|
1
|
Guess HA, Arrighi HM, Metter EJ and Fozard
JL: Cumulative prevalence of prostatism matches the autopsy
prevalence of benign prostatic hyperplasia. Prostate. 17:241–246.
1990.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lepor H, Williford W, Barry M, Haakenson C
and Jones K: The impact of medical therapy on bother due to
symptoms, quality of life and global outcome, and factors
predicting response. J Urol. 160:1358–1367. 1998.PubMed/NCBI
|
|
3
|
AUA Practice Guidelines Committee. AUA
guideline on management of benign prostatic hyperplasia (2003).
Chapter 1: Diagnosis and treatment recommendations. J Urol.
170:530–547. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Anderson JB, Roehrborn CG, Schalken JA and
Emberton M: The progression of benign prostatic hyperplasia:
Examining the evidence and determining the risk. Eur Urol.
39:390–399. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Roehrborn CG, McConnell JD, Lieber M,
Kaplan S, Geller J, Malek GH, Castellanos R, Coffield S, Saltzman
B, Resnick M, et al: Serum prostate-specific antigen concentration
is a powerful predictor of acute urinary retention and need for
surgery in men with clinical benign prostatic hyperplasia. PLESS
Study Group Urology. 53:473–480. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Deslypere JP, Young M, Wilson JD and
McPhaul MJ: Testosteronem and 5 alpha-dihydrotestosterone interact
differently with the androgen receptor to enhance transcription of
the MMTV-CAT reporter gene. Mol Cell Endocrinol. 88:15–22.
1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Welén K and Damber JE: Prostate
diseases-role of sex steroids and their inhibitors. Best Pract Res
Clin Endocrinol Metab. 25:355–367. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Griffiths K, Eaton CL, Harper ME, Peeling
B and Davies P: Steroid hormones and the pathogenesis of benign
prostatic hyperplasia. Eur Urol. 20 (Suppl 1):S68–S77.
1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Naslund M, Regan TS, Ong C and Hogue SL:
5-Alpha reductase inhibitors in men with an enlarged prostate: An
evaluation of outcomes and therapeutic alternatives. Am J Manag
Care. 14 (5 Suppl 2):S148–S153. 2008.PubMed/NCBI
|
|
10
|
McConnell JD, Bruskewitz R, Walsh P,
Andriole G, Lieber M, Holtgrewe HL, Albertsen P, Roehrborn CG,
Nickel JC, Wang DZ, et al: The effect of finasteride on the risk of
acute urinary retention and the need for surgical treatment among
men with benign prostatic hyperplasia Finasteride Long-Term
Efficacy and Safety Study Group. N Engl J Med. 338:557–563.
1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Roehrborn CG, Boyle P, Nickel JC, Hoefner
K and Andriole G: ARIA3001 ARIA3002 and ARIA3003 Study
Investigators. Efficacy and safety of a dual inhibitor of
5-alpha-reductase types 1 and 2 (dutasteride) in men with benign
prostatic hyperplasia. Urology. 60:434–441. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yin T, Qiao Z, Li Y, Li D, Jiang M, An C,
Wang F, Zuo M, Hu K and Li Q: Comparisons of the efficacy and
safety of finasteride and dutasteride for benign prostatic
hyperplasia: A network meta-analysis. Am J Ther. 24:e517–e523.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jun JEJ, Kinkade A, Tung ACH and Tejani
AM: 5α-reductase inhibitors for treatment of benign prostatic
hyperplasia: A systematic review and meta-analysis. Can J Hosp
Pharm. 70:113–119. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. Ann Intern Med.
151:264–269. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jadad AR: Randomised controlled trials: A
user's guide. BMJ Publishing Group, London, 1998.
|
|
16
|
Higgins JP and Green S (eds): Cochrane
handbook for systematic reviews of interventions. Version 5.1.0.
The Cochrane Collaboration, 2011. http://www.cochrane-handbook.orgsimplewww.cochrane-handbook.org.
Updated March 2011.
|
|
17
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.PubMed/NCBI View Article : Google Scholar
|
|
18
|
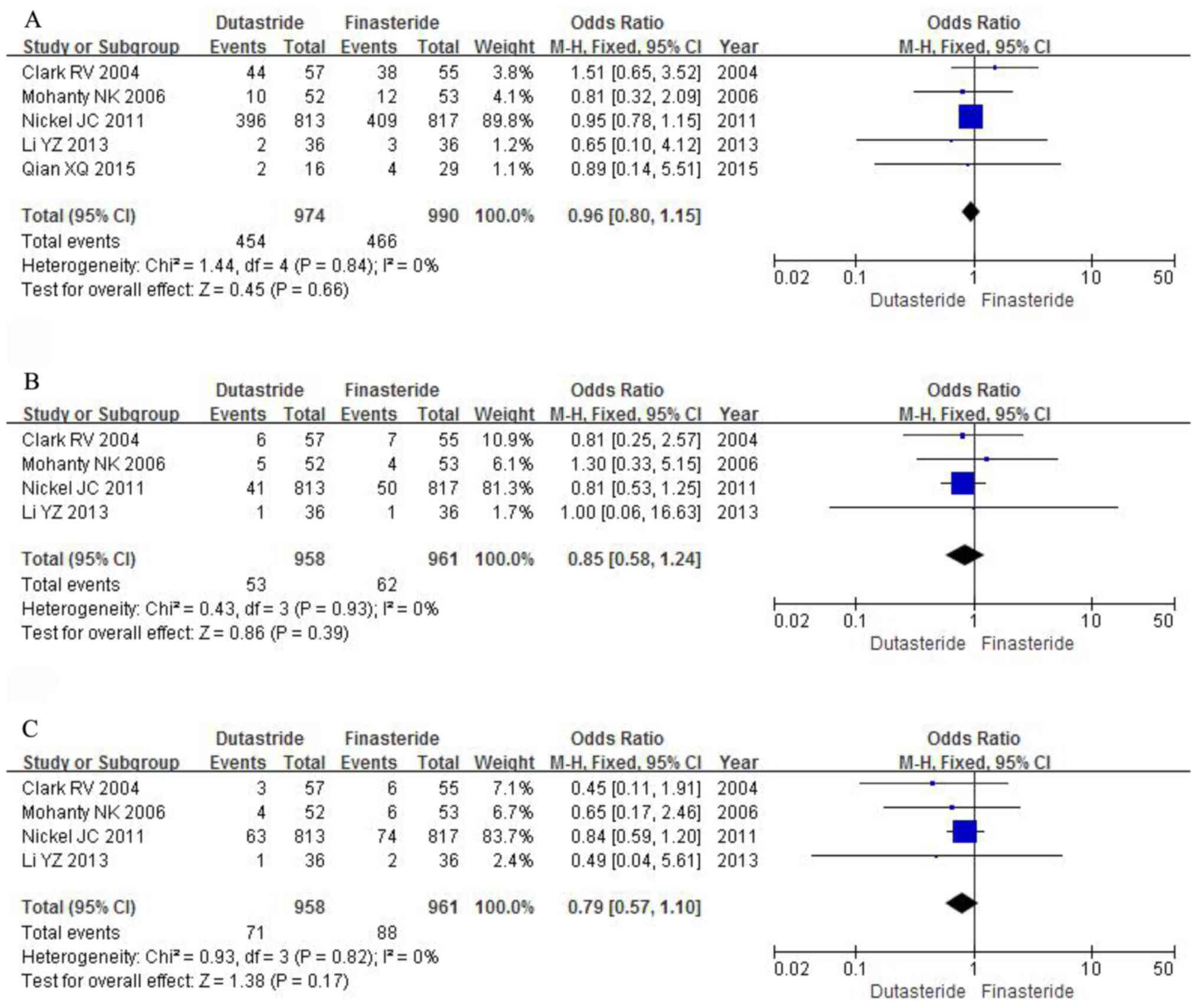
Clark RV, Hermann DJ, Cunningham GR,
Wilson TH, Morrill BB and Hobbs S: Marked suppression of
dihydrotestosterone in men with benign prostatic hyperplasia by
dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol
Metab. 89:2179–2184. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
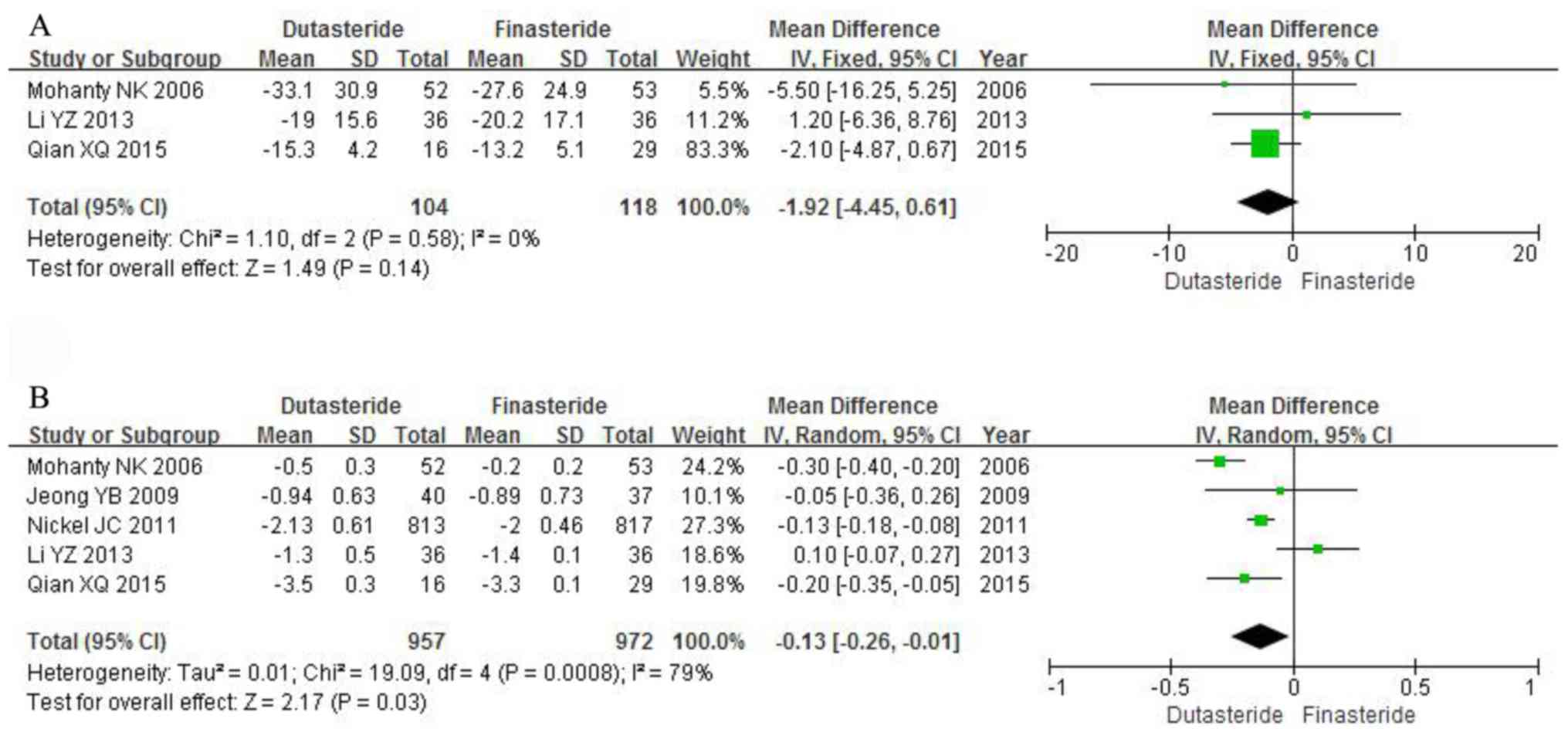
Mohanty NK, Singh UP, Sharma NK, Arora RP
and Amitabh V: A comparative study of fixed dose of tamsulosin with
finasteride vs tamsulosin with dutasteride in the management of
benign prostatic hyperplasia. Indian J Urol. 22:130–134. 2006.
|
|
20
|
Jeong YB, Kwon KS, Kim SD and Kim HJ:
Effect of discontinuation of 5alpha-reductase inhibitors on
prostate volume and symptoms in men with BPH: A prospective study.
Urology. 73:802–806. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nickel JC, Gilling P, Tammela TL, Morrill
B, Wilson TH and Rittmaster RS: Comparison of dutasteride and
finasteride for treating benign prostatic hyperplasia: The Enlarged
Prostate International Comparator Study (EPICS). BJU Int.
108:388–394. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Y and Wang J: Clinical efficacy and
safety analysis of dutasteride in treatment of benign prostatic
hyperplasia. Chinese J Androl. 27:49–55. 2013.
|
|
23
|
Qian X, Yu G, Qian Y, Xu D, Liu H, Kong X,
Zhu Y, Wang Z, Zheng J and Qi J: Efficacy of 5α-reductase
inhibitors for patients with large benign prostatic hyperplasia
(>80 ml) after transurethral resection of the prostate. Aging
Male. 18:238–243. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Girman CJ: Population-based studies of the
epidemiology of benign prostatic hyperplasia. Br J Urol. 82 (Suppl
1):S34–S43. 1998.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Emberton M, Andriole GL, de la Rosette J,
Djavan B, Hoefner K, Vela Navarrete R, Nordling J, Roehrborn C,
Schulman C, Teillac P, et al: Benign prostatic hyperplasia: A
progressive disease of aging men. Urology. 61:267–273.
2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jacobsen SJ, Jacobson DJ, Girman CJ,
Roberts RO, Rhodes T, Guess HA and Lieber MM: Natural history of
prostatism: Risk factors for acute urinary retention. J Urol.
158:481–487. 1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Corona G, Vignozzi L, Rastrelli G, Lotti
F, Cipriani S and Maggi M: Benign prostatic hyperplasia: A new
metabolic disease of the aging male and its correlation with sexual
dysfunctions. Int J Endocrinol. 2014(329456)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
O'Leary MP, Roehrborn CG and Black L:
Dutasteride significantly improves quality of life measures in
patients with enlarged prostate. Prostate Cancer Prostatic Dis.
11:129–133. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Corona G, Tirabassi G, Santi D, Maseroli
E, Gacci M, Dicuio M, Sforza A, Mannucci E and Maggi M: Sexual
dysfunction in subjects treated with inhibitors of 5α-reductase for
benign prostatic hyperplasia: A comprehensive review and
meta-analysis. Andrology. 5:671–678. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shibata Y, Arai S, Miyazawa Y, Shuto T,
Nomura M, Sekine Y, Koike H, Matsui H, Ito K and Suzuki K: Effects
of steroidal antiandrogen or 5-alpha-reductase inhibitor on
prostate tissue hormone content. Prostate. 77:672–680.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Evans HC and Goa KL: Dutasteride. Drugs
Aging. 20:905–918. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guess HA, Gromley GJ, Stoner E and
Oesterling JE: The effect of finasteride on prostate specific
antigen: Review of available data. J Urol. 155:3–9. 1996.PubMed/NCBI
|
|
33
|
Schröder FH: Review of diagnostic markers
for prostate cancer. Recent Results Cancer Res. 181:173–182.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Morgia G, Urzì D and Russo GI: 5ARI and
PSA: Evidences. Urologia. 81 (Suppl 24):S4–S11. 2014.PubMed/NCBI View Article : Google Scholar : (In Italian).
|
|
35
|
Andriole GL, Bostwick D, Brawley OW,
Gomella L, Marberger M, Montorsi F, Pettaway C, Tammela TL, Teloken
C, Tindall D, et al: The effect of dutasteride on the usefulness of
prostate specific antigen for the diagnosis of high grade and
clinically relevant prostate cancer in men with a previous negative
biopsy: Results from the REDUCE study. J Urol. 185:126–131.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Traish A, Haider KS, Doros G and Haider A:
Long-term dutasteride therapy in men with benign prostatic
hyperplasia alters glucose and lipid profiles and increases
severity of erectile dysfunction. Horm Mol Biol Clin Investig 30,
2017.
|