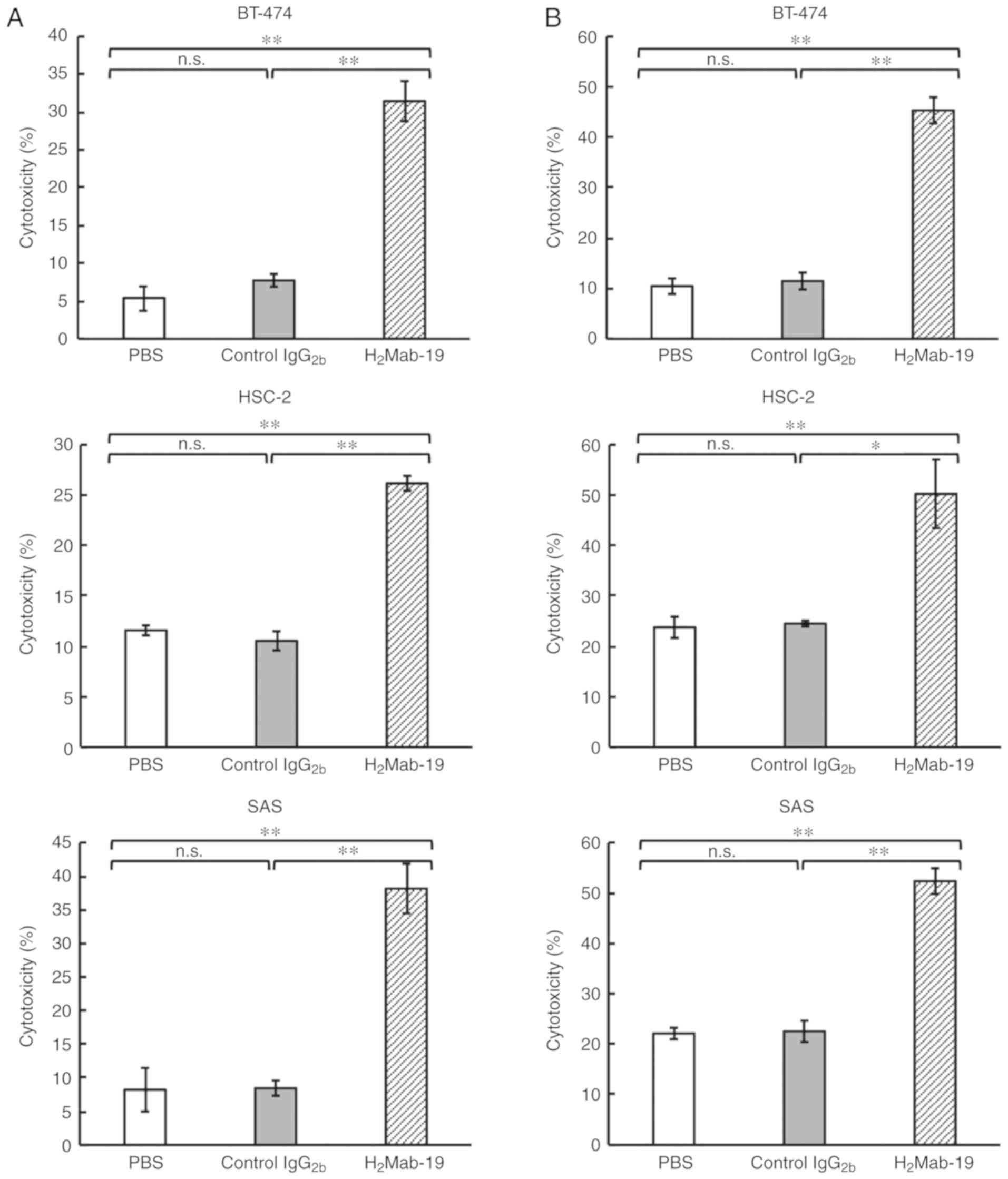
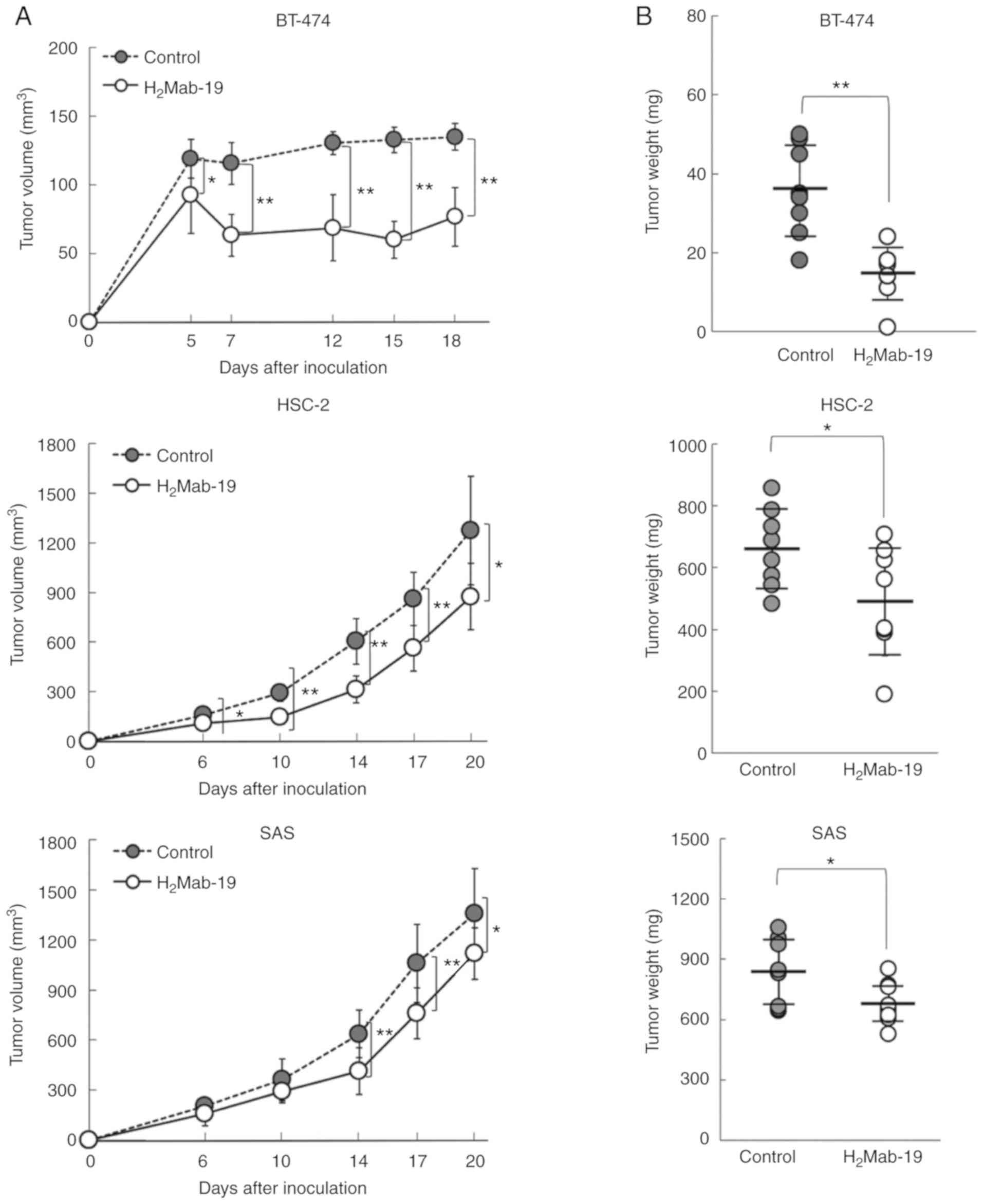
|
1
|
Howlader N, Altekruse SF, Li CI, Chen VW,
Clarke CA, Ries LA and Cronin KA: US incidence of breast cancer
subtypes defined by joint hormone receptor and HER2 status. J Natl
Cancer Inst. 106(dju055)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xie S, Zhang H, Wang X, Ge Q and Hu J: The
relative efficacy and safety of targeted agents used in combination
with chemotherapy in treating patients with untreated advanced
gastric cancer: A network meta-analysis. Oncotarget. 8:26959–26968.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Harder J, Ihorst G, Heinemann V, Hofheinz
R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S,
et al: Multicentre phase II trial of trastuzumab and capecitabine
in patients with HER2 overexpressing metastatic pancreatic cancer.
Br J Cancer. 106:1033–1038. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim EK, Kim KA, Lee CY and Shim HS: The
frequency and clinical impact of HER2 alterations in lung
adenocarcinoma. PLoS One. 12(e0171280)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Seo AN, Kwak Y, Kim DW, Kang SB, Choe G,
Kim WH and Lee HS: HER2 status in colorectal cancer: Its clinical
significance and the relationship between HER2 gene amplification
and expression. PLoS One. 9(e98528)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lambert JM and Chari RV: Ado-trastuzumab
emtansine (T-DM1): An antibody-drug conjugate (ADC) for
HER2-positive breast cancer. J Med Chem. 57:6949–6964.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Valabrega G, Montemurro F and Aglietta M:
Trastuzumab: Mechanism of action, resistance and future
perspectives in HER2-overexpressing breast cancer. Ann Oncol.
18:977–984. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Amiri-Kordestani L, Wedam S, Zhang L, Tang
S, Tilley A, Ibrahim A, Justice R, Pazdur R and Cortazar P: First
FDA approval of neoadjuvant therapy for breast cancer: Pertuzumab
for the treatment of patients with HER2-positive breast cancer.
Clin Cancer Res. 20:5359–5364. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Swain SM, Kim SB, Cortés J, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Knott A, et al: Pertuzumab, trastuzumab, and docetaxel for
HER2-positive metastatic breast cancer (CLEOPATRA study): Overall
survival results from a randomised, double-blind,
placebo-controlled, phase 3 study. Lancet Oncol. 14:461–471.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Doi T, Shitara K, Naito Y, Shimomura A,
Fujiwara Y, Yonemori K, Shimizu C, Shimoi T, Kuboki Y, Matsubara N,
et al: Safety, pharmacokinetics, and antitumour activity of
trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug
conjugate, in patients with advanced breast and gastric or
gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet
Oncol. 18:1512–1522. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nakada T, Sugihara K, Jikoh T, Abe Y and
Agatsuma T: The latest research and development into the
antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a),
for HER2 cancer therapy. Chem Pharm Bull (Tokyo). 67:173–185.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Itai S, Fujii Y, Kaneko MK, Yamada S,
Nakamura T, Yanaka M, Saidoh N, Chang YW, Handa S, Takahashi M, et
al: H2Mab-77 is a sensitive and specific anti-HER2 monoclonal
antibody against breast cancer. Monoclon Antib Immunodiagn
Immunother. 36:143–148. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kato Y, Kunita A, Abe S, Ogasawara S,
Fujii Y, Oki H, Fukayama M, Nishioka Y and Kaneko MK: The chimeric
antibody chLpMab-7 targeting human podoplanin suppresses pulmonary
metastasis via ADCC and CDC rather than via its neutralizing
activity. Oncotarget. 6:36003–36018. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kaneko MK, Yamada S, Itai S and Kato Y:
Development of an anti-HER2 monoclonal antibody H2Mab-139 against
colon cancer. Monoclon Antib Immunodiagn Immunother. 37:59–62.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fujii Y, Kaneko MK and Kato Y: MAP tag: A
novel tagging system for protein purification and detection.
Monoclon Antib Immunodiagn Immunother. 35:293–299. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Al-Saden N, Lam K, Chan C and Reilly RM:
Positron-emission tomography of HER2-positive breast cancer
xenografts in mice with 89Zr-labeled trastuzumab-DM1: A
comparison with 89Zr-labeled trastuzumab. Mol Pharm.
15:3383–3393. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kato Y and Kaneko MK: A cancer-specific
monoclonal antibody recognizes the aberrantly glycosylated
podoplanin. Sci Rep. 4(5924)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yamada S, Itai S, Nakamura T, Chang YW,
Harada H, Suzuki H, Kaneko MK and Kato Y: Establishment of
H2Mab-119, an anti-human epidermal growth factor
receptor 2 monoclonal antibody, against pancreatic cancer. Monoclon
Antib Immunodiagn Immunother. 36:287–290. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kaneko MK, Nakamura T, Honma R, Ogasawara
S, Fujii Y, Abe S, Takagi M, Harada H, Suzuki H, Nishioka Y and
Kato Y: Development and characterization of anti-glycopeptide
monoclonal antibodies against human podoplanin, using
glycan-deficient cell lines generated by CRISPR/Cas9 and TALEN.
Cancer Med. 6:382–396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ogasawara S, Kaneko MK and Kato Y:
LpMab-19 recognizes sialylated O-Glycan on Thr76 of human
podoplanin. Monoclon Antib Immunodiagn Immunother. 35:245–253.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fiedler W, Stoeger H, Perotti A, Gastl G,
Weidmann J, Dietrich B, Baumeister H, Danielczyk A, Goletz S,
Salzberg M and De Dosso S: Phase I study of TrasGEX, a
glyco-optimised anti-HER2 monoclonal antibody, in patients with
HER2-positive solid tumours. ESMO Open. 3(e000381)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hashibe M, Brennan P, Chuang SC, Boccia S,
Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova
E, et al: Interaction between tobacco and alcohol use and the risk
of head and neck cancer: Pooled analysis in the international head
and neck cancer epidemiology consortium. Cancer Epidemiol
Biomarkers Prev. 18:541–550. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tota JE, Anderson WF, Coffey C, Califano
J, Cozen W, Ferris RL, St John M, Cohen EEW and Chaturvedi AK:
Rising incidence of oral tongue cancer among white men and women in
the United States, 1973-2012. Oral Oncol. 67:146–152.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hussein AA, Helder MN, de Visscher JG,
Leemans CR, Braakhuis BJ, de Vet HCW and Forouzanfar T: Global
incidence of oral and oropharynx cancer in patients younger than 45
years versus older patients: A systematic review. Eur J Cancer.
82:115–127. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bagan J, Sarrion G and Jimenez Y: Oral
cancer: Clinical features. Oral Oncol. 46:414–417. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
30
|
Guneri P and Epstein JB: Late stage
diagnosis of oral cancer: Components and possible solutions. Oral
Oncol. 50:1131–1136. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vokes EE: Induction chemotherapy for head
and neck cancer: Recent data. Oncologist. 15 (Suppl
3)(S3-S7)2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Marcazzan S, Varoni EM, Blanco E, Lodi G
and Ferrari M: Nanomedicine, an emerging therapeutic strategy for
oral cancer therapy. Oral Oncol. 76:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Furness S, Glenny AM, Worthington HV,
Pavitt S, Oliver R, Clarkson JE, Macluskey M, Chan KK and Conway
DI: Interventions for the treatment of oral cavity and
oropharyngeal cancer: Chemotherapy. Cochrane Database Syst Rev.
CD006386:2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Naruse T, Yanamoto S, Matsushita Y,
Sakamoto Y, Morishita K, Ohba S, Shiraishi T, Yamada SI, Asahina I
and Umeda M: Cetuximab for the treatment of locally advanced and
recurrent/metastatic oral cancer: An investigation of distant
metastasis. Mol Clin Oncol. 5:246–252. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Amit M, Yen TC, Liao CT, Chaturvedi P,
Agarwal JP, Kowalski LP, Ebrahimi A, Clark JR, Kreppel M, Zöller J,
et al: Improvement in survival of patients with oral cavity
squamous cell carcinoma: An international collaborative study.
Cancer. 119:4242–4248. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hanken H, Gaudin R, Gröbe A, Fraederich M,
Eichhorn W, Smeets R, Simon R, Sauter G, Grupp K, Izbicki JR, et
al: Her2 expression and gene amplification is rarely detectable in
patients with oral squamous cell carcinomas. J Oral Pathol Med.
43:304–308. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yan M, Schwaederle M, Arguello D, Millis
SZ, Gatalica Z and Kurzrock R: HER2 expression status in diverse
cancers: Review of results from 37,992 patients. Cancer Metastasis
Rev. 34:157–164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kondo N, Ishiguro Y, Kimura M, Sano D,
Fujita K, Sakakibara A, Taguchi T, Toth G, Matsuda H and Tsukuda M:
Antitumor effect of gefitinib on head and neck squamous cell
carcinoma enhanced by trastuzumab. Oncol Rep. 20:373–378.
2008.PubMed/NCBI
|
|
41
|
Kondo N, Tsukuda M, Sakakibara A,
Takahashi H, Hyakusoku H, Komatsu M, Niho T, Nakazaki K and Toth G:
Combined molecular targeted drug therapy for EGFR and HER-2 in head
and neck squamous cell carcinoma cell lines. Int J Oncol.
40:1805–1812. 2012.PubMed/NCBI View Article : Google Scholar
|