Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor in children and adolescents. The incidence of
OS is ~4-5 per year per million people (1). Treatment for OS has made notable
progress with the development of surgery and the combination of
neoadjuvant chemotherapy. However, the problems of tumor
metastasis, recurrence and multi-chemoresistance have yet to be
solved (2). In recent years,
targeted therapy has been developed as a treatment for human
malignant tumors (3). These
therapeutic drugs always specifically target several molecules,
such as growth factor receptors or intracellular signaling proteins
that are involved with tumor proliferation, migration and/or
invasion (4,5).
Calcyclin-binding protein/Siah-1-interacting protein
(CacyBP/SIP) was initially identified in Ehrlich ascites tumor
cells as a target of calcyclin (6,7). Later,
CacyBP/SIP was defined as a binding partner for Siah-1(8). Several studies revealed that CacyBP/SIP
is wildly expressed in a range of human tissues (9), where it influences diverse cellular
processes, including proliferation (10), differentiation (11), multidrug resistance (12) and tumorigenicity (13). Other studies have indicated that
CacyBP/SIP plays a role as an oncogene with increased expression in
human glioma and colorectal cancer (10,14).
Nevertheless, CacyBP/SIP is expressed at low levels in renal cell
carcinoma and gastric cancer (15,16).
Notably, CacyBP/SIP has also been reported to serve a role in
promoting apoptosis in acute lymphocytic leukemia where it appears
to function as a tumor suppressor (13). These data indicate that the effect of
CacyBP/SIP on tumorigenic progression may differ depending on the
tumor type. It has been demonstrated that CacyBP/SIP, as a novel
phosphatase, targets ERK1/2 and MAPK p38(17). It has also been shown that CacyBP/SIP
is expressed in the nuclei of osteogenic sarcoma cells (9). However, the function of CacyBP/SIP in
OS cells remains to be elucidated.
The present study evaluated the expression of
CacyBP/SIP in OS cell lines and investigated the roles of
CacyBP/SIP in OS cell proliferation and apoptosis. It was
demonstrated that downregulation of CacyBP/SIP inhibited cellular
proliferation, and induced G1/S phase cell arrest and
cell apoptosis. All these data suggest that CacyBP/SIP may play a
key role in OS progression and that CacyBP/SIP might be a target
for OS treatment.
Materials and methods
Cell lines and regents
Saos-2, MG-63, HOS, U20S OS cells and 293T cells
were obtained from the American Type Culture Collection and
cultured in DMEM medium (Corning, Inc.). All media contained 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.),
penicillin G (100 U/ml) and streptomycin (100 μg/ml; Sigma-Aldrich;
Merck KGaA). All cell cultures were maintained as a monolayer at
37˚C in a humidified atmosphere containing 5% CO2.
Construction of recombinant lentivirus
and gene silencing
The short hairpin (sh)RNA (5'-TTACCTGACCCAGGTTG
AA-3') for human CacyBP/SIP gene was inserted into the lentivirus
expression plasmid pGCSIL-GFP (Shanghai GeneChem Co., Ltd.) and
non-silencing shRNA (5'-TTCTC CGAACGTGTCACGT-3') was used as a
negative control. For virus packaging, 0.5 µg CacyBP/SIP-shRNA
vector or control (Ctrl) vector together with 0.5 µg pHelper 1.0
and 0.5 µg pHelper 2.0 (Shanghai GeneChem Co., Ltd.), were added to
293T cells with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Saos-2 cells were transduced with either the
CacyBP/SIP-shRNA lentivirus or Ctrl lentivirus at a multiplicity of
infection of 10 for 72 h. The transduced cells expressing GFP
protein were observed by using fluorescence microscopy to determine
the transduction efficiency. CacyBP/SIP expression was confirmed by
reverse transcription-quantitative PCR (RT-qPCR) and western blot
analysis after lentivirus transduction.
RNA extraction and RT-qPCR
After 3 days of transduction, total RNA was isolated
from Saos-2, MG-63, HOS and U20S OS cells (2x105
cells/well) using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Total isolated RNA (2 µg) was used to synthesize cDNA
using reverse transcription system composed by M-MLV reverse
transcriptase (Promega Corporation), M-MLV reverse transcriptase 5X
reaction buffer (Promega Corporation), dNTPs (Promega Corporation)
and random primers (Invitrogen; Thermo Fisher Scientific, Inc.) for
60 min at 37˚C according to the manufacturer's protocol. CacyBP/SIP
mRNA expression was evaluated by RT-qPCR on Roche LightCycler 480
platform with SYBR Master Mixture (Takara Bio, Inc.). The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95˚C for 30 sec; followed by 40 cycles of denaturation at 95˚C
for 5 sec and extension at 60˚C for 30 sec. The absorbance value
was obtained at the extension stage. GAPDH was used as the internal
reference control. The following PCR primers were used: CacyBP/SIP
forward, 5'-CTCCCATTACAACGGGCTATAC-3' and reverse,
5'-GAACTGCCTTCCACAGAGATG-3'; GAPDH forward,
5'-TGACTTCAACAGCGACACCCA-3' and reverse,
5'-CACCCTGTTGCTGTAGCCAAA-3'. Data were analyzed using the
2-∆∆Cq method (18). Results were presented as CT values,
which were defined as the threshold PCR cycle number at which an
amplified product was first detected. The average CT was calculated
for both CacyBP/SIP and GAPDH, and ΔCT was determined as the ratio
of the mean of the triplicate CT values for CacyBP/SIP to the mean
of the triplicate CT values for GAPDH. Each experiment was
performed in triplicate and repeated three times.
Western blotting
Cells were washed with ice cold PBS and then lysed
in 2X lysis buffer (100 mM Tris-HCl, pH 6.8; 2% mercaptoethanol;
20% glycerinum; 4% SDS) on ice for 15 min. The lysates were
clarified by centrifugation at 12,000 x g for 15 min at 4˚C and the
supernatants were employed for further analysis. The total protein
concentration was estimated using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology). Protein samples
(20 µg) were loaded and electrophoresed in an SDS-PAGE (10% gel) at
120 mA for 1 h and subsequently transferred to PVDF membranes (EMD
Millipore) at 300 mA for 120 min. After being blocked with TBS with
Tween-20 (TBST) containing 5% (w/v) non-fat dried skim milk powder
for 24 h at 4˚C, membranes were incubated with a CacyBP/SIP
antibody (cat. no. 3354; 1:1,000) or a GAPDH antibody (cat. no.
3683; 1:1,000; both from Cell Signaling Technology, Inc.) overnight
at 4˚C. p21 antibody (ab188224; 1:1,000), cyclin-dependent kinase
(CDK) 2 antibody (ab32147; 1:1,000), CDK4 antibody (ab199728;
1:2,000), Cyclin D1 antibody (ab226977; 1:2,000), Cyclin E1
antibody (ab33911; 1:2,000), Bax antibody (ab32503; 1:2,000) and
Bcl-2 antibody (ab692; 1:500) were purchased from Abcam. After
washing with TBST, the membranes were incubated with anti-rabbit
horseradish peroxidase-conjugated secondary antibody (cat. no.
7074; 1:2,000; Cell Signaling Technology, Inc.) at room temperature
for 2 h. The membranes were analyzed and visualized by Pierce ECL
Western Blotting Substrate (cat. no. 32109; Pierce; Thermo Fisher
Scientific, Inc.). Each experiment was repeated three times. The
quantification of proteins was performed in ImageJ software
(version 1.7.9; National Institutes of Health).
Cell growth assay
Cell growth was measured using a Celigo Imaging
Cytometer (Nexcelom Bioscience LLC). Briefly, 3 days after Saos-2
cells were transfected with the negative Ctrl lentivirus or
CacyBP/SIP-shRNA lentivirus, cells in logarithmic phase were
digested, resuspended, counted and inoculated in 96-well plates for
5 days (2,000 cells/well). Cell growth rate was defined as cell
count on day n / cell count on day 1, where n=2, 3, 4 and 5. Plates
were analyzed with using the Celigo image cytometer (4.1.3.0;
Nexcelom Bioscience LLC).
MTT assay
Following a previous report (19), Saos-2 cells from different groups
(shCacyBP/SIP, shCtrl) were seeded in 96-well plates at a density
of 3,000 cells/well after 72 h of lentivirus transduction. On days
1, 3 and 5, MTT was added into each well at a final concentration
of 5 mg/ml for 4 h. Acidic isopropanol [10% SDS, 5% (v/v)
isopropanol and 0.01 mol/l HCl] was subsequently added to stop the
reaction. Absorbance was measured with an ELISA reader (Bio-Rad
Laboratories, Inc.) at a wavelength of 595 nm. Viability of cells
was calculated from absorbance. Each experiment was performed in
triplicate and repeated three times.
Colony formation assay
In order to assay monolayer colony formation, stably
transduced Saos-2 cells from shCacyBP/SIP and shCtrl groups after a
72-h transfection were seeded into 6-well plates at a density of
600 cells/well. After culture for 16 days, cells were fixed with 4%
paraformaldehyde (1 ml/well) for 30 min at room temperature. The
cells were washed with PBS and then stained with 500 µl Giemsa
staining solution at room temperature for 15 min. Colonies (>50
cells/colony) were photographed by using a fluorescence microscope
under x100 magnification (IX71; Olympus Corporation) and counted by
using the ImageJ software (version 1.7.9; National Institutes of
Health). Each experiment was performed in triplicate and repeated
three times.
Cell cycle analysis
A total of 3 days after transfection, the cells were
seeded in 60-mm-diameter plates for 72 h and were collected and
fixed and permeabilized in 70% ethanol on ice for 1 h. After
washing with PBS twice, the cells were incubated with staining
buffer (including 10 µg/ml RNase, propidium iodide) at room
temperature for 10 min and were subsequently subject to cell cycle
analysis by flow cytometry (Guava easyCyte HT; EMD Millipore). The
data were analyzed using the ModFit LT™ 3.3 software (Verity
Software House, Inc.).
Cell apoptosis detection
For the apoptosis assay, after 3 days of CacyBP/SIP
lentiviral transfection, the cells were seeded in a 6-well plate
for 72 h. Subsequently, the cells were harvested and washed with
binding buffer, resuspended in staining buffer and then incubated
with Annexin V-APC (cat. no. 88-8007; eBioscience; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions and
detected by a flow cytometer (Guava easyCyte HT; EMD Millipore).
The data were analyzed by CytoSoft 5.3.1 software (EMD
Millipore).
The Cancer Genome Atlas (TCGA)
database
The expression profiles of CacyBP/SIP as well as
clinical information of sarcoma (SARC) samples were obtained from
TCGA database (https://cancergenome.nih.gov/), including level 2
RNA-seq data from 262 SARC patients (20,21). The
K-means clustering analysis with K=2 was performed using R 3.6.1
(https://www.r-project.org/) to
categorize the patients into two groups based on the expression
levels of CacyBP/SIP. The K-means clustering is an unsupervised
clustering, mainly used to group unlabeled data, where K represents
the number of packets. Packet represents the grouping condition of
unsupervised clustering. In the present study, K=2 means clustering
into two groups after unsupervised clustering. The K-means
algorithm iteratively assigns each data point to K packets, so that
the data points are aggregated based on feature similarity
(22).
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software (SPSS, Inc.). The differences between groups were
compared using Student's t-test and data are presented as the mean
± standard deviation of three independent experiments. The log-rank
test and Kaplan-Meier curve were employed to evaluate the
association between CacyBP/SIP expression and prognosis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Lentivirus-mediated shRNA effectively
decreases CacyBP/SIP expression in Saos-2 cells
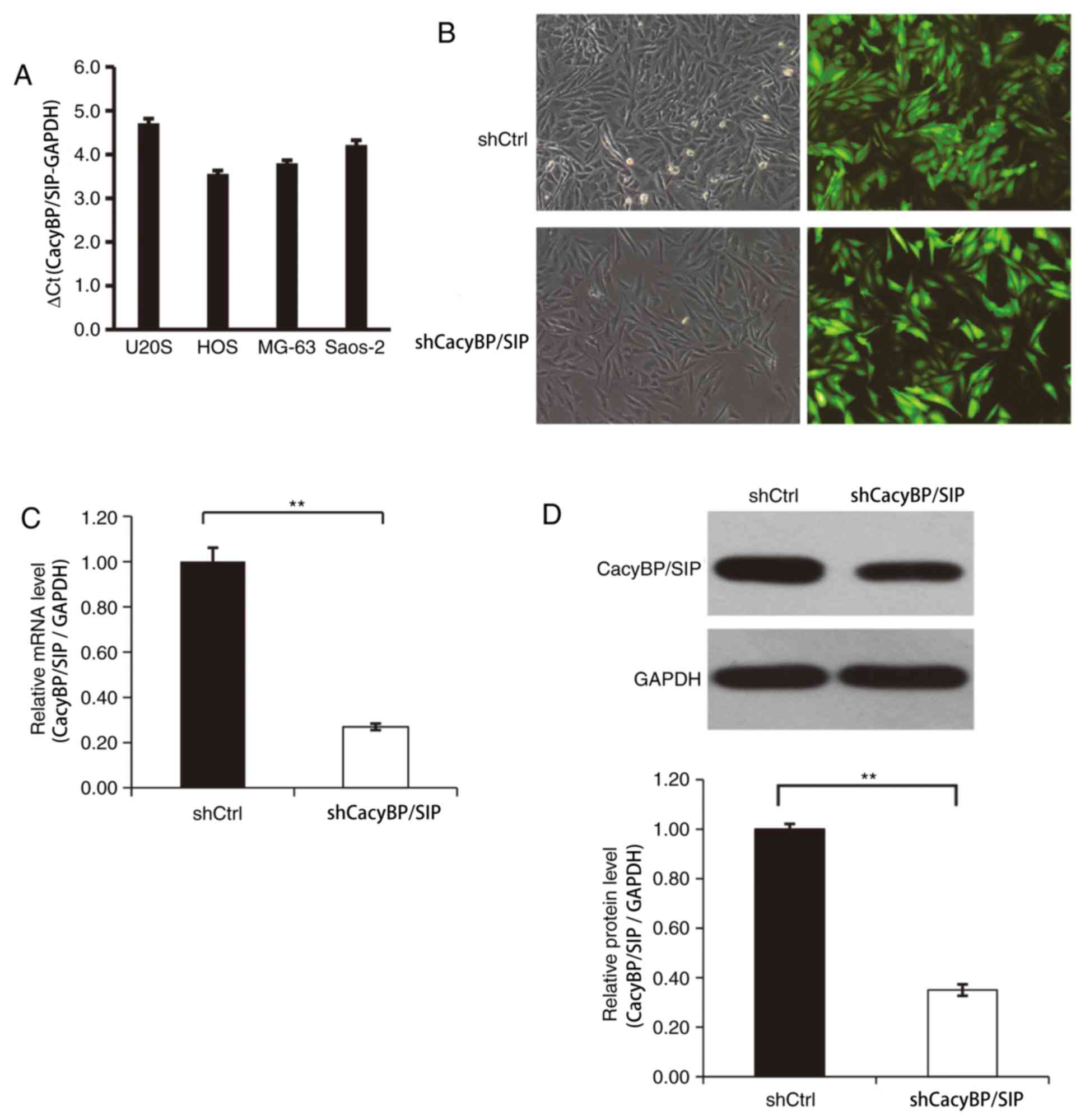
The levels of CacyBP/SIP expression in a variety of
OS cell lines was first evaluated and CacyBP/SIP mRNA expression
was detected in all four OS cell lines including Saos-2, MG-63, HOS
and U20S (Fig. 1A). After 3 days of
CacyBP/SIP lentiviral transfection, the transfection efficiency was
found to be ~80% for the CacyBP/SIP-shRNA lentivirus and the Ctrl
shRNA lentivirus (Fig. 1B). The
fluorescence level of GFP protein was observed to determine the
transfection efficiency. CacyBP/SIP mRNA level was confirmed by
RT-qPCR. The result indicted that CacyBP/SIP-shRNA lentivirus
transfection significantly reduced CacyBP/SIP expression compared
the Ctrl lentivirus-transfected cells (Fig. 1C). Decreased CacyBP/SIP protein level
was detected in CacyBP/SIP-shRNA lentivirus-transfected Saos-2
cells and the knockdown efficiency was quantitatively evaluated
(Fig. 1D).
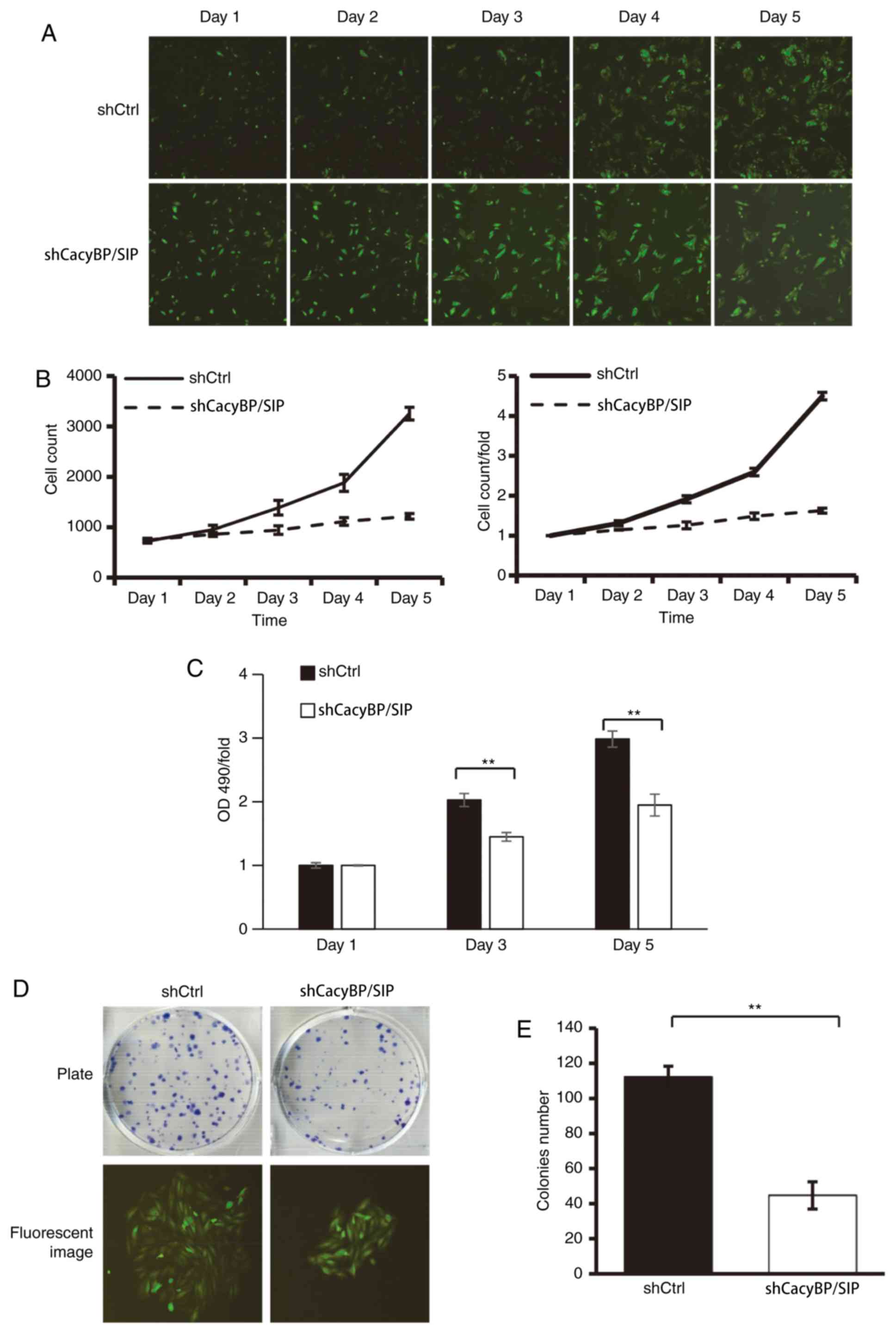
Knockdown of CacyBP/SIP inhibits
Saos-2 cell proliferation and colony formation in vitro
In order to explore the function of CacyBP/SIP on
cell growth, Saos-2 cells expressing either CacyBP/SIP-shRNA
lentivirus or Ctrl lentivirus were seeded in 96-well plates and
cell growth was monitored with a Celigo Imaging Cytometer daily for
5 days. The results demonstrated that following the downregulation
of CacyBP/SIP expression, the total number of cells remained almost
unchanged from day 2 to day 5. The cell growth rate was stalled
compared with that in the shCtrl group (Fig. 2A and B). There was a consistent result from the
MTT assay (Fig. 2C).
The colony formation was assayed to determine
CacyBP/SIP knockdown in Saos-2 and U20S cells tumorigenesis in
vitro. The results demonstrated that CacyBP/SIP knockdown in
Saos-2 cells caused a substantial reduction in colony formation
compared with the Ctrl cells (Fig.
2D). Similarly, CacyBP/SIP knockdown in U20S cells also caused
a substantial reduction in colony formation (Fig. S1). The number of colonies in the
CacyBP/SIP-shRNA lentivirus-transduced cells was significantly
decreased compared with the Ctrl group, with shCtrl 112±6 vs.
shCacyBP/SIP 45±8 in Saos-2 cells (P<0.01; Fig. 2E).
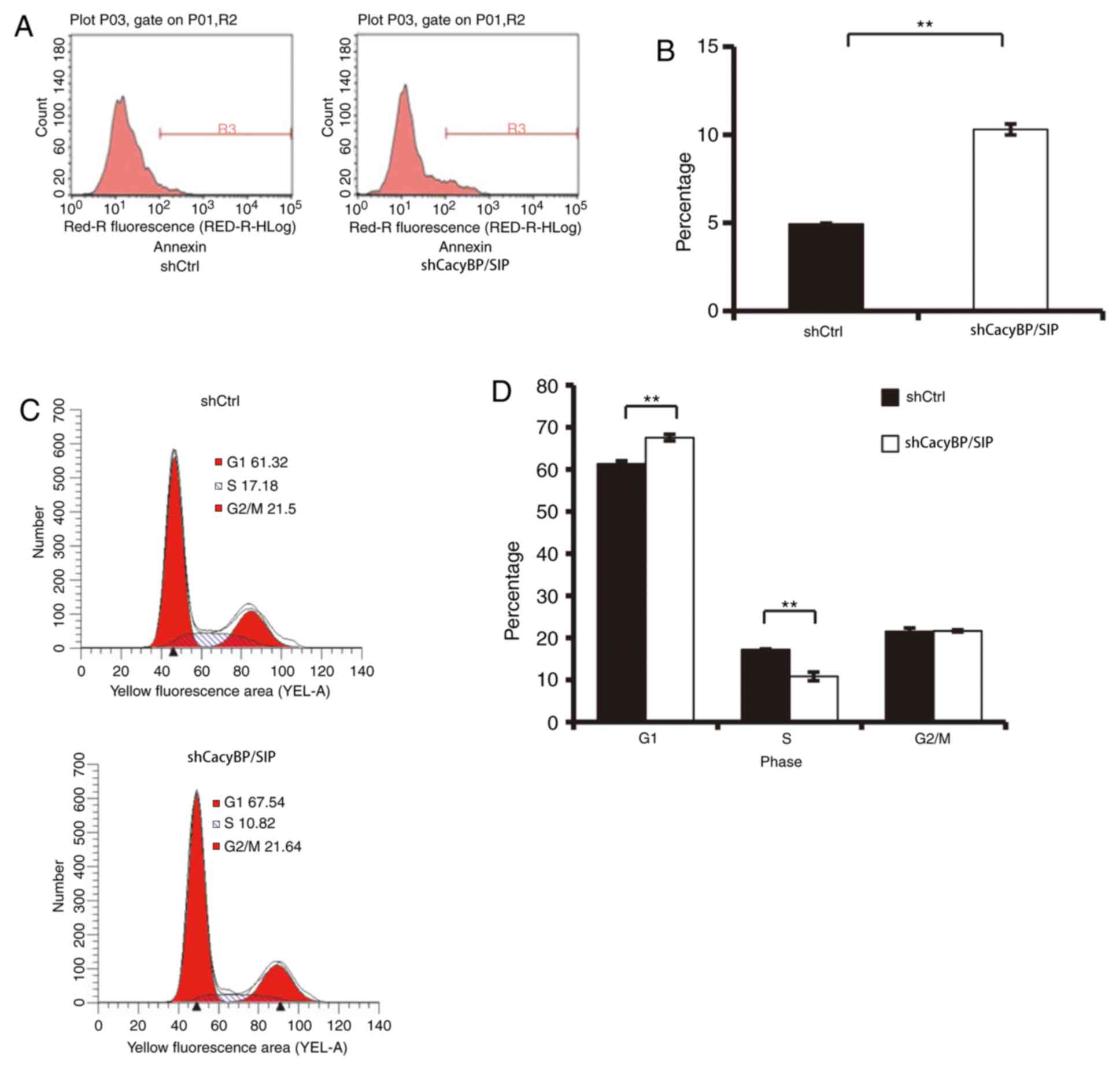
Knockdown of CacyBP/SIP induces Saos-2
cell apoptosis and cell cycle arrest
Alterations in cell proliferation is frequently
caused by cell apoptosis (23). To
further explore this mechanism, cell apoptosis was examined by
Annexin V-APC. As illustrated in Fig.
3A and B, 6 days after cell
seeding, the percentage of apoptotic Saos-2 cells was significantly
increased in the shCacyBP/SIP group compared with that in the
shCtrl group (shCtrl, 4.93±0.06%; shCacyBP/SIP 10.3±0.31%;
P<0.01). This result indicated that CacyBP/SIP may be associated
with the apoptosis of Saos-2 cells.
To further explore the mechanism by which CacyBP/SIP
promoted Saos-2 cell growth, the effects of silencing CacyBP/SIP
expression on the cell cycle were investigated by flow cytometry. A
total of 67.54±0.76% of shCacyBP/SIP cells were in the
G1 phase, compared with 61.32±0.69% of Ctrl cells
(Fig. 3C and D; P<0.01). In
addition, S phase distribution of Ctrl cells and shCacyBP/SIP cells
were 17.18±0.16 and 10.82±1.01%, respectively (Fig. 3C and D; P<0.01). The results indicated that
downregulation of CacyBP/SIP induced G1/S phase cell
cycle arrest.
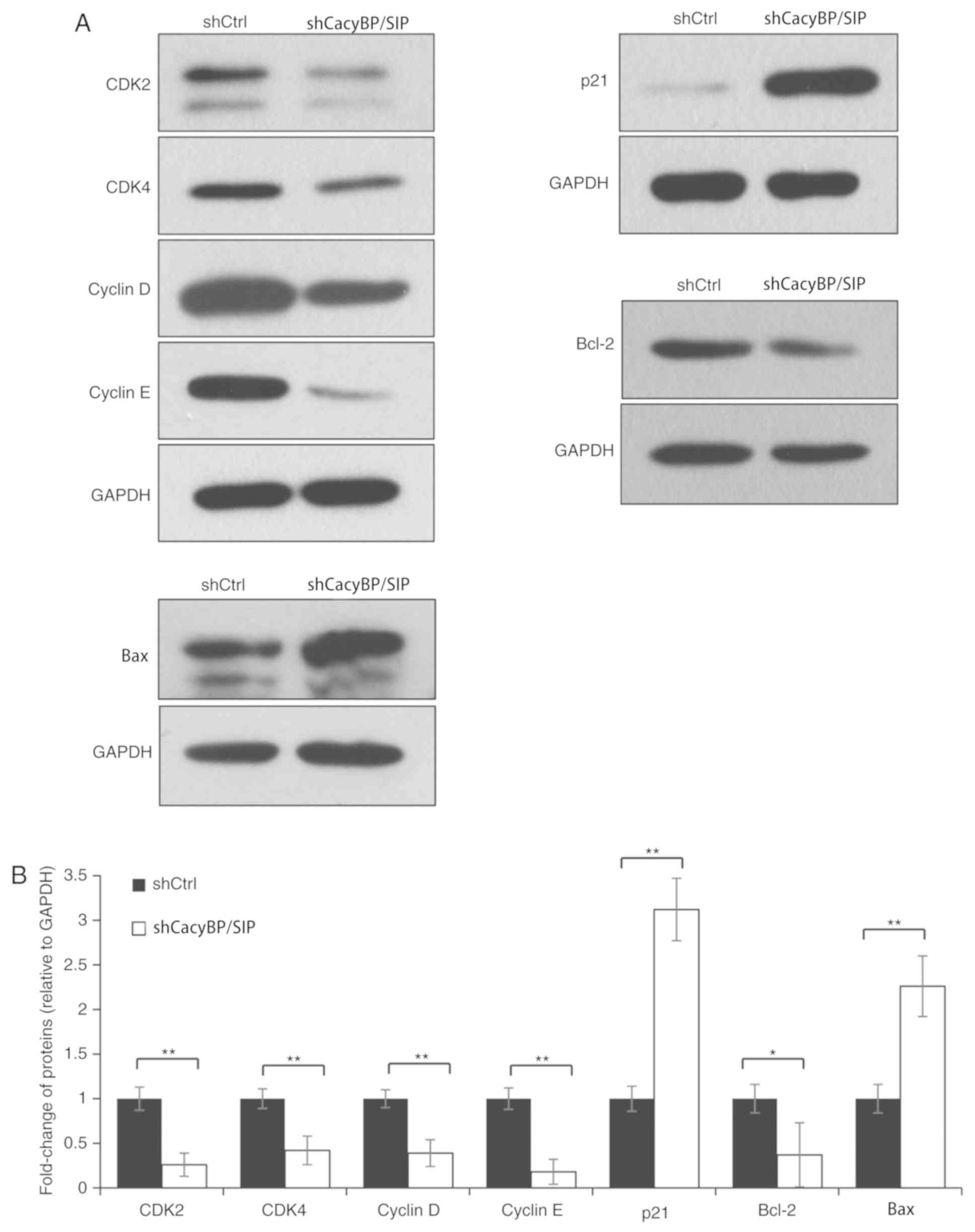
Effects of CacyBP/SIP knockdown on
CDK/cyclin/P21 and Bcl-2/Bax axis
Next, the levels of key proteins involved in the
intracellular mechanisms of the cell cycle and apoptosis were
evaluated. The data demonstrated that knockdown of CacyBP/SIP
protein was associated with an increase in p21 levels and a
decrease in CDK2, CDK4, cyclin D and cyclin E levels (P<0.01;
Fig. 4), indicating that CacyBP/SIP
arrested the process of cell cycle G1 to S phase
transition. Knockdown of CacyBP/SIP resulted in an increase of Bax
(P<0.01; Fig. 4), and decrease of
Bcl-2 (P<0.05; Fig. 4), which are
two key regulators in the apoptosis process. The results
demonstrated that CDK/cyclin/P21 axis and Bcl-2/Bax axis were
activated by CacyBP/SIP.
Prognosis analysis based on TCGA
data
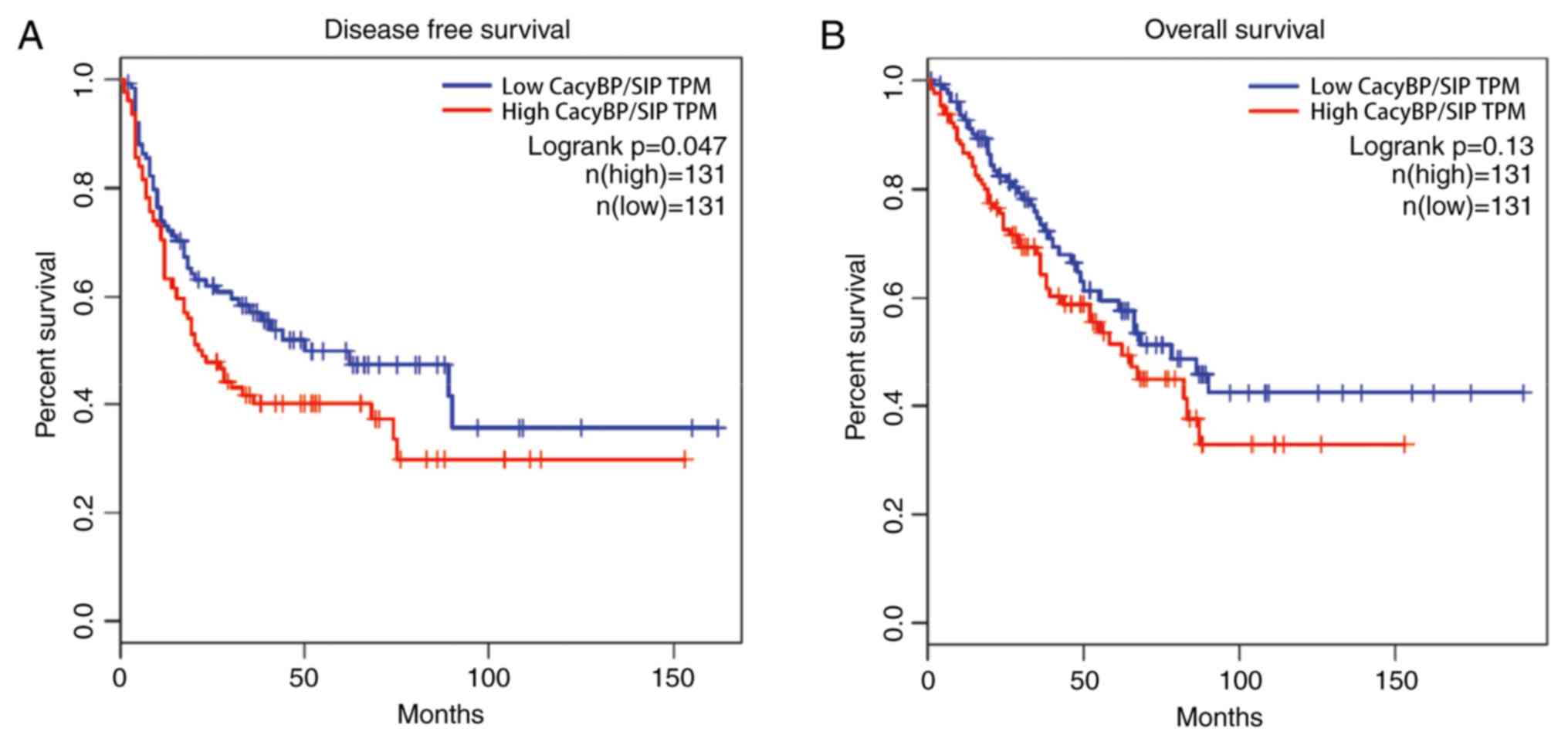
Patients with high CacyBP/SIP mRNA levels
demonstrated worse prognosis compared with patients with low
CacyBP/SIP mRNA levels, from both the disease free survival
(Fig. 5A) and overall survival
(Fig. 5B) data. The results
demonstrated that CacyBP/SIP had a tumor-driving role in TCGA
samples.
Discussion
CacyBP/SIP is an important protein in tumorigenesis
and is widely detected in a variety of human tissues, where it
regulates cell proliferation, differentiation, chemotherapy
resistance and tumorigenicity (8).
However, the exact role of CacyBP/SIP in cancer remains unclear.
CacyBP/SIP has been reported to be an oncogene in a number of
malignant tumors. In human glioma, colorectal cancer and pancreatic
cancer patients, increased expression of CacyBP/SIP was
significantly associated with tumor cell proliferation and
metastasis (10,15,24). In
addition, decreased expression of CacyBP/SIP was found in renal
cell carcinoma, gastric cancer and chronic lymphocytic leukemia
cells where it appeared to be a tumor suppressor (13,15,16,25,26).
Therefore, CacyBP/SIP cannot be regarded only as an oncogene or
tumor suppressor. Its function appears to vary according to cell
lines and the organs involved, or the different modes of tumor
progression.
In the present study, OS cell lines Saos-2, MG-63,
HOS and U20S expressed CacyBP/SIP. Lentivirus-mediated CacyBP/SIP
knockdown markedly inhibited cell proliferation in both Saos-2 and
U20S cells and induced Sao-2 cell apoptosis in vitro. The
total cell number in the shCacyBP/SIP group remained unchanged,
whilst the total cell number of shCtrl group were significantly
increased during these tested days. In addition, the cell growth
rate in the CacyBP/SIP-shRNA lentivirus-transduced group were
significantly decreased compared with the Ctrl
lentivirus-transduced group from days 2 to 5. Colony formation
assay demonstrated that CacyBP/SIP knockdown in Saos-2 cells caused
a substantial reduction in colony formation numbers. In addition,
the data demonstrated that knockdown of CacyBP/SIP inhibited the
cell cycle by inducing G1/S phase arrest. p21, also
known as CDK inhibitor 1, is capable of binding and inhibiting
numerous cyclin/CDK complexes, such as cyclin D/CKD4(27), cyclin E/CDK2(28) and cyclin A/CDK2(29). The results of the present study
demonstrated that knockdown of CacyBP/SIP protein was associated
with an increase in p21 levels and decrease in CDK2, CDK4, cyclin D
and cyclin E levels. In accordance with this result, several
studies have identified that overexpression of p21 can induce cell
cycle arrest at G1, G2 and S phases (13,30). It
has been previously reported that p21 serves as a tumor suppressor
(31). The present study indicated
that CacyBP/SIP was associated with the CDK/cyclin/P21 axis. All of
the data from the present study suggested that knockdown of
CacyBP/SIP may inhibit OS cell proliferation by regulating specific
cell factors involved in cell cycle and apoptosis processes.
Furthermore, CacyBP/SIP knockdown induced cell apoptosis. Taken
together, the present study demonstrated that CacyBP/SIP serves an
important role in OS cell proliferation and apoptosis, suggesting
an oncogenic role for CacyBP/SIP in OS. Further research is
required to explore the complex molecular mechanisms of CacyBP/SIP
in OS cells.
In conclusion, the results of the present study
suggested that the downregulation of CacyBP/SIP by shRNA could
markedly suppress proliferation and tumorigenesis by inducing
apoptosis in OS cells. To the best of the authors' knowledge, this
is the first study to investigate the role of CacyBP/SIP in OS
cells. Further investigation is required to elucidate the
mechanisms of CacyBP/SIP in OS cells. The present study revealed
the function of CacyBP/SIP in OS cells and indicated that
CacyBP/SIP might be a potential molecular target in the treatment
of OS.
Supplementary Material
Figure S1. Knockdown of CacyBP/SIP
inhibits U2OS cell proliferation and colony formation in
vitro. U2OS cells expressing either CacyBP/SIP‑shRNA lentivirus
or Ctrl lentivirus were seeded in 96‑well plates and 6‑well plates.
(A and B) Colony formation assay and (C) MTT assay were performed
to evaluate cell proliferation. Magnification, x100.
*P<0.05 and **P<0.01. CacyBP/SIP,
calcyclin‑binding protein/Siah‑1‑interacting protein; sh, short
hairpin; Ctrl, control; OD, optical density.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Hebei
Province Scientific Research Foundation for the Returned Overseas
Chinese Scholars (grant no. CY201715) and a grant from the Key
R&D Plan Project of Hebei Science and Technology Department
(grant no. 18277798D).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ, RZZ and GCZ designed all the experiments,
analyzed the data and revised the paper; MZ, RZZ, DWQ and HYC
performed experiments, drafted and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bernthal NM, Federman N, Eilber FR, Nelson
SD, Eckardt JJ, Eilber FC and Tap WD: Long-term results (>25
years) of a randomized, prospective clinical trial evaluating
chemotherapy in patients with high-grade, operable osteosarcoma.
Cancer. 118:5888–5893. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grignani G, Palmerini E, Dileo P, Asaftei
SD, D'Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F,
Casali PG, et al: A phase II trial of sorafenib in relapsed and
unresectable high-grade osteosarcoma after failure of standard
multimodal therapy: An Italian Sarcoma Group study. Ann Oncol.
23:508–516. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang Y, Yang J, Zhao N, Wang C, Kamar S,
Zhou Y, He Z, Yang J, Sun B, Shi X, et al: Progress in the
chemotherapeutic treatment of osteosarcoma. Oncol Lett.
16:6228–6237. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grignani G, Palmerini E, Ferraresi V,
D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al: Italian Sarcoma Group: Sorafenib and
everolimus for patients with unresectable high-grade osteosarcoma
progressing after standard treatment: A non-randomised phase 2
clinical trial. Lancet Oncol. 16:98–107. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Filipek A and Kuźnicki J: Molecular
cloning and expression of a mouse brain cDNA encoding a novel
protein target of calcyclin. J Neurochem. 70:1793–1798.
1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Filipek A and Wojda U: p30, a novel
protein target of mouse calcyclin (S100A6). Biochem J. 320:585–587.
1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Topolska-Woś AM, Chazin WJ and Filipek A:
CacyBP/SIP - Structure and variety of functions. Biochim Biophys
Acta. 1860:79–85. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhai H, Shi Y, Jin H, Li Y, Lu Y, Chen X,
Wang J, Ding L, Wang X and Fan D: Expression of calcyclin-binding
protein/Siah-1 interacting protein in normal and malignant human
tissues: An immunohistochemical survey. J Histochem Cytochem.
56:765–772. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi H, Gao Y, Tang Y, Wu Y, Gong H, Du J,
Zheng B, Hu J, Shi Q and Yu R: CacyBP/SIP protein is important for
the proliferation of human glioma cells. IUBMB Life. 66:286–291.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Rosińska S, Leśniak W and Filipek A:
Distinct effect of CacyBP/SIP on the ERK1/2-CREB-BDNF pathway in
undifferentiated and differentiated neuroblastoma NB2a cells.
Neurochem Int. 97:65–72. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen X, Zheng P, Xue Z, Li J, Wang W, Chen
X, Xie F, Yu Z and Ouyang X: CacyBP/SIP enhances multidrug
resistance of pancreatic cancer cells by regulation of P-gp and
Bcl-2. Apoptosis. 18:861–869. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fu C, Wan Y, Shi H, Gong Y, Wu Q, Yao Y,
Niu M, Li Z and Xu K: Expression and regulation of CacyBP/SIP in
chronic lymphocytic leukemia cell balances of cell proliferation
with apoptosis. J Cancer Res Clin Oncol. 142:741–748.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhai H, Shi Y, Chen X, Wang J, Lu Y, Zhang
F, Liu Z, Lei T and Fan D: CacyBP/SIP promotes the proliferation of
colon cancer cells. PLoS One. 12(e0169959)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ning X, Sun S, Hong L, Liang J, Liu L, Han
S, Liu Z, Shi Y, Li Y, Gong W, et al: Calcyclin-binding protein
inhibits proliferation, tumorigenicity, and invasion of gastric
cancer. Mol Cancer Res. 5:1254–1262. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun S, Ning X, Liu J, Liu L, Chen Y, Han
S, Zhang Y, Liang J, Wu K and Fan D: Overexpressed CacyBP/SIP leads
to the suppression of growth in renal cell carcinoma. Biochem
Biophys Res Commun. 356:864–871. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Topolska-Woś AM, Rosińska S and Filipek A:
MAP kinase p38 is a novel target of CacyBP/SIP phosphatase. Amino
Acids. 49:1069–1076. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chai R, Yu X, Tu S and Zheng B: Depletion
of UBA protein 2-like protein inhibits growth and induces apoptosis
of human colorectal carcinoma cells. Tumour Biol. 37:13225–13235.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Henderson T, Chen M, Darrow MA, Li CS,
Chiu CL, Monjazeb AM, Murphy WJ and Canter RJ: Alterations in
cancer stem-cell marker CD44 expression predict oncologic outcome
in soft-tissue sarcomas. J Surg Res. 223:207–214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Comprehensive and Integrated Genomic
Characterization of Adult Soft Tissue Sarcomas. Cell 171:
950-965.e928, 2017.
|
|
22
|
Hartigan JA and Wong MA: A K-means
clustering algorithm. Appl Stat. 28:100–108. 2013.
|
|
23
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Vats P, Ray K, Majumadar D, Amitabh Joseph
DA, Bayen S, Akunov A, Sarbaev A and Singh SB: Changes in
cardiovascular functions, lipid profile, and body composition at
high altitude in two different ethnic groups. High Alt Med Biol.
14:45–52. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhai HH, Meng J, Wang JB, Liu ZX, Li YF
and Feng SS: CacyBP/SIP nuclear translocation induced by gastrin
promotes gastric cancer cell proliferation. World J Gastroenterol.
20:10062–10070. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ning X, Sun S, Zhang K, Liang J, Chuai Y,
Li Y and Wang X: S100A6 protein negatively regulates
CacyBP/SIP-mediated inhibition of gastric cancer cell proliferation
and tumorigenesis. PLoS One. 7(e30185)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang Y, Wang S, Qian W, Ji D, Wang Q,
Zhang Z, Wang S, Ji B, Fu Z and Sun Y: uc.338 targets p21 and
cyclin D1 via PI3K/AKT pathway activation to promote cell
proliferation in colorectal cancer. Oncol Rep. 40:1119–1128.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Merli M, Benassi MS, Gamberi G, Ragazzini
P, Sollazzo MR, Molendini L, Magagnoli G, Ferrari C, Maltarello MC
and Picci P: Expression of G1 phase regulators in MG-63
osteosarcoma cell line. Int J Oncol. 14:1117–1121. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Adams PD, Sellers WR, Sharma SK, Wu AD,
Nalin CM and Kaelin WG Jr: Identification of a cyclin-cdk2
recognition motif present in substrates and p21-like
cyclin-dependent kinase inhibitors. Mol Cell Biol. 16:6623–6633.
1996.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Song Z, Lin J, Sun Z, Ni J and Sha Y:
RNAi-mediated downregulation of CDKL1 inhibits growth and
colony-formation ability, promotes apoptosis of human melanoma
cells. J Dermatol Sci. 79:57–63. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993.PubMed/NCBI View Article : Google Scholar
|