Introduction
As a subgroup of chronic rhinosinusitis, nasal
polyposis is a clinical manifestation with a complex pathogenesis,
which involves chronic local infection, a family history and
genetic predisposition, atopy and allergy, as well as aerodynamic
factors (1). Although nasal polyps
are only detected in a small percentage of adults, they have been
discovered to lead to headaches, facial pain, fatigue, hyposmia or
total loss of the sense of smell and taste (2). In children, the occurrence of nasal
polyps has been associated with cystic fibrosis or cilia
dysfunction (3). Unfortunately, the
subjective nature of this disease makes it difficult to quantify
successfully, and patients with nasal polyps usually fail to be
fully cured due to the complex mechanisms underlying the
pathogenesis and recurrence (2).
Mucins (MUC) are mainly synthesized by goblet cells
and submucosal glandular cells in the airways (4). The MUC5AC, MUC5B and MUC2 genes, which
have all been proven to serve a pivotal role in inflammatory
respiratory diseases (5,6), are expressed in the sinus and mixed
nasal mucus secretions of patients with nasal polyps (7). It was previously demonstrated that
downregulation of MUC1 was associated with corticosteroid
resistance in patients with nasal polyps (8). In addition, MUC5AC and MUC5B were
discovered to contribute to the pathogenesis of nasal polyposis and
the development of chronic rhinosinusitis (9,10). It
has also been suggested that elevated levels of MUC2 and MUC5B may
induce excessive mucus secretion and decreased mucociliary
clearance in patients with chronic rhinosinusitis (11). However, to the best of our knowledge,
the correlation between the expression levels of MUC5AC, MUC5B and
MUC2 and the recurrence of nasal polyps has not been extensively
investigated.
Therefore, the present study aimed to investigate
the expression levels of MUC2, MUC5AC and MUC5B in patients with
and without the recurrence of nasal polyps, in addition to
determining whether MUC expression levels were associated with
recurrence.
Materials and methods
Patients
A total of 56 patients with nasal polyps who
underwent functional endoscopic sinus surgery (FESS) at The Third
Central Hospital of Tianjin (Tianjin, China) between June 2007 and
June 2010 were included in the present study. The inclusion
criteria were as follows: Male or female patients >18 years old
and patients with suspected nasal polyps were treated surgically
and nasal polyps were confirmed by pathology. Post-operative
pathology confirmed that all of the patients had nasal polyps.
Among the 56 patients, 39 were males and 17 were females, and the
median age was 45.5 years with a range of 18-66 years. A total of
12 patients (7 males and 5 females, aged 17-56 years with a median
age of 40.5 years) without nasal polyps underwent nasal septal
reconstruction. Patients with a history of food allergy, drug
allergy, smoking, allergic rhinitis, asthma, polypectomy, or oral
or nasal spray hormone use within two months prior to surgery were
excluded. All tissue samples were stored at -80˚C. The study
protocol was approved by the Ethics Committee of The Third Central
Hospital of Tianjin (Tianjin, China) and informed consent was
obtained verbally from all patients.
Experimental procedure
Pre-operative routine medical history, pre-operative
nasal endoscopy, sinus CT examination and scoring were performed
using Lund-Mackay CT score (12).
The pre-operative endoscopic examination revealed the presence of
new translucent grape-like organisms in the nasal cavity.
Considering the presence of nasal polyps, the location and scope of
the polyps were determined by an experienced otolaryngologist, who
identified polypoid via nasal endoscopy before operation, and a
Lund-Kennedy endpoint score (13)
was given. Polypectomy and open sinus surgery were performed
according to the principles of FESS (14). During the operation, nasal polyp
tissues from the middle nasal meatus and nasal mucosal tissues
without polyps (paranasal tissues) were collected to determine the
expression levels of MUC2, MUC5AC and MUC5B. After the surgery,
patients were administered external treatment (64 µg budesonide
nasal spray, once in each nostril, twice in the morning and
evening, continuous application for ≥12 weeks) and daily nasal
flushing with saline solution for 6 months. The subjects were also
subjected to regular endoscopy examination (every 2 weeks for 6
months) and surgical cavity cleaning (every 4 weeks for 6 months),
including dry scabs and vesicles, to check for recurrent polyps. At
6 months after surgery, the majority of the recurrent polyps were
located in the operative cavity of the open ethmoid sinus or
partially open ethmoid sinus in the middle nasal meatus, rather
than completely filling the middle nasal meatus. However, there
were also other mucosal tissues besides the polyps. Thus, the
epithelialized nasal mucosal tissues were collected again to
determine the expression levels of MUC2, MUC5AC and MUC5B. At 1
year post-surgery, 43 patients were recurrence-free, whereas 13
patients demonstrated recurrence for nasal polyps. In addition, 12
normal mucosal tissue samples were collected following nasal septal
reconstruction, and MUC2, MUC5AC and MUC5B expression levels were
also detected. The patients without colds were re-examined using
nasal endoscopy after the operation. If gray litchi flesh-like
tissues were observed in the middle nasal tract, the patients were
given budesonide nasal spray treatment for 2 weeks. After 2 weeks
of nasal hormone therapy, a pathological biopsy was taken if
lychee-like tissues were still present. Pathology confirmed that
these tissues were polyp tissues, which was subsequently defined as
the recurrence of nasal polyps.
Extraction and quantitative analysis
of total RNA
The total RNA Isolation kit (Promega Corp.) was used
according to the manufacturer's protocol to extract total RNA from
tissue. Together with 1 ml RNA lysate and 7 µl β-mercaptoethanol
(Sigma-Aldrich; Merck KGaA), 0.1 g tissue was fully ground and
collected in Eppendorf tubes (Eppendorf). NaAC (2 mol/l; pH 4.0;
Sigma-Aldrich; Merck KGaA), 1 ml water-saturated phenol and 200 ml
chloroform/isoamyl alcohol (49:1; Sigma-Aldrich; Merck KGaA) were
added in succession. The mixture was fully homogenized by violent
oscillation and placed on ice for 15 min. Following centrifugation
at 4˚C for 15 min at 12,000 x g, the supernatant was carefully
collected and an equal volume of isopropanol (Thermo Fisher
Scientific, Inc.; 99.5%; HPLC grade) was added following
precipitation at -20˚C for >1 h. Subsequently, 300 µl pancreatic
RNase A (Sigma-Aldrich; Merck KGaA) was added and re-precipitation
was performed by adding an equal volume of isopropanol. After
washing with precooled 75% ethanol (Sigma-Aldrich; Merck KGaA) for
10 sec, the precipitate was dissolved in 100 ml diethyl
pyrocarbonate (DEPC)-treated water (Sigma-Aldrich; Merck KGaA). The
RNA concentration was measured using a spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
(qPCR)
First-strand complementary (c)DNA was synthesized in
a total volume of 20 µl containing 1 µg total RNA, 6.2 µl random
primer pd(N) (Roche Diagnostics), DEPC-treated water, 4 µl 5X RT
buffer (Invitrogen; Thermo Fisher Scientific, Inc.), 1 µl avian
myeloblastosis virus reverse transcriptase (Roche Diagnostics
GmbH), 2.4 µl MgCl2 (25 mM; Sangon Biotech Co., Ltd.)
and 1 µl deoxynucleoside triphosphates (10 mM). The temperature
protocol was 25˚C for 15 min, 42˚C for 80 min and 70˚C for 10 min.
qPCR was subsequently performed in a total volume of 24 µl,
containing 2 µl template, 20 µl Real-Time PCR Master Mix (SYBR
Green; Roche Diagnostics), 0.5 µl 10 µM primer (Table I) and 1 µl Taq DNA polymerase (U/µl;
AmpliTaq; PerkinElmer, Inc.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95˚C for
2 min; followed by 40 cycles at 94˚C for 60 sec and 60˚C for 45
sec. The quantification cycle (Ct) values were obtained according
to the real-time amplification curve and melting curve of each
gene. The relative expression levels of the target genes were
calculated using the 2-ΔΔCq method (15).
 | Table IPrimers used for the reverse
transcription-quantitative PCR. |
Table I
Primers used for the reverse
transcription-quantitative PCR.
| Gene | Position
(nucleotides) | Primer sequences | Size of product
(bp) |
|---|
| G3PDH | 915-935 nt | Forward
5'-GGGCATCCTGGGCTACACTGA-3' | |
| | 1057-1032 nt | Reverse
5'-CAAATTCGTTGTCATACCAGGAAATG-3' | 143 |
| MUC2 | 12884-12901 nt | Forward
5'-AACCCACACCGCCCCTGC-3' | |
| | 13256-13237 nt | Reverse
5'ACGGCCCCGTTAAGCACAGC-3' | 373 |
| MUC5AC | 2743-2763 nt | Forward
5'-ATGGAGGCTGCTGAGGGACAG-3' | |
| | 3258-3239 nt | Reverse
5'GTGGCCGTTGACCAGGACGG-3' | 516 |
| MUC5B | 15475-15494 nt | Forward
5'-GCCGACAGCAGCTTCACCGT-3' | |
| | 15617-15598 nt | Reverse
5'TTGAGGAACACGCCGCCGTC-3' | 143 |
Immunohistochemical analysis
The normal nasal mucosa in the control group and the
nasal polyp tissues were fixed in 4% neutral formalin for 24 h,
embedded in paraffin and cut into 5-µm sections for
immunohistochemical staining. The sections were deparaffinized
using Histoplast PE Paraffin at 65˚C and rehydrated using anhydrous
ethanol for 5 min, 95% ethanol for 5 min, 80% ethanol for 5 min and
75% ethanol for 5 min, and then subjected to antigen retrieval
using EDTA (pH 9.0). Deparaffinized sections were incubated with 3%
H2O2 (Sangon Biotech Co., Ltd.) for 10 min at
37˚C to inhibit endogenous peroxidase activity and subsequently
incubated with goat serum (Gibco; Thermo Fisher Scientific, Inc.)
to block non-specific binding sites. The tissue sections were
incubated with anti-mouse MUC5AC (1:200; Abcam; cat. no. ab3649),
MUC5B (1:250; Abcam; cat. no. ab87376) and MUC2 (1:200; Abcam; cat.
no. ab134119) monoclonal primary antibodies at 4˚C for 10 min.
Following the incubation with primary antibody, the sections were
washed with PBS for 2 min and incubated for 30 min with a secondary
antibody (Polyperoxidase-anti-mouse/rabbit IgG; 1:200; cat. no,
JHA01; Shanghai Jiehao Biotechnology Co., Ltd) at 37˚C. The slides
were subsequently stained with 3,3'-diaminobenzidine at 37˚C for 30
min, followed by counterstaining with hematoxylin at 37˚C for 1
min, dehydration using 75% ethanol for 5 min, 80% ethanol for 5
min, 95% ethanol for 5 min and anhydrous ethanol for 5 min) and
mounting by neutral gum seal. The yellowish to brownish in the cell
membrane or cytoplasm was considered to indicate positive cells.
Five high power fields (OLYMPUS-BX53; Olympus Corporation;
magnification, x400) were randomly selected for analysis. To
evaluate staining, a score of (a) the percentage of positive cells
in each field of view (0, no positive cells; 1, 1-29% positive
cells; 2, 30-59% positive cells; 3, ≥60% positive cells)*(b) the
staining intensity (0, non-staining; 1, faint yellow; 2, brown
yellow; 3, sepia) was considered to be a comprehensive score: <2
was negative and ≥2 was positive. The results of the staining were
assessed by two independent pathologists in a blinded manner.
Statistical analysis
All experiments were repeated in triplicate.
Statistical analysis was performed using SPSS version 16.0 software
(SPSS, Inc.). The experimental data are presented as the median and
interquartile range. In Table II,
the categorical variable sex was analyzed using a
χ2-test. Differences between two groups were determined
using a Mann-Whitney U-test. Spearman's rank correlation was used
for correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
 | Table IIAssociation between the recurrence
rate and clinicopathological features of patients with nasal
polyps. |
Table II
Association between the recurrence
rate and clinicopathological features of patients with nasal
polyps.
| Variable | Total (n=56) | Patients with
recurrence (n=13) | Patients without
recurrence (n=43) | P-value |
|---|
| Age (years) | 45.51±11.9 | 45.82±11.9 | 44.15±12.11 | 0.78 |
| Sex | | | | 0.97 |
|
Male | 39 (69.6) | 9 (69.2) | 30 (69.8) | |
|
Female | 17 (30.4) | 4 (30.8) | 13 (30.2) | |
| Previous nasal polyp
(months) | 5.65±1.62 | 6.67±1.15 | 4.51±1.24 | 0.55 |
| Lund-Mackay CT
score | 21.20±2.54 | 21.71±2.45 | 20.81±2.51 | 0.61 |
| Lund-Kennedy
endoscopy score | 11.48±0.81 | 11.84±0.98 | 0.92±0.73 | 0.71 |
| Hospital stay
(days) | 5.92±0.51 | 5.54±0.91 | 6.01±0.82 | 0.51 |
| Duration of operation
(h) | 1.55±0.35 | 1.67±0.23 | 1.35±0.26 | 0.47 |
| Post-operative
re-examinations (n) | 7.81±0.51 | 8.02±0.92 | 5.91±0.95 | 0.32 |
| Post-operative use of
nasal spray hormone (weeks) | 13.02±0.41 | 14.24±0.62 | 11.51±0.32 | 0.21 |
Results
Patient characteristics
A total of 56 patients with nasal polyps who
underwent FESS were included in the present study. The included
patients were divided into the recurrence group (n=13) and the
non-recurrence group (n=43). No significant differences were
observed in the mean age, sex, previous nasal polyp, Lund-Mackay CT
score, Lund-Kennedy endoscopy score, hospital stay, duration of the
operation, post-operative re-examinations or the post-operative use
of nasal sprays between patients with recurrence and those without
recurrence (P>0.05; Table
II).
Expression levels of MUC5AC, MUC5B and
MUC2 prior to treatment
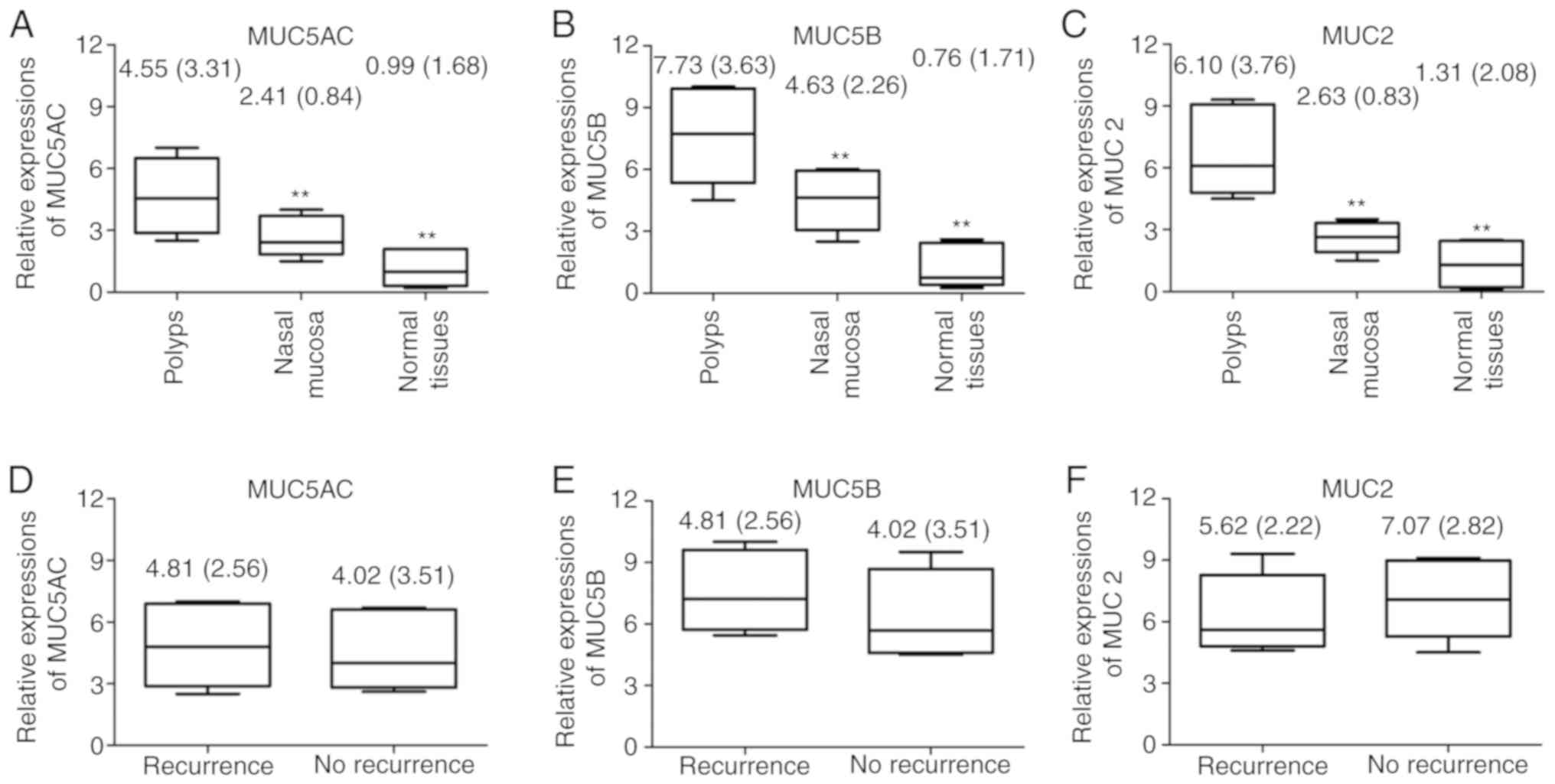
The expression levels of MUC5AC, MUC5B and MUC2 in
the nasal polyp tissues prior to treatment were significantly
upregulated compared with those in the paranasal tissues and normal
nasal mucosal tissues (P<0.01; Fig.
1A-C). However, no significant differences were identified in
the expression levels of MUC5AC, MUC5B and MUC2 mRNA between
patients with recurrent nasal polyps and those without recurrent
nasal polyps prior to treatment (P>0.05; Fig. 1D-F).
Immunohistochemical analysis of
MUC5AC, MUC5B and MUC2 expression levels
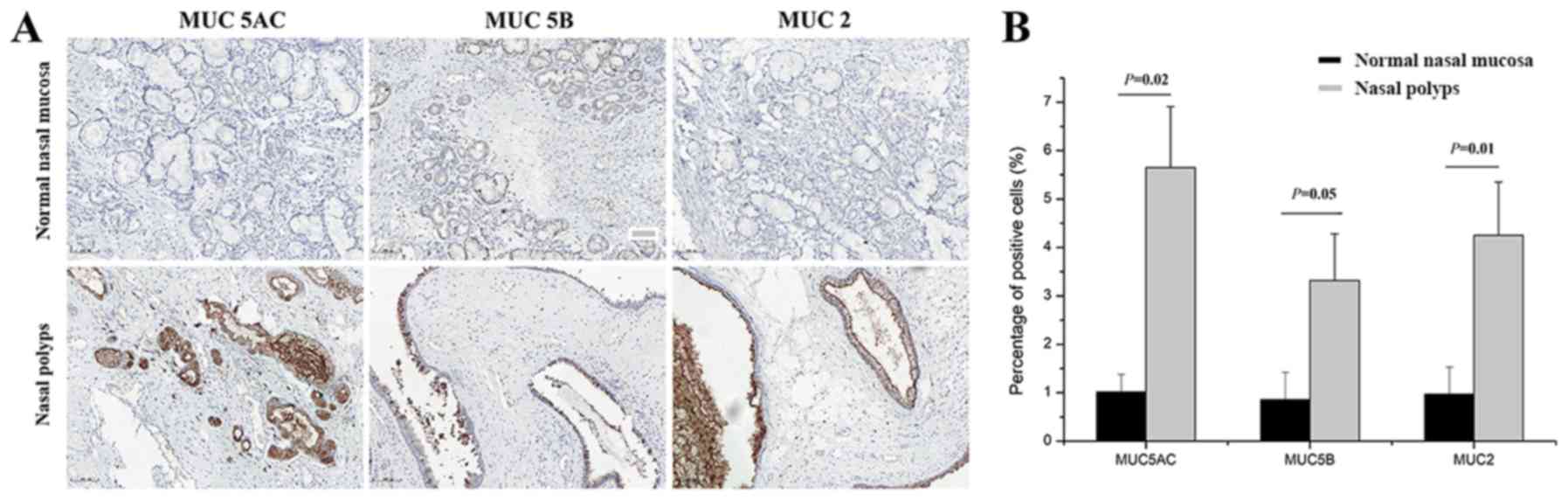
To determine the localization and expression levels
of MUC5AC, MUC5B and MUC2, immunohistochemical staining was
performed prior to treatment. Positive MUC5AC, MUC5B and MUC2
staining was mainly localized in the membrane and cytoplasm of
epithelial and goblet cells, and it was less prominent in the
glandular cells (Fig. 2A). Compared
with the normal nasal mucosal tissues, the levels of MUC5AC
(P=0.02), MUC5B (P=0.05) and MUC2 (P=0.01) were significantly
elevated in the nasal polyps (Fig.
2B).
Expression levels of MUC5AC, MUC5B and
MUC2 following treatment
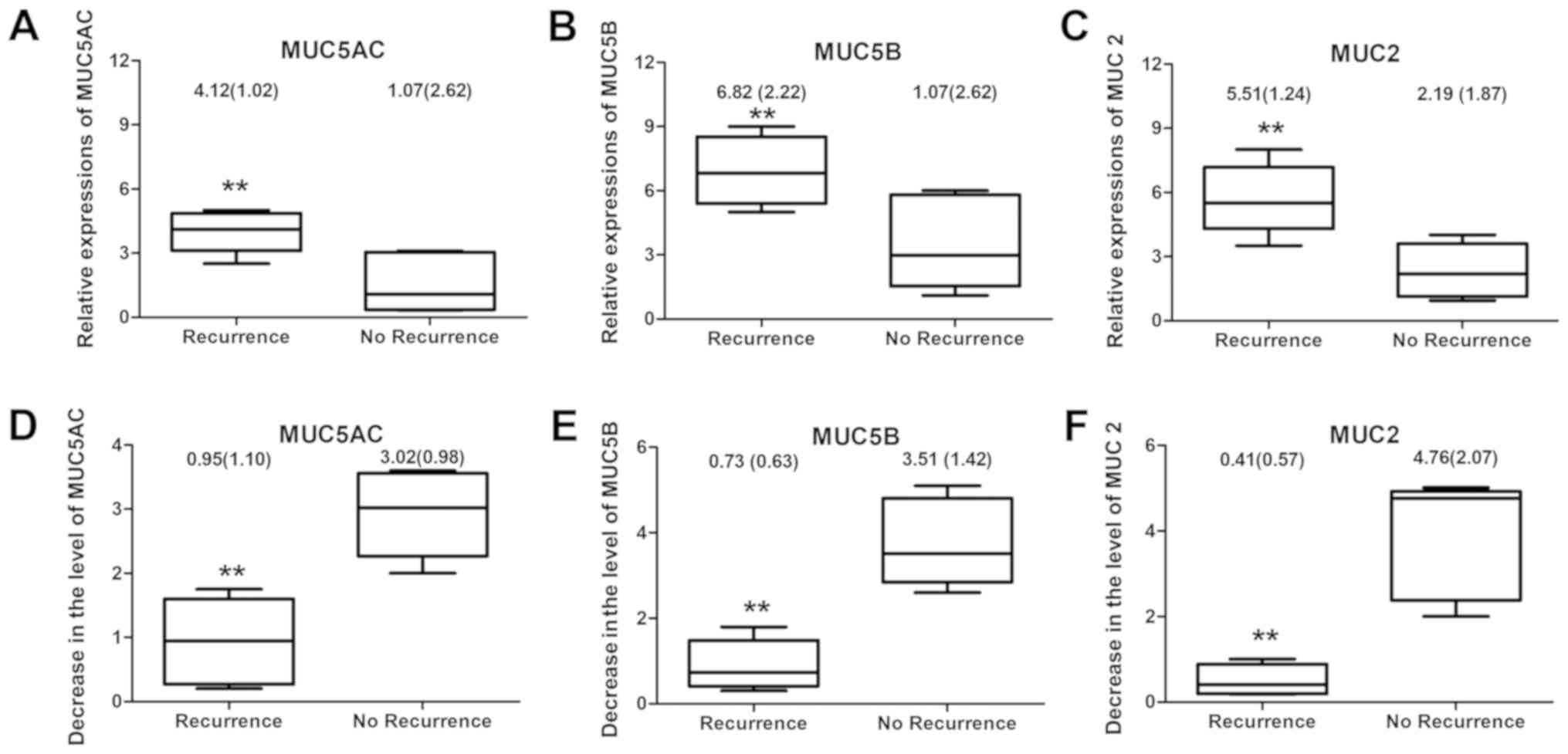
Among the 56 patients who underwent FESS and drug
treatment, 43 were observed to have complete epithelialization of
the nasal mucosa and no recurrence of nasal polyps was observed one
year later; however, 13 patients presented with recurrent nasal
polyps. Patients without recurrence of nasal polyps were discovered
to have significantly decreased expression levels of MUC5AC, MUC5B
and MUC2 compared with those in patients with recurrence of nasal
polyps at 6 months after the operation (P<0.01; Fig. 3A-C). Furthermore, a greater reduction
(at 6 months compared with the baseline) of MUC5AC, MUC5B and MUC2
expression levels was observed in patients without nasal polyp
recurrence compared with that in patients with nasal polyp
recurrence (P<0.01; Fig.
3D-F).
Correlation analysis of MUC5AC, MUC5B
and MUC2 expression levels with the recurrence rate of nasal
polyps
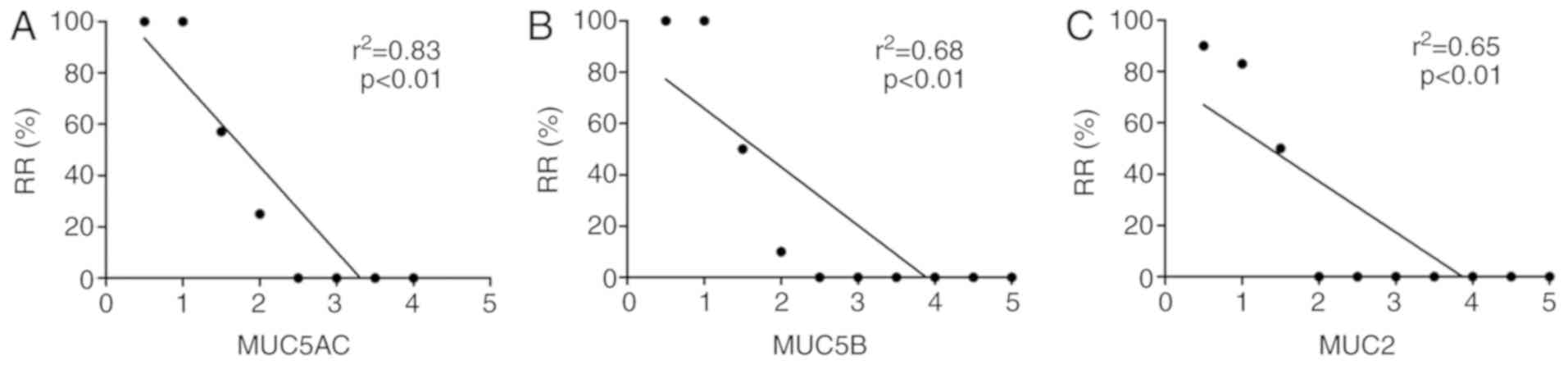
Correlation analysis of the reduction in the
expression levels of MUC5AC, MUC5B and MUC2 with the recurrence
rate of nasal polyps at one year after the operation was then
performed. The decreased values of MUC5AC (r2=0.83;
P<0.01), MUC5B (r2=0.68; P<0.01) and MUC2
(r2=0.65; P<0.01) in patients with recurrence and
without recurrence of nasal polyps at six months compared with
baseline levels were revealed to be significantly negatively
correlated with the recurrence rate of nasal polyps (Fig. 4A-C).
Discussion
Submucosal glandular cells and superficial
epithelial goblet cells produce various MUC proteins (9). Excessive secretion of mucus was
identified to be one of the major symptoms of nasal polyposis,
which may be the result of the increased quantity of mucus and/or
changes in its physical properties (16). Studies on human MUC genes, including
MUC2, MUC5AC and MUC5B, have been previously performed (17,18);
however, to the best of our knowledge, there are no detailed
studies on the role of MUC5AC, MUC5B and MUC2 in patients
presenting with nasal polyp recurrence, and their association with
the recurrence of nasal polyps has yet to be fully elucidated. To
investigate the association of MUC5AC, MUC5B and MUC2 with the
recurrence of nasal polyps, the expression levels of MUC5AC, MUC5B
and MUC2 were detected in patients with and without nasal polyp
recurrence. a correlation analysis was also performed to determine
whether these MUC genes were correlated with the recurrence rate of
nasal polyps. The results of the present study revealed that the
expression levels of MUC5AC, MUC5B and MUC2 in nasal polyp tissues
prior to treatment were significantly increased. However, following
FESS and medical treatment, significantly decreased expression
levels of MUC5AC, MUC5B and MUC2 were observed in patients without
recurrence compared with those with recurrent nasal polyps.
Correlation analysis also demonstrated that the decrease in the
expression levels of MUC5AC, MUC5B and MUC2 was significantly
negatively correlated with the recurrence rate of nasal polyps.
In a previous study, proteins in the sinonasal
secretions of pediatric patients were analyzed by mass
spectrometry, revealing that MUC5B and MUC5AC were present in the
majority of the samples, and the relative abundance of MUC5B was
indicated to be significantly higher in pediatric patients with
chronic rhinosinusitis (CRS) (19).
Kim et al (20) evaluated the
expression levels of MUC5AC and MUC5B in 20 chronic rhinosinusitis
samples and discovered that the expression levels of MUC5AC and
MUC5B mRNA were markedly increased compared with those in the
normal sinus mucosa. In addition, Poachanukoon et al
(21) demonstrated that the
expression levels of MUC2 and MUC5AC genes, as well as tumor
necrosis factor (TNF)-α production, were inhibited following
treatment with mometasone fuorate (MF) and budesonide (BUD),
indicating that MF and BUD may attenuate the pathogenic effects of
MUC2 and MUC5AC in phorbol-12-myristate-13-acetate-induced human
airway epithelial cells. The results of the present study were
consistent with these previous studies; in the present study,
increased expression levels of MUC2, MUC5AC and MUC5B were
identified in patients with recurrent nasal polyps.
In a study on 24 patients with CRS, Mao et al
(10) assessed the expression status
of MUC1, MUC2, MUC5AC and MUC5B on the bacterial biofilm (BBF; + or
-status) in patients with CRS. It was discovered that patients in
the BBF (+) CRS group had significantly increased expression levels
of MUC5AC and MUC5B in the sinus mucosa compared with those in the
BBF (-) CRS group, whereas no significant differences were observed
in the expression levels of MUC1 and MUC2 between the two groups.
Contrary to the results, the present study included 56 patients
with nasal polyps who underwent FESS, and it was discovered that
the expression levels of the MUC2 gene were significantly increased
in the patients with nasal polyp recurrence compared with those in
patients without recurrence. These different outcomes may be
explained by the differences in patients included in the two
studies.
In CRS, upregulation of MUC5AC and MUC5B was
determined in the sinus mucosa of BBF in CRS, suggesting that the
elevated expression of MUC5AC and MUC5B may serve an important role
in the pathogenesis of BBF formation (10). Infection of mucosal epithelial cells
by Shigella species was also indicated to lead to intense and acute
inflammatory bowel disease, and elevated expression levels of MUC2
and MUC5AC within 6-8 h via activating TNF-α, protein kinase C and
ERK1/2(22). Tos and Mogensen
(23) proposed the theory of
epithelial rupture during the formation of nasal polyps, in which
the infiltration and edema in the nasal mucosa resulted in the
rupture of the epithelium and the formation of granulations, which
gradually become lined with pseudostratified columnar epithelium.
Following the epithelial rupture, mucosal herniation,
re-epithelialization, new gland formation and finally, the
formation of nasal polyps was observed. Thus, in the present study,
MUC genes were investigated to determine their association with the
recurrence of nasal polyps. The results suggested that upregulated
expression levels of MUC2, MUC5AC and MUC5B were observed in
patients with nasal polyps and that the reduction in MUC5AC, MUC5B
or MUC2 expression levels was significantly negatively correlated
with the recurrence rate of nasal polyps. These results indicated
that the inflammation associated with nasal polyps may lead to the
increased expression levels of MUC5AC, MUC5B and MUC2.
Following treatment, the results of the current
study demonstrated that increased expression levels of MUC genes
remained; this is most likely due to the incomplete resistance to
current treatment or incomplete removal of nasal polyps following
an operation, the epithelization of nasal mucosa or incomplete
restoration of surface ciliary function, leading to the retention
of mucus secretions and bacteria, and the persistence of various
inflammatory factors. This would contribute to the high expression
levels of MUC genes and eventually nasal polyp recurrence.
Therefore, if the expression levels of MUC genes are not reduced,
research should focus on actively determining the causes in order
to intervene as early as possible to avoid the recurrence of
polyps.
Although the expression levels of MUC5AC, MUC5B and
MUC2 were proven to be associated with the recurrence rate of nasal
polyps, there were certain limitations to the present study. First,
the present study had a relatively small sample size, which may
introduce selection bias. In addition, it was a single-center study
and lacks external validation. Thus, further multicenter and
large-sample studies are required to validate the present
results.
In conclusion, the present study demonstrated that
the expression levels of MUC5AC, MUC5B and MUC2 were significantly
negatively correlated with the recurrence rate of nasal polyps.
These results may provide novel evidence for the diagnosis and
treatment of patients with recurrent nasal polyps.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the repository of Tianjin Third
Central Hospital (Tianjin, China) http://www.tj3zx.cn/. The datasets used and/or
analyzed during the current study are available from the
corresponding author on reasonable request.
Authors' contributions
LL performed the experiments, collected the data and
wrote the manuscript; CHY designed the experiments and revised the
manuscript; and SDT analyzed the data and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of The Third Central Hospital of Tianjin (Tianjin, China)
and informed consent was obtained verbally from all patients. The
analysis was retrospective.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pawliczak R, Lewandowska-Polak A and
Kowalski ML: Pathogenesis of nasal polyps: An update. Curr Allergy
Asthma Rep. 5:463–471. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Deal RT and Kountakis SE: Significance of
nasal polyps in chronic rhinosinusitis: Symptoms and surgical
outcomes. Laryngoscope. 114:1932–1935. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bequignon E, Dupuy L, Zerah-Lancner F,
Bassinet L, Honoré I, Legendre M, Devars du Mayne M, Escabasse V,
Crestani B, Maître B, et al: Critical evaluation of sinonasal
disease in 64 adults with primary ciliary dyskinesia. J Clin Med.
8(619)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Crystal RG, Randell SH, Engelhardt JF,
Voynow J and Sunday ME: Airway epithelial cells: Current concepts
and challenges. Proc Am Thorac Soc. 5:772–777. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Perrais M, Pigny P, Copin MC, Aubert JP
and Van Seuningen I: Induction of MUC2 and MUC5AC mucins by factors
of the epidermal growth factor (EGF) family is mediated by EGF
receptor/Ras/Raf/extracellular signal-regulated kinase cascade and
Sp1. J Biol Chem. 277:32258–32267. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Henke MO, John G, Germann M, Lindemann H
and Rubin BK: MUC5AC and MUC5B mucins increase in cystic fibrosis
airway secretions during pulmonary exacerbation. Am J Respir Crit
Care Med. 175:816–821. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ali MS, Wilson J and Pearson JP: Mixed
nasal mucus as a model for sinus mucin gene expression studies.
Laryngoscope. 112:326–331. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Milara J, Peiró T, Armengot M, Frias S,
Morell A, Serrano A and Cortijio J: Mucin 1 downregulation
associates with corticosteroid resistance in chronic rhinosinusitis
with nasal polyps. J Allergy Clin Immunol. 135:470–476.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ding GQ and Zheng CQ: The expression of
MUC5AC and MUC5B mucin genes in the mucosa of chronic
rhinosinusitis and nasal polyposis. Am J Rhinol. 21:359–366.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mao YJ, Chen HH, Wang B, Liu X and Xiong
GY: Increased expression of MUC5AC and MUC5B promoting bacterial
biofilm formation in chronic rhinosinusitis patients. Auris Nasus
Larynx. 42:294–298. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xue-Kun H, Yuan L, Jin Y, Peng L and Hong
L: Expression of MUC2 and MUC5B in ethmoid sinus mucosa of patients
with chronic rhinosinusitis. Sci Res Essays. 5:1690–1696. 2010.
|
|
12
|
Lund VJ and Kennedy DW: Staging for
rhinosinusitis. Otolaryngol Head Neck Surg. 117 (Suppl):S35–S40.
1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lund WJ and Kennedy DW: Quantification for
staging. The Staging and Therapy Group. Ann Otol Rhinol Laryngol.
167 (Suppl):S17–S21. 1995.PubMed/NCBI
|
|
14
|
Kennedy DW: Functional endoscopic sinus
surgery Technique. Arch Otolaryngol. 111:643–649. 1985.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ali MS, Wilson JA, Bennett M and Pearson
JP: Mucin gene expression in nasal polyps. Acta Otolaryngol.
125:618–624. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chang JH, Song KJ, Kim HJ, Kim JH, Kim NH
and Kim KS: Dietary polyphenols affect MUC5AC expression and
ciliary movement in respiratory cells and nasal mucosa. Am J Rhinol
Allergy. 24:e59–e62. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
İlhan Ö, Han Ü, Önal B and Çelık SY:
Prognostic significance of MUC1, MUC2 and MUC5AC expressions in
gastric carcinoma. Turk J Gastroenterol. 21:345–352.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Saieg A, Brown KJ, Pena MT, Rose MC and
Preciado D: Proteomic analysis of pediatric sinonasal secretions
shows increased MUC5B mucin in CRS. Pediatr Res. 77:356–362.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim DH, Chu HS, Lee JY, Hwang SJ, Lee SH
and Lee HM: Up-regulation of MUC5AC and MUC5B mucin genes in
chronic rhinosinusitis. Otolaryngol Head Neck Surg. 130:747–752.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Poachanukoon O, Koontongkaew S,
Monthanapisut P and Pattanacharoenchai N: Mometasone furoate
suppresses PMA-Induced MUC-5AC and MUC-2 production in human airway
epithelial cells. Tuberc Respir Dis (Seoul). 80:60–68.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Radhakrishnan P, Halagowder D and Devaraj
SN: Altered expression of MUC2 and MUC5AC in response to Shigella
infection, an in vivo study. Biochim Biophys Acta. 1770:884–889.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tos M and Mogensen C: Pathogenesis of
nasal polyps. Rhinology. 15:87–95. 1977.PubMed/NCBI
|