Introduction
Infantile haemangioma (IH) is a type of benign
vascular tumour that occurs in 3-10% of infants (1). While most of these lesions are
asymptomatic and subside by the age of 5 years, complications may
arise, including painful bleeding, ulceration or disfiguration
(2,3). IH may also induce mental distress to
the parents and children (4). An
early intervention is required in such cases to prevent future
complications.
Systematic steroids were used as a first-line
medication for the treatment of IH. However, the long-term use of
steroids may lead to serious adverse reactions, including growth
delay, adrenal cortical insufficiency and/or hypertension (5). To overcome these adverse reactions, The
Food Drug Administration (FDA) of the US approved beta (β) blockers
as the first-line medications for the management of IH in
2014(6). Propranolol is a
non-selective lipophilic β blocker proven to be effective against
His (7) by inhibiting the
proliferation and inducing the regression of the lesion during the
proliferative phase (8). However,
propranolol treatment also has certain risks, including side
effects of diarrhoea, hyperkalaemia, hypoglycaemia and bronchial
hyperreactivity. Propranolol also affects the central nervous
system (CNS) as it crosses the blood-brain barrier due to its
lipophilic nature and may cause adverse reactions, including
agitation and sleep disturbances. These undesired effects from
propranolol have led to discontinuation of therapy and regrowth of
the lesions (9-11).
Atenolol, a hydrophilic β blocker, has been used as
an alternative to propranolol in the treatment of IH (12). Atenolol has minimal safety concerns,
as it primarily acts on β1 receptors with minor effects on β2
receptors (13). As it does not act
on pulmonary β2 receptors, it may be safely used in infants with
pulmonary diseases (e.g. reactive airway conditions). Atenolol also
does not affect the pancreatic β2 receptors and does not interfere
with the glycogenolysis, gluconeogenesis or lipolysis (14). Due to its hydrophilic nature, it does
not cross the blood-brain barrier and has limited adverse reactions
when compared to propranolol (15).
Studies have reported that atenolol is as effective as propranolol
in reducing the size of the IH lesions (13,14).
Even though the morbidity profile of atenolol for
the management of IH has been established, only a few systematic
studies have compared the clinical outcomes and/or adverse effects
between these two different treatment methods (16,17). The
purpose of the present meta-analysis was to compare clinical
outcomes [recovery rate, haemangioma activity score (HAS), adverse
effects and relapse rates] between patients treated with atenolol
and those treated with propranolol in the management of IH.
Materials and methods
Search strategy
An extensive search was performed in the following
databases: Medline (PubMed) (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar
(https://scholar.google.com/),
ScienceDirect (https://www.sciencedirect.com/) and Cochrane Central
Register of Controlled Trials (CENTRAL) (https://www.cochranelibrary.com/central). In addition,
a search was performed in the following clinical trial registries:
ClinicalTrials.gov (https://clinicaltrials.gov/) and the World Health
Organization International Clinical Trials Registry Platform (WHO
ICTRP) (https://www.who.int/ictrp/search/en/). A combination
of medical subject headings and free text terms, including
‘haemangioma’, ‘atenolol’, ‘propranolol’, ‘beta blockers’, ‘adverse
events’, ‘infants’, ‘infantile haemangioma’, ‘haemangioma activity
score’ and ‘randomized controlled trial’ were searched and all
publications in English language from the database's inception to
July 2019 were retrieved.
The reference lists of primary trials obtained
through electronic searches were also checked and relevant articles
were included for review and analysis. In cases requiring
clarification or additional information, the authors of the
published trials were contacted.
Inclusion and exclusion criteria
For inclusion in the present analysis, studies were
required to fulfill all of the following criteria: i) Parallel arm
individually randomized, quasi randomized and cluster randomized
controlled trials (RCTs), and prospective/retrospective cohort
studies, ii) studies on patients with IH and iii) studies comparing
the effectiveness of atenolol and propranolol for IH
management.
All cross-over studies were excluded due to the
possibility of carryover effects. Only full-text/abstract
publications were included.
Outcome measures
The following outcome measures were assessed: HAS,
response to medication (reduction in the lesion size), adverse
events and relapse rate. Studies reporting any of the
above-mentioned outcomes and that met the inclusion criteria were
included.
Selection of studies
The literature search was performed by two
independent investigators (CW and DS) who screened the titles,
abstracts and keywords of all the retrieved citations and assessed
them for possible inclusion in the present analysis. Full-text
articles of relevant studies were obtained and further screening
was performed independently by the primary and secondary
investigators (CW and DS) to select the studies satisfying the
eligibility criteria for the present analysis. Any disagreements
between investigators during the selection process were resolved
either through consensus or consultation with another investigator
(LW). A third investigator monitored the quality of the overall
review process (LW). The Preferred Reporting Items for Systematic
Review and Meta-Analysis checklist was used for reporting in this
review (18).
Data extraction and management
The primary investigator (CW) extracted the relevant
study characteristics for review from the included studies and
included general information, including the date of extraction,
study title and authors; methods including the study design,
participants and study setting; participant's characteristics,
including the total number of participants in each arm, baseline
and post-treatment outcome measures, and inclusion and exclusion
criteria; intervention characteristics including details on the
intervention and comparison group and follow-up duration; outcomes
section, including primary and secondary outcomes, time taken for
outcome assessment and other details necessary for assessing the
risk of bias of included studies.
Primary and secondary investigators (CW and DS)
independently extracted data associated with outcome measures from
the studies included. Only extracted data from the relevant arms of
studies reporting on multiple arms in a single trial were used for
the present analyses. The primary investigator (CW) transferred the
obtained data to the statistical software RevMan 5.3 (Cochrane).
The third investigator (LW) double-checked data entries for
correctness by comparing them to the data in the studies.
Risk of bias assessment for the
studies included
The risk of bias of included RCTs was assessed by
two independent investigators (CW and DS) using the Cochrane risk
of bias tool (19). The following
domains were assessed: Random sequence generation, allocation
concealment, blinding of outcome assessment and study participants,
incomplete outcome data, selective reporting of outcomes and other
sources of bias.
For non-randomized studies, the risk of bias
assessment tool for non-randomized studies (20) was used with the following domains:
Selection of participants, confounding variables, intervention
measures, blinding of outcome assessment, incomplete outcome data
and selective outcome reporting.
For each of the above-mentioned domains, the risk of
bias was graded as low (if adequate information was provided), as
high (if the information was inadequate or not performed) or as
unclear (if the information was missing).
Statistical analysis
For continuous outcome (HAS), the mean and standard
deviation (SD) reported at baseline and follow-up were obtained. In
studies where change in mean and SD scores from baseline were
reported, they were extracted directly. If change scores were not
reported, manual calculation was performed using the following
method:
Mean (change) was calculated as mean (after)-mean
(before). Since the data were paired, equal variances were assumed
for baseline and follow-up data. n1 and n2
were the number of participants at baseline and follow-up,
respectively, while s1 and s2 were the
standard deviations of baseline and follow-up, respectively.
The square of the SD was multiplied with the degrees
of freedom: (n1-1)s12. This was
repeated for the outcome:
(n2-1)s22. The two equations were
added together and divided by the total degrees of freedom:
The standard error (SE) of the difference between
means was as follows:
SD (change) was calculated with the equation: SD=SE
x √(sample size). Mean (change) and SD (change) of both groups were
then entered into meta-analysis software. Finally, pooled estimates
were reported as Mean Difference (MD) with 95% CI.
For dichotomous variables including the response
rate, adverse effects and relapse rate, the numbers of events and
of participants in each group were obtained to estimate the pooled
effect size in terms of odds ratios (most studies included were
retrospective in nature).
Appropriate analyses were performed based on the
level of randomization (either individual or clustered). No cluster
randomized trials satisfying the eligibility criteria were
identified and therefore, they did not require appropriate
clustering adjustments. A random-effects model with inverse
variance was utilized (21).
χ2 tests of heterogeneity to assess
inter-study variance and I2 statistics were applied to
quantify inconsistencies (19).
Heterogeneity was classified according to I2 as mild
(I2<25%) moderate (I2 between 25 and 75%)
or substantial (I2>75%). Study-specific and pooled
estimates were represented graphically through forest plots with
random-effects model. None of the outcomes exhibited significant
heterogeneity. Hence, subgroup analysis or meta-regression was not
required for the present study.
Publication bias was not assessed, as the outcomes
did not have the required number of studies (minimum of 10 studies)
to assess the publication bias.
Results
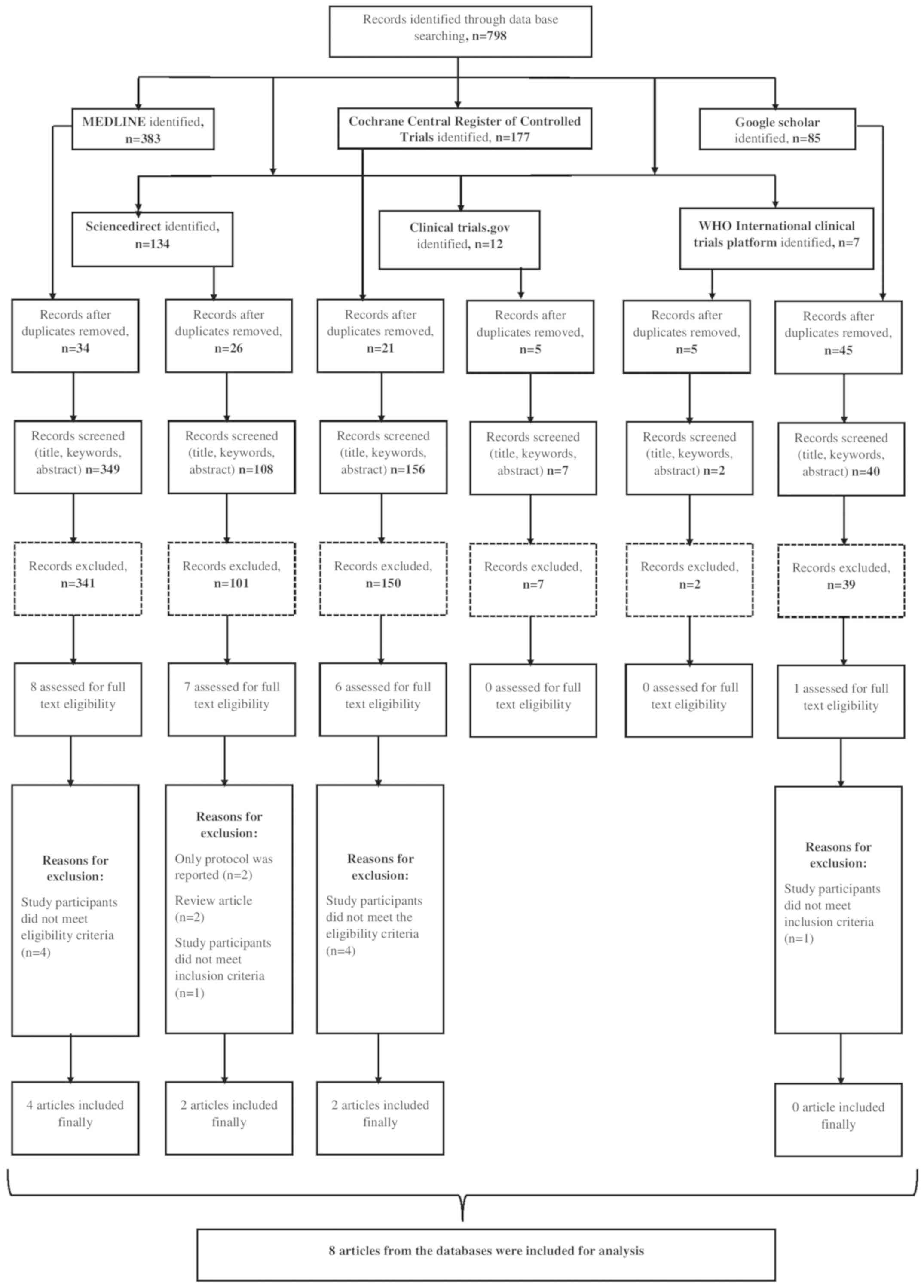
Study selection
A systematic search to retrieve studies that
directly compared the effectiveness of atenolol or propranolol for
the management of IH from the dates of database inception until
July 2019 was performed. A total of 798 citations, 383 studies
retrieved from Medline, 134 from ScienceDirect, 177 from CENTRAL,
85 from Google Scholar, 12 from ClinicalTrials.gov and 7 from WHO ICTRP were
identified (Fig. 1). After the first
screening stage (title, abstract and keywords), 22 relevant studies
were retrieved and their full texts were reviewed for eligibility.
Simultaneously, the bibliographies of the studies retrieved were
reviewed and 4 more relevant studies were identified. Finally, data
from 8 studies with 608 participants satisfying the inclusion
criteria were analysed (13,14,22-27).
Characteristics of studies
included
Table I lists the
characteristics of the studies analysed. Two studies were RCTs
(13,27), 3 were prospective (23,25,26) and
the remaining studies were retrospective studies (14,22,24).
Most of the studies were performed in Asian countries [China (2
studies) (23,26) and India (1 study)] (27) and the others were performed in
American and European countries. The mean age of the study
participants ranged from 2 to 6.4 months in the atenolol group and
that in the propranolol group ranged from 3 to 6 months. Of the 608
participants, 250 were in the atenolol cohort and 358 were in the
propranolol group. The sample sizes in the studies (both groups
together) ranged from 23 to 173, while the sample size in the
atenolol group ranged from 7 to 82 patients and that in the
propranolol group ranged from 10 to 98. Among the 8 studies
included, 6 reported on response to medication (reduction in the
lesion size) (13,22-26),
2 reported on HAS (14,27), 2 reported on relapse rate (13,25) and
6 reported on adverse effects following treatment (13,14,23-25,27).
 | Table ICharacteristics of the studies
included (n=8). |
Table I
Characteristics of the studies
included (n=8).
| First author
(year) | Country | Study design | Sample size in
atenolol arm | Sample size in the
propranolol arm | Interventions | Follow-up | Mean age of the
study participants in atenolol arm | Mean age of the
study participants in propranolol arm | (Refs.) |
|---|
| Araya et al
(2014) | Chile | Randomized
controlled trial | 13 | 10 | Atenolol: 1
mg/kg/day for 6 months in a single daily dose; Propranolol: 2
mg/kg/day in 3 daily doses for 6 months | Follow-up at 2
weeks, 4 weeks, and then monthly until 6 months of treatment were
completed | Not given
separately (mean participants, 5.2 months) | (13) |
| Ashraf (2019) | India | Randomized
controlled trial | 20 | 20 | Atenolol: 1
mg/kg/day for 9 months; Propranolol: 2 mg/kg/day for 9 months | Follow-up at
monthly intervals until end of treatment at 9 months | 3.3 months | 4.8 months | (27) |
| Bayart et al
(2017) | US | Retrospective
non-inferiority study | 27 | 53 | Atenolol: 0.5
mg/kg/day until 12-15 months of age or until complete response;
Propranolol: 2 mg/kg/day until 12-15 months of age or until
complete response | Baseline and
post-treatment assessment done | 2 months | 3 months | (14) |
| Dakoutrou et
al (2019) | Greece | Prospective | 26 | 28 | Atenolol: 0.5
mg/kg/day, increased up to 2 mg/kg; Propranolol: 2 mg/kg/day
divided into two doses Both continued until macroscopic regression
of lesion or no response after 1 month of initiation of
treatment | Follow-up was
performed 1 week after the initial check and monthly
thereafter | 3.63 months | 5.95 months | (25) |
| De Graff et
al (2013) | Netherlands | Retrospective | 30 | 28 | Atenolol: Starting
dose, 0.5 mg/kg/day (once daily). After 1 week of treatment, the
atenolol dosage was increased to 1 mg/kg/day. Propranolol: Average
dosage 2 mg/kg/day | Follow-up at 2-8
weeks (t1) and 11-24 weeks | Provided only for
atenolol group (median age, 6.4 months) | (24) |
| Sharma et al
(2013) | Canada | Retrospective | 7 | 98 | Atenolol: 1.6
mg/kg/day Propranolol: 1.5 mg/kg/day | Premature,
follow-up weekly; 2-12 weeks, follow-up bi-weekly; >12 weeks,
follow-up every 2-3 weeks | Not given
separately (mean age of total participants, 3.3 months) | (22) |
| Sun et al
(2018) | China | Prospective | 82 | 91 | Both drugs until 24
weeks; dosage not given | First week was
followed up each day, followed by once a month. At 6 months after
commencement of treatment, the response was compared between the
two groups | Not specified | (23) |
| Wang et al
(2016) | China | Prospective | 45 | 30 | Atenolol: 1
mg/kg/day once a day for 24 consecutive weeks; Propranolol: 2
mg/kg/day in 3 doses daily for 24 consecutive weeks | Follow-up at 1, 4,
12 and 24 weeks after treatment | Not specified | (26) |
Methodological quality of the studies
included
Assessments of risk of bias were performed
separately for RCTs and non-randomized studies (Table IIA and B, respectively). There were no patients
lost to follow-up reported in any of the studies included. The two
RCTs (13,27) included in the present meta-analysis
had low risks of bias in almost all of the domains. Among the
non-randomized studies (14,22-26),
all of the studies had a low risk of bias regarding selection of
participants, intervention measures, incomplete outcome data and
unclear risks of selective reporting of outcomes (protocols not
published), or blinding of outcome assessment (not mentioned in the
studies). Furthermore, three out of six studies had high risks of
bias with respect to confounding variables.
 | Table IIRisk of bias assessment. |
Table II
Risk of bias assessment.
| A, Randomized
studies (n=2) |
|---|
| Study | Random sequence
generation | Allocation
concealment | Blinding of the
participants, outcome assessment | Incomplete outcome
data | Selective reporting
of outcomes | Other risk of
bias | (Refs.) |
|---|
| Araya et al
(2014) | Low risk | Low risk | Low risk | Low risk | Unclear risk | Low risk | (13) |
| Ashraf et al
(2019) | Low risk | Low risk | Low risk | Low risk | Unclear risk | Low risk | (27) |
| B, Non-randomized
studies (n=6) |
| Study | Selection of
participants | Confounding
variables | Intervention
measures | Blinding of the
outcome assessment | Incomplete outcome
data | Selective reporting
of outcomes | (Refs.) |
| Bayart et al
(2017) | Low risk | High risk | Low risk | Unclear risk | Low risk | Unclear risk | (14) |
| Dakoutrou et
al (2019) | Low risk | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | (25) |
| De Graff et
al (2013) | Low risk | High risk | Low risk | Unclear risk | Low risk | Unclear risk | (24) |
| Sharma et al
(2013) | Low risk | High risk | Low risk | Unclear risk | Low risk | Unclear risk | (22) |
| Sun et al
(2018) | Low risk | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | (23) |
| Wang et al
(2016) | Low risk | Low risk | Low risk | Unclear risk | Low risk | Unclear risk | (26) |
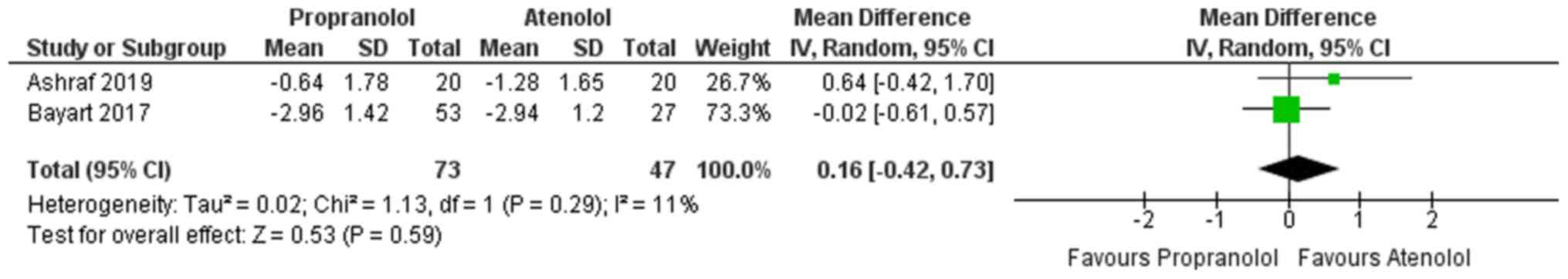
HAS
A total of two studies reported on HAS for the two
groups (atenolol and propranolol) (14,27).
Fig. 2 presents the pooled MD in the
HAS at 0.16 (95% CI, -0.42 to 0.73). This indicates that the
evidence is not conclusive to determine which method results in a
greater improvement in HAS. Furthermore, no significant
heterogeneity was identified in the studies included reporting on
HAS (I2=11%, P=0.29).
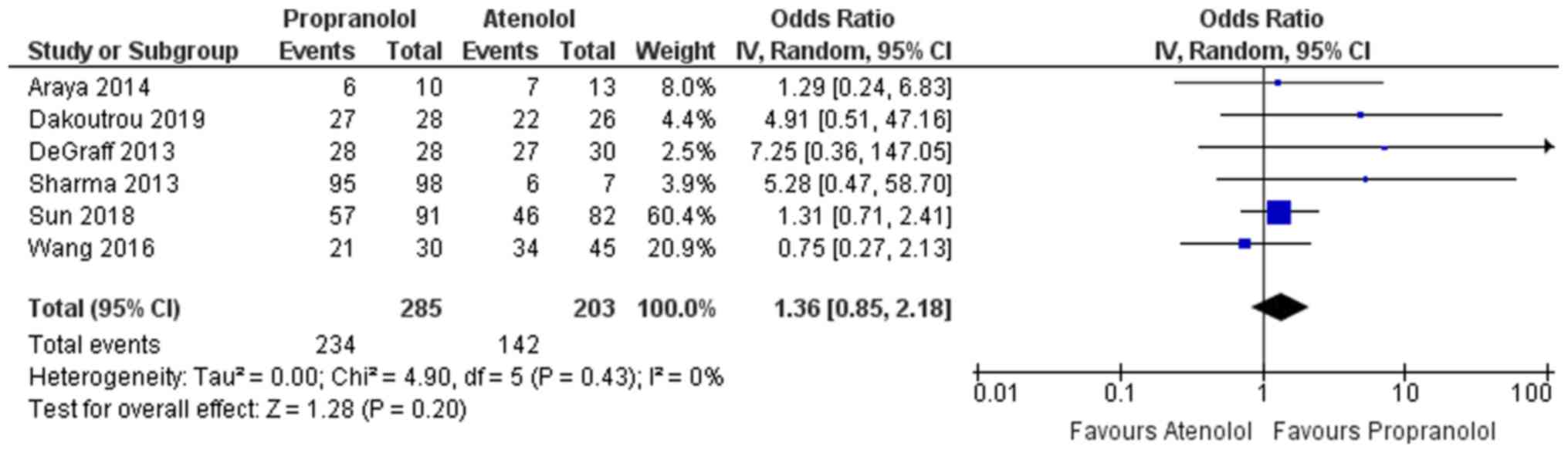
Response to medication
Among the studies included, 6 reported on the
response rate or reduction in the lesion size following intake of
the medication in the two groups (atenolol and propranolol)
(13,22-26).
Apart from the study by Wang et al (26), all of the other studies (13,14,22-25,27)
indicated that propranolol was favoured, as it had a higher
response rate when compared to the atenolol group. The overall
pooled odds ratio (OR) in the propranolol arm was 1.36, indicating
these infants had a 1.36 times greater odds of having complete
response (reduction in lesion size) following the medication than
those in the atenolol group (Fig.
3). However, the confidence of this pooled estimate crossed the
null value of 1 (95% CI, 0.85-2.18) and the result was not
statistically significant. Furthermore, no heterogeneity among the
studies reporting the response rate with I2=0% was
identified. The χ2 test for heterogeneity also indicated
the absence of significant heterogeneity among the studies
reporting on the response rate (P=0.43).
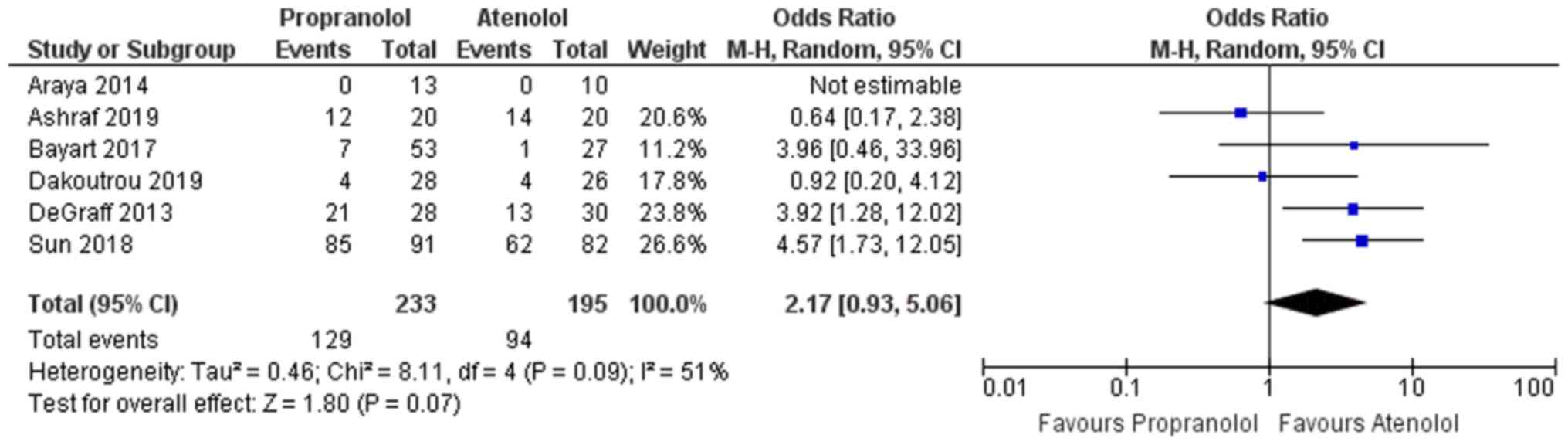
Adverse effects
A total of 6 studies reported on adverse effects
following the medication in the two groups (13,14,23-25,27).
Except for the studies by Ashraf et al (27) and Dakoutrou et al (25), all other studies reported that
infants taking propranolol had a greater chance of developing
adverse effects following medication when compared with infants
taking atenolol. The overall pooled OR in the propranolol group was
2.17, indicating these infants had a 2.17 times higher odds of
developing adverse reactions following the medication than those in
the atenolol group (Fig. 4).
However, the confidence of this pooled estimate crossed the null
value (95% CI, 0.93-5.06) and the result was not statistically
significant. Furthermore, moderate heterogeneity was present among
the studies reporting on adverse effects with I2=51%.
The χ2 test for heterogeneity indicated the absence of
significant heterogeneity among the studies reporting on adverse
effects (P=0.07).
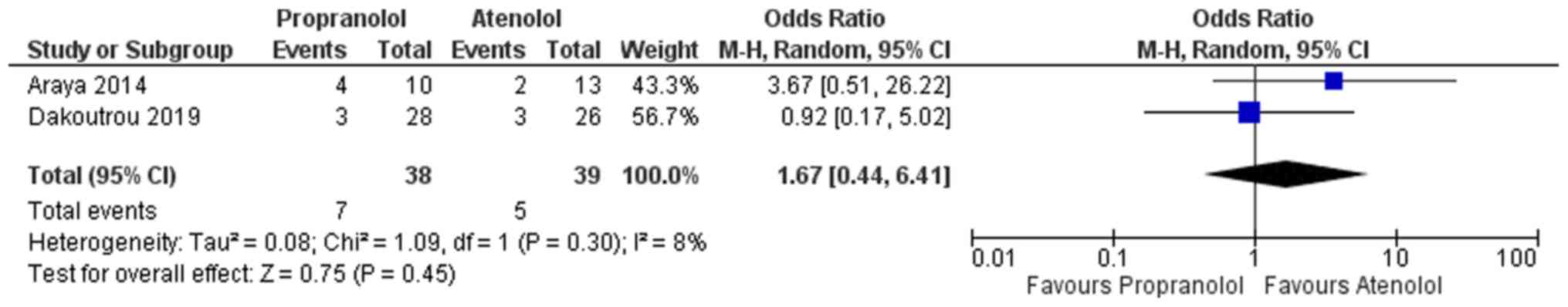
Relapse rate
A total of 2 studies reported on the relapse rate
following successful completion of treatment for the two groups
(atenolol and propranolol) (13,25). The
overall pooled OR in the propranolol arm was 1.67, indicating that
these infants had a 1.67 times higher odds of relapse of IH
following successful completion of medication than those in the
atenolol group (Fig. 5). Similar to
the above, the confidence of this pooled estimate crossed the null
value (95% CI, 0.44-6.41) and the result was not statistically
significant. Furthermore, mild heterogeneity among the studies
reporting on adverse effects with I2=8% was obtained.
The χ2 test for heterogeneity indicated an absence of
significant heterogeneity among the studies reporting on adverse
effects (P=0.45).
Discussion
The management of IH has varied historically and β
blockers have been the mainstay of treatment with a complete
response rate of ~60% (2). Each of
the β blockers used in the present analysis has its own advantages
and disadvantages. There is a lack of systematic and high-quality
studies assessing the effectiveness of these β blockers directly.
Hence, the present analysis was performed to compare the efficacies
of atenolol and propranolol, in terms of clinical outcomes
including the HAS and response rate, adverse effects and relapse
rate among infants with haemangioma. The best possible evidence
available to date was compiled in order to compare these
medications.
A total of 8 studies comprising 608 participants
were selected for inclusion in the present analysis. Of these, only
2 studies were RCTs and 3 were prospective studies, while the
remainder were retrospective in nature. Most of the studies had
either low or unclear bias risks. No substantial heterogeneity was
identified among the reported outcomes in the studies. Hence,
subgroup analysis or meta-regression was not performed to explore
the source of heterogeneity. Except for the response to medication,
all other outcomes (HAS, adverse reactions and relapse rate) were
better for the atenolol group than the propranolol group. However,
no conclusive or significant evidence for any of these outcomes was
obtained, as the confidence limit crossed the null value in all of
the outcomes assessed. The results from this analysis suggested
that atenolol may be non-inferior to propranolol treatment in the
management of IH. In almost all of the studies, atenolol was used
at a dose of 0.5-1 mg/kg/day and propranolol at a dose of 2
mg/kg/day. However, the optimal dose of atenolol and propranolol
remains to be determined, as there is a lack of dose-response
studies.
The major strengths of the present study include the
comprehensive literature search and the broad search strategy to
include all of the relevant up-to-date publications. To the best of
our knowledge, the present study was the first review directly
comparing the clinical outcomes and adverse reaction profile
between atenolol and propranolol for the management of IH. Two
previous reviews (16,17) comparing propranolol with various
other interventions have only included one study by Ábarzúa-Araya
et al (13) to directly
compare atenolol and propranolol.
The present review has certain limitations. Only 2
RCTs were included among the 8 studies. Since certain studies were
retrospective in nature, no causal associations between the
interventions and the outcomes can be inferred. Hence, more trials
of adequate size are required to be performed to gather more
evidence. It was not possible to assess publication bias, as the
number of studies included in the review was <10 (minimum
requirement to perform funnel plot or Egger's test) (19). There was insufficient information to
determine the optimal dose for propranolol or atenolol, the optimal
schedule or factors responsible for regrowth of IH. Finally, most
of the studies included in the present review were performed in
high-income countries, which may limit the generalizability of the
results to other geographical regions.
The results of the present study had certain
implications towards clinical practice. Atenolol may be
non-inferior to the propranolol treatment in the management of IH.
Propranolol is widely used as a first-line drug in the management
of complications. Previous evidence has indicated that propranolol
has potential adverse effects on the development of the CNS of
infants. It is known to negatively influence psychomotor function
or the memory of infants. In addition, bronchial-associated adverse
effects (e.g. bronchial hypersensitivity) have been reported
propranolol users. With the current evidence, clinicians are able
to use atenolol in place of propranolol depending on the patient's
profile (i.e. if the infants require medication having a minimal
effect on bronchus or CNS) or it may be used as an alternative if
the infant on propranolol develops side effects. However,
uncertainties regarding the efficacy and safety persist, as most of
the studies have an inadequate sample size that limits the power of
the studies.
Apart from efficacy and safety concerns, questions
regarding the dose-response association to determine the optimal
dose, schedule and factors responsible for regrowth or relapse of
IH following treatment require further exploration. To obtain
conclusive evidence on these factors, more RCTs or prospective
studies with larger sample sizes are required to strengthen the
evidence for recommendations on how to best treat infants with
haemangioma, as β blockers are the only FDA-approved drug for this
condition.
To summarize, atenolol may be non-inferior to
propranolol in the management of IH with respect to clinical
outcomes and adverse reactions. However, more RCTs with larger
sample sizes are required to derive conclusive evidence towards
efficacy, safety and dose-response association of atenolol and
propranolol.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL designed the study. C Wu, DS, LW, JL, C Wang and
LG were involved in literature search and data interpretation. C Wu
and DS were responsible for the data analysis. ZL prepared the
manuscript. LG edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frieden IJ, Haggstrom AN, Drolet BA,
Mancini AJ, Friedlander SF, Boon L, Chamlin SL, Baselga E, Garzon
MC, Nopper AJ, et al: Infantile hemangiomas: Current knowledge,
future directions Proceedings of a research workshop on infantile
hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr
Dermatol. 22:383–406. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Darrow DH, Greene AK, Mancini AJ and
Nopper AJ: Section On Dermatology, Section On Otolaryngology-Head
And Neck Surgery, and Section On Plastic Surgery. Diagnosis and
management of infantile hemangioma. Pediatrics. 136:e1060–1104.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Drolet BA, Frommelt PC, Chamlin SL,
Haggstrom A, Bauman NM, Chiu YE, Chun RH, Garzon MC, Holland KE,
Liberman L, et al: Initiation and use of propranolol for infantile
hemangioma: Report of a consensus conference. Pediatrics.
131:128–140. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Csoma ZR, Dalmády S, Ábrahám R, Rózsa T,
Rácz K and Kemény L: Infantile haemangioma: Clinical and
demographic characteristics, experiences in the treatment. Orv
Hetil. 158:1535–1544. 2017.PubMed/NCBI View Article : Google Scholar : (In Hungarian).
|
|
5
|
Maturo S and Hartnick C: Initial
experience using propranolol as the sole treatment for infantile
airway hemangiomas. Int J Pediatr Otorhinolaryngol. 74:323–325.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Léauté-Labrèze C, Hoeger P,
Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, Phillips RJ,
Caceres H, Lopez Gutierrez JC, Ballona R, et al: A randomized,
controlled trial of oral propranolol in infantile hemangioma. N
Engl J Med. 372:735–746. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Léaute-Labrèze C, Boccara O,
Degrugillier-Chopinet C, Mazereeuw-Hautier J, Prey S, Lebbé G,
Gautier S, Ortis V, Lafon M, Montagne A, et al: Safety of oral
propranolol for the treatment of infantile hemangioma: A systematic
review. Pediatrics. 138(e20160353)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang L, Wu HW, Yuan W and Zheng JW:
Propranolol therapy for infantile hemangioma: Our experience. Drug
Des Devel Ther. 11:1401–1408. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Raphael MF, Breur JM, Vlasveld FA, Elbert
NJ, Liem YT, Kon M, Breugem CC and Pasmans SG: Treatment of
infantile hemangiomas: Therapeutic options in regard to side
effects and adverse events-a review of the literature. Expert Opin
Drug Saf. 15:199–214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Léauté-Labrèze C, Harper JI and Hoeger PH:
Infantile haemangioma. Lancet. 390:85–94. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chang L, Gu Y, Yu Z, Ying H, Qiu Y, Ma G,
Chen H, Jin Y and Lin X: When to stop propranolol for infantile
hemangioma. Sci Rep. 7(43292)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Raphaël MF, de Graaf M, Breugem CC,
Pasmans SGMA and Breur JMPJ: Atenolol: A promising alternative to
propranolol for the treatment of hemangiomas. J Am Acad Dermatol.
65:420–421. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ábarzúa-Araya A, Navarrete-Dechent CP,
Heusser F, Retamal J and Zegpi-Trueba MS: Atenolol versus
propranolol for the treatment of infantile hemangiomas: A
randomized controlled study. J Am Acad Dermatol. 70:1045–1049.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bayart CB, Tamburro JE, Vidimos AT, Wang L
and Golden AB: Atenolol versus propranolol for treatment of
infantile hemangiomas during the proliferative phase: A
retrospective noninferiority study. Pediatr Dermatol. 34:413–421.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Q, Xiang B, Chen S and Ji Y: Efficacy
and safety of oral atenolol for the treatment of infantile
haemangioma: A systematic review. Australas J Dermatol. 60:181–185.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu X, Qu X, Zheng J and Zhang L:
Effectiveness and safety of oral propranolol versus other
treatments for infantile hemangiomas: A meta-analysis. PLoS One.
10(e0138100)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Novoa M, Baselga E, Beltran S, Giraldo L,
Shahbaz A, Pardo-Hernandez H and Arevalo-Rodriguez I: Interventions
for infantile haemangiomas of the skin. Cochrane Database Syst Rev.
4(CD006545)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. J Clin Epidemiol.
62:e1–e34. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Higgins JP and Green S (eds.): Cochrane
Handbook for Systematic Reviews of Interventions. John Wiley &
Sons, Ltd., Chichester, 2008.
|
|
20
|
Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS,
Hahn S, Jang BH and Son HJ: Testing a tool for assessing the risk
of bias for nonrandomized studies showed moderate reliability and
promising validity. J Clin Epidemiol. 66:408–414. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rao G, Lopez-Jimenez F, Boyd J, D'Amico F,
Durant NH, Hlatky MA, Howard G, Kirley K, Masi C, Powell-Wiley TM,
et al: Methodological standards for meta-analyses and qualitative
systematic reviews of cardiac prevention and treatment studies: A
scientific statement from the American heart association.
Circulation. 136:e172–e194. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma VK, Fraulin FO, Dumestre DO, Walker
L and Harrop AR: Beta-blockers for the treatment of problematic
hemangiomas. Can J Plast Surg. 21:23–28. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun L, Sun B, Ma Y, et al: Comparison of
the efficacy and safety of propranolol and atenolol in the
treatment of infant hemangioma. Progress Modern Biomed. 18:108–112.
2018.
|
|
24
|
de Graaf M, Raphael MF, Breugem CC, Knol
MJ, Bruijnzeel-Koomen CA, Kon M, Breur JM and Pasmans SG: Treatment
of infantile haemangiomas with atenolol: Comparison with a
historical propranolol group. J Plast Reconstr Aesthet Surg.
66:1732–1740. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dakoutrou M, Alexopoulos A, Miligkos M,
Georgiadou E, Kanaka-Gantenbein C and Kakourou T: Atenolol
treatment for severe infantile hemangiomas: Comparison with a
propranolol group of our centre. J Eur Acad Dermatol Venereol.
33:e199–e200. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang Q, Xiang B, Ji Y, Li F, Xu Z and
Zhong L: Propranolol versus atenolol in the treatment of infantile
hemangioma: A comparative study. Chin J Dermatol. 49:683–687.
2016.
|
|
27
|
Ashraf R, Mahajan R, Abas M, Handa S,
Sinha A, De D and Sachdev N: Comparing the clinical, radiologic and
biochemical effectiveness of atenolol and propranolol in the
treatment of infantile hemangioma-a randomized controlled trial.
Social Science Research Network, Rochester, NY, 2019. https://ssrn.com/abstract=3353380.
|