Introduction
Spinal cord injury (SCI) is a serious condition with
high mortality and poor prognosis following spinal trauma,
including primary and secondary injury. Primary injury often occurs
during the first 48 h after injury while the secondary injury,
which occurs a few days to several months after SCI, is more
destructive and considered as a determining factor of the repair of
damaged neurons (1). The tumor
growth factor β (TGF-β)/Smad pathway serves an important role in
secondary SCI (2). Growth
differentiation factor-11 (GDF-11), a member of the TGF-β
superfamily, is recognized by activin type II receptor A and -B,
and phosphorylates Smad2 and -3. Following phosphorylation, Smad2/3
binds to Smad4 to form a complex, which regulates gene expression
and accumulates in the nucleus. A previous study suggested that the
GDF-11/Smads pathway was associated with cell growth,
differentiation and homeostasis (3).
Notably, Shi and Liu (3)
demonstrated a slower neuronal differentiation rate in
GDF-11-/- mouse embryos during spinal cord development.
GDF-11 signals produced by newly differentiated neurons act as a
feedback signal on the adjacent progenitors to promote cell cycle
exit, inhibit proliferation, and thus facilitate temporal
progression of neurogenesis in the developing spinal cord (4). This indicates that GDF-11 may play a
role in neurogenesis in the spinal cord.
Pyroptosis is a caspase-1-dependent, inflammatory
form of programmed cell death (5).
Several previous studies have only focused on the role of
pyroptosis in macrophages and dendritic cells (6-9).
However, a recent study demonstrated that the pyroptosis pathway
may also play a role in nerve cells (10). Pyroptosis-associated proteins,
including apoptosis-associated speck-like protein containing a
caspase-activating recruitment domain (ASC), caspase-1 and
interleukin (IL)-1β, may play a role in the innate immune response
against bacterial infection or trauma. The inflammasome is formed
after injury of the central nervous system via absent in melanoma-2
(AIM-2) (11) or NOD-like receptors
protein (NLRP)-1(12), which induce
cleavage of pro-caspase-1. Activated caspase-1 serves a key role in
pyroptosis through the induction of IL-1β and IL-18, which are
involved in the regulation of inflammation (13).
Therefore, the pyroptosis pathway may be involved in
the pathological process of SCI and may be a therapeutic target for
alleviating secondary injury. The aim of the present study was to
investigate the roles of the GDF-11/Smad and pyroptosis pathways in
SCI during the second injury period, and to determine the
underlying mechanism.
Materials and methods
Animals and design
The present study used ICR wild-type mice (6-8 week
old; male; n=120 in total) purchased from The Animals Center of
China Medical University. The mice were housed in a 12-h light/dark
cycle at 26˚C with 50% humidity and free access to food and water,
except where otherwise indicated. SCI was established by artery
clamp through clamping of the T10 level spinal cord after
laminectomy for 1 min according to a previous study by Marques
et al (14). Control mice
received a sham-operation, which included a laminectomy without
SCI. Ethical standards of China Medical University were followed,
and the present study was approved by the local Animal Committee of
China Medical University.
ICR wild-type mice were randomly divided into six
groups (n=10 per group). The mice of the normal saline (N) +
control (con) group were treated with 20 µl normal saline at T10
level through a local intraspinal epidural injection after
sham-operation. After SCI was established, the mice of the N+sci
group were treated with 20 µl normal saline at the injured level
through local injection. The mice of the caspase-1 (C) + con group
were treated with caspase-1 inhibitor (cat. no. sc-358878; Santa
Cruz Biotechnology, Inc.; 20 µg in 20 µl normal saline) at the T10
level of the spinal cord after sham-operation. The SCI mice in the
C+sci group were also injected with caspase-1 inhibitor at the T10
level. The mice of the Smad3 inhibitor (S) + con group were treated
with Smad3 inhibitor (cat. no. sc-222318; Santa Cruz Biotechnology,
Inc.; 20 µg in 20 µl normal saline) at the T10 level through local
injection, and the mice in the S+sci group were treated with Smad3
inhibitor at the injured level after SCI. Basso Mouse Scale (BMS)
scores (15) were used to assess the
recovery of the injured mice during the first 2 weeks after
operation. The behaviors of the mice were observed and the degree
of SCI was assessed by BMS scores prior to sacrifice. Behavioral
changes, including significant decrease of body temperature,
respiratory depression and bradycardia in SCI mice were considered
as humane endpoints where the mice would be sacrificed by the staff
of The Animal Department of China Medical University. The mice were
sacrificed and the injured level of the spinal cord was harvested
on the 14th day postoperatively, except where otherwise indicated.
All animal procedures were performed to minimize suffering in
accordance with the guidelines established by The Animal
Experimental Committee.
Western blot analysis
The spinal cord tissues (a length of 6 mm including
the injured tissue) were homogenized in Laemmli buffer (cat. no.
1610737; Bio-Rad Laboratories, Inc.), and the proteins (50 µg per
lane determined using a Bradford Protein Assay kit; cat. no. P0006;
Beyotime Institute of Biotechnology) were separated by 10%
SDS-PAGE, then transferred to PVDF membranes. The membranes were
blocked with 1% BSA at room temperature for 1 h and incubated
sequentially with primary antibodies (1:1,000 dilution; room
temperature; 2 h) and secondary antibodies (1:2,000 dilution; room
temperature; 1 h). β-actin was used as the internal control.
Western blotting was performed using antibodies against caspase-1
(cat. no. sc-56036; Santa Cruz Biotechnology, Inc.), IL-1β (cat.
no. 12703; Cell Signaling Technology, Inc.), GDF-11 (cat. no.
sc-81952; Santa Cruz Biotechnology, Inc.), Smad4 (cat. no. sc-7966;
Santa Cruz Biotechnology, Inc.), NLRP1 (cat. no. QC49289; Sigma
Aldrich; Merck KGaA), AIM-2 (cat. no. ab180655; Abcam), ASC (cat.
no. sc-22514-R; Santa Cruz Biotechnology, Inc.) and β-actin (cat.
no. sc-47778; Santa Cruz Biotechnology, Inc.). HRP-conjugated goat
anti rabbit IgG (cat. no. ZB-2301; OriGene Technologies, Inc.) or
HRP-conjugated goat anti mouse IgG (cat. no. ZB-2305; OriGene
Technologies, Inc.) were used as secondary antibodies. ECL Western
Blotting Substrate (cat. no. 32106; Thermo Fisher Scientific, Inc.)
was used as the visualization reagent (Image Lab V5.2.1; Bio-Rad
Laboratories, Inc.).
Immunohistochemistry and
immunofluorescence
Spinal cords were fixed overnight with 4%
formaldehyde in PBS (pH 7.2) at room temperature, carefully
isolated, embedded in paraffin and cut into 5-µm sections. The
sections were first incubated with 3% BSA at room temperature for 1
h to prevent endogenous interference. The sections were then
incubated with primary antibodies (1:100 dilution) at room
temperature for 2 h, followed by secondary antibodies (1:200
dilution) labeled with fluorescence or horseradish peroxidase (room
temperature; 1 h). The following primary antibodies were used for
analysis: Caspase-1 (cat. no. sc-56036; Santa Cruz Biotechnology,
Inc.), GDF-11 (cat. no. sc-81952; Santa Cruz Biotechnology, Inc.),
Smad2/3 (cat. no. 5678; Cell Signaling Technology, Inc.), NLRP1
(cat. no. QC49289; Sigma-Aldrich; Merck KGaA) and AIM-2 (cat. no.
ab180655; Abcam). The following secondary antibodies were used for
analysis: HRP-conjugated goat anti rabbit IgG (cat. no. ZB-2301;
OriGene Technologies, Inc.), HRP-conjugated goat anti mouse IgG
(cat. no. ZB-2305; OriGene Technologies, Inc.), Cy3-conjugated goat
anti-mouse IgG (cat. no. ab97035; Abcam) and FITC-conjugated goat
anti-rabbit IgG (cat. no. ab6717; Abcam). Images were acquired
using fluorescence microscopy (magnification, x400). Representative
images were obtained from at least two different sections.
Statistical analysis
The data are presented as the mean ± standard
deviation. Data were analyzed using Student's t-test, except for
multiple comparisons, where ANOVA was used instead. Tukey's post
hoc test was used following ANOVA. P<0.05 was considered to
indicate a statistically significant difference. All analyses were
performed using SPSS software (V16.0; SPSS, Inc.) and all
experiments were repeated three times.
Results
Pyroptosis signaling pathway may be
involved in SCI during the recovery period
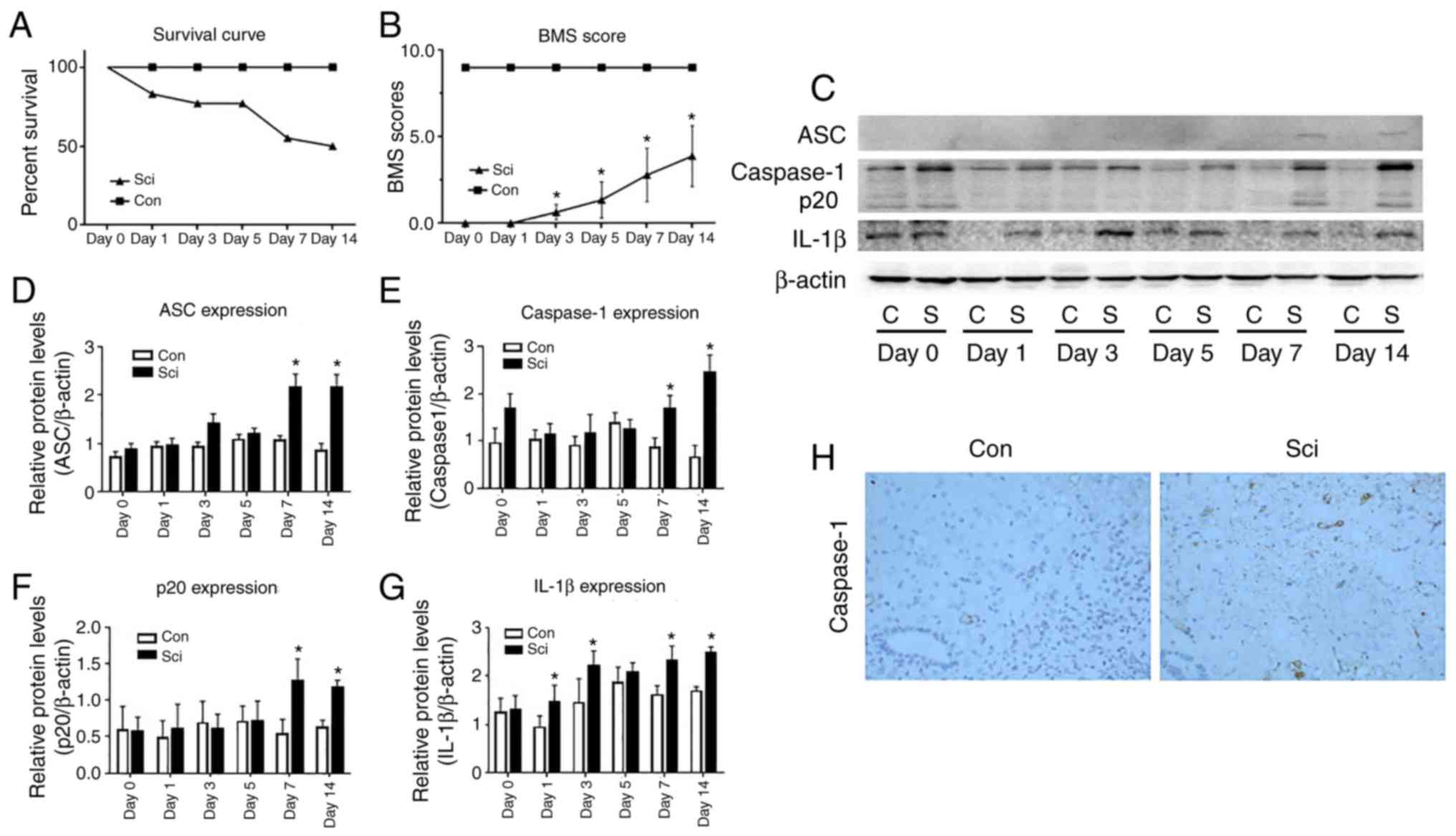
No mice died in the con group and the survival rate
of this group was 100% over 2 weeks. After the sham operation, hind
limb dysfunction of this group was not observed and the BMS scores
were 9. In the SCI group, the survival rate decreased to 77.78% at
day 3 following surgery (Fig. 1A),
and a slight ankle movement was observed in 7 of the 9 mice in this
group. The mean BMS score was 0.64±0.44. On day 5 after surgery,
significant ankle joint activity was observed in the majority of
mice in the SCI group, while a large range of active ankle joint
activity and occasionally landing on the instep was observed in 3
of the 9 mice. The mean BMS score was increased to 1.36±1.05 and
the survival rate of the SCI group was reduced to 55.56% compared
with the con group. Limb dysfunction observed in mice from the SCI
group gradually stabilized between days 7 and 14 after SCI, and the
mean BMS score increased to 3.89±1.76. The BMS scores were
significantly different between the SCI and con groups between days
3 and 14 (Fig. 1B).
ASC and caspase-1 were highly expressed in the
spinal cords of mice in the SCI group at days 7 and 14 (Fig. 1D and E). By contrast, ASC and caspase-1 were
hardly expressed in the spinal cord of the con group (Fig. 1C). In addition, the caspase-1 p20
subunit was detected in the SCI group (Fig. 1F), but not in the con group at days 7
and 14. The expression of IL-1β was significantly increased in the
SCI group, compared with the con group at days 1, 3, 7 and 14
(P<0.05; Fig. 1C and G).
The expression of caspase-1 at the T10 spinal cord
level was detected by immunohistochemical staining at day 14 after
surgery. It was identified that caspase-1 was highly expressed and
predominantly distributed in the anterior horn of the spinal cord
in SCI group mice, while it was rarely expressed in the con group
(Fig. 1H).
SCI is alleviated by caspase-1
inhibitors and Smad3 inhibitors
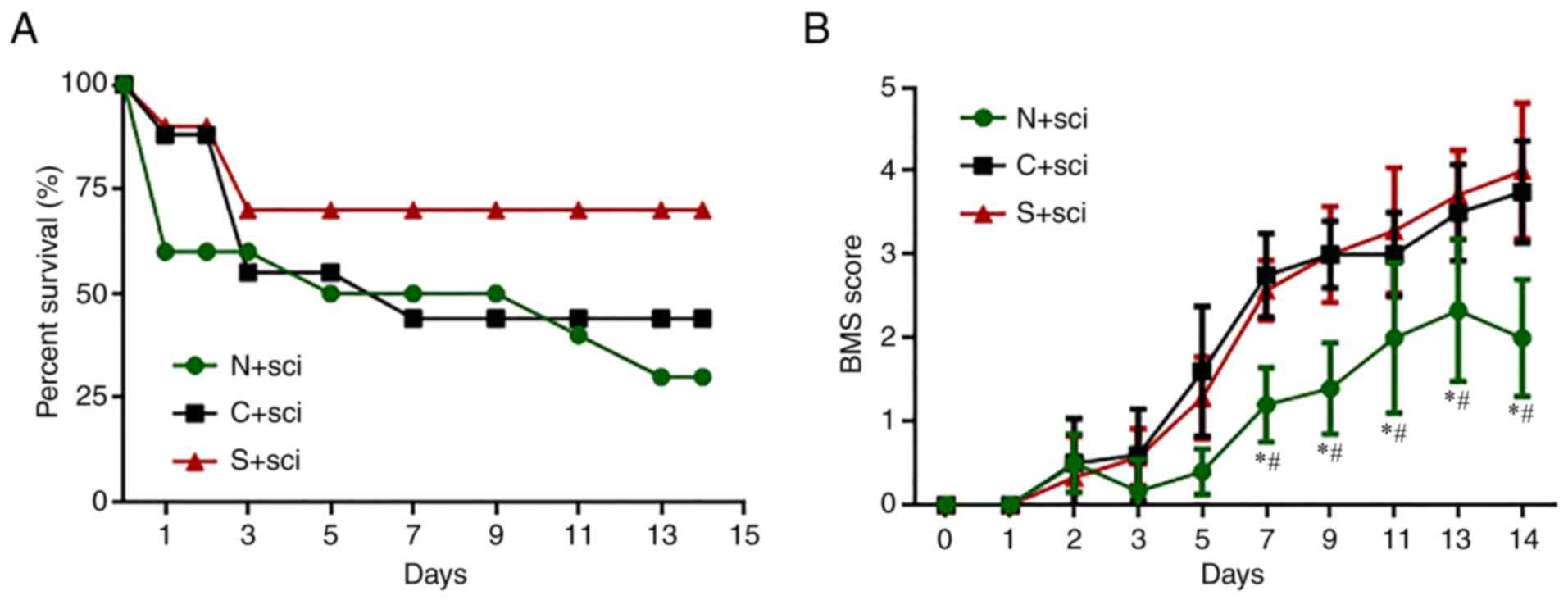
At the first 48 h after SCI, the survival rate was
reduced markedly to 60% in the N+sci group, 90% in the S+sci group
and the 88% in C+sci group (Fig.
2A). There were no obvious differences in the survival rates of
the C+sci and N+sci groups between days 4 and 14. However, the
survival rate of the S+sci group was markedly higher than the other
two groups (Fig. 2A).
The BMS score was 0 for all three SCI groups at the
first day after operation. The scores then gradually increased
during the first 5 days and there were no significant differences
among the three groups (P>0.05). The BMS scores of the S+sci and
C+sci groups were markedly increased between days 7 and 14,
compared with the N+sci group (Fig.
2B). There were no significant differences in the BMS scores
between the C+sci and S+sci groups.
Smad3 inhibitors inhibit
caspase-1-induced pyroptosis of neurons in SCI mice
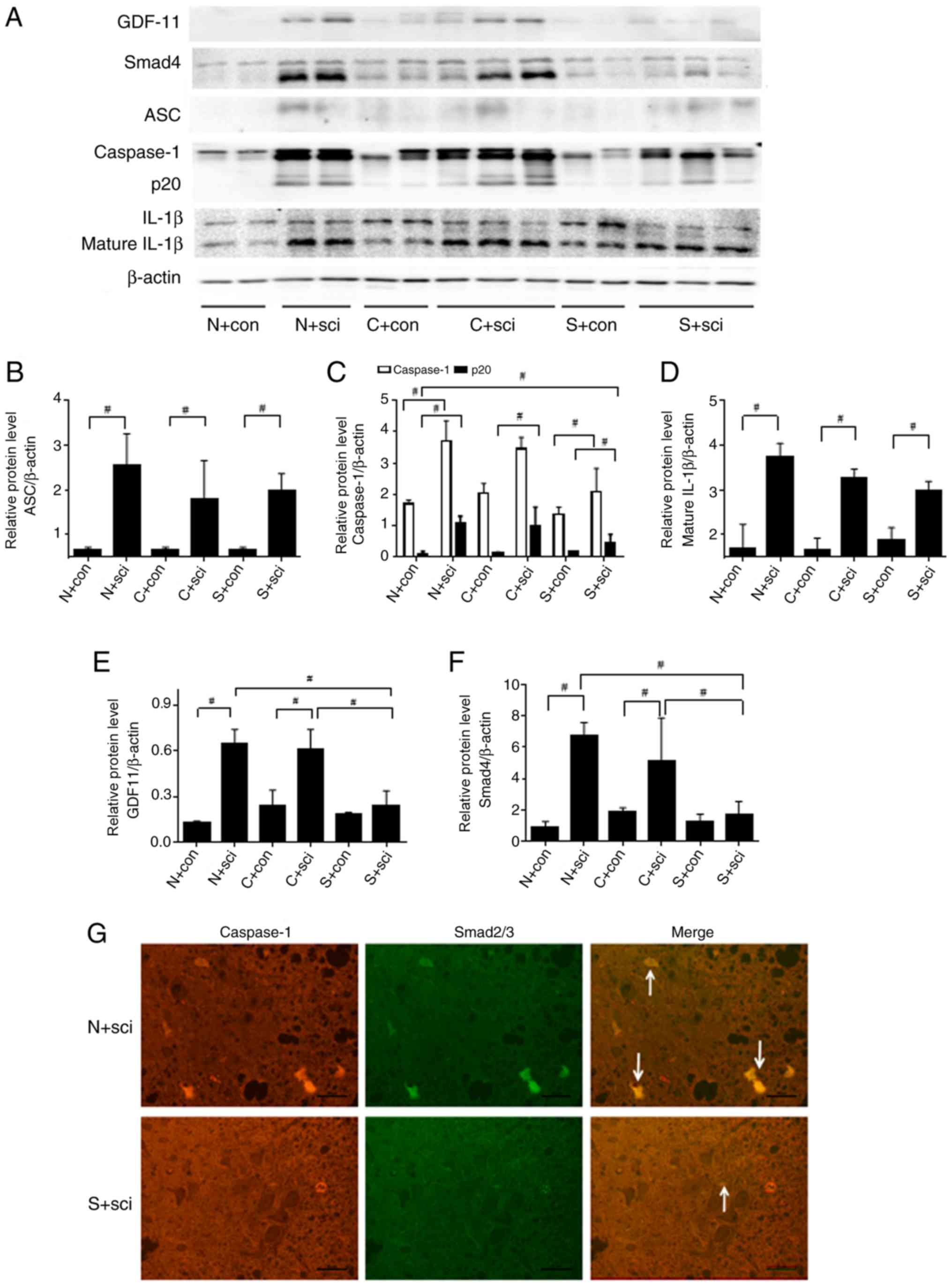
Markers of the pyroptosis signaling pathway,
including ASC, p20 and mature IL-1β, were expressed at low levels
in the three control groups. No significant difference was
identified in the expression of ASC between the three SCI groups
(Fig. 3B). Mature IL-1β in SCI mice
was downregulated following treatment with caspase-1 inhibitor
compared with the N+sci group (Fig.
3A). The expression levels of p20 and IL-1β were significantly
decreased in the S+sci group, compared with the N+sci group
(P<0.05; Fig. 3C-D). The
caspase-1 fragment p20 was detectable following SCI in the N+sci
group, and downregulated in the S+sci group compared with the other
two SCI groups (Fig. 3A and C).
The expression levels of GDF-11 and Smad4 were
increased after SCI in the N+sci group and expressed at low levels
in the S+sci group, compared with the other two SCI groups at day
14 after injury (Fig. 3A). No
significant differences were identified in the expression levels of
GDF-11 and Smad4 between the C+sci and N+sci groups (Fig. 3A, E
and F).
Co-immunostaining demonstrated that Smad2/3
expression was enriched in caspase-1-expressing neurons at the
ventricornu of the N+sci group. The expression of Smad2/3 and
caspase-1 could be inhibited and the number of pyroptotic neurons
was decreased by treating the SCI mice with the Smad3 inhibitor
(Fig. 3G).
AIM-2 or NLRP1 may not be targets of
the GDF-11/Smads pathway in the regulation of pyroptosis in
neurons
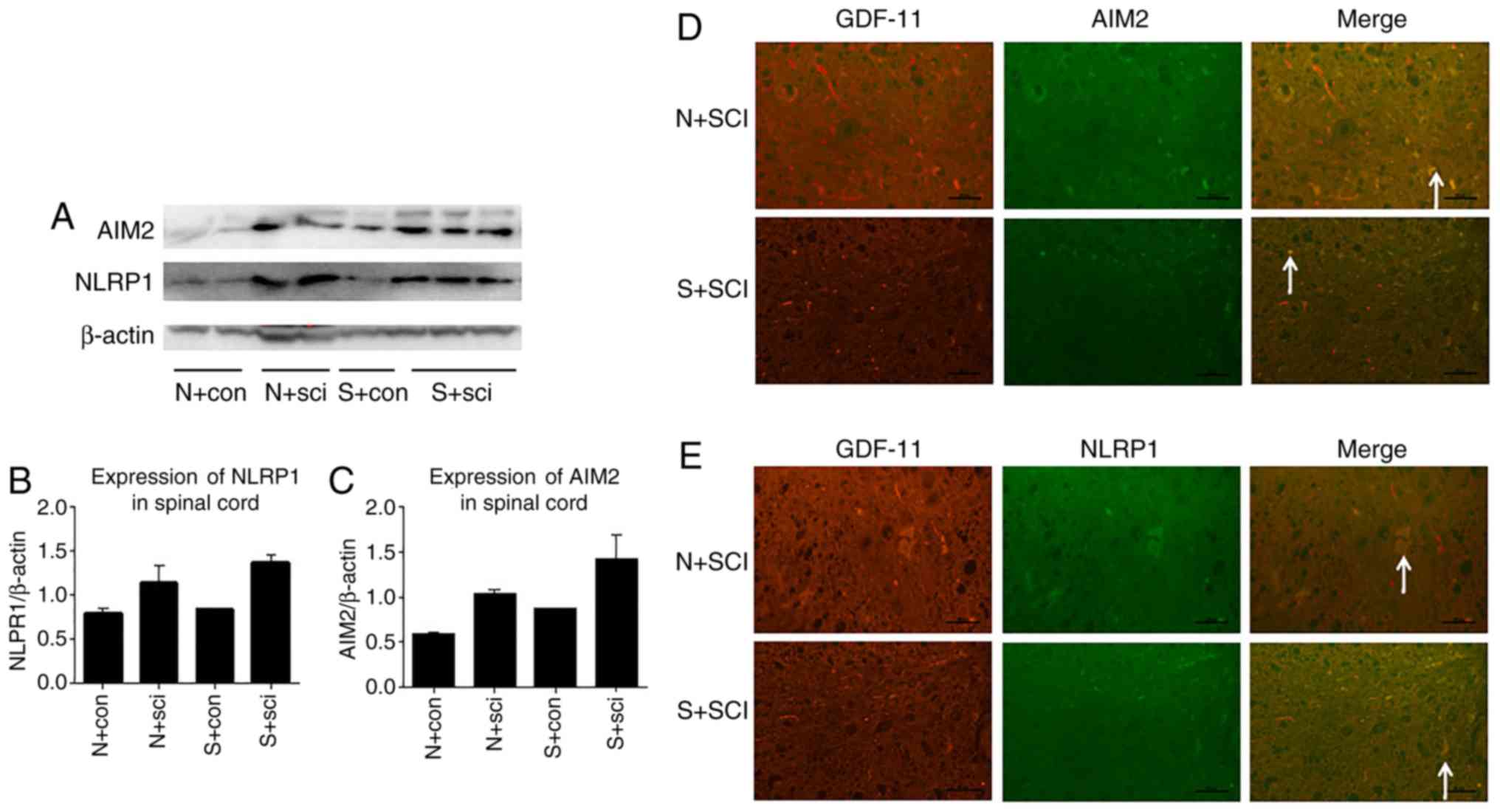
The expression levels of NLRP1 and AIM-2 were
markedly increased in the N+sci and S+sci groups, compared with the
respective controls, while there were no significant differences in
NLRP1 and AIM-2 expression between these two groups (Fig. 4). Co-immunostaining of GDF-11 with
AIM-2 or NLRP1 in ventricornu demonstrated no association between
GDF-11 and AIM-2 or NLRP1 (Fig. 4D
and E).
Discussion
Pyroptosis is a distinct form of programmed cell
death triggered by the activation of the ASC/caspase-1 pathway.
Unlike apoptosis, cells undergoing pyroptosis enhance the release
of the pro-inflammatory factors IL-1β and IL-18 (16,17).
Previous studies demonstrated that the pyroptosis pathway mediates
host defense and induces a secondary immunological response to
infectious diseases or damage (18,19).
Pro-caspase-1, a large protein of ~51 kDa, is activated by ASC and
cleaves into the activated form p20 (~20 kDa) to induce pyroptosis.
de Rivero Vaccari et al (19)
suggested that ASC expression increased and caspase-1 was activated
in rats following SCI, compared with control rats during primary
injury. It was suggested that neuronal pyroptosis may serve an
important role in SCI. The present study demonstrated that
pyroptosis of spinal cord neurons may be involved in SCI during the
recovery period. Thus, inhibition of neuronal pyroptosis could
alleviate secondary SCI and may be an underlying therapeutic target
for SCI.
In the present study, the highest mortality in the
N+sci group was observed 24 to 72 h after SCI, which could be
attributed to secondary injury of the spinal cord, including nerve
cell death, the release of inflammatory cytokines, calcium overload
and peroxidation which often gradually occurs at 24 to 72 h after
injury (20-22).
In a previous study by Li et al (23), the caspase inhibitor zVAD-fmk (10 µg
in 10 µl vehicle) could block the caspase-1 pathway in C57BL/6 mice
(20-25 g) following SCI.
To understand the role of the pyroptosis signaling
pathway in SCI, the present study focused on ICR mice (30-35 g)
treated with caspase-1 inhibitor according to the previous study by
Li et al (23). Following
SCI, the survival rate of mice treated with the caspase-1 inhibitor
was markedly improved during the first 2 days, compared with the
control group. The BMS scores were also markedly higher with
caspase-1 inhibition, compared with the control. This suggested
that SCI could be alleviated by inhibiting the pyroptosis signaling
pathway via caspase-1. Inhibition of the pyroptosis pathway might
reduce the injury of nerve cells during secondary injury, decrease
the release of inflammatory cytokines, including IL-1β, and
suppress neuronal pyroptosis.
The member of the TGF-β superfamily GDF-11 is
involved in cell growth and differentiation (4). A previous study demonstrated that
GDF-11 was associated with neurogenesis, and spinal cord neural
progenitor cells that do not express GDF-11 fail to exit the cell
cycle, resulting in alteration of neural subtypes (3). GDF-11 exerts its function by
interacting with its receptors to induce phosphorylation and
activation of Smad2 and Smad3. Smad2/3 assembles with Smad4 to form
complexes in the nucleus that regulate target genes (24-26).
The present study demonstrated that the expression levels of GDF-11
and Smad4 increased significantly in N+sci and C+sci group,
compared with the respective control groups, indicating that the
GDF-11/Smads pathway is activated following SCI. To understand the
role of the GDF-11/Smads signaling pathway in SCI, a Smad3
inhibitor was used in the early injury period to inhibit this
pathway. In the present study, the survival rate of mice treated
with Smad3 inhibitor was marked improved and BMS scores were higher
from days 7 to 14 after spinal cord injured, compared with that of
the N+sci group. Therefore, SCI could be alleviated by blocking the
GDF-11/Smads pathway. Compared with the control group, the
expression levels of p20 and IL-1β in the Smad3-inihitor-treated
group mice significantly decreased, which suggested that the
pyroptosis signaling pathway in SCI mice could be suppressed by a
Smad3 inhibitor. There may be an underlying association between the
GDF-11/Smads and pyroptosis pathways in SCI during the recovery
period. Therefore, Smad3 inhibitors may serve a role in alleviating
SCI in mice by reducing neuronal pyroptosis induced by
caspase-1.
Tamai R and Kiyoura (27) demonstrated that alendronate could
augment lipid A-induced IL-1β release and
Smad3/NLRP-3/ASC-dependent J774.1 cell death, and that Smad3
inhibition could suppress alendronate-augmented caspase-1 and IL-1β
production. These previous results were consistent with the
findings of the present study. While the previous study focused on
macrophage pyroptosis and investigated the role of NRLP-3, a marker
of the macrophage-mediated inflammation, the present study examined
pyroptosis in nerve cells.
AIM2 and NLRP1 are components of inflammasomes in
neurons, which contribute to the activation of pro-caspase-1 and
the induction of pyroptosis (11,12). To
understand the regulation of the Smad3 inhibitor in the pyroptosis
pathway of neurons, NLRP1 and AIM-2 expression in SCI mice was
assessed in the present study. There were no differences in the
NLRP1 and AIM-2 expression levels between the Smad3 inhibitor and
the control groups, which suggested that AIM-2 and NLRP1 may not be
the targets of the GDF-11/Smads pathway that serve a role in the
regulation of neural cell pyroptosis. The expression levels of
caspase-1 and Smad2/3 in the spinal cord were detected by
immunofluorescence co-staining. Smad2/3 and caspase-1 were strongly
co-expressed in neurons undergoing pyroptosis in the control group.
The expressions of Smad2/3 and caspase-1, as well as the extent of
pyroptosis, were decreased by treating SCI mice with the Smad3
inhibitor. This suggested that Smad3 may directly regulate the
expression of caspase-1 and affect pyroptosis.
In summary, the present study demonstrated that
inhibition of the GDF-11/Smads pathway could relieve SCI by
directly downregulating caspase-1 to reduce pyroptosis of neurons
during the recovery period after SCI. The underlying mechanism of
this remains unclear and requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from
Shengjing Hospital in 2015 and a Li Jiesou Intestinal Barrier
Research Grant (grant no. LJS-201812C). This work was also
supported by a grant from Health Care Big Data Research from China
Medical University (grant no. HMB201902102).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ carried out the experiment, participated in the
analysis and interpretation of data and drafted the manuscript. YF
participated in the analysis of the data and performed the
statistical analysis. GT conceived the study and participated in
its design and coordination. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the local Animal
Committee of China Medical University. Ethical standards of China
Medical University were followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang P, Zhang L, Zhu L, Chen F, Zhou S,
Tian T, Zhang Y, Jiang X, Li X, Zhang C, et al: The change tendency
of PI3K/Akt pathway after spinal cord injury. Am J Transl Res.
7:2223–2232. 2015.PubMed/NCBI
|
|
2
|
Nori S, Nakamura M and Okano H: Chapter 2.
Plasticity and regeneration in the injured spinal cord after cell
transplantation therapy. Prog Brain Res. 231:33–56. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shi Y and Liu JP: Gdf11 facilitates
temporal progression of neurogenesis in the developing spinal cord.
J Neurosci. 31:883–893. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gokoffski KK, Wu HH, Beites CL, Kim J, Kim
EJ, Matzuk MM, Johnson JE, Lander AD and Calof AL: Activin and
GDF11 collaborate in feedback control of neuroepithelial stem cell
proliferation and fate. Development. 138:4131–4142. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fink SL and Cookson BT: Apoptosis,
pyroptosis, and necrosis: Mechanistic description of dead and dying
eukaryotic cells. Infect Immun. 73:1907–1916. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miao EA, Rajan JV and Aderem A:
Caspase-1-induced pyroptotic cell death. Immunol Rev. 243:206–214.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen L, Liu X, Yu X, Ren R, Wang C, Zhao
R, Meng G, Li S and Zhou X: Chlamydia muridarum Infection of
Macrophages Stimulates IL-1β Secretion and Cell Death via
Activation of Caspase-1 in an RIP3-Independent Manner. BioMed Rese
Int. 2017(1592365)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Suzuki T, Franchi L, Toma C, Ashida H,
Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C and Nuñez G:
Differential regulation of caspase-1 activation, pyroptosis, and
autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS
Pathog. 3(e111)2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Eichholz K, Bru T, Tran TT, Fernandes P,
Welles H, Mennechet FJ, Manel N, Alves P, Perreau M and Kremer EJ:
Immune-Complexed Adenovirus Induce AIM2-Mediated Pyroptosis in
Human Dendritic Cells. PLoS Pathog. 12(e1005871)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li R, Zhang LM and Sun WB: Erythropoietin
rescues primary rat cortical neurons from pyroptosis and apoptosis
via Erk1/2-Nrf2/Bach1 signal pathway. Brain Res Bull. 130:236–244.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hornung V, Ablasser A, Charrel-Dennis M,
Bauernfeind F, Horvath G, Caffrey DR, Latz E and Fitzgerald KA:
AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating
inflammasome with ASC. Nature. 458:514–518. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu W, Zhang Y, Wu W, Yin Y, Huang D, Wang
Y and Li W and Li W: Chronic glucocorticoids exposure enhances
neurodegeneration in the frontal cortex and hippocampus via NLRP-1
inflammasome activation in male mice. Brain Behav Immun. 52:58–70.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jorgensen I, Lopez JP, Laufer SA and Miao
EA: IL-1β, IL-18, and eicosanoids promote neutrophil recruitment to
pore-induced intracellular traps following pyroptosis. Eur J
Immunol. 46:2761–2766. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Marques SA, Garcez VF, Del Bel EA and
Martinez AM: A simple, inexpensive and easily reproducible model of
spinal cord injury in mice: Morphological and functional
assessment. J Neurosci Methods. 177:183–193. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Basso DM, Fisher LC, Anderson AJ, Jakeman
LB, McTigue DM and Popovich PG: Basso Mouse Scale for locomotion
detects differences in recovery after spinal cord injury in five
common mouse strains. J Neurotrauma. 23:635–659. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Awad F, Assrawi E, Louvrier C, Jumeau C,
Georgin-Lavialle S, Grateau G, Amselem S, Giurgea I and Karabina
SA: Inflammasome biology, molecular pathology and therapeutic
implications. Pharmacol Ther. 187:133–149. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Winkler S, Hedrich CM and Rösen-Wolff A:
Caspase-1 regulates autoinflammation in rheumatic diseases. Z
Rheumatol. 75:265–275. 2016.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
18
|
Cai R, Liu L, Luo B, Wang J, Shen J, Shen
Y, Zhang R, Chen J and Lu H: Caspase-1 Activity in CD4 T Cells Is
Downregulated Following Antiretroviral Therapy for HIV-1 Infection.
AIDS Res Hum Retroviruses. 33:164–171. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
de Rivero Vaccari JP, Lotocki G, Marcillo
AE, Dietrich WD and Keane RW: A molecular platform in neurons
regulates inflammation after spinal cord injury. J Neurosci.
28:3404–3414. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Young W: Secondary injury mechanisms in
acute spinal cord injury. J Emerg Med. 11 (Suppl 1):13–22.
1993.PubMed/NCBI
|
|
21
|
Oyinbo CA: Secondary injury mechanisms in
traumatic spinal cord injury: A nugget of this multiply cascade.
Acta Neurobiol Exp (Wars). 71:281–299. 2011.PubMed/NCBI
|
|
22
|
Impellizzeri D, Mazzon E, Paterniti I,
Esposito E and Cuzzocrea S: Effect of fasudil, a selective
inhibitor of Rho kinase activity, in the secondary injury
associated with the experimental model of spinal cord trauma. J
Pharmacol Exp Ther. 343:21–33. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li M, Ona VO, Chen M, Kaul M, Tenneti L,
Zhang X, Stieg PE, Lipton SA and Friedlander RM: Functional role
and therapeutic implications of neuronal caspase-1 and -3 in a
mouse model of traumatic spinal cord injury. Neuroscience.
99:333–342. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rochette L, Zeller M, Cottin Y and Vergely
C: Growth and differentiation factor 11 (GDF11): Functions in the
regulation of erythropoiesis and cardiac regeneration. Pharmacol
Ther. 156:26–33. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Duran J, Troncoso MF, Lagos D, Ramos S,
Marin G and Estrada M: GDF11 Modulates Ca2+-Dependent
Smad2/3 Signaling to Prevent Cardiomyocyte Hypertrophy. Int J Mol
Sci. 19(1508)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu Q, Tu ML, Li CJ, Zhang L, Jiang TJ, Liu
T and Luo XH: GDF11 Inhibits Bone Formation by Activating Smad2/3
in Bone Marrow Mesenchymal Stem Cells. Calcif Tissue Int.
99:500–509. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tamai R and Kiyoura Y: Alendronate
augments lipid A-induced IL-1β release and
Smad3/NLRP3/ASC-dependent cell death. Life Sci. 198:8–17.
2018.PubMed/NCBI View Article : Google Scholar
|