ADHD is characterized by inattention, motor
hyperactivity and impulsivity, affecting childhood and adolescence
until adulthood. ADHD is a childhood-onset neurodevelopmental
disorder with a worldwide prevalence of 1.4-3.0% (1). ADHD is associated with substance
misuse, oppositional defiant disorder, conduct disorder,
depression, post-traumatic stress disorder (PTSD), school or
occupational failure and criminality, and these comorbidities may
even lead to increased mortality in adulthood (2,3). Half of
the patients with ADHD have impairing symptoms persisting into
adolescence and 30-60% into adulthood (4). Therefore, the pathogenesis and causes
of ADHD warrant more attention.
The etiology of ADHD is complex, and genetic and
environmental factors have a role in it (1). ADHD is a familial disorder with high
heritability that ranges between 60 and 90% (5). Psychosocial risks, such as low income,
family adversity and hostile parenting, are strongly related to
ADHD and other psychiatric disorders (6). The relative risk of ADHD is 5-9 in
first-degree relatives of probands with ADHD (5). Several different classes of genomic
variants have been identified to be associated with ADHD (6). Candidate gene studies have revealed the
effects of genes associated with monoamine neurotransmitter systems
(1). The composite genetic risk
scores and copy number variants exhibit a significant overlap
between ADHD and schizophrenia and mood disorders (7). In addition, environmental factors are
significant risk factors for ADHD. Several lines of clinical
evidence suggest that prenatal and perinatal factors, environmental
toxins, and dietary and psychosocial factors may be potential risk
factors for ADHD (8). In-utero
exposure to maternal stress, cigarettes, alcohol, prescribed drugs
(e.g., paracetamol) and illegal drugs were reported to be
associated with ADHD (9).
Psychosocial risks, including low income, family adversity and
harsh or hostile parenting, have also been demonstrated to be
associated with ADHD and several other psychiatric disorders, such
as autism spectrum disorder (ASD) and obsessive-compulsive disorder
(10-12).
The occurrence of ADHD is based on the combined effects of genetic
and environmental factors.
Although ADHD is a heterogeneous disorder and the
pathogenesis has not been fully elucidated, studies in animal
models have suggested the involvement of dopaminergic,
noradrenergic and serotoninergic neurotransmission (11,13).
Structural and functional abnormalities in the cortical and
subcortical regions of the brain are also considered to be
characteristic of ADHD. For instance, a study including imaging
data of >3,000 patients with ADHD suggested that the volume of
the nucleus accumbens, amygdala, caudate nucleus, hippocampus and
putamen was reduced (14).
Methylphenidate (MPH), the first-line medical treatment for ADHD,
may cause side effects, including depression, compulsion and loss
of appetite (15). Furthermore, a
certain proportion of patients taking MPH did not achieve the
expected outcomes (16). To improve
the treatment of ADHD, it may thus be worthwhile to gain novel
insight into the pathological mechanisms.
Neuroinflammation acts as a double-edged sword,
which is an epiphenomenon following neuronal cell damage and also
an inherent host-defense mechanism to protect and restore the
normal structure and function of the brain against infection and
injury, contributing to the recovery of impaired neurons and to the
occurrence and aggravation of neurodegeneration (17). Neuroinflammation, particularly when
persistent, has an important role in central nervous system (CNS)
disorders, including neuroimmune diseases, neurodegenerative
diseases and other neuropsychiatric diseases, such as multiple
sclerosis (MS) (18), Parkinson's
disease (PD) (19), Alzheimer's
disease (AD) (20), stroke (21), depression (22), autism (23), schizophrenia (24) and chronic pain (25). Neuroinflammation differs from
inflammation at other sites with no dendritic cells involved.
Microglia and mast cells (MCs), which are natural immune cells of
the CNS, are mainly involved in the occurrence of neuroinflammation
(26). Astrocytes are also involved
in neuroinflammation (27).
Although the role of MCs is overlooked compared with
microglia, MCs remain an important factor in the immune signaling
pathway (29). MCs, the effector
cells of the innate immune system, are derived from hematopoietic
stem cells and multifunctional antigen-presenting cells and have a
pivotal role in immunoglobulin type E (IgE)-associated allergic and
inflammation-associated diseases (35). Despite their low numbers in most
organs, MCs are present in both healthy and disease states. MCs are
the first line of defense against invading pathogens and are
distributed in almost all organs and vascularized tissues (36). Blood MCs express CD34 and contain
cytoplasmic granules filled with heparin and histamine, the latter
of which is released after binding to IgE. Unlike other
myeloid-derived cells, tissue MCs have a hematopoietic
developmental lineage (37,38). During MC development, immature
lineage progenitors enter the circulation and are recruited to
peripheral tissues by endothelial cells, regulating the appearance
of granules with proteases (37,38).
Human MCs may be classified into mucosal and connective tissue
types according to the type of proteases present in their
cytoplasmic granules; the mucosal type contains tryptase, whereas
the connective tissue type contains both tryptase and chymase
(39). MCs act as first responders
and environmental ‘sensors’ to interact with other cellular
elements involved in physiological and immune responses, promoting
the neuroinflammation process (40).
MCs are present in various areas of the brain and meninges.
Although less distributed in the brain, they are generally found in
the subthalamic nucleus, choroid plexus and the parenchyma of the
hypothalamic region (41). The
pathogenic roles of MCs were indicated to extend from allergic
disease to autoimmune diseases and carcinogenesis (42-47).
The most common way through which MCs perform their
function is degranulation. The activation of the inflammatory
process results in a rapid release of MC granules into the
interstitium. MC granules contain pre-formed and newly synthesized
reactive chemicals known as MC mediators. These mediators include
histamine, tryptase, chymase, interleukin families, tumor necrosis
factor-α (TNF-α), serotonin, heparin, proteoglycans, vascular
endothelial growth factor (VEGF), prostaglandins, leukotrienes,
chemokines and growth factors, several of these are unique to MCs
(42,48). Studies have indicated that MC
degranulation may cause cognitive dysfunction (49). Large-scale MC degranulation may cause
fatal anaphylaxis; however, most physiological functions of MCs,
including regulation of inflammatory processes, occur without
complete degranulation (50). MCs
are phenotypically and functionally heterogeneous. The pathways and
results of MC activation are multifaceted. In addition to IgE, MCs
may also be activated through a number of other stimuli, including
trauma, other immunoglobulins, complements, toll-like receptors
(TLRs), neuropeptides, cytokines, chemokines and other inflammatory
products, causing mast cell activation and leading to the selective
release of mediators and/or stimulating T-cell proliferation,
differentiation and migration (51,52). A
characteristic of MC physiology that has been overlooked is that
MCs are able to secrete mediators via differential or selective
release without significant degranulation. This process may be
regulated by the action of distinct protein kinases on a unique
phosphoprotein (53). MCs undergo
changes in the core of the electron-dense granules but without
overt degranulation, a process that has been termed as activation,
intragranular activation or piecemeal degranulation (54). MCs are essential for the pathogenesis
of numerous inflammatory diseases, but this effect may only be
achieved if MCs release selective mediators without degranulation,
which may otherwise cause allergic reactions (52). Under normal circumstances, the brain
does not express IgE receptor (FcεRI), since the brain does not
display any allergic reactions and IgE does not cross the
blood-brain barrier (BBB) under normal conditions (55).
The ways in which the mediators are secreted depend
on the given stimuli and microenvironmental conditions. For
instance, serotonin may be selectively released without histamine
or arachidonic acid metabolites (56). The combination of TLR4 and mast cells
does not cause degranulation but results in the secretion of
inflammation-associated mediators. TLR4 binds to the co-receptors
CD14 and MD-2 expressed by MCs. Subsequently, activation by myeloid
differentiation primary response protein MyD88 innate immune signal
transduction adaptor results in activation of interleukin (IL)
receptor-associated kinase family members and pyruvate
dehydrogenase kinase isoform 1, mitogen-activated protein kinases
(MAPKs) p38 and JNK and to phospholipase A2(57). TLR4 also binds to lipopolysaccharides
(LPS) and induces TNF-α release without degranulation (58). LPS induces secretion of IL-5, IL-10
and IL-13 but not granulocyte-macrophage colony-stimulating factor,
IL-1 or leukotriene C4 (LTC4) (58). The selective release of IL-6 occurs
in the MC response to LPS, provided the presence of the PI3K
inhibitor wortmannin or stem cell factors (59). Corticotropin-releasing hormone (CRH)
was demonstrated to stimulate the selective release of VEGF without
degranulation and histamine or tryptase release from the human
leukemic mast cell line HMC-1 and human umbilical cord
blood-derived mast cells (60).
Neurotensin (NT) induces expression of CRH receptor (CRHR)-1 on MCs
and NT and CRH are released under stress via NT-CRH crosstalk
(61). IL-1 stimulates human MCs to
selectively release IL-6 without degranulation, via a unique
process utilizing 40-80 nm vesicles unrelated to the length of
secretory granules (800-1,000 nm) (62). IL-33 may serve as a potent activator
of MCs and was reported to promote MC survival, maturation,
migration and adhesion, and to selectively produce a variety of
pro-inflammatory cytokines, including IL-4, IL-5, IL-6, IL-8 and
IL-13 and chemokines including macrophage inflammatory protein-1α
and monocyte chemoattractant protein 1 (MCP-1) (63,64).
IL-33 enhances the role of the pro-inflammatory peptide substance P
in stimulating human MCs to secrete high levels of VEGF and TNF via
the interaction of neurokinin 1 and ST2 receptors without
concomitant secretion of tryptase (65). In the presence of stem cell factor,
IL-33 may also induce TNF production in MCs via a MAPK-activated
protein kinases 2 and 3, ERK1/2- and PI3K-dependent pathways
(66). Understanding the selective
release of mediators may explain how MCs participate in numerous
biological processes and how they are capable of exerting both
immunostimulatory and immunosuppressive effects.
Microglia and MCs are the two most important cell
types mediating and regulating neuroinflammation in the brain.
There is a close association between MCs and glial cells. MCs are
generally clustered near the glia in neuroinflammatory conditions
to recruit and activate other inflammatory cells, where
neuroinflammation already occurs in the brain. The contribution of
MCs and glia to neuroinflammation is strongly influenced by the
likelihood of their crosstalk and pathological exacerbation
(29). MCs may interact with
microglia and astrocytes via the complement system, proteases, TLRs
and chemokines. MCs may participate in the migration and activation
of glia, thereby affecting the release of inflammatory mediators.
The expression of ligand-receptor pairings may be upregulated under
inflammatory conditions, facilitating chemotactic actions through
contact between MC and glia (27).
For instance, C5a, the chemoattractant anaphylatoxin peptide and
its receptor CD88 are upregulated in the glia of inflammatory CNS
tissues (67-69).
Complementary expression of the C5a receptor on activated MCs
produces an intense chemoattractant signal to the C5a peptide and
intense crosstalk between C5a and TLR4, which also has a role in
neuroinflammation (67-69).
TLRs are a major class of pattern recognition receptors involved in
innate immunity. TLRs are associated with groups of pathogens
recognized by innate immune system cells, including microglia and
MCs, and act as a bridge between non-specific and specific immunity
(70). Upregulation of C-C motif
chemokine 5 (CCL5; also known as RANTES) by MC activation leads to
a pro-inflammatory response in microglia, releasing IL-6 and CCL5,
which in turn promotes chemokine expression in MC (71). IL-33 is an activator of MCs and IL-33
release from astrocytes may activate brain MCs and microglia
(72). The binding of IL-33 to MC
receptors leads to the secretion of IL-6, IL-13 and MCP-1 to
regulate microglia activity. Furthermore, IL-33 may be stimulated
from microglia pre-activated with pathogen-associated molecular
patterns via TLRs (73,74). Together, MC protease and matrix
metalloproteinase (MMP) activate p38, ERK1/2, MAPKs and
transcription factors including NF-κB in astrocytes, microglia and
MCs (75). IL-6 and TNF-α released
from microglia upregulate protease-activated receptor 2 (PAR2)
expression in MCs, causing MC activation and TNF-α release
(76). MC tryptase may induce the
release of pro-inflammatory mediators such as TNF-α, IL-6 and
reactive oxygen species (ROS) via the PAR2/MAPK/NF-κB signaling
pathway and activation of PAR2 receptors on MCs, which then
contributes to the development of microglia-mediated inflammation
in the brain (77). IL-6 induces
IL-13 release from MCs, affecting the expression of TLR2/TLR4.
Furthermore, TNF-α upregulates PAR2 expression in MCs and enhances
PAR2-mediated MC activation and degranulation (78-80).
C-X-C chemokine receptor type 4 (CXCR4; also known as stromal
cell-derived factor 1) is an MC chemotaxin and studies have
indicated that CXCR4 is upregulated in hypoxia and ischemia,
promoting the migration and activation of microglia (81). In addition to microglia, astrocytes
sharing a perivascular localization with MCs maintain the viability
of MCs. Astrocytes express histamine receptors and release
cytokines/chemokines through Rho-family
GTPases/Ca2+-dependent protein kinase C isoforms, MAPK,
NF-κB and signal transducer and activator of transcription 1
(82-84).
These trigger MC degranulation and enhance CD40L and CD40 surface
expression, leading to further inflammation (82-84).
Both microglia and astrocytes express histamine receptor
H1 (HRH1), HRH2 and
HRH3 and MCs may affect the activity of microglia and
astrocytes through these receptors (85,86). An
in vitro study has indicated that MC proteases may induce
demyelination and apoptosis of oligodendrocytes, while myelin
promotes MC degranulation (87).
Several experiments have confirmed the relationship between MCs and
glia. Co-culture of microglia and HMC-1 cells revealed that
activated HMC-1 cells stimulate the activation of microglia and
subsequent production of pro-inflammatory factors TNF-α and
IL-6(88). MC degranulator compound
48/80 induces microglia activation and inflammatory cytokine
production, triggering an acute brain inflammatory response.
However, the MC stabilizer cromolyn inhibits this effect, reduces
inflammatory cytokines and inhibits the MAPK, AKT and NF-κB
signaling pathways. Furthermore, cromolyn inhibits HRH1,
HRH4, protease activity, PAR2 and TLR4 in microglia
(49,89). Incubation of astrocytes and neurons
with 1-methyl-4-phenylpyridinium, glia maturation factor (GMF),
mouse MC protease-6 (MMCP-6) and MMCP-7 increased PAR-2 expression,
suggesting contact between MCs and astrocytes (90).
The connection between MCs and neurons mainly occurs
through peripheral interactions. A number of studies have revealed
the association between MCs and neurons in CNS neuroinflammation.
In the brain, the co-localization of MCs and neurons provides a
basis for neuroimmunological interactions. Cell adhesion molecule-1
(CADM1), expressed by mature hippocampal neurons, may have an
important role in the development of MC neuron interactions
(91). In the CNS, MC-derived
products may enter adjacent neurons to insert their granular
contents, a process known as granulation. In this way, MCs change
the internal environment of neurons, presenting a novel form of
neuroimmunological interaction (92). In addition, MCs express a series of
neurotransmitter receptors, which may be directly activated,
enhanced [neurokinin 1 receptor (NK1R), NK2R, NK3R and VIP receptor
type 2] or inhibited (acetylcholine receptor) (93,94).
Furthermore, it was reported that activated MCs enhanced
excitotoxic damage to 60% when co-cultured with hippocampal
neurons. In N-methyl-D-aspartate receptor-mediated synaptic
neurotransmission, MC-derived histamine directly increases the
death of hippocampal neurons (95).
Tryptase released by MCs may directly activate proteinase-activated
receptors on neurons and MC-derived TNF-α has a vital role in
neuronal development, cell survival, synaptic plasticity and ionic
homeostasis in the CNS (96). These
MC-neuron interactions are thought to be involved in the
pathogenesis of numerous neuroinflammatory diseases.
The association between chronic stress and
neuroinflammation has been confirmed by numerous studies. MCs have
a vital role in the mechanism of brain damage caused by chronic
stress on the brain. A variety of psychological and physiological
stresses may lead to changes in the expression, distribution and
activity of MCs in the CNS. Stress and pro-inflammatory cytokines
activate the HPA axis, thus leading to an increase in CRH and
arginine vasopressin release from the paraventricular nucleus of
the hypothalamus. HPA axis activation also enhances the expression
of CRH receptors, vascular permeability and MC activation (97). CRH released from MCs activates MCs
and glia in the CNS in an autocrine and paracrine manner in the
context of stress and neuroinflammation (98). In turn, activation of CNS MCs
activates the HPA axis. MCs are located near CRH-positive neurons
in the median eminence and are closely linked to
corticotropin-releasing factor receptors, which may be activated by
CRH (99). This may be closely
associated with the meningeal vasodilation and increased secretion
of cytokines during meningeal inflammation in migraines (46). Cao et al (100) indicated that intravesical stress,
CRH, MC activation and VEGFs have a crucial role in the
stress-induced deterioration of inflammation, which may provide
insight into the mechanism of brain stress. MC activation and CRH
release increase BBB permeability, leading to further brain damage
and contributing to chronic neuroinflammation in the brain
(60,101). Microglia express CRH receptors and
activation of microglia by CRH leads to the release of harmful
inflammatory mediators in psychiatric diseases, such as AD and pain
(102,103). Human MCs synthesize and secrete CRH
and express functional CRH receptors (CRHR1 and CRHR2) (104). CRHR1-mediated activation of
microglia induces microglia proliferation, TNF-α release and
activation of MAPK. CRHR1 also mediates stress-induced MC
degranulation (105). CRH release
from activated MCs may also activate glial cells in
neurodegenerative diseases such as AD (103,106).
Stressful conditions, including trauma or hypoxia, also activate
peripheral MCs, which in turn activate CRH and substance P
pathways, leading to BBB leakage and glial activation, causing
further neuroinflammation and neurodegeneration (107). CRH concentrations are higher in
brain regions prone to developing a pathology of AD (108). Elevated cortisol levels and HPA
axis dysfunction are implicated in chronic stress, which releases
amyloid beta (Aβ) that causes and/or worsens AD (109). CRHR1 antagonists have been
indicated to decrease stress-mediated oxidative damage, prevent
cognitive damage and loss of dendritic cells and reduce Aβ
deposition in the brain (110).
These results confirm the correlation between CRH and AD. Other
neuropeptides, including NT, may work with CRH to enhance MC
activation and release of excessive inflammatory mediators under
stress (61). CRH may enhance VEGF
release from human MCs and induce FcεRI expression in MCs, and this
effect may be blocked by the natural flavone luteolin (111). CRH is also implicated in the
pathogenesis of PD. Emotional chronic stress, which is closely
associated with CRH, enhances glial activation and aggravates
neuronal death through inflammation in the substantia nigra of the
brain of patients with PD (107).
Furthermore, observations in animal models of PD indicate that
stress-induced striatal damage may subsequently worsen motor
symptoms (112).
The BBB is composed of functional cerebral blood
vessels, which create a stable CNS environment and protect brain
parenchymal cells from harmful substances in the immune cells and
blood. The BBB consists of tightly connected endothelial junctions
and several intact transmembrane proteins, including claudin and
occludin, that ensure its integrity. The basal lamina, which is
part of the extracellular matrix, connects the endothelial cells of
the BBB to adjacent cell layers (113). BBB destruction involves the
accumulation of multiple vascular and neurotoxic molecules within
the brain parenchyma, decreased cerebral blood flow and hypoxia
(114). MCs are present in the dura
mater and meninges, as well as on the cerebral side of the BBB, and
MCs are in contact with the distal ends of the astrocytes (115). MCs may cross the BBB and
blood-spinal cord barrier when the barrier is damaged by CNS
pathologies. Inflammatory factors released by MC activation,
including histamine, tryptase, chymotrypsin and TNF-α, may regulate
BBB permeability (116).
Furthermore, TNF-α induces the expression of intercellular adhesion
molecule 1 (ICAM-1) and allows leukocytes to enter the affected
tissues in the brain (117). The me
chanism by which MCs destroy the BBB and promote basal layer
degradation may involve vascular activity and matrix degradation
components of MCs. MCs affect the integrity of the BBB through
MMPs, whose enzymatic activity may be regulated by tissue MMP
inhibitors. These include histamine and protease chymase, trypsin
and cathepsin G (118). Cathepsin G
activates MMPs, which degrade most of the protein components of the
neurovascular matrix (118). In
cerebral ischemic disease, MC degranulation increases, and brain
MCs affect the activation of acute microvascular gelatinases (MMP-2
and -9) by releasing proteases to affect BBB destruction. In
addition, elevated levels of VEGF may cause BBB rupture, vascular
leakage and edema, which in turn causes stroke (119,120).
This process extravasates glutamate and albumin, activates
astrocytes, alters K+ homeostasis in the brain
parenchyma and leads to excessive neuronal excitation and
inflammatory cell entry (119,120).
In experimental autoimmune encephalomyelitis (EAE), activation of
meningeal MCs leads to TNF-α production and early neutrophil
recruitment (121). This promotes
local BBB destruction, allowing initial immune cells to enter the
CNS and aggravate neuroinflammation (121). An in vitro study revealed
that TNF-α induces downregulation of tight junction proteins
occludin, claudin-5 and vascular endothelial-cadherin via an
increase in ROS, which leads to increased paracellular permeability
(122). IL-6 participates in the
effect of TNF-α on endothelial monolayers. TNF-α upregulates the
expression of ICAM-1 and vascular cell adhesion molecule-1 on brain
microvascular endothelial cells (123). ICAM-1 is involved in leukocyte
adhesion to the endothelium and its upregulation and
leukocyte-mediated BBB breakdown are one of the pathological
mechanisms and characteristics of various brain inflammatory
diseases, including MS (123).
Brain MCs may induce post-operative cognitive dysfunction by
destabilizing the BBB and acute stress may cause BBB breakdown by
activating MCs (88,124). In addition to cerebral ischemia,
BBB destruction has also been detected in dementia, motor neuron
disease, MS, AD and other neuropsychiatric disorders (125-128).
Substance P, which is released following traumatic brain injury or
under stress, activates MCs and glia, releasing neuroinflammatory
mediators and increasing BBB permeability (129). The release of CRH from MCs
contributes to the subsequent release of various neuroinflammatory
and neurotoxic mediators, leading to BBB rupture and glial cell
activation, chronic neuroinflammation in the brain and causing
autism (130). Cromoglycate, a
MC-stabilizing agent, reversed BBB destruction, brain edema and
neutrophil recruitment post-ischemia by inhibiting MC activation in
a stroke model (131).
There appears to be a high comorbidity between ADHD
and allergic, inflammatory and autoimmune diseases. Epidemiological
studies revealed that allergic diseases or conditions are closely
associated with psychological and behavioral problems in pre-school
children (132). A prospective
birth cohort study examining the association between atopic eczema
(AE) and ADHD was conducted. The results of the study indicated
that children with AE were susceptible to ADHD, which was more
obvious when they were at a younger age (133). Among early preterm-born children,
systemic inflammation during the first post-natal month appears to
increase the risk of teacher-identified ADHD characteristics
(134). A prospective cohort study
of 23,645 patients in Denmark suggested that a personal or maternal
history of autoimmune disease was linked to a high risk of ADHD
(135). A cross-sectional study
involving 2,500,118 individuals in Norway indicated that ADHD was
associated with psoriasis and Crohn's disease among females
(136).
Several clinical studies have reported elevated
levels of pro-inflammatory factors in the blood of children with
ADHD. A clinical trial in Taiwan involving 216 children with ADHD
and 216 non-ADHD controls indicated that the levels of hemoglobin
and 5-hydroxytryptamine receptor 3A (5-HT) in fasting venous blood
were significantly lower in children with ADHD compared with
controls, whereas IgE and eosinophil counts were elevated compared
with controls (137). A genome-wide
association analysis identified a link between ADHD and the gene
encoding IL-1 receptor antagonist (138). An association study of 546 patients
with ADHD and 546 controls demonstrated an association between
cytokine family ciliary neurotrophic factor receptor and both adult
and childhood ADHD (139).
Inflammatory processes may also increase the risk of ADHD in obese
individuals and peripheral inflammatory factor levels may aggravate
the severity of ADHD core symptoms (140,141).
The incidence of obesity and neuropsychiatric diseases has risen
rapidly over the past three decades in the US (142,143).
Epidemiological studies indicated that maternal obesity and
metabolic dysfunction increase the risk of ADHD, ASD, anxiety,
depression, schizophrenia and food addiction via the
neuroinflammatory pathway (144,145).
A review revealed that microbiota-gut-brain axis interactions
affect the pathogenesis of a variety of inflammation-associated
disorders, including mood disorders, ASD, ADHD, MS and obesity
(146). Evidence suggested that
patients displaying symptoms of ADHD have higher serum cytokine
levels compared with normal controls, including IL-1β, IL-2, IL-6,
IL-10, IL-13, IL-16, interferon (IFN)-γ and TNF-α (147-150).
An investigation on serum cytokine levels in children with ADHD
indicated that Purkinje cell antibodies were associated with the
ADHD group, suggesting that neuro-antibodies and cytokines may
contribute to ADHD (151). Patients
with ADHD administered MPH had lower cytokine levels compared with
those of unmedicated patients with ADHD (152). These data suggested that patients
with ADHD may be in a high inflammatory state and ADHD treatment
reduces cytokine levels. However, most studies involving a number
of cases only identified a slight association between ADHD and
peripheral inflammation but did not further explore the underlying
mechanisms. Several retrospective studies have indicated that
perinatal infection, preterm birth and low birth weight are closely
associated with the risk of ADHD-like symptoms (150,153).
White matter injury caused by preterm birth is associated with
maternal inflammation, perinatal infections and oxygen supply
interruption, and occurs through the activation of glia,
excitotoxicity and oxidative stress. Inflammation and hypoxia in
this process are risk factors for ASD, ADHD and other psychological
disorders (154). However, several
studies found no evidence supporting a link between ADHD and
inflammation in the brain. In a study on depression and anxiety in
from the Netherlands including 2,307 subjects indicated that there
was no evidence that ADHD development was associated with
dysregulation of inflammatory markers, and there was no interaction
between ADHD symptoms and stress-associated affective disorders
(155). Examination of the early
gestational maternal C-reactive protein in maternal serum and the
risk of ADHD in offspring suggests a lack of correlation (156). In addition to clinical studies,
several animal studies have identified or confirmed the association
between ADHD and inflammation. Kozlowska et al (157) concluded that there is an
interaction between neurological and immune systems in ADHD
pathogenesis. This conclusion was reached by examining the
concentration of cytokines, chemokines, oxidative stress markers,
metabolic parameters, steroid hormones and steroidogenic enzymes in
the serum and/or tissues of spontaneously hypertensive rats (ADHD
model) and Wistar Kyoto rats (control animals).
Several pieces of evidence indirectly revealed a
possible association between ADHD and inflammation. For instance,
vitamin D has a significant protective effect on inflammation,
oxidative stress and certain neurotrophic factors. Neurotransmitter
and vitamin D levels are lower in patients with ADHD compared with
those in healthy children (158).
Dietary antioxidant treatment may have a positive effect on nerve
damage caused by inflammation, oxidative stress and immune
dysfunction in ADHD (159). Iron
deficiency is considered to be a possible physiological etiology in
subsets of patients with ADHD and serum ferritin may be affected by
a variety of conditions, including inflammatory status (160). A randomized double-blinded
controlled trial revealed that children with ADHD have lower blood
levels of long-chain polyunsaturated fatty acids (PUFAs) compared
with children with no ADHD. Furthermore, following PUFA
supplementation, children with ADHD exhibited improvements in
ADHD-associated symptoms, thus supporting a link to pathways
responsible for inflammation in the body (161).
The intestines have a profound effect on the entire
body including the brain, and the role of gut-brain connections has
gradually been discovered. Food allergy is a common condition in
children and adolescents and is suggested to be one of the
gastrointestinal tract triggers for numerous psychological and
psychiatric conditions including depression, anxiety and ADHD
(162,163). A study indicated that the majority
of food allergies/intolerances are mediated by IgE. Following
continuous food exposure, allergens may bind conjugated IgE to
induce MC degranulation and the secretion of inflammatory
mediators, including cytokines, histamines, leukotrienes and
prostaglandin (164). IgE-mediated
allergic reactions are referred to as immediate type
hypersensitivities. In non-IgE-mediated reactions, the allergic
response may be mediated by Ig-free light chains or other cells
such as eosinophils, T cells and mast cells. Cell-mediated food
allergy, which is classified as delayed-type hypersensitivity, does
not involve Igs and symptom onset is observed from 1 h to days
after ingestion of the food protein (165,166).
Food intake may affect the behavior of children with ADHD through a
mechanism that involves a non-IgE-mediated, cell-mediated or
non-allergic response (167). A
cross-sectional study from China suggested that early food
allergies in school-age children are associated with ADHD (168). Children with ADHD reacted severely
to allergenic foods including cow's milk, wheat and eggs (167,169).
Studies have pointed out that for certain patients with ADHD,
dietary restrictions may provide significant benefits (170). A case study reported on a
7-year-old boy with ADHD and severe IgG-mediated food allergy who
presented reduced IgG antibody levels and improved behavior with
dietary supplement intervention (171). However, several studies obtained
negative results regarding the association between ADHD with food
allergies (172). One potential
reason for this may be the complexity of the IgE immune response to
food allergens in the gastrointestinal tract falling between the
tolerance and sensitization mechanisms (173). Based on conflicting results of
research, it was hypothesized that ADHD is not caused by allergic
reactions, but that ADHD itself is a (non-)allergic
hypersensitivity disorder (174,175).
Food-derived allergens trigger a hypersensitivity reaction that
causes ADHD-like symptoms, possibly via an IgE or non-IgE allergic
response or a non-allergic mechanism (174). Food allergy is closely linked to
MCs. MCs express various substances that may trigger enteric
neurons, including tryptase, histamine, 5-HT, nerve growth factor
and TNF-α (176). Allergic
reactions in the intestines may affect behavior through intestinal
mast cells, which may trigger intestinal neurons to transmit
information to the CNS via afferent sensory pathways (177). Activated MCs increase IL-6
production through the mTOR pathway (178). IL-6 was indicated to induce
behavioral defects and is enhanced in post-mortem brains of
patients with ASD (179). In
addition, gut microbes affect host social behavior through the
alteration of brain neural circuits and food allergies may affect
behaviors through gut bacteria. For instance, the
bacteroidete/firmicute ratio was increased in children with ASD and
propionic acid produced by gut bacteria may increase locomotor
activities and stereotyped behavior (180,181).
Although the possible association between MCs and
ADHD has not been previously reported, the role of MCs in other
brain disorders has been confirmed. For instance, studies have
suggested that patients suffering from PTSD have immune disorders
with an excessive inflammatory response. Patients with PTSD exhibit
chronic stress responses along with low-grade inflammation in the
body. Furthermore, MCs are also dysregulated in combat soldiers
(182). The number of MCs in the
skin, gastrointestinal tract and respiratory tract of patients with
PTSD is higher compared with that in individuals without PTSD
(183). In addition, patients with
PTSD have high levels of CRH and inflammatory factors, including
serum IL-6, IL-1β, TNF-α and IFN-γ. Increased expression of these
factors may be linked to MC activation (184). MC-derived substance P markedly
contributes to pain in patients with PTSD (185). Furthermore, PTSD is an important
risk factor for several autoimmune and MC-associated diseases,
including MS, rheumatoid arthritis, thyroiditis, lupus
erythematosus and IBD (186).
Multiple lines of evidence suggested that MC activation accelerates
the pathogenesis of AD in high-risk brain injury, trauma, stress
and PTSD. MCs are one of the first type of brain cell involved in
the pathogenesis of AD and respond early to Aβ formation (187). GMF regulates neuroinflammation via
the NACHT, LRR and PYD domains-containing protein 3 inflammasome in
brains of patients with AD (188).
Enhanced IL-33 and GMF expression was observed in the vicinity of
amyloid plaques and neurofibrillary tangles in human AD brains
(189). In AD, increased ROS
activates MCs to release inflammatory mediators and several
MC-derived inflammatory mediators were reported to be involved in
the pathogenesis and severity of AD (190). Mitochondrial uncoupling proteins
(UCPs) are implicated in neurodegenerative diseases and MCs express
UCP2 and UCP4(191). The
concentration of CRH is higher in areas prone to AD-associated
pathological changes (108). In the
brain of a rat model of AD, chymotrypsin-like proteases surrounded
the meninges and Aβ highly accumulated in cortical blood vessels,
and these proteases are thought to affect Aβ accumulation (192). MS is a chronic inflammatory disease
of the CNS, characterized by demyelination, immune cell
infiltration and axonal damage. MCs are present in perivascular
demyelinating lesions associated with immune cell infiltration and
in the CNS parenchyma and leptomeninges of patients with MS
(193). MCs may regulate the
transport of inflammatory cells through the BBB, thereby exerting
effects on MS and EAE (107,121).
Levels of histamine and tryptase are elevated in the cerebrospinal
fluid of patients with MS (194).
MCs may also be involved in the pathogenesis of ASD. Serum and
brain NT levels as are elevated in patients with ASD, which may
cause MC activation (195). TNF-α,
IL-6, MCP-1 and granulocyte macrophage colony-stimulating factor
were significantly increased in the brain tissue of patients with
ASD (179). Inflammation may induce
depression through different pathways. Elevated kynurenine levels
are associated with depression in humans. Kynurenine promotes
IgE-mediated reactions of MCs, including degranulation,
LTC4 release and IL-13 production through activation of
phospholipase C-γ1, Akt, MAPK p38 and intracellular calcium release
in an aryl hydrocarbon receptor-dependent manner, possibly
modulating MC responses (196).
Mastocytosis is a rare disease characterized by the accumulation
and activation of MCs, with a prevalence of depression ranging from
40-70% (197). A study of 54
patients with mastocytosis identified the role of MCs in the
tryptophan (TRP) catabolic pathway leading to depression. The
levels of TRP and serotonin were significantly lower in patients
with mastocytosis compared with healthy subjects, with higher
indoleamine-2,3-dioxygenase activity and higher levels of kynurenic
and quinolinic acids (198). This
demonstrated a TRP metabolism disorder in mastocytosis, and its
association with perceived stress and depression, thereby
indicating a close association between MCs and the development of
depression.
Various pieces of evidence suggested that ADHD may
be a neuroinflammatory disease and is closely linked to the
activation of MCs. The role of neuroinflammation and MCs in various
neuropsychiatric diseases, including ASD, AD, PD and depression,
has been elucidated. However, no studies have assessed the role of
MCs in ADHD. In the present review, it was hypothesized that ADHD
is a neuroinflammatory disease in which MCs have an important role.
The association between other brain diseases and MCs and the
inflammation-associated signal cascade induced by MC activation
allow for the hypothesis that MCs may induce the development and
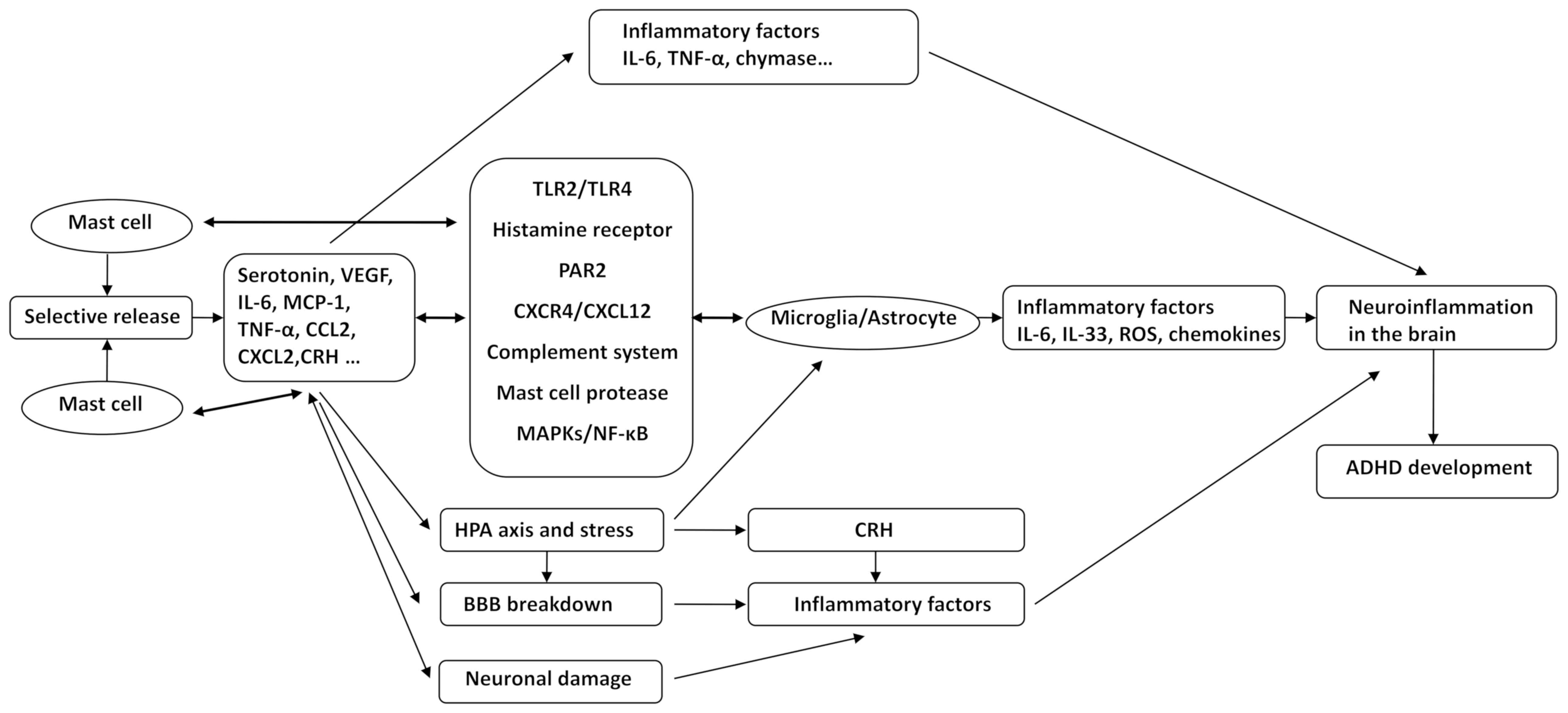
progression of ADHD through the following mechanisms: i) MCs
selectively release various neuroinflammatory mediators, including
IL-6, TNF-α, CRH and MCP-1. ii) Microglial and astrocyte activation
by MCs via CD40L, TLR2/TLR4, HRH, PAR2, CXCR4/CXCL12, the
complement system, MC protease, MAPKs and NF-κB, causes an
increased release of IL-6, IL-33, TNF-α, ROS and other inflammatory
factors; in turn, glia affect the activation of MCs through the
above-mentioned pathways. These pathological processes trigger and
exacerbate the state of inflammation in the brain. iii) MCs mediate
alterations of co-localized neurons through CADM1, enhancing
neuroimmune responses through a process called transgranulation.
Mediators released from MCs affect neurodevelopment in the CNS,
cause neuronal damage and trigger neuroinflammation. iv) Chronic
stress-mediated activation of the HPA axis, which enhances CRH
receptor expression and CNS MC activation, leads to microglia
activation, increased BBB permeability and release of inflammatory
mediators. v) Inflammatory mediators released by MC activation,
including histamine, tryptase, chymase and TNF-α, result in
increased expression of MMPs, VEGF, ICAM-1, as well as decreased
expression of occludin and claudin-5 and destruction of BBB
integrity. Inflammatory factors and inflammatory cells then enter
the brain tissue, aggravating neuroinflammation in the brain and
causing the occurrence and progression of ADHD (Fig. 1). Our group will endeavor to explore
and validate these hypotheses using clinical and in vivo
experiments.
With the enhanced requirement for life quality,
behavioral disorders such as ADHD are gaining increased attention.
However, at present, there is no consensus on the etiology,
pathogenesis and effective treatment of ADHD. The association
between ADHD and immunity or inflammation has recently been
discovered, but the underlying mechanisms have remained to be
elucidated. Previous studies (34,41,49,199)
have reported that MC activation is an important mechanism in the
progression of neuroinflammatory diseases. MCs are of significance
and easily overlooked in the immune system of the CNS. Based on the
study of ADHD and inflammation, as well as the association between
MCs and other neuropsychiatric diseases, it is reasonable to
speculate that MC-meditated neuroinflammation has a vital role in
ADHD. MC activation may lead to the selective release of
inflammatory factors and also affect the function of glial cells
via a number of ways, which in turn promotes the occurrence of CNS
neuroinflammation. In addition, brain MCs interact with neurons,
the BBB and the HPA axis, aggravating neuroinflammation and
disrupting brain function. MCs may promote CNS inflammation through
various pathways, further leading to the occurrence and
exacerbation of ADHD. In the present review, this hypothesis was
discussed based on previously published studies. To the best of our
knowledge, the association between MCs and ADHD appears to lack
sufficient evidence at present and this hypothesis is worthy of
further investigation using clinical studies and well-designed
experiments. The present study provided a perspective of
inflammatory mechanisms being accountable for ADHD. This hypothesis
may expand the current understanding of the onset of ADHD and
provide a novel target for the treatment of the condition.
The authors thank Dr Rongyi Zhou (The First
Affiliated Hospital of Henan University of Chinese Medicine,
Zhengzhou, China) and Dr Jichao Sun (The First Affiliated Hospital
of Guangxi University of Chinese Medicine, Nanning, China) for
their helpful advice, supervision and early studies in the field of
ADHD.
The present study was supported by the National
Natural Science Foundation of China (grant no. 81674023 and
81873342).
Not applicable.
XH conceived and designed the topic of the review.
YS and ML performed the literature search and wrote the manuscript.
HY and TC reviewed and edited the manuscript. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Thapar A and Cooper M: Attention deficit
hyperactivity disorder. Lancet. 387:1240–1250. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dalsgaard S, Ostergaard SD, Leckman JF,
Mortensen PB and Pedersen MG: Mortality in children, adolescents,
and adults with attention deficit hyperactivity disorder: A
nationwide cohort study. Lancet. 385:2190–2196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Howlett JR, Campbell-Sills L, Jain S,
Heeringa SG, Nock MK, Sun X, Ursano RJ and Stein MB: Attention
deficit hyperactivity disorder and risk of posttraumatic stress and
related disorders: A prospective longitudinal evaluation in U.S.
army soldiers. J Trauma Stress. 31:909–918. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ahmed R, Borst JM, Yong CW and Aslani P:
Do parents of children with attention-deficit/hyperactivity
disorder (ADHD) receive adequate information about the disorder and
its treatments? A qualitative investigation. Patient Prefer
Adherence. 8:661–670. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hawi Z, Cummins TD, Tong J, Johnson B, Lau
R, Samarrai W and Bellgrove MA: The molecular genetic architecture
of attention deficit hyperactivity disorder. Mol Psychiatry.
20:289–297. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Posner J, Polanczyk GV and Sonuga-Barke E:
Attention-deficit hyperactivity disorder. Lancet. 395:450–462.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Williams NM, Zaharieva I, Martin A,
Langley K, Mantripragada K, Fossdal R, Stefansson H, Stefansson K,
Magnusson P, Gudmundsson OO, et al: Rare chromosomal deletions and
duplications in attention-deficit hyperactivity disorder: A
genome-wide analysis. Lancet. 376:1401–1408. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Thapar A, Cooper M, Eyre O and Langley K:
What have we learnt about the causes of ADHD? J Child Psychol
Psychiatry. 54:3–16. 2013.
|
|
9
|
Thompson JM, Waldie KE, Wall CR, Murphy R
and Mitchell EA: ABC study group: Associations between
acetaminophen use during pregnancy and ADHD symptoms measured at
ages 7 and 11 years. PLoS One. 9(e108210)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Harold GT, Leve LD, Barrett D, Elam K,
Neiderhiser JM, Natsuaki MN, Shaw DS, Reiss D and Thapar A:
Biological and rearing mother influences on child ADHD symptoms:
Revisiting the developmental interface between nature and nurture.
J Child Psychol Psychiatry. 54:1038–1046. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Waltes R, Freitag CM, Herlt T, Lempp T,
Seitz C, Palmason H, Meyer J and Chiocchetti AG: Impact of
autism-associated genetic variants in interaction with
environmental factors on ADHD comorbidities: An exploratory pilot
study. J Neural Transm (Vienna). 126:1679–1693. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Grisham JR, Fullana MA, Mataix-Cols D,
Moffitt TE, Caspi A and Poulton R: Risk factors prospectively
associated with adult obsessive-compulsive symptom dimensions and
obsessive-compulsive disorder. Psychol Med. 41:2495–2506.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Russell VA: Overview of animal models of
attention deficit hyperactivity disorder (ADHD). Curr Protoc
Neurosci. 9:9–35. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hoogman M, Bralten J, Hibar DP, Mennes M,
Zwiers MP, Schweren LSJ, van Hulzen KJE, Medland SE, Shumskaya E,
Jahanshad N, et al: Subcortical brain volume differences in
participants with attention deficit hyperactivity disorder in
children and adults: A cross-sectional mega-analysis. Lancet
Psychiatry. 4:310–319. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gurkan K, Bilgic A, Turkoglu S, Kilic BG,
Aysev A and Uslu R: Depression, anxiety and obsessive-compulsive
symptoms and quality of life in children with attention-deficit
hyperactivity disorder (ADHD) during three-month methylphenidate
treatment. J Psychopharmacol. 24:1810–1818. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wilens TE: Effects of methylphenidate on
the catecholaminergic system in attention-deficit/hyperactivity
disorder. J Clin Psychopharmacol. 28 (3 Suppl 2):S46–S53.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lyman M, Lloyd DG, Ji X, Vizcaychipi MP
and Ma D: Neuroinflammation: The role and consequences. Neurosci
Res. 79:1–12. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Milo R, Korczyn AD, Manouchehri N and
Stüve O: The temporal and causal relationship between inflammation
and neurodegeneration in multiple sclerosis. Mult Scler.
4(1352458519886943)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Varley J, Brooks DJ and Edison P: Imaging
neuroinflammation in Alzheimer's disease and other dementias:
Recent advances and future directions. Alzheimers Dement.
11:1110–1120. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bradburn S, Murgatroyd C and Ray N:
Neuroinflammation in mild cognitive impairment and Alzheimer's
disease: A meta-analysis. Ageing Res Rev. 50:1–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jayaraj RL, Azimullah S, Beiram R, Jalal
FY and Rosenberg GA: Neuroinflammation: Friend and foe for ischemic
stroke. J Neuroinflammation. 16(142)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Woelfer M, Kasties V, Kahlfuss S and
Walter M: The role of depressive subtypes within the
neuroinflammation hypothesis of major depressive disorder.
Neuroscience. 403:93–110. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Matta SM, Hill-Yardin EL and Crack PJ: The
influence of neuroinflammation in autism spectrum disorder. Brain
Behav Immun. 79:75–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Calabrese V, Giordano J, Crupi R, Di Paola
R, Ruggieri M, Bianchini R, Ontario ML, Cuzzocrea S and Calabrese
EJ: Hormesis, cellular stress response and neuroinflammation in
schizophrenia: Early onset versus late onset state. J Neurosci Res.
95:1182–1193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huh Y, Ji RR and Chen G:
Neuroinflammation, bone marrow stem cells, and chronic pain. Front
Immunol. 8(1014)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lucas SM, Rothwell NJ and Gibson RM: The
role of inflammation in CNS injury and disease. Br J Pharmacol. 147
(Suppl 1):S232–S240. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Skaper SD, Facci L, Zusso M and Giusti P:
An inflammation-centric view of neurological disease: Beyond the
neuron. Front Cell Neurosci. 12(72)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Colonna M and Butovsky O: Microglia
function in the central nervous system during health and
neurodegeneration. Annu Rev Immunol. 35:441–468. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Skaper SD, Giusti P and Facci L: Microglia
and mast cells: Two tracks on the road to neuroinflammation. FASEB
J. 26:3103–3117. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang CJ, Jiang M, Zhou H, Liu W, Wang C,
Kang Z, Han B, Zhang Q, Chen X, Xiao J, et al: TLR-stimulated IRAKM
activates caspase-8 inflammasome in microglia and promotes
neuroinflammation. J Clin Invest. 128:5399–5412. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xiong XY, Liu L and Yang QW: Functions and
mechanisms of microglia/macrophages in neuroinflammation and
neurogenesis after stroke. Prog Neurobiol. 142:23–44.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Colombo E and Farina C: Astrocytes: Key
regulators of neuroinflammation. Trends Immunol. 37:608–620.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Montoya A, Elgueta D, Campos J, Chovar O,
Falcón P, Matus S, Alfaro I, Bono MR and Pacheco R: Dopamine
receptor D3 signalling in astrocytes promotes neuroinflammation. J
Neuroinflammation. 16(258)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kempuraj D, Selvakumar GP, Thangavel R,
Ahmed ME, Zaheer S, Raikwar SP, Iyer SS, Bhagavan SM,
Beladakere-Ramaswamy S and Zaheer A: Mast cell activation in brain
injury, stress, and post-traumatic stress disorder and Alzheimer's
disease pathogenesis. Front Neurosci. 11(703)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Krystel-Whittemore M, Dileepan KN and Wood
JG: Mast cell: A multi-functional master cell. Front Immunol.
6(620)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gilfillan AM, Austin SJ and Metcalfe DD:
Mast cell biology: Introduction and overview. Adv Exp Med Biol.
716:2–12. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Prussin C and Metcalfe DD: 4. IgE, mast
cells, basophils, and eosinophils. J Allergy Clin Immunol. 111
(Suppl 2):S486–S494. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Vyas H and Krishnaswamy G: Paul Ehrlich's
‘Mastzellen’-from aniline dyes to DNA chip arrays: A historical
review of developments in mast cell research. Methods Mol Biol.
315:3–11. 2006.PubMed/NCBI
|
|
39
|
Irani AM and Schwartz LB: Human mast cell
heterogeneity. Allergy Proc. 15:303–308. 1994.PubMed/NCBI View Article : Google Scholar
|
|
40
|
da Silva EZ, Jamur MC and Oliver C: Mast
cell function: A new vision of an old cell. J Histochem Cytochem.
62:698–738. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Polyzoidis S, Koletsa T, Panagiotidou S,
Ashkan K and Theoharides TC: Mast cells in meningiomas and brain
inflammation. J Neuroinflammation. 12(170)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kritikou E, Kuiper J, Kovanen PT and Bot
I: The impact of mast cells on cardiovascular diseases. Eur J
Pharmacol. 778:103–115. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sant GR, Kempuraj D, Marchand JE and
Theoharides TC: The mast cell in interstitial cystitis: Role in
pathophysiology and pathogenesis. Urology. 69 (Suppl 4):S34–S40.
2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Suurmond J, van der Velden D, Kuiper J,
Bot I and Toes RE: Mast cells in rheumatic disease. Eur J
Pharmacol. 778:116–124. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Theoharides TC, Donelan J,
Kandere-Grzybowska K and Konstantinidou A: The role of mast cells
in migraine pathophysiology. Brain Res Brain Res Rev. 49:65–76.
2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Theoharides TC, Angelidou A, Alysandratos
KD, Zhang B, Asadi S, Francis K, Toniato E and Kalogeromitros D:
Mast cell activation and autism. Biochim Biophys Acta. 1822:34–41.
2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Boyce JA: Mast cells and eicosanoid
mediators: A system of reciprocal paracrine and autocrine
regulation. Immunol Rev. 217:168–185. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Dong H, Wang Y, Zhang X, Zhang X, Qian Y,
Ding H and Zhang S: Stabilization of brain mast cells alleviates
LPS-induced neuroinflammation by inhibiting microglia activation.
Front Cell Neurosci. 13(191)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Forsythe P: Mast cells in neuroimmune
interactions. Trends Neurosci. 42:43–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Theoharides TC, Kempuraj D, Tagen M, Conti
P and Kalogeromitros D: Differential release of mast cell mediators
and the pathogenesis of inflammation. Immunol Rev. 217:65–78.
2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Theoharides TC and Cochrane DE: Critical
role of mast cells in inflammatory diseases and the effect of acute
stress. J Neuroimmunol. 146:1–12. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Theoharides TC, Sieghart W, Greengard P
and Douglas WW: Antiallergic drug cromolyn may inhibit histamine
secretion by regulating phosphorylation of a mast cell protein.
Science. 207:80–82. 1980.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dvorak AM: Basophils and mast cells:
Piecemeal degranulation in situ and ex vivo: A possible mechanism
for cytokine-induced function in disease. Immunol Ser. 57:169–271.
1992.PubMed/NCBI
|
|
55
|
Pang X, Letourneau R, Rozniecki JJ, Wang L
and Theoharides TC: Definitive characterization of rat hypothalamic
mast cells. Neuroscience. 73:889–902. 1996.PubMed/NCBI View Article : Google Scholar
|
|
56
|
van Haaster CM, Engels W, Lemmens PJ,
Hornstra G, van der Vusse GJ and Heemskerk JW: Differential release
of histamine and prostaglandin D2 in rat peritoneal mast cells:
Roles of cytosolic calcium and protein tyrosine kinases. Biochim
Biophys Acta. 1265:79–88. 1995.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lee CC, Avalos AM and Ploegh HL: Accessory
molecules for Toll-like receptors and their function. Nat Rev
Immunol. 12:168–179. 2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
McCurdy JD, Olynych TJ, Maher LH and
Marshall JS: Cutting edge: Distinct Toll-like receptor 2 activators
selectively induce different classes of mediator production from
human mast cells. J Immunol. 170:1625–1629. 2003.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sismanopoulos N, Delivanis DA,
Alysandratos KD, Angelidou A, Therianou A, Kalogeromitros D and
Theoharides TC: Mast cells in allergic and inflammatory diseases.
Curr Pharm Des. 18:2261–2277. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Cao J, Cetrulo CL and Theoharides TC:
Corticotropin-releasing hormone induces vascular endothelial growth
factor release from human mast cells via the cAMP/protein kinase
A/p38 mitogen-activated protein kinase pathway. Mol Pharmacol.
69:998–1006. 2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Alysandratos KD, Asadi S, Angelidou A,
Zhang B, Sismanopoulos N, Yang H, Critchfield A and Theoharides TC:
Neurotensin and CRH interactions augment human mast cell
activation. PLoS One. 7(e48934)2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kandere-Grzybowska K, Letourneau R,
Kempuraj D, Donelan J, Poplawski S, Boucher W, Athanassiou A and
Theoharides TC: IL-1 induces vesicular secretion of IL-6 without
degranulation from human mast cells. J Immunol. 171:4830–4836.
2003.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Bawazeer MA and Theoharides TC: IL-33
stimulates human mast cell release of CCL5 and CCL2 via MAPK and
NF-kB, inhibited by methoxyluteolin. Eur J Pharmacol.
865(172760)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Theoharides TC and Leeman SE: Effect of
IL-33 on de novo synthesized mediators from human mast cells. J
Allergy Clin Immunol. 143(451)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Taracanova A, Tsilioni I, Conti P, Norwitz
ER, Leeman SE and Theoharides TC: Substance P and IL-33
administered together stimulate a marked secretion of IL-1β from
human mast cells, inhibited by methoxyluteolin. Proc Natl Acad Sci
USA. 115:E9381–E9390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Drube S, Kraft F, Dudeck J, Muller AL,
Weber F, Gopfert C, Meininger I, Beyer M, Irmler I, Hafner N, et
al: MK2/3 are pivotal for IL-33-induced and mast cell-dependent
leukocyte recruitment and the resulting skin inflammation. J
Immunol. 197:3662–3668. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gasque P, Singhrao SK, Neal JW, Gotze O
and Morgan BP: Expression of the receptor for complement C5a (CD88)
is up-regulated on reactive astrocytes, microglia, and endothelial
cells in the inflamed human central nervous system. Am J Pathol.
150:31–41. 1997.PubMed/NCBI
|
|
68
|
Pundir P, MacDonald CA and Kulka M: The
novel receptor C5aR2 is required for C5a-mediated human mast cell
adhesion, migration, and proinflammatory mediator production. J
Immunol. 195:2774–2787. 2015.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yuan B, Fu F, Huang S, Lin C, Yang G, Ma
K, Shi H and Yang Z: C5a/C5aR pathway plays a vital role in brain
inflammatory injury via initiating fgl-2 in intracerebral
hemorrhage. Mol Neurobiol. 54:6187–6197. 2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Perkins DJ and Vogel SN: Inflammation:
Species-specific TLR signalling-insight into human disease. Nat Rev
Rheumatol. 12:198–200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Skaper SD: Impact of Inflammation on the
blood-neural barrier and blood-nerve interface: From review to
therapeutic preview. Int Rev Neurobiol. 137:29–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yasuoka S, Kawanokuchi J, Parajuli B, Jin
S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T and Suzumura A:
Production and functions of IL-33 in the central nervous system.
Brain Res. 1385:8–17. 2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Burnstock G, Krugel U, Abbracchio MP and
Illes P: Purinergic signalling: From normal behaviour to
pathological brain function. Prog Neurobiol. 95:229–274.
2011.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Chakraborty S, Kaushik DK, Gupta M and
Basu A: Inflammasome signaling at the heart of central nervous
system pathology. J Neurosci Res. 88:1615–1631. 2010.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kempuraj D, Thangavel R, Selvakumar GP,
Ahmed ME, Zaheer S, Raikwar SP, Zahoor H, Saeed D, Dubova I, Giler
G, et al: Mast cell proteases activate astrocytes and glia-neurons
and release interleukin-33 by activating p38 and ERK1/2 MAPKs and
NF-κB. Mol Neurobiol. 56:1681–1693. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhang S, Zeng X, Yang H, Hu G and He S:
Mast cell tryptase induces microglia activation via
protease-activated receptor 2 signaling. Cell Physiol Biochem.
29:931–940. 2012.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Pietrzak A, Wierzbicki M, Wiktorska M and
Brzezinska-Blaszczyk E: Surface TLR2 and TLR4 expression on mature
rat mast cells can be affected by some bacterial components and
proinflammatory cytokines. Mediators Inflamm.
2011(427473)2011.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zhang H, Lin L, Yang H, Zhang Z, Yang X,
Zhang L and He S: Induction of IL-13 production and upregulation of
gene expression of protease activated receptors in P815 cells by
IL-6. Cytokine. 50:138–145. 2010.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Zhang H, Yang H and He S: TNF increases
expression of IL-4 and PARs in mast cells. Cell Physiol Biochem.
26:327–336. 2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Wang X, Li C, Chen Y, Hao Y, Zhou W, Chen
C and Yu Z: Hypoxia enhances CXCR4 expression favoring microglia
migration via HIF-1alpha activation. Biochem Biophys Res Commun.
371:283–288. 2008.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Wang Y, Huang J, Li Y and Yang GY: Roles
of chemokine CXCL12 and its receptors in ischemic stroke. Curr Drug
Targets. 13:166–172. 2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Dong Y and Benveniste EN: Immune function
of astrocytes. Glia. 36:180–190. 2001.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Kim DY, Hong GU and Ro JY: Signal pathways
in astrocytes activated by cross-talk between of astrocytes and
mast cells through CD40-CD40L. J Neuroinflammation.
8(25)2011.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Seeldrayers PA, Levin LA and Johnson D:
Astrocytes support mast cell viability in vitro. J Neuroimmunol.
36:239–243. 1992.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Dong H, Zhang W, Zeng X, Hu G, Zhang H, He
S and Zhang S: Histamine induces upregulated expression of
histamine receptors and increases release of inflammatory mediators
from microglia. Mol Neurobiol. 49:1487–1500. 2014.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Mele T and Juric DM: Identification and
pharmacological characterization of the histamine H3 receptor in
cultured rat astrocytes. Eur J Pharmacol. 720:198–204.
2013.PubMed/NCBI
|
|
87
|
Medic N, Vita F, Abbate R, Soranzo MR,
Pacor S, Fabbretti E, Borelli V and Zabucchi G: Mast cell
activation by myelin through scavenger receptor. J Neuroimmunol.
200:27–40. 2008.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Zhang S, Dong H, Zhang X, Li N, Sun J and
Qian Y: Cerebral mast cells contribute to postoperative cognitive
dysfunction by promoting blood brain barrier disruption. Behav
Brain Res. 298(Pt B):158–166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Dong H, Zhang X, Wang Y, Zhou X, Qian Y
and Zhang S: Suppression of brain mast cells degranulation inhibits
microglial activation and central nervous system inflammation. Mol
Neurobiol. 54:997–1007. 2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Kempuraj D, Selvakumar GP, Thangavel R,
Ahmed ME, Zaheer S, Kumar KK, Yelam A, Kaur H, Dubova I, Raikwar
SP, et al: Glia maturation factor and mast cell-dependent
expression of inflammatory mediators and proteinase activated
receptor-2 in neuroinflammation. J Alzheimers Dis. 66:1117–1129.
2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Hagiyama M, Furuno T, Hosokawa Y, Iino T,
Ito T, Inoue T, Nakanishi M, Murakami Y and Ito A: Enhanced
nerve-mast cell interaction by a neuronal short isoform of cell
adhesion molecule-1. J Immunol. 186:5983–5992. 2011.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Wilhelm M, Silver R and Silverman AJ:
Central nervous system neurons acquire mast cell products via
transgranulation. Eur J Neurosci. 22:2238–2248. 2005.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kulka M, Sheen CH, Tancowny BP, Grammer LC
and Schleimer RP: Neuropeptides activate human mast cell
degranulation and chemokine production. Immunology. 123:398–410.
2008.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Masini E, Fantozzi R, Conti A, Blandina P,
Brunelleschi S and Mannaioni PF: Mast cell heterogeneity in
response to cholinergic stimulation. Int Arch Allergy Appl Immunol.
77:184–185. 1985.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Skaper SD, Facci L, Kee WJ and Strijbos
PJ: Potentiation by histamine of synaptically mediated
excitotoxicity in cultured hippocampal neurones: A possible role
for mast cells. J Neurochem. 76:47–55. 2001.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Ossovskaya VS and Bunnett NW:
Protease-activated receptors: Contribution to physiology and
disease. Physiol Rev. 84:579–621. 2004.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Claes SJ: Corticotropin-releasing hormone
(CRH) in psychiatry: From stress to psychopathology. Ann Med.
36:50–61. 2004.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Karagkouni A, Alevizos M and Theoharides
TC: Effect of stress on brain inflammation and multiple sclerosis.
Autoimmun Rev. 12:947–953. 2013.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Cao J, Papadopoulou N, Kempuraj D, Boucher
WS, Sugimoto K, Cetrulo CL and Theoharides TC: Human mast cells
express corticotropin-releasing hormone (CRH) receptors and CRH
leads to selective secretion of vascular endothelial growth factor.
J Immunol. 174:7665–7675. 2005.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Cao J, Boucher W, Kempuraj D, Donelan JM
and Theoharides TC: Acute stress and intravesical
corticotropin-releasing hormone induces mast cell dependent
vascular endothelial growth factor release from mouse bladder
explants. J Urol. 176:1208–1213. 2006.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Rozniecki JJ, Sahagian GG, Kempuraj D, Tao
K, Jocobson S, Zhang B and Theoharides TC: Brain metastases of
mouse mammary adenocarcinoma is increased by acute stress. Brain
Res. 1366:204–210. 2010.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Kritas SK, Caraffa A, Antinolfi P, Saggini
A, Pantalone A, Rosati M, Tei M, Speziali A, Saggini R, Pandolfi F,
et al: Nerve growth factor interactions with mast cells. Int J
Immunopathol Pharmacol. 27:15–19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Kempuraj D, Mentor S, Thangavel R, Ahmed
ME, Selvakumar GP, Raikwar SP, Dubova I, Zaheer S, Iyer SS and
Zaheer A: Mast cells in stress, pain, blood-brain barrier,
neuroinflammation and Alzheimer's disease. Front Cell Neurosci.
13(54)2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Papadopoulou NG, Oleson L, Kempuraj D,
Donelan J, Cetrulo CL and Theoharides TC: Regulation of
corticotropin-releasing hormone receptor-2 expression in human cord
blood-derived cultured mast cells. J Mol Endocrinol. 35:R1–R8.
2005.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Ayyadurai S, Gibson AJ, D'Costa S, Overman
EL, Sommerville LJ, Poopal AC, Mackey E, Li Y and Moeser AJ:
Frontline science: Corticotropin-releasing factor receptor subtype
1 is a critical modulator of mast cell degranulation and
stress-induced pathophysiology. J Leukoc Biol. 102:1299–1312.
2017.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Kempuraj D, Papadopoulou NG, Lytinas M,
Huang M, Kandere-Grzybowska K, Madhappan B, Boucher W,
Christodoulou S, Athanassiou A and Theoharides TC:
Corticotropin-releasing hormone and its structurally related
urocortin are synthesized and secreted by human mast cells.
Endocrinology. 145:43–48. 2004.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Kempuraj D, Thangavel R, Selvakumar GP,
Zaheer S, Ahmed ME, Raikwar SP, Zahoor H, Saeed D, Natteru PA, Iyer
S and Zaheer A: Brain and peripheral atypical inflammatory
mediators potentiate neuroinflammation and neurodegeneration. Front
Cell Neurosci. 11(216)2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Pedersen WA, McCullers D, Culmsee C,
Haughey NJ, Herman JP and Mattson MP: Corticotropin-releasing
hormone protects neurons against insults relevant to the
pathogenesis of Alzheimer's disease. Neurobiol Dis. 8:492–503.
2001.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Morgese MG, Schiavone S and Trabace L:
Emerging role of amyloid beta in stress response: Implication for
depression and diabetes. Eur J Pharmacol. 817:22–29.
2017.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Zhang C and Rissman RA:
Corticotropin-releasing factor receptor-1 modulates biomarkers of
DNA oxidation in Alzheimer's disease mice. PLoS One.
12(e181367)2017.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Asadi S and Theoharides TC:
Corticotropin-releasing hormone and extracellular mitochondria
augment IgE-stimulated human mast-cell vascular endothelial growth
factor release, which is inhibited by luteolin. J
Neuroinflammation. 9(85)2012.PubMed/NCBI View Article : Google Scholar
|
|
112
|
de Pablos RM, Herrera AJ, Espinosa-Oliva
AM, Sarmiento M, Munoz MF, Machado A and Venero JL: Chronic stress
enhances microglia activation and exacerbates death of nigral
dopaminergic neurons under conditions of inflammation. J
Neuroinflammation. 11(34)2014.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Strbian D, Kovanen PT,
Karjalainen-Lindsberg ML, Tatlisumak T and Lindsberg PJ: An
emerging role of mast cells in cerebral ischemia and hemorrhage.
Ann Med. 41:438–450. 2009.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Profaci CP, Munji RN, Pulido RS and
Daneman R: The blood-brain barrier in health and disease: Important
unanswered questions. J Exp Med. 217(e20190062)2020.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Silver R and Curley JP: Mast cells on the
mind: New insights and opportunities. Trends Neurosci. 36:513–521.
2013.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Skaper SD: Mast cell-glia dialogue in
chronic pain and neuropathic pain: Blood-brain barrier
implications. CNS Neurol Disord Drug Targets. 15:1072–1078.
2016.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Lindsberg PJ, Strbian D and
Karjalainen-Lindsberg ML: Mast cells as early responders in the
regulation of acute blood-brain barrier changes after cerebral
ischemia and hemorrhage. J Cereb Blood Flow Metab. 30:689–702.
2010.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Tchougounova E, Lundequist A, Fajardo I,
Winberg JO, Abrink M and Pejler G: A key role for mast cell chymase
in the activation of pro-matrix metalloprotease-9 and pro-matrix
metalloprotease-2. J Biol Chem. 280:9291–9296. 2005.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Lapilover EG, Lippmann K, Salar S,
Maslarova A, Dreier JP, Heinemann U and Friedman A: Peri-infarct
blood-brain barrier dysfunction facilitates induction of spreading
depolarization associated with epileptiform discharges. Neurobiol
Dis. 48:495–506. 2012.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Mattila OS, Strbian D, Saksi J,
Pikkarainen TO, Rantanen V, Tatlisumak T and Lindsberg PJ: Cerebral
mast cells mediate blood-brain barrier disruption in acute
experimental ischemic stroke through perivascular gelatinase
activation. Stroke. 42:3600–3605. 2011.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Christy AL, Walker ME, Hessner MJ and
Brown MA: Mast cell activation and neutrophil recruitment promotes
early and robust inflammation in the meninges in EAE. J Autoimmun.
42:50–61. 2013.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Rochfort KD and Cummins PM:
Cytokine-mediated dysregulation of zonula occludens-1 properties in
human brain microvascular endothelium. Microvasc Res. 100:48–53.
2015.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Liu H, Luiten PG, Eisel UL, Dejongste MJ
and Schoemaker RG: Depression after myocardial infarction:
TNF-α-induced alterations of the blood-brain barrier and its
putative therapeutic implications. Neurosci Biobehav Rev.
37:561–572. 2013.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Esposito P, Gheorghe D, Kandere K, Pang X,
Connolly R, Jacobson S and Theoharides TC: Acute stress increases
permeability of the blood-brain-barrier through activation of brain
mast cells. Brain Res. 888:117–127. 2001.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Alvarez JI, Cayrol R and Prat A:
Disruption of central nervous system barriers in multiple
sclerosis. Biochim Biophys Acta. 1812:252–264. 2011.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Bell RD, Winkler EA, Sagare AP, Singh I,
LaRue B, Deane R and Zlokovic BV: Pericytes control key
neurovascular functions and neuronal phenotype in the adult brain
and during brain aging. Neuron. 68:409–427. 2010.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Henkel JS, Beers DR, Wen S, Bowser R and
Appel SH: Decreased mRNA expression of tight junction proteins in
lumbar spinal cords of patients with ALS. Neurology. 72:1614–1616.
2009.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Khalil MH, Silverman AJ and Silver R: Mast
cells in the rat brain synthesize gonadotropin-releasing hormone. J
Neurobiol. 56:113–124. 2003.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Corrigan F, Mander KA, Leonard AV and Vink
R: Neurogenic inflammation after traumatic brain injury and its
potentiation of classical inflammation. J Neuroinflammation.
13(264)2016.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Theoharides TC, Asadi S and Patel AB:
Focal brain inflammation and autism. J Neuroinflammation.
10(46)2013.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Strbian D, Karjalainen-Lindsberg ML,
Tatlisumak T and Lindsberg PJ: Cerebral mast cells regulate early
ischemic brain swelling and neutrophil accumulation. J Cereb Blood
Flow Metab. 26:605–612. 2006.PubMed/NCBI View Article : Google Scholar
|
|
132
|
van der Schans J, Pleiter JC, de Vries TW,
Schuiling-Veninga CC, Bos JH, Hoekstra PJ and Hak E: Association
between medication prescription for atopic diseases and
attention-deficit/hyperactivity disorder. Ann Allergy Asthma
Immunol. 117:186–191. 2016.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Genuneit J, Braig S, Brandt S, Wabitsch M,
Florath I, Brenner H and Rothenbacher D: Infant atopic eczema and
subsequent attention-deficit/hyperactivity disorder-a prospective
birth cohort study. Pediatr Allergy Immunol. 25:51–56.
2014.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Allred EN, Dammann O, Fichorova RN, Hooper
SR, Hunter SJ, Joseph RM, Kuban K, Leviton A, O'Shea TM and Scott
MN: ELGAN Study ADHD symptoms writing group for the ELGAN Study
Investigators: Systemic inflammation during the first postnatal
month and the risk of attention deficit hyperactivity disorder
characteristics among 10 year-old children born extremely preterm.
J Neuroimmune Pharmacol. 12:531–543. 2017.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Nielsen PR, Benros ME and Dalsgaard S:
Associations between autoimmune diseases and
attention-deficit/hyperactivity disorder: A nationwide study. J Am
Acad Child Adolesc Psychiatry. 56:234–240. 2017.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Hegvik TA, Instanes JT, Haavik J,
Klungsoyr K and Engeland A: Associations between
attention-deficit/hyperactivity disorder and autoimmune diseases
are modified by sex: A population-based cross-sectional study. Eur
Child Adolesc Psychiatry. 27:663–675. 2018.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Wang LJ, Yu YH, Fu ML, Yeh WT, Hsu JL,
Yang YH, Chen WJ, Chiang BL and Pan WH: Attention
deficit-hyperactivity disorder is associated with allergic symptoms
and low levels of hemoglobin and serotonin. Sci Rep.
8(10229)2018.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Segman RH, Meltzer A, Gross-Tsur V, Kosov
A, Frisch A, Inbar E, Darvasi A, Levy S, Goltser T, Weizman A and
Galili-Weisstub E: Preferential transmission of interleukin-1
receptor antagonist alleles in attention deficit hyperactivity
disorder. Mol Psychiatry. 7:72–74. 2002.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Ribases M, Hervas A, Ramos-Quiroga JA,
Bosch R, Bielsa A, Gastaminza X, Fernandez-Anguiano M, Nogueira M,
Gomez-Barros N, Valero S, et al: Association study of 10 genes
encoding neurotrophic factors and their receptors in adult and
child attention-deficit/hyperactivity disorder. Biol Psychiatry.
63:935–945. 2008.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Cortese S and Vincenzi B: Obesity and
ADHD: Clinical and neurobiological implications. Curr Top Behav
Neurosci. 9:199–218. 2012.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Cortese S, Angriman M, Comencini E,
Vincenzi B and Maffeis C: Association between inflammatory
cytokines and ADHD symptoms in children and adolescents with
obesity: A pilot study. Psychiatry Res. 278:7–11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Boyle CA, Boulet S, Schieve LA, Cohen RA,
Blumberg SJ, Yeargin-Allsopp M, Visser S and Kogan MD: Trends in
the prevalence of developmental disabilities in US children,
1997-2008. Pediatrics. 127:1034–1042. 2011.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Olfson M, Blanco C, Wang S, Laje G and
Correll CU: National trends in the mental health care of children,
adolescents, and adults by office-based physicians. JAMA
Psychiatry. 71:81–90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Rivera HM, Christiansen KJ and Sullivan
EL: The role of maternal obesity in the risk of neuropsychiatric
disorders. Front Neurosci. 9(194)2015.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Sullivan EL, Riper KM, Lockard R and
Valleau JC: Maternal high-fat diet programming of the
neuroendocrine system and behavior. Horm Behav. 76:153–161.
2015.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Petra AI, Panagiotidou S, Hatziagelaki E,
Stewart JM, Conti P and Theoharides TC: Gut-microbiota-brain axis
and its effect on neuropsychiatric disorders with suspected immune
dysregulation Clin. Ther. 37:984–995. 2015.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Anand D, Colpo GD, Zeni G, Zeni CP and
Teixeira AL: Attention-deficit/hyperactivity disorder and
inflammation: What does current knowledge tell Us? A systematic
review. Front Psychiatry. 8(228)2017.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Darwish AH, Elgohary TM and Nosair NA:
Serum interleukin-6 level in children with attention-deficit
hyperactivity disorder (ADHD). J Child Neurol. 34:61–67.
2019.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Donfrancesco R, Nativio P, Borrelli E,
Giua E, Andriola E, Villa MP and DI Trani M: Serum cytokines in
paediatric neuropsychiatric syndromes: Focus on attention deficit
hyperactivity disorder. Minerva Pediatr: 2016 (Epub ahead of
print).
|
|
150
|
O'Shea TM, Joseph RM, Kuban KC, Allred EN,
Ware J, Coster T, Fichorova RN, Dammann O and Leviton A: ELGAN
Study Investigators: Elevated blood levels of inflammation-related
proteins are associated with an attention problem at age 24 mo in
extremely preterm infants. Pediatr Res. 75:781–787. 2014.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Donfrancesco R, Nativio P, Di Benedetto A,
Villa MP, Andriola E, Melegari MG, Cipriano E and Di Trani M:
Anti-yo antibodies in children with ADHD: First results about serum
cytokines. J Atten Disord 2016 (Epub ahead of print).
|
|
152
|
Oades RD, Dauvermann MR, Schimmelmann BG,
Schwarz MJ and Myint AM: Attention-deficit hyperactivity disorder
(ADHD) and glial integrity: S100B, cytokines and kynurenine
metabolism-effects of medication. Behav Brain Funct.
6(29)2010.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Rand KM, Austin NC, Inder TE, Bora S and
Woodward LJ: Neonatal infection and later neurodevelopmental risk
in the very preterm infant. J Pediatr. 170:97–104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
154
|
van Tilborg E, Heijnen CJ, Benders MJ, van
Bel F, Fleiss B, Gressens P and Nijboer CH: Impaired
oligodendrocyte maturation in preterm infants: Potential
therapeutic targets. Prog Neurobiol. 136:28–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Vogel SWN, Bijlenga D, Verduijn J, Bron
TI, Beekman ATF, Kooij JJS and Penninx BWJH:
Attention-deficit/hyperactivity disorder symptoms and
stress-related biomarkers. Psychoneuroendocrinology. 79:31–39.
2017.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Chudal R, Brown AS, Gyllenberg D,
Hinkka-Yli-Salomaki S, Sucksdorff M, Surcel HM, Upadhyaya S and
Sourander A: Maternal serum C-reactive protein (CRP) and offspring
attention deficit hyperactivity disorder (ADHD). Eur Child Adolesc
Psychiatry. 29:239–247. 2020.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Kozlowska A, Wojtacha P, Rowniak M,
Kolenkiewicz M and Huang ACW: ADHD pathogenesis in the immune,
endocrine and nervous systems of juvenile and maturating SHR and
WKY rats. Psychopharmacology (Berl). 236:2937–2958. 2019.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Saedisomeolia A, Samadi M, Gholami F,
Seyedi M, Effatpanah M, Hashemi R, Abdolahi M and Honarvar NM:
Vitamin d's molecular action mechanism in attention-deficit/
hyperactivity disorder: A review of evidence. CNS Neurol Disord
Drug Targets. 17:280–290. 2018.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Verlaet AAJ, Maasakkers CM, Hermans N and
Savelkoul HFJ: Rationale for dietary antioxidant treatment of ADHD.
Nutrients. 10(E405)2018.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Cortese S and Angriman M:
Attention-deficit/hyperactivity disorder, iron deficiency, and
obesity: Is there a link? Postgrad Med. 126:155–170.
2014.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Kean JD, Camfield D, Sarris J, Kras M,
Silberstein R, Scholey A and Stough C: A randomized controlled
trial investigating the effects of PCSO-524, a patented oil extract
of the New Zealand green lipped mussel (Perna canaliculus), on the
behaviour, mood, cognition and neurophysiology of children and
adolescents (aged 6-14 years) experiencing clinical and
sub-clinical levels of hyperactivity and inattention: Study
protocol ACTRN12610000978066. Nutr J. 12(100)2013.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Ghunaim N, Gronlund H, Kronqvist M,
Gronneberg R, Soderstrom L, Ahlstedt S and van Hage-Hamsten M:
Antibody profiles and self-reported symptoms to pollen-related food
allergens in grass pollen-allergic patients from northern Europe.
Allergy. 60:185–191. 2005.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Zhou L, Chen L, Li X, Li T, Dong Z and
Wang YT: Food allergy induces alteration in brain inflammatory
status and cognitive impairments. Behav Brain Res. 364:374–382.
2019.PubMed/NCBI View Article : Google Scholar
|
|
164
|
de Theije CG, Bavelaar BM, Lopes da Silva
S, Korte SM, Olivier B, Garssen J and Kraneveld AD: Food allergy
and food-based therapies in neurodevelopmental disorders. Pediatr
Allergy Immunol. 25:218–226. 2014.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Sarlus H, Höglund CO, Karshikoff B, Wang
X, Lekander M, Schultzberg M and Oprica M: Allergy influences the
inflammatory status of the brain and enhances tau-phosphorylation.
J Cell Mol Med. 16:2401–2412. 2012.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Niggemann B, Reibel S, Roehr CC, Felger D,
Ziegert M, Sommerfeld C and Wahn U: Predictors of positive food
challenge outcome in non-IgE-mediated reactions to food in children
with atopic dermatitis. J Allergy Clin Immunol. 108:1053–1058.
2001.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Milosz M, Demkow U and Wolanczyk T:
Relation between attention-deficit hyperactivity disorder and
IgE-dependent allergy in pediatric patients. Adv Exp Med Biol.
1096:105–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Jiang X, Shen C, Dai Y, Jiang F, Li S,
Shen X, Hu Y and Li F: Early food allergy and respiratory allergy
symptoms and attention-deficit/hyperactivity disorder in Chinese
children: A cross-sectional study. Pediatr Allergy Immunol.
29:402–409. 2018.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Boris M and Mandel FS: Foods and additives
are common causes of the attention deficit hyperactive disorder in
children. Ann Allergy. 72:462–468. 1994.PubMed/NCBI
|
|
170
|
Verlaet AA, Noriega DB, Hermans N and
Savelkoul HF: Nutrition, immunological mechanisms and dietary
immunomodulation in ADHD. Eur Child Adolesc Psychiatry. 23:519–529.
2014.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Ritz BW and Lord RS: Case study: The
effectiveness of a dietary supplement regimen in reducing
IgG-mediated food sensitivity in ADHD. Altern Ther Health Med.
11:72–75. 2005.PubMed/NCBI
|
|
172
|
Miyazaki C, Koyama M, Ota E, Swa T, Mlunde
LB, Amiya RM, Tachibana Y, Yamamoto-Hanada K and Mori R: Allergic
diseases in children with attention deficit hyperactivity disorder:
A systematic review and meta-analysis. BMC Psychiatry.
17(120)2017.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Johnston LK, Chien KB and Bryce PJ: The
immunology of food allergy. J Immunol. 192:2529–2534.
2014.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Pelsser LM, Buitelaar JK and Savelkoul HF:
ADHD as a (non) allergic hypersensitivity disorder: A hypothesis.
Pediatr Allergy Immunol. 20:107–112. 2009.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Suwan P, Akaramethathip D and Noipayak P:
Association between allergic sensitization and attention deficit
hyperactivity disorder (ADHD). Asian Pac J Allergy Immunol.
29:57–65. 2011.PubMed/NCBI
|
|
176
|
Rijnierse A, Nijkamp FP and Kraneveld AD:
Mast cells and nerves tickle in the tummy: Implications for
inflammatory bowel disease and irritable bowel syndrome. Pharmacol
Ther. 116:207–235. 2007.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Gui XY: Mast cells: A possible link
between psychological stress, enteric infection, food allergy and
gut hypersensitivity in the irritable bowel syndrome. J
Gastroenterol Hepatol. 13:980–989. 1998.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Hirano T, Taga T, Nakano N, Yasukawa K,
Kashiwamura S, Shimizu K, Nakajima K, Pyun KH and Kishimoto T:
Purification to homogeneity and characterization of human B-cell
differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci USA.
82:5490–5494. 1985.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Li X, Chauhan A, Sheikh AM, Patil S,
Chauhan V, Li XM, Ji L, Brown T and Malik M: Elevated immune
response in the brain of autistic patients. J Neuroimmunol.
207:111–116. 2009.PubMed/NCBI View Article : Google Scholar
|
|
180
|
Needham BD, Tang W and Wu WL: Searching
for the gut microbial contributing factors to social behavior in
rodent models of autism spectrum disorder. Dev Neurobiol.
78:474–499. 2018.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Meeking MM, MacFabe DF, Mepham JR, Foley
KA, Tichenoff LJ, Boon FH, Kavaliers M and Ossenkopp KP: Propionic
acid induced behavioural effects of relevance to autism spectrum
disorder evaluated in the hole board test with rats. Prog
Neuropsychopharmacol Biol Psychiatry. 97(109794)2020.PubMed/NCBI View Article : Google Scholar
|
|
182
|
Zass LJ, Hart SA, Seedat S, Hemmings SM
and Malan-Muller S: Neuroinflammatory genes associated with
post-traumatic stress disorder: Implications for comorbidity.
Psychiatr Genet. 27:1–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Nakamura Y, Ishimaru K, Shibata S and
Nakao A: Regulation of plasma histamine levels by the mast cell
clock and its modulation by stress. Sci Rep.
7(39934)2017.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Passos IC, Vasconcelos-Moreno MP, Costa
LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhaes PV,
Kapczinski F and Kauer-Sant'Anna M: Inflammatory markers in
post-traumatic stress disorder: A systematic review, meta-analysis,
and meta-regression. Lancet Psychiatry. 2:1002–1012.
2015.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Geracioti TD Jr, Carpenter LL, Owens MJ,
Baker DG, Ekhator NN, Horn PS, Strawn JR, Sanacora G, Kinkead B,
Price LH and Nemeroff CB: Elevated cerebrospinal fluid substance p
concentrations in posttraumatic stress disorder and major
depression. Am J Psychiatry. 163:637–643. 2006.PubMed/NCBI View Article : Google Scholar
|
|
186
|
O'Donovan A, Cohen BE, Seal KH, Bertenthal
D, Margaretten M, Nishimi K and Neylan TC: Elevated risk for
autoimmune disorders in Iraq and afghanistan veterans with
posttraumatic stress disorder. Biol Psychiatry. 77:365–374.
2015.PubMed/NCBI View Article : Google Scholar
|
|
187
|
Hendriksen E, van Bergeijk D, Oosting RS
and Redegeld FA: Mast cells in neuroinflammation and brain
disorders. Neurosci Biobehav Rev. 79:119–133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Ahmed ME, Iyer S, Thangavel R, Kempuraj D,
Selvakumar GP, Raikwar SP, Zaheer S and Zaheer A: Co-localization
of glia maturation factor with NLRP3 inflammasome and autophagosome
markers in human Alzheimer's disease brain. J Alzheimers Dis.
60:1143–1160. 2017.PubMed/NCBI View Article : Google Scholar
|
|
189
|
Xiong Z, Thangavel R, Kempuraj D, Yang E,
Zaheer S and Zaheer A: Alzheimer's disease: Evidence for the
expression of interleukin-33 and its receptor ST2 in the brain. J
Alzheimers Dis. 40:297–308. 2014.PubMed/NCBI View Article : Google Scholar
|
|
190
|
Shaik-Dasthagirisaheb YB and Conti P: The
role of mast cells in Alzheimer's disease. Adv Clin Exp Med.
25:781–787. 2016.PubMed/NCBI View Article : Google Scholar
|
|
191
|
Tagen M, Elorza A, Kempuraj D, Boucher W,
Kepley CL, Shirihai OS and Theoharides TC: Mitochondrial uncoupling
protein 2 inhibits mast cell activation and reduces histamine
content. J Immunol. 183:6313–6319. 2009.PubMed/NCBI View Article : Google Scholar
|
|
192
|
Nelson RB, Siman R, Iqbal MA and Potter H:
Identification of a chymotrypsin-like mast cell protease in rat
brain capable of generating the N-terminus of the Alzheimer amyloid
beta-protein. J Neurochem. 61:567–577. 1993.PubMed/NCBI View Article : Google Scholar
|
|
193
|
Ibrahim MZ, Reder AT, Lawand R, Takash W
and Sallouh-Khatib S: The mast cells of the multiple sclerosis
brain. J Neuroimmunol. 70:131–138. 1996.PubMed/NCBI View Article : Google Scholar
|
|
194
|
Russi AE and Brown MA: The meninges: New
therapeutic targets for multiple sclerosis. Transl Res.
165:255–269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
195
|
Theoharides TC, Asadi S, Panagiotidou S
and Weng Z: The ‘missing link’ in autoimmunity and autism:
Extracellular mitochondrial components secreted from activated live
mast cells. Autoimmun Rev. 12:1136–1142. 2013.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Kawasaki H, Chang HW, Tseng HC, Hsu SC,
Yang SJ, Hung CH, Zhou Y and Huang SK: A tryptophan metabolite,
kynurenine, promotes mast cell activation through aryl hydrocarbon
receptor. Allergy. 69:445–452. 2014.PubMed/NCBI View Article : Google Scholar
|
|
197
|
Hermine O, Lortholary O, Leventhal PS,
Catteau A, Soppelsa F, Baude C, Cohen-Akenine A, Palmerini F,
Hanssens K, Yang Y, et al: Case-control cohort study of patients'
perceptions of disability in mastocytosis. PLoS One.
3(e2266)2008.PubMed/NCBI View Article : Google Scholar
|
|
198
|
Georgin-Lavialle S, Moura DS, Salvador A,
Chauvet-Gelinier JC, Launay JM, Damaj G, Cote F, Soucie E,
Chandesris MO, Barete S, et al: Mast cells' involvement in
inflammation pathways linked to depression: Evidence in
mastocytosis. Mol Psychiatry. 21:1511–1516. 2016.PubMed/NCBI View Article : Google Scholar
|
|
199
|
Couturier N, Zappulla JP, Lauwers-Cances
V, Uro-Coste E, Delisle MB, Clanet M, Montagne L, Van der Valk P,
Bö L and Liblau RS: Mast cell transcripts are increased within and
outside multiple sclerosis lesions. J Neuroimmunol. 195:176–185.
2008.PubMed/NCBI View Article : Google Scholar
|