Introduction
Papillary thyroid carcinoma (PTC) is a common type
of thyroid malignancy, accounting for 74-80% of all cases of
thyroid cancer, and the incidence has kept increasing in recent
years (1). In addition to PTC, other
malignant tumors of the thyroid gland include follicular carcinomas
(FTC; moderate malignancy; prevalence of 11-15%), undifferentiated
carcinomas (ATC; high malignancy; prevalence of 1-2%) and medullary
carcinomas (MTC; prevalence of 3-8%). PTC is clinically
characterized by obscure symptoms, slow disease development and
relatively low mortality (2). With
the application of color Doppler ultrasound imaging, the
time-intensity curve of the tumors may be obtained using
quantitative parameter analysis of ultrasound contrast dynamic
imaging, as well as the association between the parameters and the
expression levels of P53 and Ki67 in the tumors (3). Information on the blood supply of
tissues and organs, together with the evidence of vascular invasion
or metastasis, is important for tumor staging, recurrence analysis
and prognostication (4,5). On the other hand, FTC is a type of
thyroid cancer that occurs relatively rarely, which may be divided
into invasive and non-invasive subtypes. The clinical
manifestations, diagnostic methods and treatment methods are
basically similar to those of PTC. Furthermore, ATC accounts for
1-2% of thyroid tumor cases, with low disease incidence but high
degree of malignancy. The growth and development of ATC is rapid,
featured by strong local invasiveness and a high distant metastasis
rate. The prognosis of ATC is dismal, and at present, no effective
treatment is available. Furthermore, MTC is derived from thyroid
follicular cells (also known as C cells) that secrete calcitonin.
The major cause of MTC is mutation of the RET proto-oncogene and
the disease prognosis is generally poor, while it is frequently
closely associated with the blood calcitonin level. The present
study reported on the clinical incidence, disease manifestations
and examination results of patients with PTC and determined
prognostic factors.
Over the past years, relatively less invasive
methods that do not cause excessive scarring have gradually
replaced routine surgical resection as the clinical application.
Ultrasound-guided radiofrequency ablation (RFA) is a type of
minimally invasive treatment, which causes local hyperthermia to
induce necrosis in the tumor tissue, not only representing an
effective disease treatment but also meeting the patients'
aesthetic requirements (6). However,
although it is a minimally invasive treatment method,
ultrasound-guided RFA may still cause certain damage to the body
and tissue.
Recently, with the rapid development of molecular
biology, the molecular biological indicators for PTC have gradually
become a research focus (7). P53 and
Ki67 are the major indicators for malignant tumors (8,9).
Therefore, the establishment of specific tumor markers for PTC are
of important significance in the clinic to improve the diagnosis
and treatment of the disease.
A high incidence and therapeutic value of PTC have
been observed in the clinic, and compared with other types of
thyroid tumor, the prognosis for PTC is relatively favorable. In
the present study, the association between the features of the
contrast-enhanced ultrasound (CEUS) and the biological indicators
for PTC were further analyzed. In addition, the ultrasound
performance of PTC, as well as the disease recurrence after RFA,
were analyzed and discussed. Furthermore, possible influencing
factors on the prognosis of PTC were determined.
Materials and methods
Study subjects
A total of 72 patients with PTC (22 males and 50
females; average age, 48.7±4.5 years; age range, 28-70 years), who
received treatment at Taihe Hospital, Hubei University of Medicine,
from February 2016 to January 2018, were included in the present
study. The follow-up visits lasted for 18 months. The inclusion
criteria were as follows (10): i)
Patients receiving ultrasound-guided RFA for PTC at our hospital.
The indications should be in accordance with at least one of the
following conditions: a) Patients having a lesion with a diameter
of <2 cm or aesthetics-affecting tumor convexity; b) patients
having multiple tumors (<3 lesions) with mild infiltration and
lymph node metastasis around the tumor, who would not consider
surgical resection; ii) patients with complete pre-operative
ultrasound examination data; iii) patients with complete follow-up
data; and iv) patients who were able to be staged according to the
TNM Clinical Staging Criteria of thyroid papillary carcinoma from
the American Joint Committee on Cancer (AJCC; 7th edition, 2017)
(11). Based on TNM staging, the
patients at the T1 stage were included and subjected to clinical
treatment. The exclusion criteria were as follows (5): i) Patients with a previous history of
thyroid disease or abnormal vocal cord function on the
contralateral side of the lesion; ii) patients with a history of
serious diseases that required long-term medication; iii) patients
with unclear pathological findings or previous history of psychosis
or neuropathy; iv) patients having received relevant treatments
that may affect the observation indicators; and v) patients with
severe heart, liver and/or kidney damage that affected the
metabolism, or severe coagulation mechanism disorders. The present
study was approved by the ethics committee of Taihe Hospital, Hubei
University of Medicine, and all patients had provided written
informed consent.
Tumor puncture biopsy
All patients underwent the routine ultrasound
examination of the neck and thyroid prior to treatment. The lesions
were located on the body surface and the needling depth was
determined. For the conventional disinfection, local infiltration
anesthesia and puncturing, following local anesthesia, a disposable
7# needle was inserted to the subcutaneous positioning
point. The puncturing direction of the 18G needle was observed by
and adjusted according to the real-time ultrasound, until the
needle tip was located in the lesion area at the sagittal and
coronal section. The 2-3 tissue samples (1-2 cm in length) were
obtained, which were placed in formalin solution, followed by
pathological analysis.
CEUS
For the CEUS, the SonoVue contrast agent was used
(Bracco), diluted with 5 ml 0.9% NaCl solution, which was
administered through the anterior elbow vein as a bolus injection
(2.4 ml). The whole process of the contrast perfusion (including
peaking and withdrawal) was dynamically observed. Furthermore, the
lesion's enhancement level, enhancement pattern and its association
with surrounding tissues, were observed. The time-intensity curve
was drawn and the parameters, including the peak value (PEAK), mean
transit time (MTT), initial increasing time and peak time, were
obtained. The image data were read and analyzed by two physicians
with ultrasound experience of >10 years.
Ablation treatment
RFA was performed with the Celon AG RFA system
(Olympus Corp.), equipped with an 18G bipolar RFA needle with a 9-
or 15-mm electrode, and the output frequency was 5-8 W. Under the
guidance of ultrasound, 1% lidocaine hydrochloride was injected
subcutaneously and around the thyroid anterior capsule for local
anesthesia. Furthermore, 20-40 ml NaCl (0.9%) was injected in the
area around the thyroid capsule. After isolating the thyroid from
the surrounding tissues (including large blood vessels, nerves,
trachea, esophagus and muscles), the 18G RFA needle was placed
inside the mass under the guidance of color Doppler ultrasound,
with the Philips IU22 color Doppler ultrasound imaging system
(Philips Ultrasound), with the line array probe of L3-9 and
frequency of 3-9 MHz, or L5-12 and frequency of 5-12 MHz. The
mobile ablation technique was applied to ablate the mass, with the
from-inside-to-outside and from-shallow-to-deep mode, until the
lesion was completely covered by the hyperechoic gasification zone.
After the ablation, CEUS was performed during the operation to
evaluate whether the ablation was sufficient.
Pathological analysis
The obtained tissues were fixed with conventional
neutral formalin (at room temperature for 12-24 h), dehydrated, and
embedded in paraffin. For immunohistochemical staining for P53 and
Ki67, the tissue section was dewaxed and treated with xylene and
alcohol (for 3 rounds, 5 min per round), followed by washing with
distilled water and PBS. According to standard protocols, after
antigen retrieval, the section was incubated with 3% hydrogen
peroxide at room temperature for 5 min, and then washed with PBS
for 3 times (2 min each time). The sections were then incubated
with mouse anti-human P53 monoclonal antibody (1:100 dilution;
Zhongshang Goldenbridge-BIO) and mouse anti-human Ki-67 monoclonal
antibody (1:100 dilution; Zhongshang Goldenbridge-BIO),
respectively, at room temperature for 1-2 h. The sections were
subsequently treated with Reagents 1 and 2 within the kit
(REF.10128105P-G, P/N80302-3101; Hangzhou Shitai Biotech Co.,
Ltd.), at room temperature for 20 min each time. Diaminobenzidine
chromogenic droplets were used to treat the tissue section,
followed by the hematoxylin counterstaining for 5 min. After
treatment with alcohol and xylene, the sections were observed under
a microscope. The data were analyzed and scored according to the
following criteria: Ratio of cells with positive staining <10%,
negative (-), and ≥10%, positive (+), where 10-25% was considered
as weakly positive (1 point), 25-50% as moderately positive (2
points) and >50% as strongly positive (3 points). The staining
was performed and the results were analyzed by two pathologists (Lu
Jian, Department of Pathology, Taihe Hospital, Hubei University of
Medicine, Shiyan City, Hubei, China; and Dandan Zou, Attending
Physician, Department of Pathology, People's Hospital of Longhua
District, Shenzhen, Guangdong, China), with senior professional
titles.
Statistical analysis
SPSS 17.0 software (SPSS, Inc.) was used for
statistical analysis. Count data were expressed as % and were
analyzed with the χ2 test. Measurement data were
expressed as the mean ± standard deviation. The measurement data
fitting a normal distribution (The ratio of the sample median to
the arithmetic mean, and the relationship between the arithmetic
mean and the standard deviation, were used for the normal
distribution), were compared with the Student's t-test or analysis
of variance (with the Bonferroni post-hoc test), while the
measurement data with an anomalous distribution were compared with
the rank-sum test followed by the Conover post-hoc test.
Multivariate analysis of parameters associated with recurrence was
performed using logistic regression analysis with the stepwise
backward method. Non-normally distributed measurement data and
non-parametric grading data were analyzed by Spearman correlation
analysis. The non-parametric data were compared using the
χ2 test. P<0.05 was considered to indicate
statistical significance.
Results
Imaging performance and time-intensity
curve analysis of PTC on CEUS
The clinicopathological characteristics of the
patients were provided in Table SI.
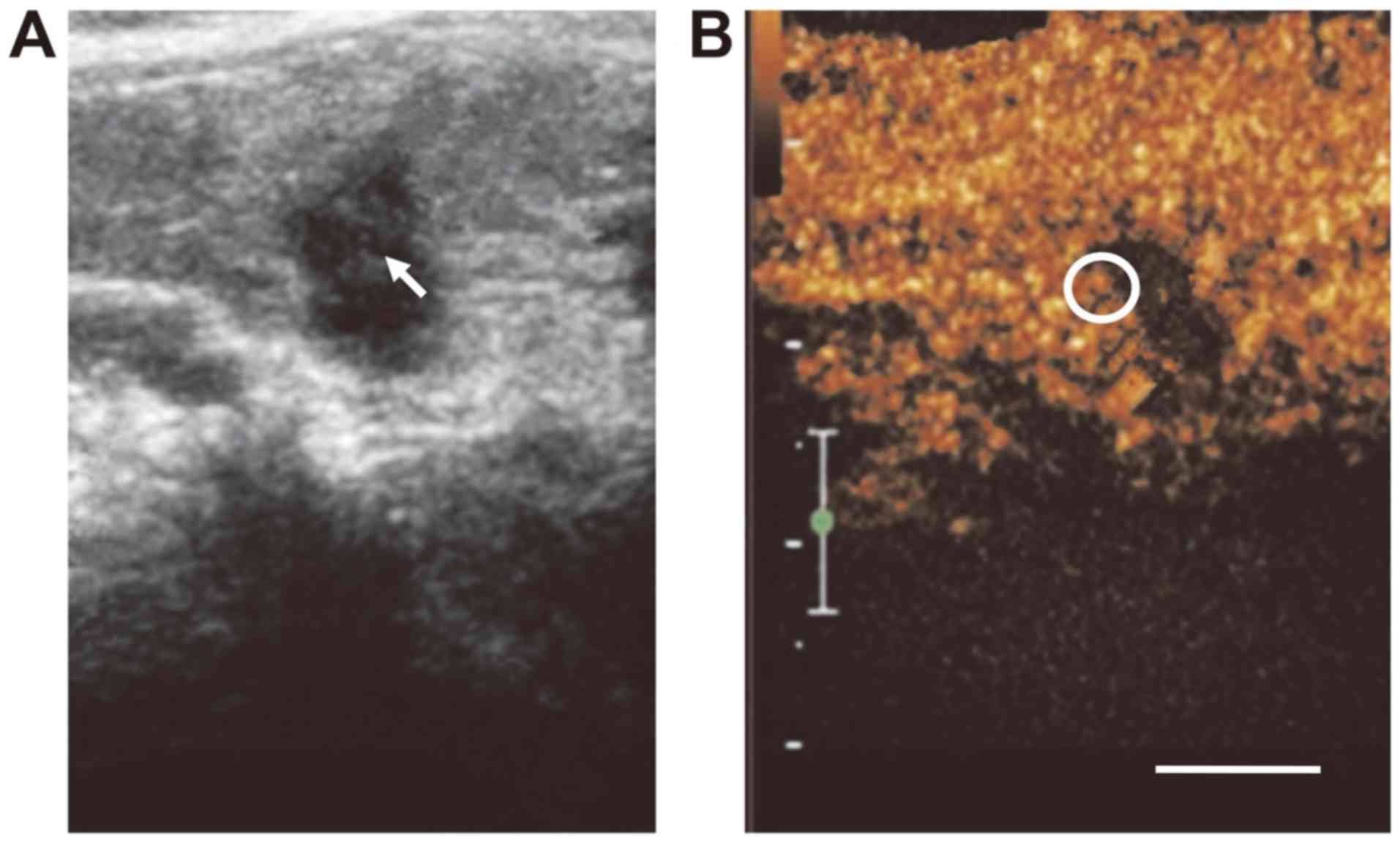
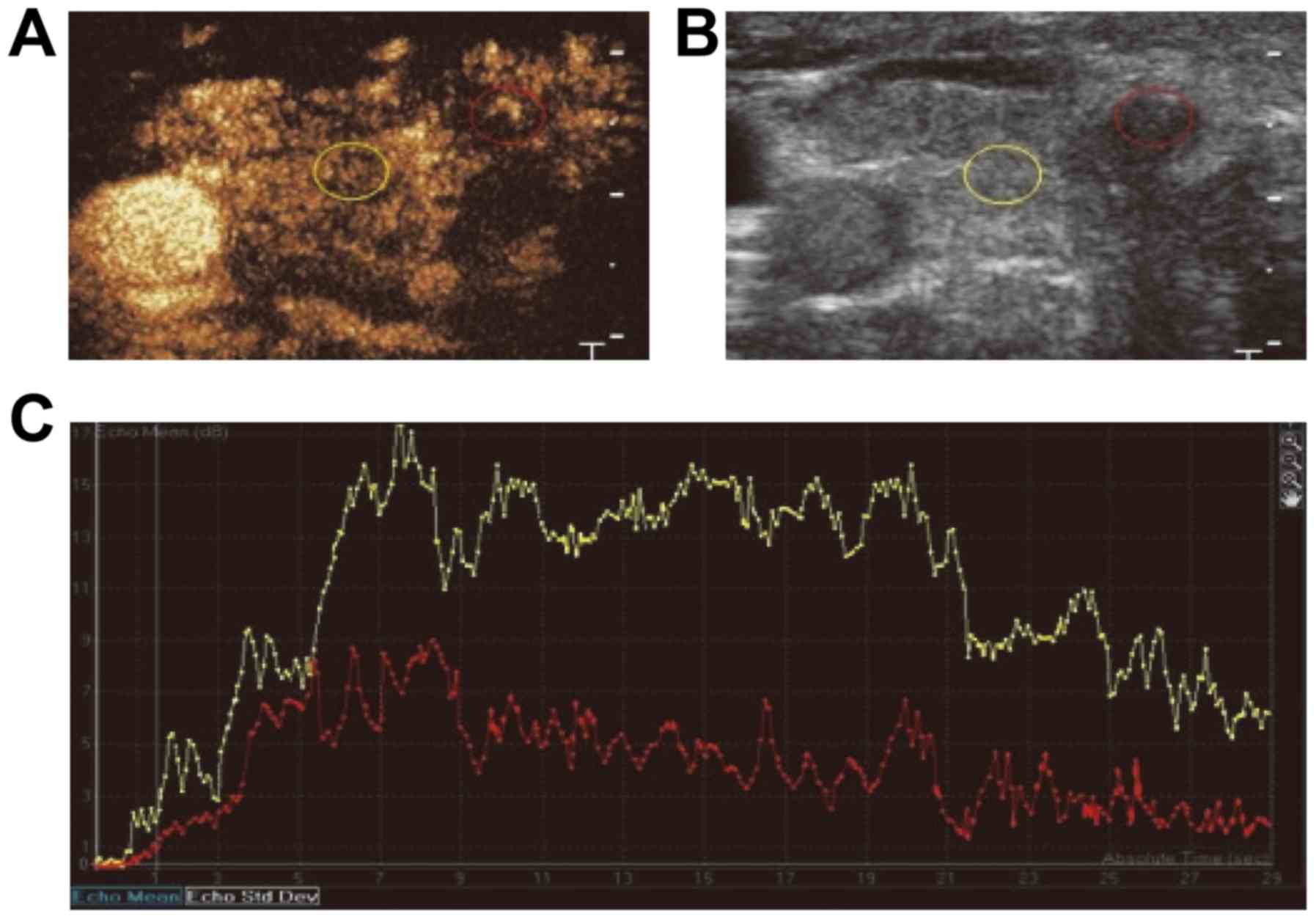
For PTC, the CEUS indicated that the lesion had an irregular shape
and no clear boundary, with no obvious or uneven enhancement, as
well as the focal perfusion defect regions (Figs. 1 and 2A and B).
Compared with the normal surrounding thyroid tissues, the carcinoma
lesion exhibited a slow perfusion pattern. In the lesion tissue,
the initial increasing time was prolonged and the enhancement level
was lower compared with that in the surrounding thyroid tissue. For
the carcinoma lesions, the rising slope of the curve was relatively
flat, slowly reaching the peak, which then gradually regressed.
During the whole enhancement process, the enhancement level for the
PTC was lower than that for the normal thyroid tissue. Furthermore,
the peak intensity for the normal thyroid tissue was higher than
that for the PTC tissue, and the MTT for the PTC was significantly
longer than that for the normal tissue (Fig. 2C; Table
I).
 | Table ITime-intensity curve analysis of CEUS
for papillary thyroid carcinoma. |
Table I
Time-intensity curve analysis of CEUS
for papillary thyroid carcinoma.
| CEUS | PTC tissue | P-value | Normal surrounding
tissue |
|---|
| Initial increasing
time | 15.32±2.73 | <0.05 | 11.44±1.26 |
| Peak time | 11.23±3.01 | <0.05 | 21.16±3.53 |
| Regression time | 30.0±4.24 | | 29.06±3.98 |
| Mean transit
time | 54.14±14.26 | <0.05 | 53.63±13.27 |
| Peak intensity | 37.06±9.07 | <0.05 | 40.6±9.45 |
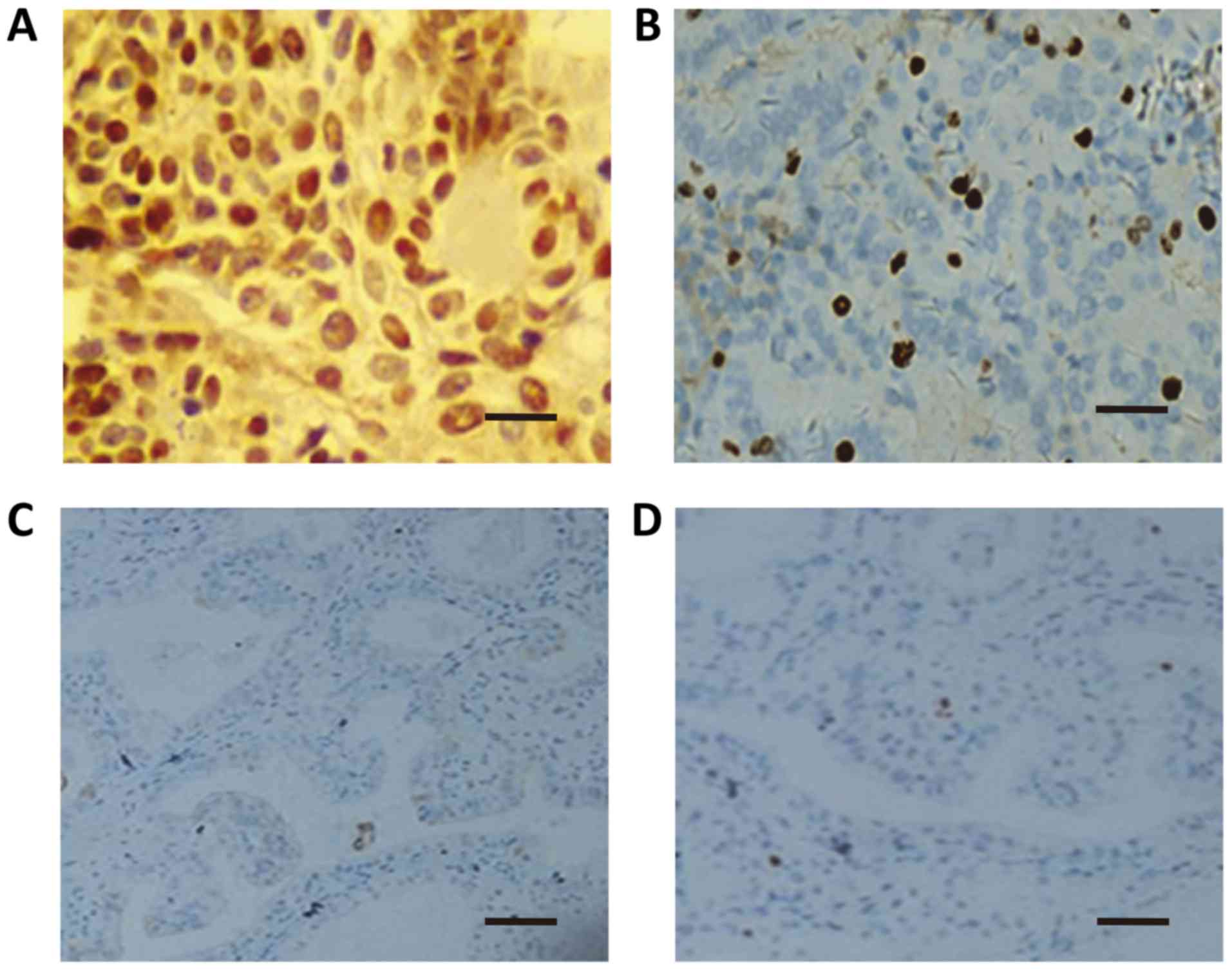
Correlation between biological
indicators and clinical features of PTC
For the PTC group, the positive rates of P53 and
Ki67 were significantly associated with age (<45 years), local
infiltration, lymph node metastasis, and lesion numbers
(P<0.05). However, the positive rates were not related to the
gender, tumor size, histological type, or differentiation degree
(P>0.05; Table II; Fig. 3).
 | Table IIComparison of P53- and Ki67-positive
rates in patients with papillary thyroid carcinoma with different
clinicopathological features. |
Table II
Comparison of P53- and Ki67-positive
rates in patients with papillary thyroid carcinoma with different
clinicopathological features.
| Parameter | N | P53 (+) | χ2 | P-value | Ki67 (+) | χ2 | P-value |
|---|
| Age (years) | | | 4.47 | 0.03 | | 5.51 | 0.01 |
|
≥45 | 24 | 22 (91.66) | | | 20 (83.33) | | |
|
<45 | 48 | 19 (39.58) | | | 15 (31.25) | | |
| Sex | | | 0.137 | 0.711 | | 0.16 | 0.68 |
|
Male | 22 | 16 (72.72) | | | 18 (81.81) | | |
|
Female | 50 | 42 (84.0) | | | 35 (70.0) | | |
| Tumor size (cm) | | | 0.12 | 0.72 | | 0.13 | 0.71 |
|
≥1,
<2 | 19 | 14 (73.68) | | | 15 (78.94) | | |
|
<1 | 53 | 45 (84.9) | | | 36 (67.92) | | |
| Local
infiltration | | | 4.13 | 0.04 | | 4.85 | 0.02 |
|
Yes | 32 | 31 (96.87) | | | 30 (93.75) | | |
|
No | 40 | 18 (45.0) | | | 16 (40.0) | | |
| Lymph node
metastasis | | | | | | | |
|
Yes | 25 | 23 (92.0) | 3.89 | 0.04 | 22 (88.0) | 6.79 | 0.00 |
|
No | 47 | 20 (42.55) | | | 14 (29.78) | | |
| Multiplicity of
lesions | | | | | | | |
|
Multiple | 31 | 10 (32.25) | 6.32 | 0.01 | 8 (25.8) | 7.57 | 0.00 |
|
Single | 41 | 38 (92.68) | | | 36 (87.80) | | |
| Degree of
differentiation | | | | | | | |
|
Well | 57 | 47 (82.45) | 8.31 | 0.36 | 40 (70.17) | 1.16 | 0.28 |
|
Poor | 15 | 8 (53.33) | | | 6 (40.0) | | |
Correlation between CEUS parameters
and expression of P53 and Ki67 in PTC
For the patients with PTC, the initial increasing
time, peak time and MTT were significantly negatively associated
with the expression levels of P53 and Ki67 in the PTC tissues
(P<0.05). In addition, the peak intensity was positively
associated with the expression of P53 and Ki67 (P<0.05).
Furthermore, the regression time was negatively associated with the
expression of P53 and Ki67 in the PTC tissues, but with no
statistical significance (P>0.05; Table III).
 | Table IIICorrelation between contrast-enhanced
ultrasound parameters and expression of P53 and Ki67 in papillary
thyroid carcinoma. |
Table III
Correlation between contrast-enhanced
ultrasound parameters and expression of P53 and Ki67 in papillary
thyroid carcinoma.
| | P53 | Ki67 |
|---|
| Parameter | r | P-value | r | P-value |
|---|
| Initial increasing
time | -0.28 | 0.01 | -0.52 | <0.01 |
| Peak time | -0.32 | <0.01 | -0.26 | 0.02 |
| Peak intensity | 0.26 | 0.02 | 0.39 | 0.01 |
| Mean transit
time | -0.40 | <0.01 | -0.25 | 0.02 |
| Regression
time | -1.64 | 0.16 | -0.01 | 0.87 |
Analysis of risk factors associated
with recurrence after RFA
Multivariate logistic regression analysis was
performed to determine the association of CEUS parameters and
clinicopathological features (used as independent variables) with
recurrence at 18 months after RFA (used as the dependent variable).
The results revealed that the presence of lymph node metastasis
[odds ratio (OR): 0.010, 95% CI:<0.001-0.063], age (OR: 0.853,
95% CI: 0.757-0.960), local infiltration (OR: 0.080, 95% CI:
0.007-0.895), peak intensity (OR: 0.828, 95% CI: 0.701-0.979) and
MTT (OR: 0.755, 95% CI: 0.649-0.879) were significant factors for
predicting recurrence (P<0.05), with negative partial regression
coefficients indicating a negative association based on the risk
factor assessment form (Table IV).
These results suggest that lymph node metastasis, age, local
infiltration, peak intensity and MTT are major risk factors for
recurrence after RFA.
 | Table IVAnalysis of risk factors for
recurrence after radiofrequency ablation. |
Table IV
Analysis of risk factors for
recurrence after radiofrequency ablation.
| | | | | | | Exp (β) 95% CI |
|---|
| Parameter | Regression
coefficient β | SE | Wald
χ2 | Sig. | Odds ratio Exp
(β) | Lower limit | Upper limit |
|---|
| Mean transit
time | -0.281 | 0.077 | 13.239 | <0.001 | 0.755 | 0.649 | 0.879 |
| Peak intensity | -0.189 | 0.085 | 4.895 | 0.027 | 0.828 | 0.701 | 0.979 |
| Lymph node
metastasis | -6.722 | 2.016 | 11.116 | 0.001 | 0.001 | <0.001 | 0.063 |
| Age | -0.159 | 0.061 | 6.908 | 0.009 | 0.853 | 0.757 | 0.960 |
| Local
infiltration | -2.525 | 1.232 | 4.201 | 0.040 | 0.080 | 0.007 | 0.895 |
| Constant | 33.410 | 9.140 | 13.362 | <0.001 |
3.236x1014 | | |
Discussion
Thyroid cancer is one of the most common malignant
tumor types of the endocrine system, accounting for 1-5% of such
cases (12). The total incidence has
kept increasing rapidly worldwide (13,14). At
present, the diagnosis of most thyroid tumor cases depends on the
histopathological results. The tumor staging was performed based on
the results of the pathological puncture biopsy, according to the
staging criteria from the AJCC (7th edition, 2017). However, the
diagnosis of PTC is relatively difficult based on the imaging
technique, in terms of distinguishing between benign and malignant
papillary nodular hyperplasia. CEUS, also known as ultrasound
microcirculation angiography, is a non-invasive imaging technique,
which may be used to dynamically observe the microvascular
perfusion of tumors in real-time and sensitively reflect the status
of blood vessels and microvessels in the tumor tissues,
contributing to the identification of malignant tumors and
diagnosis of the disease. Ultrasound imaging has been widely
applied in the detection of abdominal organs, including the liver
and kidney (15,16), and has also been applied in the
detection of small organs, including the thyroid and breast.
Considering the detection and prognosis prediction of PTC, there
are still no effective specific molecular markers (17). Due to the enhanced tumor cell growth
activity, the tumor grows rapidly. Therefore, P53 and Ki67 are
considered to be reliable indicators for the detection of the
proliferation activity of tumor cells (18). Previous studies have reported that
P53 and Ki67 are associated with the development, metastasis and
prognosis of various malignant tumor types (19,20).
In the RFA treatment, high-heat energy is
transferred to destroy the tumor tissues, while inducing relatively
less damage to normal tissues surrounding the lesion. When applied
to patients with PTC, RFA treatment may preserve thyroid function.
Compared with traditional surgery, RFA treatment induces less
inhibitory effects on the immune response, which is beneficial for
disease recovery (21,22). Therefore, in the clinic, it is of
great significance to develop a more sensitive, efficient and
convenient diagnostic method for PTC, which, together with the
clinical features based on cytology and pathology, provides
effective markers for the disease.
It has been reported that CEUS of thyroid carcinoma
is always characterized by a fast-in and fast-out pattern, mainly
due to the increased and/or dysfunctional internal vessels, uneven
distribution and uneven vessel diameters in the thyroid carcinoma,
which may lead to the formation of a large number of arteriovenous
fistula. However, the present results suggested that the initial
increasing time in the CEUS for PTC was later, while the
enhancement level and PEAK were lower than those of the surrounding
normal tissues. However, there were no significant differences in
the regression time between the PTC and normal surrounding tissues.
The CEUS of PTC exhibited the slow-in and low-enhancement pattern
and the initial increasing time, peak time and MTT in the PTC
lesions was significantly negatively correlated with the expression
levels of P53 and Ki67 in the tumor tissues. Furthermore, the
regression time in PTC was also negatively associated with the
expression levels of P53 and Ki67 in the tumor, but with no
statistical significance. This phenomenon was probably due to the
fact that there were plenty of newly generated blood vessels in the
tumor tissue (23-25),
and the malignant growth destroyed various tissue structures
(including the blood vessels), resulting in different degrees of
cirrhosis, necrosis and liquefaction. At the same time, the blood
vessels are chaotic and irregular, and the peripheral blood vessels
are finer, leading to increased blood flow resistance and,
subsequently, inefficient arrival of the contrast agents.
Furthermore, at the early stage, the tumor vascular bed and
arteriovenous fistula are not formed and the blood supply is not
sufficient, which may be associated with the delayed contrast
perfusion. The delayed contrast perfusion in the lesion tissue,
together with the sufficient blood supply in the normal surrounding
thyroid tissue, contributes to the obvious contrast in the
perfusion patterns.
P53 and Ki67 participate in the regulation of the
cell cycle and apoptosis through various pathways, which are also
involved in the proliferation, infiltration, and metastasis of
tumors. They are important indicators reflecting the activity of
tissue cells. Studies have indicated that the positive expression
rates of P53 and Ki67 in the PTC are 88.4 and 66.7%, respectively
(26-28).
Furthermore, it has been suggested that the expression levels of
P53 and Ki67 are able to reflect the proliferative activity of
thyroid tumor cells, which may contribute to the determination of
the biological behavior of thyroid carcinoma. In the present study,
the results suggested that P53 and Ki67 were positively expressed
in PTC tissues, which were significantly associated with age
(<45 years; which has been updated to 45-55 years in the 8th
edition of the Clinical Staging Criteria of TNM from the AJCC),
local infiltration, lymph node metastasis and number of lesions,
rather than the gender, tumor size, histological type or degree of
differentiation. These results provide evidence for the
pathogenesis, development and prognosis of PTC in the clinic.
Analysis of recurrence after RFA indicated that the presence of
lymph node metastasis, age <45 years, local infiltration, peak
intensity and MTT were major factors influencing post-treatment
recurrence, which was in line with the results from a previous
study (28). Early recurrence is
closely associated with the biological behavior of tumors,
including tumor cell proliferation, as well as structural changes
within the tumors. Along with the tumor growth, the vessels within
the tumor lesions are destroyed and re-constructed, with an
increased proportion of nourishing arteries within the tumor, which
is linked to higher recurrence at a later stage (25).
In the present study, 72 patients were followed up
for 18 months. During the follow-up period, no recurrence was
reported after RFA. The relatively short follow-up period and
limited sample size represent limitations for the prediction of
disease recurrence. Furthermore, no stratification analysis was
performed based on the disease staging, which may have affected the
results. Previous studies concerning the ultrasound examination of
PTC are mainly clinical studies or basic research articles. As
another limitation, the TNM stage may have had an impact on the
disease prognosis. As the conventional RFA ablation range was 2-4
cm, only T1 cases were included in the present study and the
inclusion of N\M was moderately adjusted. The prognosis for cases
of other TNM stages after RFA treatment will be investigated in
further in-depth studies in the future.
P53/Ki67 are common tumor markers, which have,
however, not been suggested as indicators for the clinical
diagnosis according to the clinical guidelines for thyroid cancer,
and have not been implemented in hospitals. The present study was
also in line with previous basic studies on the clinical
application of P53/Ki67 in diagnosing this type of tumor. At the
same time, due to the numerous types of thyroid cancer, further
in-depth studies should be performed focusing on the analysis of
P53/Ki67 contents in different thyroid cancer types in the future.
In addition, in fact, exact tumor markers for PTC still remain to
be determined. Most of the markers are still at the clinical
research stage. It may be possible that better markers will be
available in the future. These issues should be addressed in
further in-depth studies.
In conclusion, the present results indicated that
CEUS provided additional information for the diagnosis of PTC. CEUS
was characterized by delayed enhancement, low perfusion, weak
development or uneven perfusion performance. The slow-in, low
enhancement pattern (rather than the fast-in and fast-out, high
enhancement pattern) may provide additional information and
quantitative analysis for the diagnosis of malignant thyroid tumors
and distinguishing them from benign lesions. The present results
confirmed that P53 and Ki67 were highly expressed in PTC tissues,
which may contribute to the current knowledge on the pathogenesis
and development of PTC and may be utilized for the prognostication
of patients. Furthermore, the ultrasound-guided RFA was able to
effectively control PTC lesions and the quantitative parameters
from the ultrasound and clinicopathological features proved to be
effective predictive factors for disease recurrence after RFA. The
present results provide further knowledge on gene mutations
associated with PTC based on immunohistochemistry and in the
future, genetic analysis may provide evidence to benefit the
clinical treatment. Based on the performance of the ultrasound
imaging, the estimation of biological characteristics and
associated risk factors may provide an accurate evaluation and
powerful evidence for the clinical diagnosis and treatment of
PTC.
Supplementary Material
Table SI. Basic clinical statistics of
patients.
Acknowledgements
The authors are grateful to Prof. Wenjun Zhang (the
Chief Physician; Department of Ultrasound, Taihe Hospital, Hubei
University of Medicine, Shiyan, China) for the kind assistance with
the study design, data collection and analysis and manuscript
preparation.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DW, XZ and ML contributed to the study design,
experiment performance, data collection and analysis, and
manuscript preparation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Taihe Hospital, Hubei University of Medicine, and all
patients had provided written informed consent.
Patient consent for publication
All patients had provided written the informed
consent and consent for publication.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
He C, Zhang J and Xing Y: Research
progress in diagnosis and treatment of papillary thyroid carcinoma.
Modern Diagnosis Treat. 17:290–292. 2006.(In Chinese).
|
|
2
|
Fu D, Zhu Y, Shao W, et al: Clinical
analysis of 124 cases of papillary microcarcinoma. Chin J Curr Adv
Gen Surg. 16:157–158. 2013.(In Chinese).
|
|
3
|
Sun P and Liang D: Effects of
ultrasound-guided radiofrequency ablation and postoperative stress
on thyroid micropapillary carcinoma. Chin J Curr Adv Gen Surg.
21:217–222. 2018.
|
|
4
|
Zhou Q and Jiang Z: Value of
Contrast-enhanced ultrasound in diagnosis of thyroid papillary
carcinoma. Chin J Ultrasound Med. 27:595–597. 2011.
|
|
5
|
Wang F, Chen M, Huang Y, Gao Y, Qiu Y,
Wang B and Chang C: Ultrasonograpbic characteristics of thyroid
carcinoma and analysis of the misdiagnosed cases. Chin J
Ultrasonography. 19:134–137. 2010.(In Chinese).
|
|
6
|
Gao X and Zhang Y, Sun Y and Zhang Y:
Clinical efficacy and adverse reaction analysis of laparoscopic
surgery for colon; cancer. Chin J Curr Adv Gen Surg. 18:738–740.
2015.(In Chinese).
|
|
7
|
Liu F, Xia Q, Qiu J, et al: Expression and
clinical significance of C-myb and P53 in papillary thyroid
carcinoma. J Oncol. 21:320–324. 2015.
|
|
8
|
Dong L, Peng T, Liu N, Shuang L and Xudong
S: Expression and clinical significance of ER, PR and Her2/neu in
papillary thyroid carcinoma. J Modern Oncol. 23:3576–3579.
2015.
|
|
9
|
Jiang WF, Wang YX and Cheng M: Expression
and relationship of XIAP, P53 and Ki67 in papillary thyroid
carcinoma. Beijing Med J. 34:971–974. 2012.(In Chinese).
|
|
10
|
Tang X, Cui D, Chi J, Wang Z, Wang T, Zhai
B and Li P: Evaluation of the safety and efficacy of radiofrequency
ablation for treating benign thyroid nodules. J Cancer. 8:754–760.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang L, Dong Y, Hu S, et al: The impact
of American Cancer Joint Committee Revised Thyroid Cancer Staging
System (8th edition) on staging of papillary thyroid carcinoma.
Chin Oncol. 7:491–496. 2018.(In Chinese). PubMed/NCBI View Article : Google Scholar
|
|
12
|
Song J, Yang Z and Wang HM: Domestic
progress in the diagnosis of differentiated thyroid carcinoma.
World Latest Med Infor. 18:64–65. 2016.(In Chinese).
|
|
13
|
Xhaard C, Rubino C, Cléro E, Maillard S,
Ren Y, Borson-Chazot F, Sassolas G, Schvartz C, Colonna M, Lacour
B, et al: Menstrual and reproductive factors in the risk of
differentiated thyroid carcinoma in young women in France: A
population-based case-control study. Am J Epidemiol. 180:1007–1017.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tafani M, De Santis E, Coppola L, Perrone
GA, Carnevale I, Russo A, Pucci B, Carpi A, Bizzarri M and Russo
MA: Biodging hypoxin, inflammation and estrogen receptors in the
thyroid cancer progression. Biomed Pharmacother. 68:1–5.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen X and Zhao B: Contrast-enhanced
ultrasound in diagnosis and treatment of liver diseases. Chin J Med
Imaging Technol. 21:484–487. 2005.
|
|
16
|
Du L and Li F: Application of
contrast-enhanced ultrasound in the diagnosis of renal occupied
lesions. Chin J Ultrasonography. 15:813–815. 2006.
|
|
17
|
Wang L and Yang W: Expression and clinical
significance of p53, C-myc and CerbB-2 in papillary thyroid
carcinoma. Chongqing Med. 45:77–80. 2016.
|
|
18
|
Wang YQ and Qiu XG: Expressions of p53,
RUNX3 and Ki67 proteins in papillary thyroid carcinoma and clinical
significance. Clin Med. 33:3–4. 2013.
|
|
19
|
de Azambuja E and Cardoso F: Ki-67 as
prognostic marker in early breast cancer: A meta-analysis of
published studies involving 12, 155 patients. Br J Cancer.
96:1504–1513. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Viale G, Regan MM, Mastropasqua MG,
Maffini F, Maiorano E, Colleoni M, Price KN, Golouh R, Perin T,
Brown RW, et al: Predictive value of tumor Ki-67 expression in two
randomized trials of adjuvant chemoendocrine therapy for
Node-negative breast cancer. J Natl Cancer Inst. 100:207–212.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yeh CC, Lin JT, Jeng LB, Charalampos I,
Chen TT, Lee TY, Wu MS, Kuo KN, Liu YY and Wu CY: Hepatic resection
for hepatocellular carcinoma patients on hemodialysis for Uremia: A
nationwide cohort study. World J Surg. 37:2402–2409.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Argalia G, De Bernardis S, Mariani D,
Abbattista T, Taccaliti A, Ricciardelli L, Faragona S, Gusella PM
and Giuseppetti GM: Ultrasonographic contrast agent: Evaluation of
time-intensity curves in the characterisation of solitary thyroid
nodules. Radiol Med. 103:407–13. 2002.(In English, Italian).
PubMed/NCBI
|
|
23
|
Shi X, Zheng X, Guo H, Li C, Peng M, Zhou
G, Jiang Y, Yan S and Yu Y: Contrast analysis on contrast-enhanced
ultrasonography and pathology of thyroid lumps. Zhejiang Practical
Med. 12:87–88. 2007.(In Chinese).
|
|
24
|
Bartolotta TV, Midiri M, Galia M, Runza G,
Attard M, Savoia G, Lagalla R and Cardinale AE: Qualitative and
quantitative evaluation of solitary thyroid nodules with
contrast-enhanced ultrasound: Initial results. Eur Radiol.
16:2234–2241. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen Q: Diagnostic value of high frequency
ultrasound in benign and malignant thyroid nodules. Chin Imaging J
Integrated Traditional Western Med. 10:545–546. 2012.
|
|
26
|
Zhao L, Lin J, Shi B, et al: Expression of
P53, Ki67, galectin-3, HBME-1, 34βE12 and CK19 in papillary thyroid
carcinoma and its clinicopathological significance. Basic Clin Med.
32:1202–1206. 2012.
|
|
27
|
Huang H: Expression of CK19, Ki67 and VEGF
and their clinical significances in papillary thyroid carcinoma. J
Nanchang Univ (Med Sci). 50:24–27. 2010.
|
|
28
|
Jia W and Yin C: Expressions of p53, Her-2
and Ki67 in thyroid papillary carcinoma and their clinical
significance. China J Modern Med. 28:33–37. 2018.
|