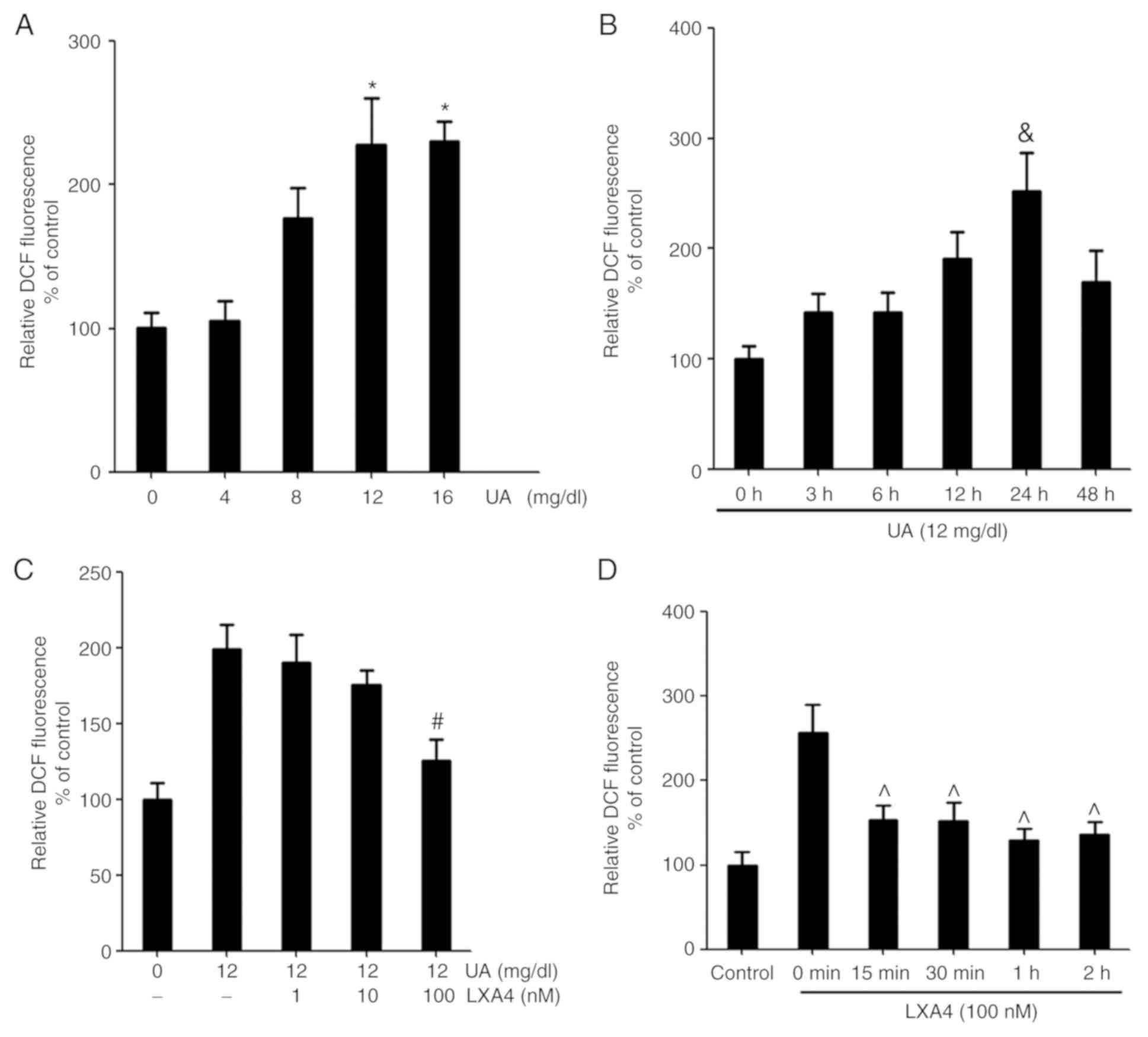
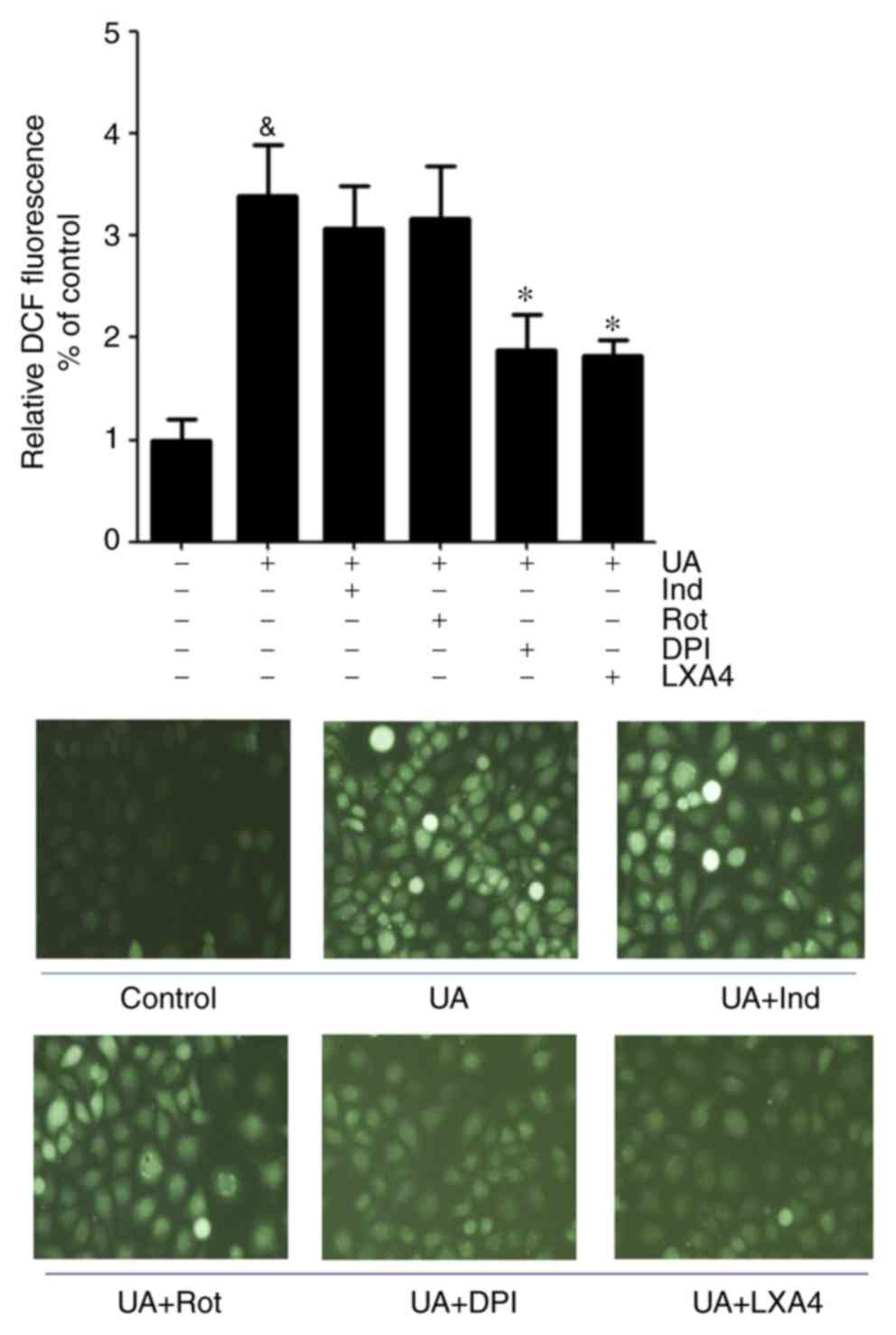
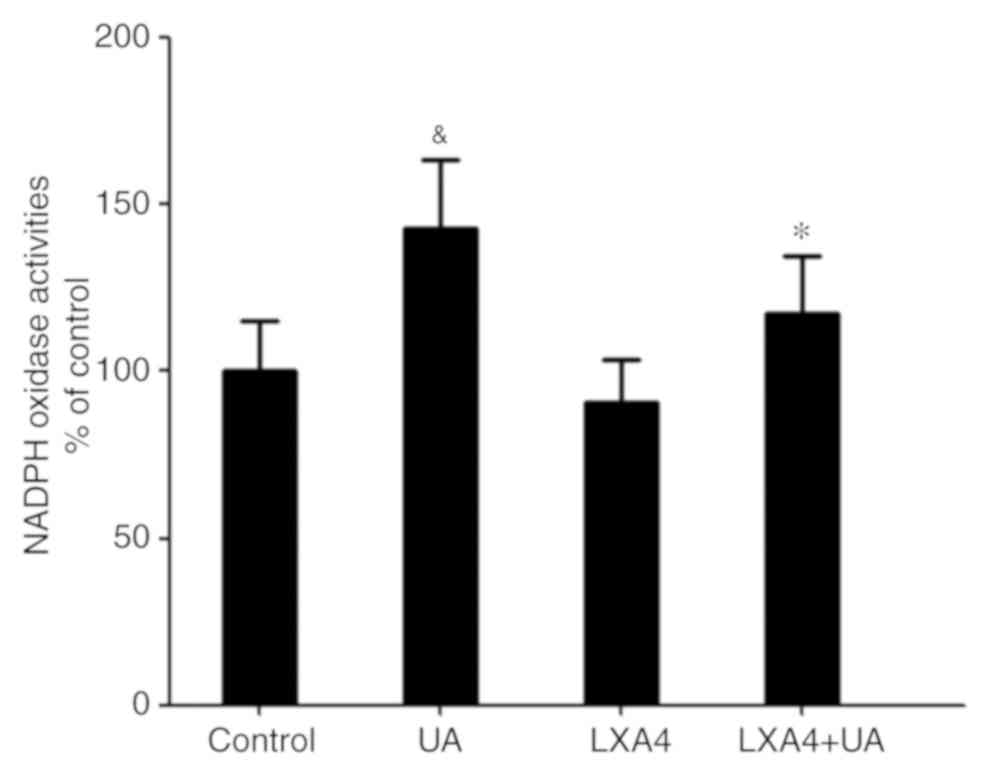
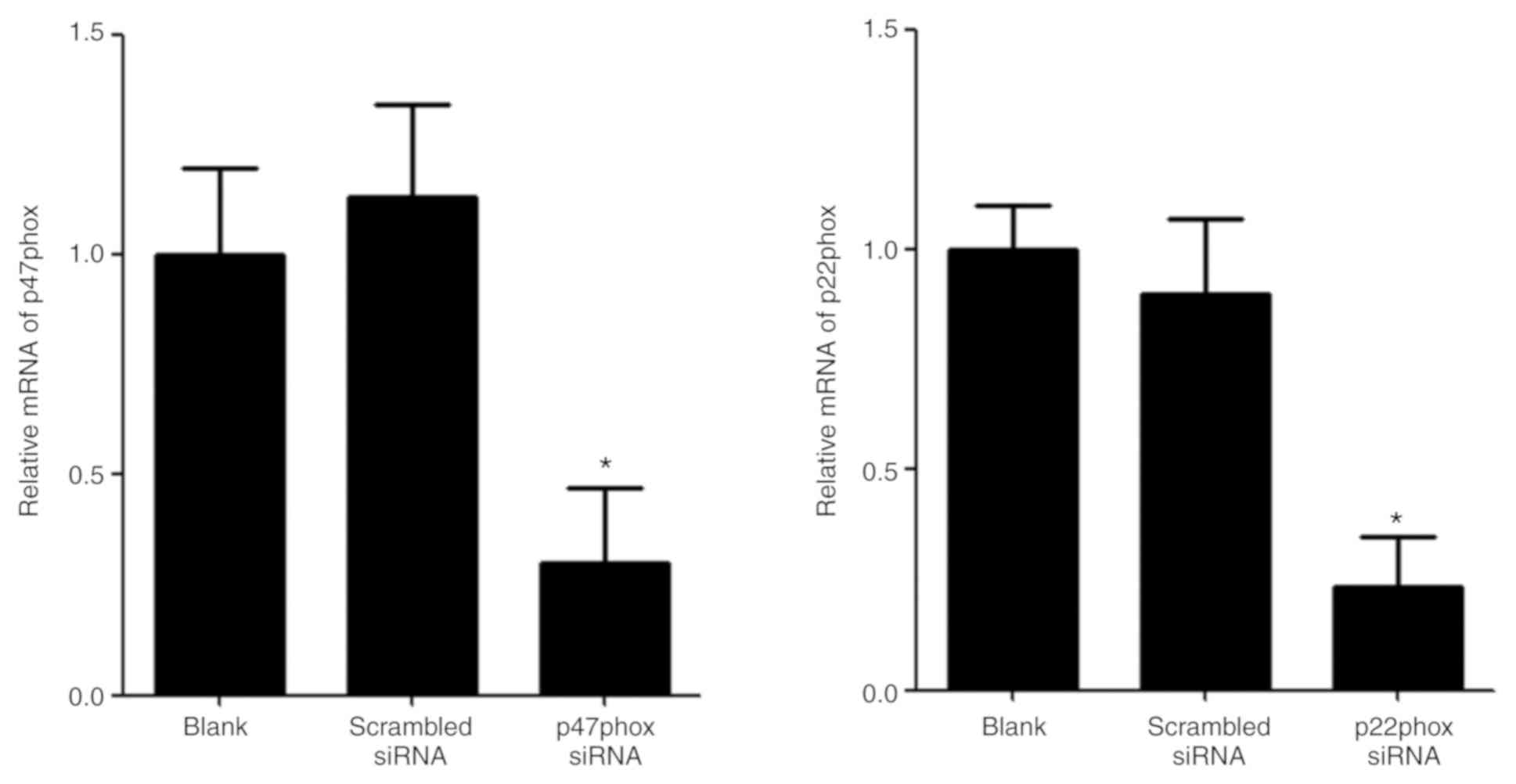
|
1
|
Liu CW, Chen KH, Tseng CK, Chang WC, Wu YW
and Hwang JJ: The dose-response effects of uric acid on the
prevalence of metabolic syndrome and electrocardiographic left
ventricular hypertrophy in healthy individuals. Nutr Metab
Cardiovasc Dis. 29:30–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Puddu P, Puddu GM, Cravero E, Vizioli L
and Muscari A: Relationships among hyperuricemia, endothelial
dysfunction and cardiovascular disease: Molecular mechanisms and
clinical implications. J Cardiol. 59:235–242. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li M, Hou W, Zhang X, Hu L and Tang Z:
Hyperuricemia and risk of stroke: A systematic review and
meta-analysis of prospective studies. Atherosclerosis. 232:265–270.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ogura T, Matsuura K, Matsumoto Y, Mimura
Y, Kishida M, Otsuka F and Tobe K: Recent trends of hyperuricemia
and obesity in Japanese male adolescents, 1991 through 2002.
Metabolism. 53:448–453. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mortada I: Hyperuricemia, type 2 diabetes
mellitus, and hypertension: An emerging association. Curr Hypertens
Rep. 19(69)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mishima M, Hamada T, Maharani N, Ikeda N,
Onohara T, Notsu T, Ninomiya H, Miyazaki S, Mizuta E, Sugihara S,
et al: Effects of uric acid on the NO production of HUVECs and its
restoration by urate lowering agents. Drug Res (Stuttg).
66:270–274. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lepetsos P and Papavassiliou AG:
ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys
Acta. 1862:576–591. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rasheed NO, Ahmed LA, Abdallah DM and
El-Sayeh BM: Nephro-toxic effects of intraperitoneally injected
EGCG in diabetic mice: Involvement of oxidative stress,
inflammation and apoptosis. Sci Rep. 7(40617)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Minutoli L, Puzzolo D, Rinaldi M, Irrera
N, Marini H, Arcoraci V, Bitto A, Crea G, Pisani A, Squadrito F, et
al: ROS-mediated NLRP3 inflammasome activation in brain, heart,
kidney, and testis ischemia/reperfusion injury. Oxid Med Cell
Longev. 2016(2183026)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kadowaki D, Sakaguchi S, Miyamoto Y,
Taguchi K, Muraya N, Narita Y, Sato K, Chuang VT, Maruyama T,
Otagiri M, et al: Direct radical scavenging activity of
benzbromarone provides beneficial antioxidant properties for
hyperuricemia treatment. Biol Pharm Bull. 38:487–492.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matsuzawa A, Saegusa K, Noguchi T,
Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K
and Ichijo H: ROS-dependent activation of the TRAF6-ASK1-p38
pathway is selectively required for TLR4-mediated innate immunity.
Nat Immunol. 6:587–592. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Chen Z, Zhou Q, Zou D, Tian Y, Liu B,
Zhang Y and Wu Z: Chloro-benzoquinones cause oxidative DNA damage
through iron-mediated ROS production in Escherichia coli.
Chemosphere. 135:379–386. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kuzkaya N, Weissmann N, Harrison DG and
Dikalov S: Interactions of peroxynitrite with uric acid in the
presence of ascorbate and thiols: Implications for uncoupling
endothelial nitric oxide synthase. Biochem Pharmacol. 70:343–354.
2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xie H, Sun J, Chen Y, Zong M, Li S and
Wang Y: EGCG attenuates uric acid-induced inflammatory and
oxidative stress responses by medicating the NOTCH pathway. Oxid
Med Cell Longev. 2015(214836)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Z, Sheng Y, Liu C, Li K, Huang X, Huang
J and Xu K: Nox4 has a crucial role in uric acid-induced oxidative
stress and apoptosis in renal tubular cells. Mol Med Rep.
13:4343–4348. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ives A, Nomura J, Martinon F, Roger T,
LeRoy D, Miner JN, Simon G, Busso N and So A: Xanthine
oxidoreductase regulates macrophage IL1β secretion upon NLRP3
inflammasome activation. Nat Commun. 6(6555)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sautin YY, Nakagawa T, Zharikov S and
Johnson RJ: Adverse effects of the classic antioxidant uric acid in
adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am
J Physiol Cell Physiol. 293:C584–C596. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tang L, Xu Y, Wei Y and He X: Uric acid
induces the expression of TNF-α via the ROS-MAPK-NF-κB signaling
pathway in rat vascular smooth muscle cells. Mol Med Rep.
16:6928–6933. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chandrasekharan JA and Sharma-Walia N:
Lipoxins: Nature's way to resolve inflammation. J Inflamm Res.
8:181–192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Weiss GA, Troxler H, Klinke G, Rogler D,
Braegger C and Hersberger M: High levels of anti-inflammatory and
pro-resolving lipid mediators lipoxins and resolvins and declining
docosahexaenoic acid levels in human milk during the first month of
lactation. Lipids Health Dis. 12(89)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu C, Guan H, Cai C, Li F and Xiao J:
Lipoxin A4 suppresses osteoclastogenesis in RAW264.7 cells and
prevents ovariectomy-induced bone loss. Exp Cell Res. 352:293–303.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nascimento-Silva V, Arruda MA,
Barja-Fidalgo C and Fierro IM: Aspirin-triggered lipoxin A4 blocks
reactive oxygen species generation in endothelial cells: A novel
antioxidative mechanism. Thromb Haemost. 97:88–98. 2007.PubMed/NCBI
|
|
23
|
Peshavariya HM, Taylor CJ, Goh C, Liu GS,
Jiang F, Chan EC and Dusting GJ: Annexin peptide Ac2-26 suppresses
TNFα-induced inflammatory responses via inhibition of
Rac1-dependent NADPH oxidase in human endothelial cells. PLoS One.
8(e60790)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Griendling KK, Minieri CA, Ollerenshaw JD
and Alexander RW: Angiotensin II stimulates NADH and NADPH oxidase
activity in cultured vascular smooth muscle cells. Circ Res.
74:1141–1148. 1994.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maeda M, Hayashi T, Mizuno N, Hattori Y
and Kuzuya M: Intermittent high glucose implements stress-induced
senescence in human vascular endothelial cells: Role of superoxide
production by NADPH oxidase. PLoS One. 10(e0123169)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jaquet V, Scapozza L, Clark RA, Krause KH
and Lambeth JD: Small-molecule NOX inhibitors: ROS-generating NADPH
oxidases as therapeutic targets. Antioxid Redox Signal.
11:2535–2552. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Belambri SA, Rolas L, Raad H,
Hurtado-Nedelec M, Dang PM and El-Benna J: NADPH oxidase activation
in neutrophils: Role of the phosphorylation of its subunits. Eur J
Clin Invest. 48 (Suppl 2)(e12951)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mohandas R, Sautina L, Beem E, Schuler A,
Chan WY, Domsic J, McKenna R, Johnson RJ and Segal MS: Uric acid
inhibition of dipeptidyl peptidase IV in vitro is dependent on the
intracellular formation of triuret. Exp Cell Res. 326:136–142.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Song C and Zhao X: Uric acid promotes
oxidative stress and enhances vascular endothelial cell apoptosis
in rats with middle cerebral artery occlusion. Biosci Rep.
38(BSR20170939)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW,
Kim KC, Lee J, Lee MY, Lee SM, Kang DH, et al: Uric acid induces
endothelial dysfunction by vascular insulin resistance associated
with the impairment of nitric oxide synthesis. FASEB J.
28:3197–3204. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhou Y, Zhao M, Pu Z, Xu G and Li X:
Relationship between oxidative stress and inflammation in
hyperuricemia: Analysis based on asymptomatic young patients with
primary hyperuricemia. Medicine (Baltimore).
97(e13108)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xia F, Wang C, Jin Y, Liu Q, Meng Q, Liu K
and Sun H: Luteolin protects HUVECs from TNF-α-induced oxidative
stress and inflammation via its effects on the Nox4/ROS-NF-κB and
MAPK pathways. J Atheroscler Thromb. 21:768–783. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kantarci A, Aytan N, Palaska I, Stephens
D, Crabtree L, Benincasa C, Jenkins BG, Carreras I and Dedeoglu A:
Combined administration of resolvin E1 and lipoxin A4 resolves
inflammation in a murine model of Alzheimer's disease. Exp Neurol.
300:111–120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y,
Hao H, Tang K, Yi P, Liu M, et al: Pleiotropic regulation of
macrophage polarization and tumorigenesis by formyl peptide
receptor-2. Oncogene. 30:3887–3899. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Biala AK, Dhingra R and Kirshenbaum LA:
Mitochondrial dynamics: Orchestrating the journey to advanced age.
J Mol Cell Cardiol. 83:37–43. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Uddin MJ, Werfel TA, Crews BC, Gupta MK,
Kavanaugh TE, Kingsley PJ, Boyd K, Marnett LJ and Duvall CL:
Fluorocoxib A loaded nanoparticles enable targeted visualization of
cyclooxygenase-2 in inflammation and cancer. Biomaterials.
92:71–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lian S, Xia Y, Khoi PN, Ung TT, Yoon HJ,
Kim NH, Kim KK and Jung YD: Cadmium induces matrix
metalloproteinase-9 expression via ROS-dependent EGFR, NF-кB, and
AP-1 pathways in human endothelial cells. Toxicology. 338:104–116.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang YP, Wu Y, Li LY, Zheng J, Liu RG,
Zhou JP, Yuan SY, Shang Y and Yao SL: Aspirin-triggered lipoxin A4
attenuates LPS-induced pro-inflammatory responses by inhibiting
activation of NF-κB and MAPKs in BV-2 microglial cells. J
Neuroinflammation. 8(95)2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Park IH, Hwang HM, Jeon BH, Kwon HJ, Hoe
KL, Kim YM and Ryoo S: NADPH oxidase activation contributes to
native low-density lipoprotein-induced proliferation of human
aortic smooth muscle cells. Exp Mol Med. 47(e168)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ter Horst EN, Hahn NE, Geerts D, Musters
RJP, Paulus WJ, van Rossum AC, Meischl C, Piek JJ, Niessen HWM and
Krijnen PAJ: p47phox-dependent reactive oxygen species stimulate
nuclear translocation of the foxO1 transcription factor during
netabolic inhibition in cardiomyoblasts. Cell Biochem Biophys.
76:401–410. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen JX, Zeng H, Lawrence ML, Blackwell TS
and Meyrick B: Angiopoietin-1-induced angiogenesis is modulated by
endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol.
291:H1563–H1572. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chakraborti S, Sarkar J and Chakraborti T:
Role of PLD-PKCζ signaling axis in p47phox phosphorylation for
activation of NADPH oxidase by angiotensin II in pulmonary artery
smooth muscle cells. Cell Biol Int. 43:678–694. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Macías Pérez ME, Hernández Rodríguez M,
Cabrera Pérez LC, Fragoso-Vázquez MJ, Correa-Basurto J,
Padilla-Martínez II, Méndez Luna D, Mera Jiménez E, Flores Sandoval
C, Tamay Cach F, et al: Aromatic regions govern the recognition of
NADPH oxidase inhibitors as diapocynin and its analogues. Arch
Pharm (Weinheim). 350(1700041)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Carlo T, Kalwa H and Levy BD:
15-Epi-lipoxin A4 inhibits human neutrophil superoxide anion
generation by regulating polyisoprenyl diphosphate phosphatase 1.
FASEB J. 27:2733–2741. 2013.PubMed/NCBI View Article : Google Scholar
|