Introduction
Knee osteoarthritis (KOA) is a chronic degenerative
joint inflammation that cannot be cured. It is also the most common
adult joint disease in the world, which is more common in the
middle-aged and the elderly (1).
According to the statistics, the prevalence of KOA in men aged over
65 years is ~13%, while that in women is ~24% (2). There are many studies on the treatment
of KOA. However, so far, there is no way to cure this disease, most
of the patients were operated with joint replacement surgery to
relieve pain (3). Once KOA gets into
the terminal stage, the function of knee joint in moving will be
seriously damaged, resulting in physical imbalance, pain and
dysfunction, as well as depression, which will indirectly aggravate
the disease, and have a negative impact on the quality of life of
middle-aged and elderly patients with KOA (4-6). In
addition, the lack of specific clinical manifestations make the
disease hard to establish in the early diagnosis of KOA (7). Therefore, it is of great significance
to explore the biomarkers of the course of KOA for early diagnosis
and prognosis evaluation.
Toll-like receptor (TLR) is a type I transmembrane
protein, which plays a guiding role in the recognition of invasive
pathogenic microorganisms in immune response (8,9).
Interferon regulatory factor (IRF) is a necessary regulator of
differentiation and maturation of T cells, B cells and plasma cells
(10), and is also a group of
transcription factors that can regulate interferon and its
signaling pathway (11). Both play
an important role in virus defense, immune regulation, cell growth
and apoptosis, and regulation of many inflammatory diseases
(12). According to literature, TLR
may drive many chronic inflammatory molecules in arthritis,
including KOA, and Toll-like receptor-4 (TLR-4), Toll-like
receptor-5 (TLR-5) are TLR expressed in plasma membrane. Once
activated, they can activate downstream signal transduction
pathways with cohesion molecules to activate IRF, a
pro-inflammatory and joint destruction medium (13,14). In
the study of Nair et al (15), the expression of TLR-4 in synovial
cells of patients with early KOA was significantly higher than that
of healthy people. There was a certain correlation between TLR-4
and KOA, suggesting that TLR-4 may be a potential therapeutic
target for KOA. In the report by Chamberlain et al (16), there was a positive correlation
between TLR-5 and the severity of KOA, suggesting that TLR-5 can be
used as a new marker for the diagnosis of knee arthritis. Yang
et al (17), reported
interferon regulatory factor 4 (IRF4), as a nonspecific
transcription factor of Th17 cells, involved in the regulation of
Th17 cell differentiation. By interfering with the expression of
IRF4 and Th17 cell differentiation and IL-17 production can be
significantly reduced, thus reducing disease inflammation (18). However, there is little research on
the role of TLR-4, TLR-5 and IRF4 in KOA. A previous study on KOA
pro-inflammatory cytokines showed that a large number of matrix
metalloproteinases, including matrix metalloproteinase-1 (MMP-1),
can be produced in KOA chondrocytes and synoviocytes, and the
pro-inflammatory cytokine interleukin-1β (IL-1β), interleukin-6
(IL-6), and tumor necrosis factor-α (TNF-α) can also be secreted in
synoviocytes, suggesting MMP-1, IL-1β, IL-6, TNF-α may be involved
in the occurrence and progression of KOA (19). It has been reported that cytokines
involved in the regulation of KOA inflammation also affect the
pathogenesis of KOA through angiogenesis and chemotaxis. Studies on
cytokine gene polymorphisms contribute to the determination of
susceptibility to KOA in populations and KOA, and identification of
KOA and rheumatoid arthritis (20,21). The
induction of KOA is mainly related to the metabolic imbalance of
cartilage cells and the high expression of pro-inflammatory
cytokines. The treatment and diagnosis can start with restoring the
metabolic level of cartilage cells and improving inflammatory
response (22). Therefore,
chondrocyte metabolic balance and inflammatory response markers
play an important role in the occurrence and progression of
KOA.
In this study, the expression of TLR-4, TLR-5 and
IRF4 in serum of patients with KOA were detected, and the value of
combined detection of serum TLR-4, TLR-5 and IRF4 in the diagnosis
and prognosis of KOA was evaluated, and the correlation between
TLR-4, TLR-5 and IRF4 and cartilage metabolism and inflammatory
response markers was analyzed.
Materials and methods
General information
Patients with KOA admitted to Puyang Hospital of
Traditional Chinese Medicine (Puyang, China) from May 2016 to June
2018 were selected as subjects. In the study group, 68 patients
with KOA were included, including 19 males and 49 females. They
were aged 41-80 years, with a mean age of 54.38±13.89 years. There
were 10 patients with suspected mild KOA, 18 patients with mild
KOA, 20 patients with moderate KOA, and 20 patients with severe
KOA. The control group included 49 healthy people with physical
examination in Puyang Hospital of Traditional Chinese Medicine at
the same time, including 15 males and 34 females. The controls were
aged 40-80 years, with a mean age of 55.15±15.69 years. The
guardians of the subjects were given full information regarding the
study and signed and informed consent form. The study was approved
by the Ethics Committee of Puyang Hospital of Traditional Chinese
Medicine.
Inclusion and exclusion criteria
Inclusion criteria: The patients in the study group
met the diagnostic criteria of osteoarthritis of the American
Society of Rheumatology (23), they
were aged 40 to 80 years, and all of them were treated for the
first time. The inclusion criteria were applied to the study group.
Exclusion criteria: Patients with communication disorders, with
incomplete clinical information, with complications of heart,
liver, kidney and other important organs, with long-term use of
non-steroidal anti-inflammatory drugs, cartilage protective drugs,
painkillers, with contra-indications for the drugs used in this
study, with history of knee trauma surgery, knee joint infection,
knee deformity, metabolic osteopathy, unequal length of lower
extremities, history of knee tumor, were excluded.
Test methods
Venous blood (5 ml) of the two groups was drawn on
an empty stomach in the morning, and the blood was placed in
constant 37˚C temperature in a water bath. After 30 min, the blood
was taken out and placed in the centrifuge tube for centrifugation
at 1,500 x g and 4˚C for 10 min; the supernatant was taken out, and
then stored in the refrigerator at -70˚C. The serum was removed
from the freezer, dissolved in a refrigerator at 4˚C, and
completely dissolved at room temperature. The concentrations of
TLR-4, TLR-5, IRF4, IL-1β and IL-6, MMP-1, TNF-α in serum of two
groups of patients were detected by enzyme linked immunosorbent
assay (ELISA) (24). The ELISA kit
was purchased from Xinfan Biotechnology Co., Ltd. Sample well to be
tested, standard well and blank well were set up. No enzyme-labeled
reagent and sample were added to the blank well, and 100 µl samples
or standard substances were added to the remaining wells. After
mixing, the enzyme-labeled plate was covered with film and
incubated at 37˚C for 2 h. Each well was emptied, shaken, and
dried, 100 µl of working fluid A was added to each well, covered
with film, and incubated at 37˚C for 1 h. Each well was emptied,
shaken, and dried, and washing liquid was added to wash the plate 3
times. Each well was added with 100 µl of working liquid B, covered
with film, and incubated at 37˚C for 1 h. Each well was emptied,
shaken, and dried, and washing liquid was added to wash the plate 3
times. Each well was added with 90 µl of substrate solution,
covered with film, and placed at 37˚C to develop color for 10-15
min. Solution (50 µl) was added to each well to end the reaction.
SAF-680T enzyme labeling instrument (Shanghai Bajiu Industrial Co.,
Ltd.) was used to detect the OD value of each well at 450 nm
wavelength.
Statistical analysis
The count data were expressed by [n (%)] and were
compared using the χ2 test. The measurement data were
expressed by [mean ± standard deviation (SD)] and were analyzed
using t-test. GraphPad Prism 7 was used to draw graphs of the
expression level. The comparison between the two groups was made by
independent sample t-test, and one-way ANOVA followed by Bonferroni
test used for comparison of multiple groups. Correlation graphs
were drawn with GraphPad Prism 7. Correlation between cytokine and
grading was analyzed using Spearman correlation coefficient.
Correlation between serum TLR-4, TLR-5, IRF4 and cytokines was
analyzed using Pearson's correlation coefficient. The receiver
operating characteristic (ROC) curve was used to analyze the single
diagnostic efficacy. On the base of the analysis of single
diagnostic efficacy, SPSS 20.0 (IBM Corp.) was used for the
bivariate regression analysis of the combined diagnostic efficacy.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of general information
between two groups of people
There was no significant difference in sex, age,
body mass index (BMI), smoking history, place of residence,
educational level, eating habits, alcohol abuse or marital status
between the two groups (P>0.05) (Table I).
 | Table IComparison of general information
between the two groups [n (%)], mean ± SD. |
Table I
Comparison of general information
between the two groups [n (%)], mean ± SD.
| Group | Control group | Study group | t/χ2
value | P-value |
|---|
| Sex | | | 0.099 | 0.754 |
|
Male | 15 (30.61) | 19 (27.94) | | |
|
Female | 34 (69.39) | 49 (72.06) | | |
| Age (years) | 55.15±15.69 | 54.38±13.89 | 0.280 | 0.780 |
| BMI
(kg/m2) | 22.04±1.95 | 22.56±2.19 | 1.326 | 0.188 |
| Smoking | | | 0.696 | 0.404 |
|
Yes | 31 (63.27) | 48 (70.59) | | |
|
No | 18 (36.73) | 20 (29.41) | | |
| Resident place | | | 0.461 | 0.497 |
|
City | 24 (48.98) | 29 (42.65) | | |
|
Countryside | 25 (51.02) | 39 (57.35) | | |
| Degree of
education | | | 2.535 | 0.111 |
|
≥High
school | 23 (46.94) | 42 (61.76) | | |
|
<High
school | 26 (53.06) | 26 (38.24) | | |
| Eating habits | | | 2.195 | 0.139 |
|
Light | 34 (69.39) | 38 (55.88) | | |
|
Spicy | 15 (30.61) | 30 (44.12) | | |
| Alcohol
consumption | | | 0.451 | 0.502 |
|
Yes | 15 (30.61) | 17 (25.00) | | |
|
No | 34 (69.39) | 51 (75.00) | | |
| Marital status | | | 1.293 | 0.524 |
|
Married | 44 (88.75) | 56 (80.00) | | |
|
Unmarried | 4 (8.75) | 10 (15.00) | | |
| Bereft of
spouse | 1 (2.50) | 2 (5.00) | | |
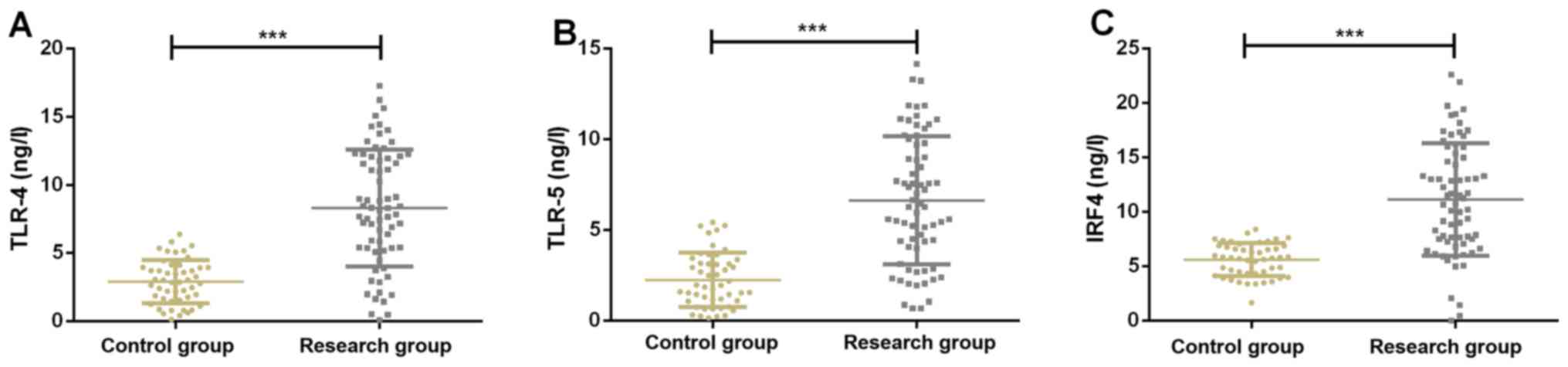
Expression of serum TLR-4, TLR-5 and
IRF4 of study group and control group
Expression of serum TLR-4 in the study group and the
control group were, respectively, 9.52±5.45 and 2.58±1.76 ng/l.
Expression of serum TLR-5 in the study group and the control group
were, respectively, 9.23±6.24 and 2.01±1.55 ng/l. Expression of
serum IRF4 in the study group and the control group were,
respectively, 15.22±7.75 and 5.89±1.26 ng/l, respectively.
Expression of serum TLR-4, TLR-5 and IRF4 in the study group was
significantly higher than those in the control group (P<0.001)
(Fig. 1).
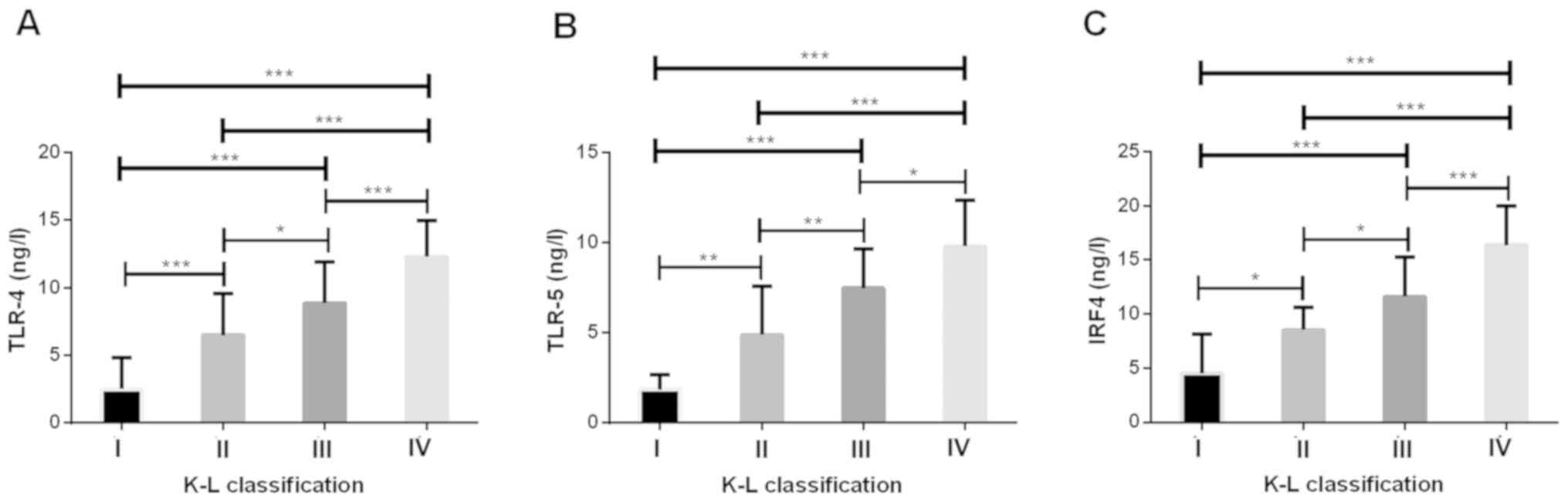
Serum TLR-4, TLR-5 and IRF4 levels in
patients with different K-L grades
The K-L (Kellgren-Lawrence) (25) scoring system is the most widely used
system for KOA, using X-ray photographs to determine the severity
of the condition. Grade 0 represents normal knee joint; Grade I
represents suspected mild KOA; Grade II represents mild KOA; Grade
III represents moderate KOA, and grade IV represents severe KOA.
Among our patients, there were 10 in grade I, 18 in grade II, 20 in
grade III and 20 in grade IV. Compared with grade I of K-L, the
levels of serum TLR-4, TLR-5 and IRF4 in patients of grade II, III
and IV in the study group were significantly higher (P<0.05).
Compared with grade II of K-L, the levels of serum TLR-4, TLR-5 and
IRF4 in patients of grade III and IV in the study group were
significantly higher (P<0.05). Compared with grade III of K-L,
serum TLR-4, TLR-5 and IRF-4 levels were significantly higher in
patients of grade IV in the study group (P<0.05). The levels of
serum TLR-4, TLR-5 and IRF4 in the study group increased with the
increase of K-L grade (Fig. 2).
Correlation between serum TLR-4, TLR-5
and IRF4 levels and different K-L grades
According to the K-L grades, the K-L grades are
grade I to 1, grade II to 2, grade III to 3 and grade IV to 4. The
results of Spearman correlation coefficient showed that there was a
positive correlation between serum TLR-4, TLR-5, IRF4 and K-L grade
(r=0.734, P<0.001, r=0.701, P<0.001, r=0.784, P<0.001)
(Fig. 3).
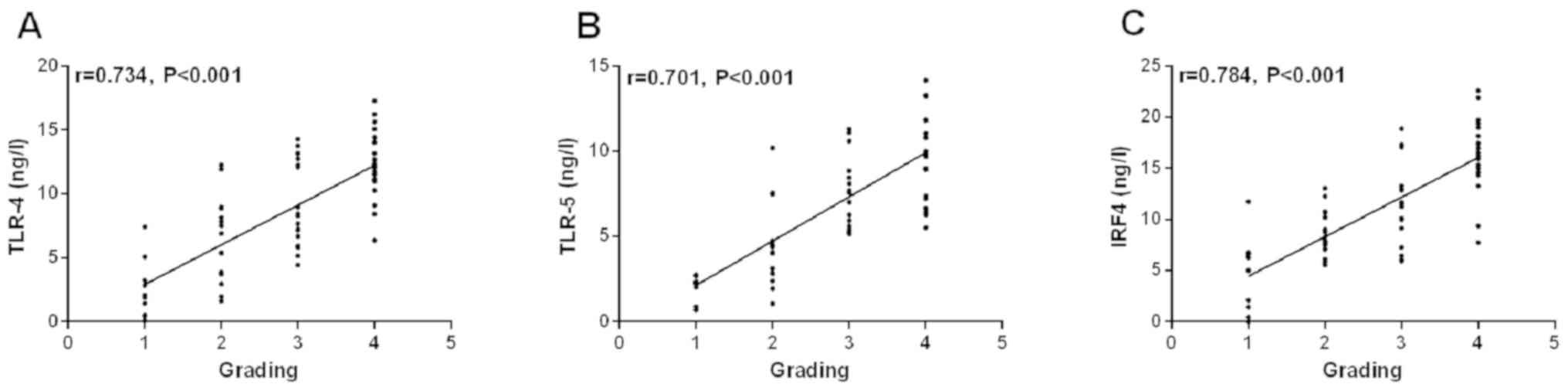 | Figure 3There is a positive correlation
between serum TLR-4 (A), TLR-5 (B), IRF4 (C) and K-L grades. There
is a positive correlation between serum TLR-4 (A), TLR-5 (B), IRF4
(C) and K-L grades (r=0.734, P<0.001; r=0.701, P<0.001;
r=0.784, P<0.001). TLR-4, toll-like receptor-4; TLR-5, toll-like
receptor-5; IRF4, interferon regulatory factor 4. |
Diagnostic efficacy of serum TLR-4,
TLR-5 and IRF4 levels in KOA
ROC curve of serum TLR-4, TLR-5 and IRF4 levels was
drawn for the diagnosis of KOA. The AUC of serum TLR-4 in the
diagnosis of KOA was 0.865 (95% CI, 0.796-0.934), the cut-off value
was 5.38 ng/l; the diagnostic sensitivity was 76.47%, and the
specificity was 93.88%. The AUC of serum TLR-5 in the diagnosis of
KOA was 0.843 (95% CI, 0.801-0.929), the cut-off value was 4.05
ng/l; the diagnostic sensitivity was 73.29%, and the specificity
was 87.76%. The AUC of serum IRF4 in the diagnosis of KOA was 0.861
(95% CI, 0.792-0.930), the cut-off value was 7.68 ng/l; the
diagnostic sensitivity was 72.06%, and the specificity was 95.92%.
The AUC of KOA diagnosed by TLR-4, TLR-5 and IRF4 was 0.971 (95%
CI, 0.959-1.004), the cut-off value was 0.63; the diagnostic
sensitivity was 94.12%, and the specificity was 97.96 (Table II and Fig. 4).
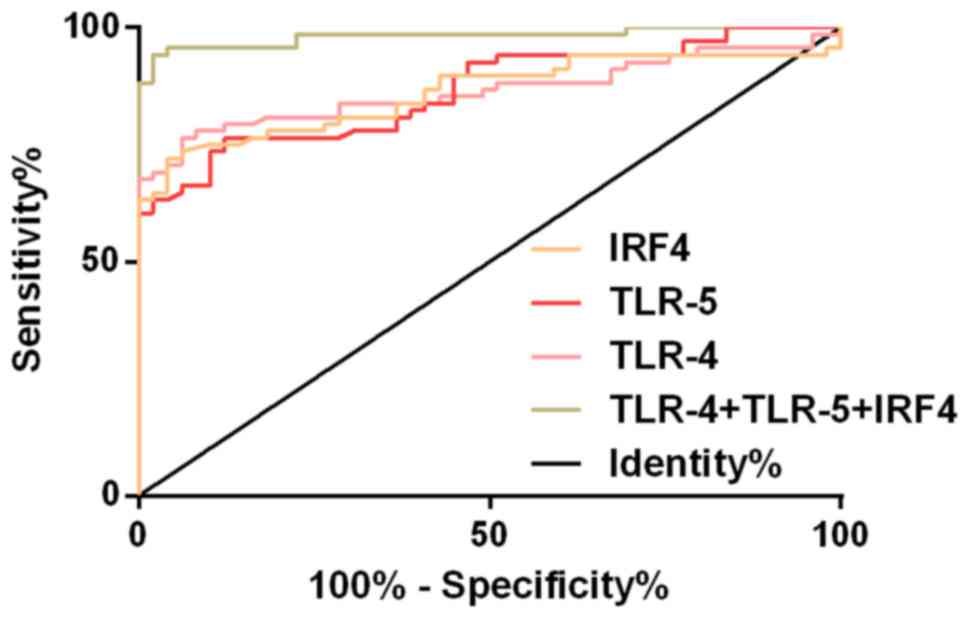 | Figure 4ROC curves of KOA diagnosed by serum
TLR-4, TLR-5 and IRF-4. The AUC of serum TLR-4 in the diagnosis of
KOA was 0.865, the cut-off value was 5.38 ng/l, the sensitivity was
76.47%, and the specificity was 93.88%. The AUC of serum TLR-5 in
the diagnosis of KOA was 0.843, the cut-off value was 4.05 ng/l,
the sensitivity was 73.29%, and the specificity was 87.76%. The AUC
of serum IRF4 in the diagnosis of KOA was 0.861, the cut-off value
was 7.68 ng/l, the sensitivity was 72.06%, and the specificity was
95.92%. The AUC of serum TLR-4, TLR-5 and IRF4 in the combined
diagnosis of KOA was 0.971, the cut-off value was 0.63, the
sensitivity was 94.12%, and the specificity was 97.96%. ROC,
receiver operating characteristic; KOA, knee osteoarthritis; TLR-4,
toll-like receptor-4; TLR-5, toll-like receptor-5; IRF4, interferon
regulatory factor 4. |
 | Table IIDiagnostic efficacy of serum TLR-4,
TLR-5 and IRF4 levels in KOA. |
Table II
Diagnostic efficacy of serum TLR-4,
TLR-5 and IRF4 levels in KOA.
| Group | AUC | 95% CI | Standard error | Cut-off value | Sensitivity
(%) | Specificity
(%) |
|---|
| TLR-4 | 0.865 | 0.796-0.934 | 0.035 | 5.38 ng/l | 76.47 | 93.88 |
| TLR-5 | 0.843 | 0.801-0.929 | 0.032 | 4.05 ng/l | 73.29 | 87.76 |
| IRF4 | 0.861 | 0.792-0.930 | 0.035 | 7.68 ng/l | 72.06 | 95.92 |
|
TLR-4+TLR-5+IRF4 | 0.971 | 0.959-1.004 | 0.012 | 0.63 | 94.12 | 97.96 |
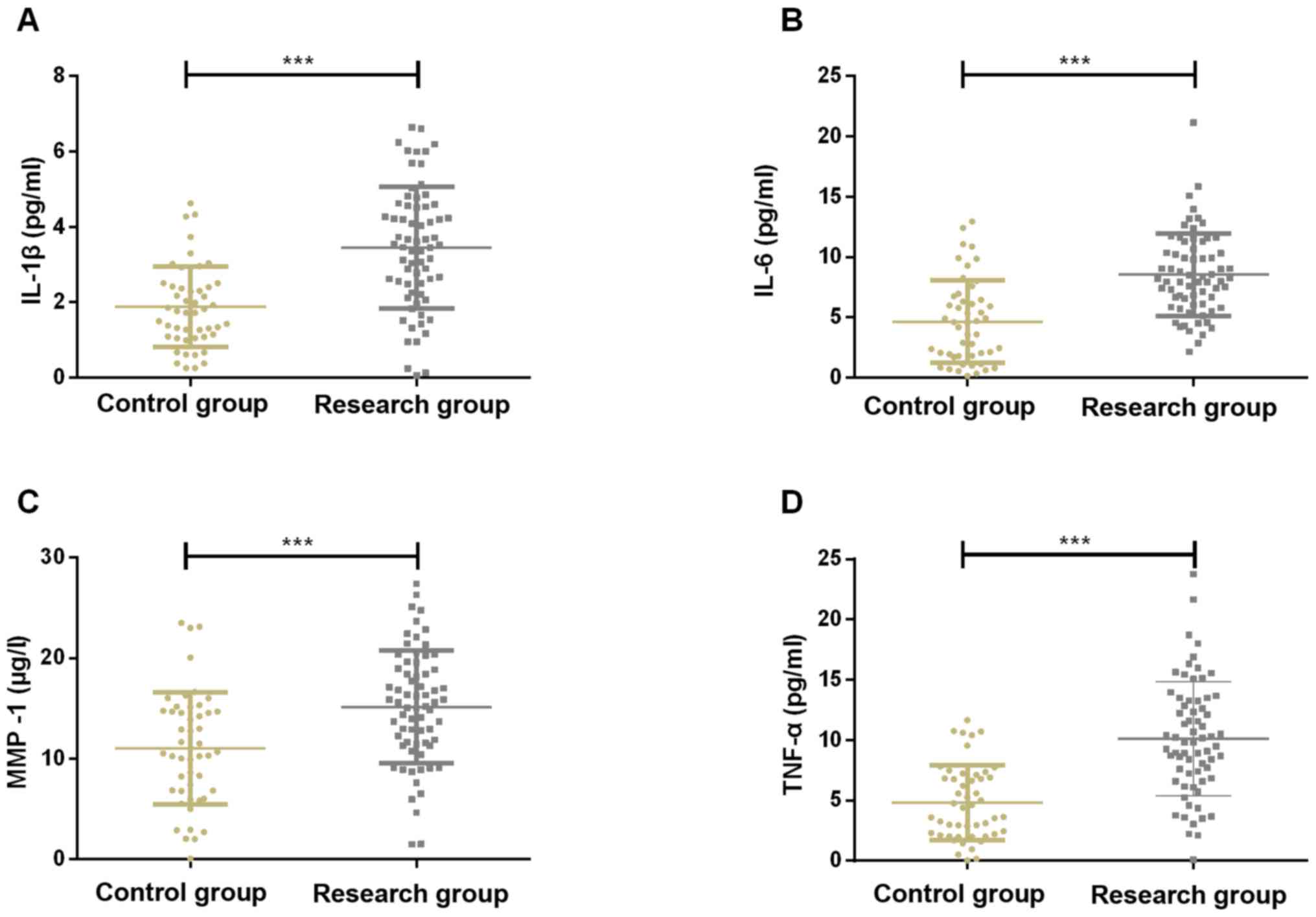
Comparison of serum IL-1β, IL-6, MMP-1
and TNF-α levels between control group and study group (mean ±
SD)
The levels of serum IL-1β, IL-6, MMP-1 and TNF-α in
the study group were significantly higher than those in the control
group (P<0.001). Pearson's correlation analysis showed that the
concentrations of serum TLR-4, TLR-5 and IRF4 were positively
correlated with the concentrations of serum IL-1β, IL-6, MMP-1 and
TNF-α, respectively (r=0.748, P<0.001, r=0.720, P<0.001,
r=0.735, P<0.001, r=0.742, P<0.001, r=0.807, P<0.001,
r=0.799, P<0.001, r=0.811, P<0.001, r=0.822, P<0.001,
r=0.758, P<0.001, r=0.752, P<0.001, r=0.779, P<0.001,
r=0.765, P<0.001) (Figs. 5 and
6).
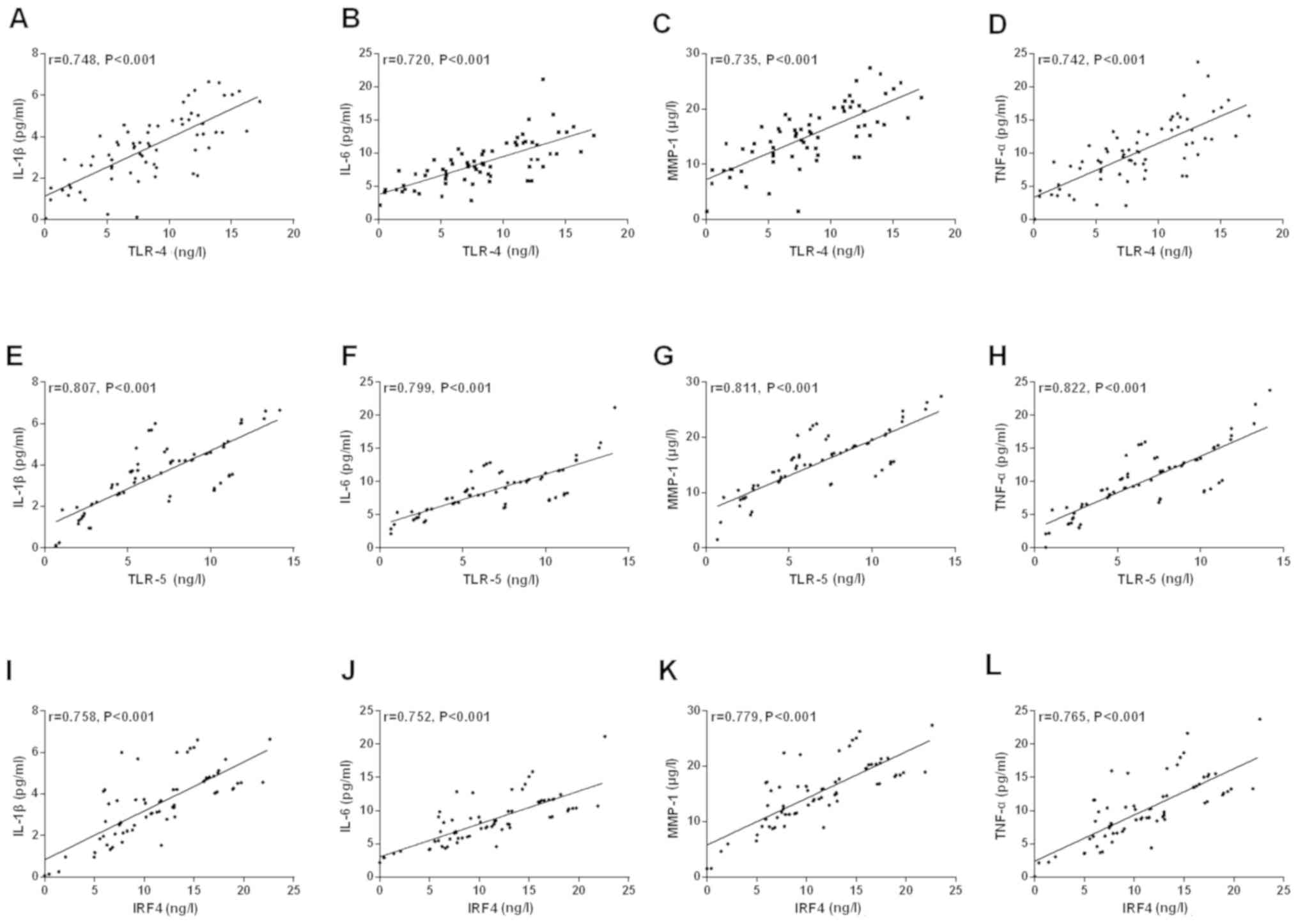 | Figure 6Correlation of serum TLR-4, TLR-5 and
IRF4 expression in the study group with IL-1β, IL-6, MMP-1 and
TNF-α levels. (A-D) A positive correlation between serum TLR-4 and
IL-1β and IL-6, MMP-1, TNF-α, respectively (r=0.748, P<0.001;
r=0.720, P<0.001; r=0.735, P<0.001; r=0.742, P<0.001).
(E-H) The relative expression of TLR-5 in serum was positively
correlated with the levels of IL-1β and IL-6, MMP-1, TNF-α,
respectively (r=0.807, P<0.001; r=0.799, P<0.001; r=0.811,
P<0.001; r=0.822, P<0.001). (I-L) Relative expression of IRF4
in serum was positively correlated with the levels of IL-1β and
IL-6, MMP-1, TNF-α, respectively (r=0.758, P<0.001; r=0.752,
P<0.001; r=0.779, P<0.001; r=0.765, P<0.001). TLR-4,
toll-like receptor-4 (TLR-4); TLR-5, toll-like receptor-5; IRF4,
interferon regulatory factor 4; IL-1β, interleukin 1β; IL-6,
interleukin-6; MMP-1, matrix metalloproteinase-1; TNF-α, tumor
necrosis factor-α. |
Discussion
KOA is a common chronic joint disease in middle-aged
and elderly people, and its incidence and disability rate in the
world are extremely high, which poses a serious threat to the
health of middle-aged and elderly patients (26). At present, magnetic resonance imaging
(MRI) has been used to evaluate the degree of cartilage injury in
early KOA. Although the specificity is high, the cost is high, and
the sensitivity needs to be improved (27). Therefore, it is important to explore
biomarkers with high specificity and sensitivity for the early
diagnosis of KOA.
Increasing number of studies have shown that TLR and
IRF play a potential clinical role in the key regulation of KOA
cartilage cells and inflammatory factors, although the initiation
and progression of KOA have not yet been clarified (28). There have been many studies on
cartilage cells and inflammatory factors of KOA in TLR-4 and IRF-4.
In the study of Liang et al (29), TLR-4 is highly expressed in type 2
diabetes-related KOA chondrocytes and promotes catabolic and
inflammatory processes. The regulatory mechanism may be related to
the activation of the nuclear factor κB (NF-κB) pathway by TLR-4
under the stimulation of hyperglycemia. In the study of Jin et
al (30), isofraxidin in KOA
competitive inhibition of TLR-4/ bone marrow differentiation
protein-2 (MD-2) complex formation, inhibit TLR-4/NF-κB signal
cascade, and reduce the level of serum inflammatory cytokines in
mouse KOA model. IRF4 is based on ROR gamma t, Th17 as a bridge to
establish an indirect relationship with KOA pro-inflammatory factor
IL-17. Mudter et al (31)
found that IRF4 can directly bind to IL-17 promoter and induce
IL-17 gene expression, resulting in KOA pro-inflammatory factor
IL-17(32). The mechanism of TLR-5
in KOA is mainly mediated by signals related to inflammatory
reaction. Lim et al (33)
found that TLR-2 ligand FSL-1 and TLR-5 ligand flagellin mediated
inflammatory response through MyD88/TRAF 6/NF-κB-dependent signal.
We speculate that TLR-5 may be an important cytokine in
osteoarthritis. TLR-5 and NK-κB signaling pathway are related to
KOA, and the damage of articular cartilage is related to the high
expression of TLR-5. However, in the past, most of the TLR and IRF
were concentrated in the mechanism of KOA (34). The diagnostic value of the combined
detection of TLR-4, TLR-5 and IRF4 in KOA remains to be
confirmed.
In this study, the relative expression of serum
TLR-4, TLR-5 and IRF4 in the study group were significantly higher
than those in the control group, and the sensitivity and
specificity of the three methods in the diagnosis of KOA were 94.12
and 97.96%, respectively. This indicates that TLR-4, TLR-5 and IRF4
may be involved in the occurrence and progression of KOA, and the
combined detection of KOA has a good predictive value for the early
diagnosis of KOA. In the study of Miller et al (35) on the regulation of TLR on KOA, TLR is
not only involved in promoting the pathological changes of KOA
joint tissue, but also mediates the pain mechanism of KOA through
its signaling pathway. In the study of the relationship between
high-level TLR-4 and articular cartilage destruction in patients
with KOA by Ospelt et al (36), the high expression of TLR-4 in serum
of patients with early KOA accelerated the process of articular
cartilage damage and increased the risk of disability. In the study
of Lee et al (37) on the
treatment of KOA involving IRF4, IRF4 was involved in a new path of
pathological development of inflammatory KOA; the therapeutic
inhibition of IRF4 is beneficial to alleviate pain and disease
progression in KOA patients. We speculate that TLR-4, TLR-5 and
IRF4 may play a role in the development of KOA. Further studies
have shown that TLR-4 and TLR-5 combined with IRF4 have some
predictive value for the severity of patients with KOA. Therefore,
TLR-4, TLR-5 and IRF4 may play a key role in the diagnosis and
severity assessment of patients with KOA. In the study on the
activity of KOA diseases by Ding et al (38), IL-37 was positively correlated with
the activity of KOA, and inhibited the production of
pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α in
synovial cells, suggesting that IL-37 may also be a potential
therapeutic target for KOA inflammation.
Inflammatory mediators IL-1β, IL-6, MMP-1 and TNF-α
are very important in the occurrence, progression and pathological
changes of KOA. IL-1β can trigger the increase of IL-6, MMP-1,
while IL-6 is related to cartilage synthesis and catabolism; MMP-1
is related to apoptosis of cartilage cells, and TNF-α is related to
the function or molecular dysfunction of articular cartilage cells
(39-41).
Zheng et al (42) reported
that purple rivet can inhibit IL-1β induced inflammatory response
in human osteoarthritis cartilage cells and slow the development of
osteoarthritis in mice. Zeng et al (43) found that the levels of MMP-1, MMP-2
and MMP-9 protein in patients with KOA were higher than those in
the control group. Matrix metalloproteinases aggravated the
inflammatory reaction and degradation of cartilage matrix by
enhancing apoptosis of cartilage cells and inhibiting the
metabolism of cartilage matrix. In the report of Stannus et
al (44), TNF-α and IL-6 changes
are associated with increased loss of medial and lateral tibial
cartilage volume, and serum IL-6 and TNF-α levels are associated
with knee cartilage loss in the elderly, suggesting that low levels
of inflammation play a role in the pathogenesis of KOA. In this
study, the levels of serum IL-1β and IL-6, MMP-1, TNF-α in the
study group were higher than those in the control group, and TLR-4,
TLR-5 and IRF4 were positively correlated with IL-1β, IL-6, MMP-1
and TNF-α, respectively. Therefore, TLR-4, TLR-5 and IRF4 may be
related to cartilage apoptosis and inflammatory factor markers in
KOA, but the specific mechanism needs to be further explored.
This study confirmed the role of combined detection
of TLR-4, TLR-5 and IRF4 in the early diagnosis and prognosis
evaluation of KOA.
In conclusion, the combined detection of TLR-4,
TLR-5 and IRF4 can diagnose early KOA and evaluate the poor
prognosis of patients with KOA, which is related to IL-1β, IL-6,
MMP-1 and TNF-α.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH, XC and XianyinW wrote the manuscript, analyzed
and interpreted the patient general data. ZS and XiaobingW
performed ELISA. ZZ and HW were responsible for observation
indicator analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Puyang Hospital of Traditional Chinese Medicine (Puyang, China).
Patients who participated in this research had complete clinical
data. Signed informed consents were obtained from the patients
and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen H, Zheng X, Huang H, Liu C, Wan Q and
Shang S: The effects of a home-based exercise intervention on
elderly patients with knee osteoarthritis: A quasi-experimental
study. BMC Musculoskelet Disord. 20(160)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Peixoto JG, de Souza Moreira B, Diz JBM,
Timoteo EF, Kirkwood RN and Teixeira-Salmela LF: Analysis of
symmetry between lower limbs during gait of older women with
bilateral knee osteoarthritis. Aging Clin Exp Res. 31:67–73.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gigis I, Fotiadis E, Nenopoulos A, Tsitas
K and Hatzokos I: Comparison of two different molecular weight
intra-articular injections of hyaluronic acid for the treatment of
knee osteoarthritis. Hippokratia. 20:26–31. 2016.PubMed/NCBI
|
|
4
|
Clynes MA, Jameson KA, Edwards MH, Cooper
C and Dennison EM: Impact of osteoarthritis on activities of daily
living: Does joint site matter? Aging Clin Exp Res. 31:1049–1056.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Paik J, Duggan ST and Keam SJ: Correction
to: Triamcinolone aceonide extended-release: A review in
osteoarthritis pain of the knee. Drugs. 79(691)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Akintayo RO, Yerima A, Olaosebikan HB,
Uhunmwangho C and Akpabio AA: How much gloom is in groans?
Depression and its determinants in Nigerian patients with knee
osteoarthritis: A multi-center cross-sectional study. Clin
Rheumatol. 38:1971–1978. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Camacho Encina M, Calamia V, Picchi
Figueira F, Van Duine J, Qiu J, Ruiz Romero C, LaBaer J and Blanco
F: Discovery of an autoantibody profile for the early diagnosis of
knee osteoarthritis using NAPPA: Data from the osteoarthritis
initiative. Osteoarthritis Cartilage. 26:S39–S40. 2018.
|
|
8
|
Ryan MD, Roulston C, de Felipe P, Odon V,
Tilsner J and Luke GA: The potential consequences for cell
signaling by a class of NOD-like receptor proteins (NLRs) bearing
an N-terminal signal sequence. J Cell Signal. 2(148)2017.
|
|
9
|
Kandahari AM, Yang X, Dighe AS, Pan D and
Cui Q: Recognition of immune response for the early diagnosis and
treatment of osteoarthritis. J Immunol Res.
2015(192415)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu WD, Pan HF, Ye DQ and Xu Y: Targeting
IRF4 in autoimmune diseases. Autoimmun Rev. 11:918–924.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schoenemeyer A, Barnes BJ, Mancl ME, Latz
E, Goutagny N, Pitha PM, Fitzgerald KA and Golenbock DT: The
interferon regulatory factor, IRF5, is a central mediator of
toll-like receptor 7 signaling. J Biol Chem. 280:17005–17012.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Petrasek J, Dolganiuc A, Csak T,
Kurt-Jones EA and Szabo G: Type I interferons protect from
Toll-like receptor 9-associated liver injury and regulate IL-1
receptor antagonist in mice. Gastroenterology. 140:697–708.e4.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mullen LM, Chamberlain G and Sacre S:
Pattern recognition receptors as potential therapeutic targets in
inflammatory rheumatic disease. Arthritis Res Ther.
17(122)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang W, Wang H, Li YS and Luo W: Role of
vasoactive intestinal peptide in osteoarthritis. J Biomed Sci.
23(63)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nair A, Kanda V, Bush-Joseph C, Verma N,
Chubinskaya S, Mikecz K, Glant TT, Malfait AM, Crow MK, Spear GT,
et al: Synovial fluid from patients with early osteoarthritis
modulates fibroblast-like synoviocyte responses to toll-like
receptor 4 and toll-like receptor 2 ligands via soluble CD14.
Arthritis Rheum. 64:2268–2277. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chamberlain ND, Vila OM, Volin MV, Volkov
S, Pope RM, Swedler W, Mandelin AM II and Shahrara S: TLR5, a novel
and unidentified inflammatory mediator in rheumatoid arthritis that
correlates with disease activity score and joint TNF-α levels. J
Immunol. 189:475–483. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang C, He D, Yin C and Tan J: Inhibition
of interferon regulatory factor 4 suppresses Th1 and Th17 cell
differentiation and ameliorates experimental autoimmune
encephalomyelitis. Scand J Immunol. 82:345–351. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chang MW, Cook AD, Nutt SL, Hamilton JA
and Lacey DC: Interferon regulatory factor 4 and inflammation.
Cytokine. 70(33)2014.
|
|
19
|
Lana JF, Macedo A, Ingrao ILG, Huber SC,
Santos GS and Santana MHA: Leukocyte-rich PRP for knee
osteoarthritis: Current concepts. J Clin Orthop Trauma. 10 (Suppl
1):S179–S182. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rogoveanu OC, Calina D, Cucu MG, Burada F,
Docea AO, Sosoi S, Stefan E, Ioana M and Burada E: Association of
cytokine gene polymorphisms with osteoarthritis susceptibility. Exp
Ther Med. 16:2659–2664. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang R, Yang X, Wang J, Han L, Yang A,
Zhang J, Zhang D, Li B, Li Z and Xiong Y: Identification of
potential biomarkers for differential diagnosis between rheumatoid
arthritis and osteoarthritis via integrative genome-wide gene
expression profiling analysis. Mol Med Rep. 19:30–40.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Trachana V, Mourmoura E, Papathanasiou I
and Tsezou A: Understanding the role of chondrocytes in
osteoarthritis: Utilizing proteomics. Expert Rev Proteomics.
16:201–213. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hochberg MC, Altman RD, Brandt KD, Clark
BM, Dieppe PA, Griffin MR, Moskowitz RW and Schnitzer TJ: American
College of Rheumatology: Guidelines for the medical management of
osteoarthritis. Part I. Osteoarthritis of the hip. Arthritis Rheum.
38:1535–1540. 1995.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kraus VB, Catterall J, Soderblom E,
Moseley MA and Suchindran S: Development of a serum biomarker panel
highly predictive of knee osteoarthritis progression.
Osteoarthritis Cartilage. 24(S20)2016.
|
|
25
|
Brandt KD, Fife RS, Braunstein EM and Katz
B: Radiographic grading of the severity of knee osteoarthritis:
Relation of the Kellgren and Lawrence grade to a grade based on
joint space narrowing, and correlation with arthroscopic evidence
of articular cartilage degeneration. Arthritis Rheum. 34:1381–1386.
1991.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Norman B, Pedoia V, Noworolski A, Link TM
and Majumdar S: Applying densely connected convolutional neural
networks for staging osteoarthritis severity from plain
radiographs. J Digit Imaging. 32:471–477. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahmed U, Anwar A, Savage RS, Thornalley PJ
and Rabbani N: Protein oxidation, nitration and glycation
biomarkers for early-stage diagnosis of osteoarthritis of the knee
and typing and progression of arthritic disease. Arthritis Res
Ther. 18(250)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen D, Shen J, Zhao W, Wang T, Han L,
Hamilton JL and Im HJ: Osteoarthritis: Toward a comprehensive
understanding of pathological mechanism. Bone Res.
5(16044)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liang H, Wang H, Luo L, Fan S, Zhou L, Liu
Z, Yao S, Zhang X, Zhong K, Zhao H and Zha Z: Toll-like receptor 4
promotes high glucose-induced catabolic and inflammatory responses
in chondrocytes in an NF-κB-dependent manner. Life Sci.
228:258–265. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jin J, Yu X, Hu Z, Tang S, Zhong X, Xu J,
Shang P, Huang Y and Liu H: Isofraxidin targets the TLR4/MD-2 axis
to prevent osteoarthritis development. Food Funct. 9:5641–5652.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mudter J, Yu J, Zufferey C, Brüstle A,
Wirtz S, Weigmann B, Hoffman A, Schenk M, Galle PR, Lehr HA, et al:
IRF4 regulates IL-17A promoter activity and controls RORγ
t-dependent Th17 colitis in vivo. Inflamm Bowel Dis. 17:1343–1358.
2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Brüstle A, Heink S, Huber M, Rosenplänter
C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T and Lohoff M:
The development of inflammatory T(H)-17 cells requires
interferon-regulatory factor 4. Nat Immunol. 8:958–966.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Lim R, Barker G and Lappas M: The TLR2
ligand FSL-1 and the TLR5 ligand Flagellin mediate pro-inflammatory
and pro-labour response via MyD88/TRAF6/NF-κB-dependent signalling.
Am J Reprod Immunol. 71:401–417. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kwa MQ, Nguyen T, Huynh J, Ramnath D, De
Nardo D, Lam PY, Reynolds EC, Hamilton JA, Sweet MJ and Scholz GM:
Interferon regulatory factor 6 differentially regulates Toll-like
receptor 2-dependent chemokine gene expression in epithelial cells.
J Biol Chem. 289:19758–19768. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Miller RE, Scanzello CR and Malfait AM: An
emerging role for Toll-like receptors at the neuroimmune interface
in osteoarthritis. Semin Immunopathol. 41:583–594. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ospelt C, Brentano F, Rengel Y, Stanczyk
J, Kolling C, Tak PP, Gay RE, Gay S and Kyburz D: Overexpression of
toll-like receptors 3 and 4 in synovial tissue from patients with
early rheumatoid arthritis: Toll-like receptor expression in early
and longstanding arthritis. Arthritis Rheum. 58:3684–3692.
2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee MC, Saleh R, Achuthan A, Fleetwood AJ,
Förster I, Hamilton JA and Cook AD: CCL17 blockade as a therapy for
osteoarthritis pain and disease. Arthritis Res Ther.
20(62)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ding L, Hong X, Sun B, Huang Q, Wang X,
Liu X, Li L, Huang Z and Liu D: IL-37 is associated with
osteoarthritis disease activity and suppresses proinflammatory
cytokines production in synovial cells. Sci Rep.
7(11601)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Haseeb A, Ansari MY and Haqqi TM:
Harpagoside suppresses IL-6 expression in primary human
osteoarthritis chondrocytes. J Orthop Res. 35:311–320.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang C, Wang P, Jiang P, Lv Y, Dong C,
Dai X, Tan L and Wang Z: Upregulation of lncRNA HOTAIR contributes
to IL-1β-induced MMP overexpression and chondrocytes apoptosis in
temporomandibular joint osteoarthritis. Gene. 586:248–253.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li ZC, Han N, Li X, Li G, Liu YZ, Sun GX,
Wang Y, Chen GT and Li GF: Decreased expression of microRNA-130a
correlates with TNF-α in the development of osteoarthritis. Int J
Clin Exp Pathol. 8:2555–2564. 2015.PubMed/NCBI
|
|
42
|
Zheng W, Zhang H, Jin Y, Wang Q, Chen L,
Feng Z, Chen H and Wu Y: Butein inhibits IL-1β-induced inflammatory
response in human osteoarthritis chondrocytes and slows the
progression of osteoarthritis in mice. Int Immunopharmacol.
42:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zeng GQ, Chen AB, Li W, Song JH and Gao
CY: High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis.
Genet Mol Res. 14:14811–14822. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Stannus O, Jones G, Cicuttini F,
Parameswaran V, Quinn S, Burgess J and Ding C: Circulating levels
of IL-6 and TNF-α are associated with knee radiographic
osteoarthritis and knee cartilage loss in older adults.
Osteoarthritis Cartilage. 18:1441–1447. 2010.PubMed/NCBI View Article : Google Scholar
|