Introduction
Benign prostatic hyperplasia (BPH) is a primary
etiology of dysplasia of the prostate that occurs mainly in the
elderly and is characterized by nonmalignant hypertrophy of the
prostate gland due to unrestricted proliferation of epithelial and
smooth muscle cells positioned in the transition region of the
prostate gland encircling the urethra (1,2).
Patients with BPH may suffer from frequent micturition, interrupted
urine flow, sense of incomplete bladder emptying and a high risk of
acute urinary retention, which impact the quality of life (3,4).
Changes of androgen levels are considered to be a
crucial factor for the development of prostate growth with age
(5). Dihydrotestosterone (DHT), an
important constituent among the androgens, may be synthesized from
testosterone by 5α-reductase (5AR) in the prostate gland (6). With the reduction in testosterone
levels, the excessive expression of DHT triggers the proliferation
of prostate epithelial and mesenchymal cells, leading to the
development of BPH (7,8). To inhibit this process, 5AR inhibitors
(5ARI) are administered to lower the serum concentration of DHT,
controlling the normal growth of the prostate itself and the
progression of BPH (9). Two 5ARIs
are available: finasteride and dutasteride. Finasteride is a
selective inhibitor of type 2 5ARI, whereas dutasteride inhibits
type 1 and 2 5ARI (10,11). Dutasteride, due to the additional
target, induces a theoretically greater reduction in DHT and
improvement in clinical presentation compared with finasteride.
However, two previous meta-analyses comparing the efficacy of the
two drugs limited their search due to a lack of standard analysis
of clinical data on various aspects and provided ambiguous results
on whether finasteride and dutasteride exhibited any clinically
significant differences (12,13).
The present study was an updated meta-analysis
aiming to compare the efficacy and safety of 0.5 mg dutasteride and
5 mg finasteride (the doses widely used in the clinic) in treating
BPH during a treatment period of at least 6 months.
Materials and methods
Protocol
The present systematic review of randomized
controlled trials (RCTs) was performed using the Preferred
Reporting Items for Systematic Reviews and Meta-analyses checklist
(14).
Information sources and literature
search
The MEDLINE (January 1992 to December 2018), EMBASE
(January 1995 to December 2018) and the Cochrane controlled trials
register databases were searched to compare the effects of
dutasteride and finasteride in BPH treatment. The following search
terms were used: [‘finasteride’ (MeSH terms) OR ‘finasteride’ (all
fields)] AND [‘dutasteride’ (MeSH terms) OR ‘dutasteride’ (all
fields)] AND [‘prostatic hyperplasia’ (MeSH terms) OR [‘prostatic’
(all fields) AND ‘hyperplasia’ (all fields)] OR ‘prostatic
hyperplasia’ (all fields) OR [‘benign’ (all fields) AND ‘prostatic’
(all fields) AND ‘hyperplasia’ (all fields)] OR ‘benign prostatic
hyperplasia’ (all fields). All publications were browsed
independently by all authors. The study was limited to published
research with no restrictions on language. Reviews and summaries
presented at meetings were excluded. Authors were contacted to
obtain further information when necessary. The references of
relevant publications were also searched.
Inclusion criteria and trial
selection
The inclusion criteria for the publications were as
follows: i) Dutasteride vs. finasteride in treating BPH were
evaluated; ii) the content and associated data of the publication
were available; iii) the data provided by the publication were
valid and valuable, including the overall number of events and
valuable results for each indicator; iv) the design of the study
was that of an RCT; v) the treatment duration was ≥6 months. If the
results of a trial were published by two or more studies, the
latest publication was selected. However, if a group of patients
was included in two or more studies, each of the studies may have
been analyzed in the present study. It was also checked whether the
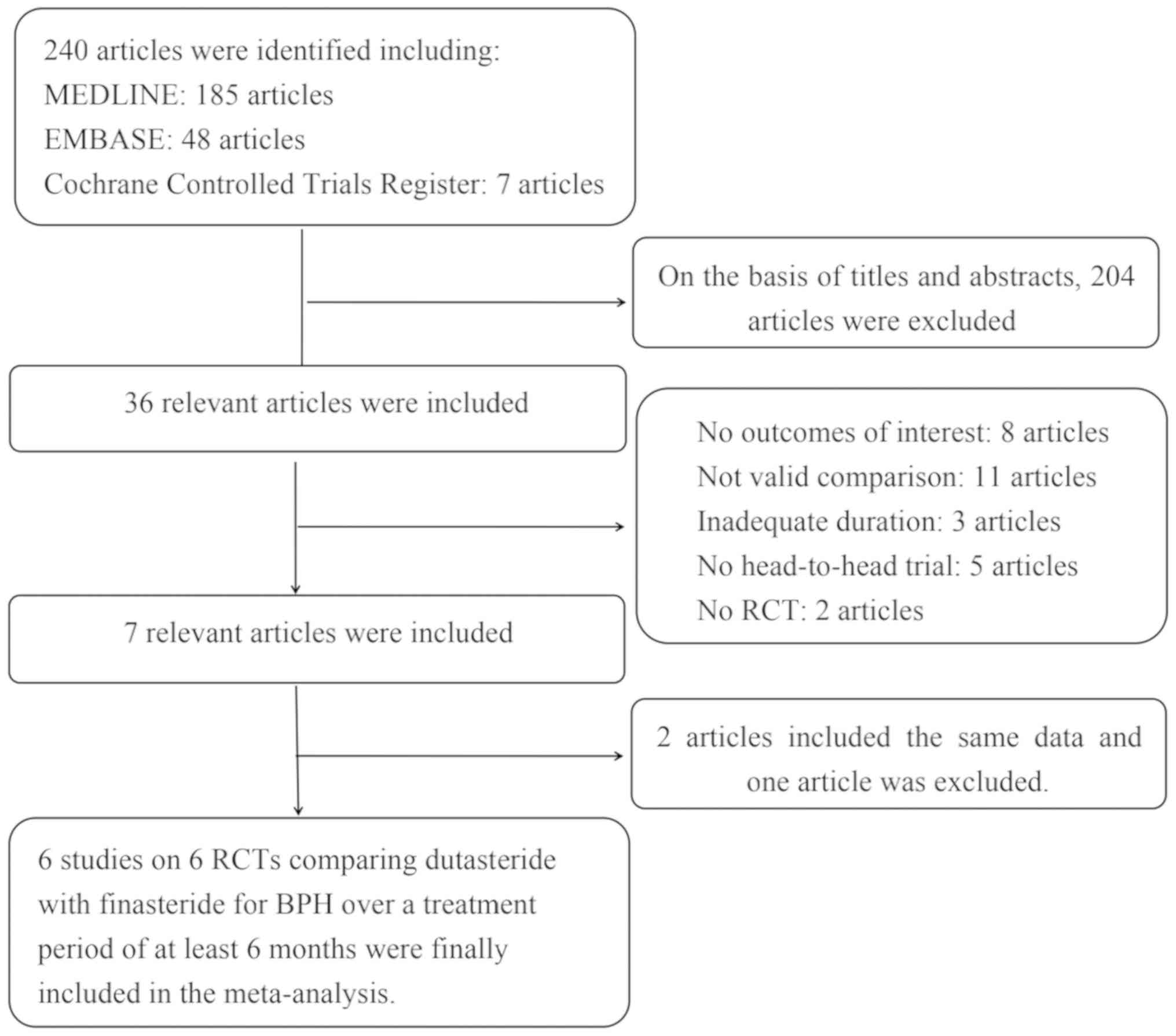
cohorts overlapped and no overlaps were found. The flow diagram of
the study selection and elimination is presented in Fig. 1.
Quality assessment methods
The quality of the studies selected was evaluated
using the Jadad scale (15). In
addition, a number of strategies of assessment were applied to
determine the quality of individual studies, including the
distribution method of participants, blinding regarding the
distribution process, double-blinding and the number of patients
lost at follow-up. Subsequently, individual studies were assessed
following the principles derived from the Cochrane Handbook for
Systematic Reviews of Interventions v5.10(16). Each publication was evaluated and
three quality classification standards were assigned: a) When a
study satisfied the majority of the quality criteria, it was
considered to have a low probability of bias; b) when the quality
criteria were partially satisfied or unclear, the study was
considered to have a moderate probability of bias; and c) when the
criteria were barely satisfied, the study was considered to have a
high probability of bias. All authors participated in the quality
assessment of the RCTs retrieved and eventually agreed with the
results of the assessment. All reviewers independently assessed
whether each study satisfied the criteria and extracted the data
from the selected studies. Any discrepancies were recorded,
discussed and settled by negotiation.
Data extraction
Two authors independently collected data from the
publications based on predetermined criteria. The following usable
data were extracted from the studies included: i) Publication year;
ii) the first author's name; iii) details on patient treatment; iv)
number of participants; and v) international prostate symptom score
(IPSS), prostate volume (PV), maximum urine flow rate (Qmax),
post-void residual volume (PVRV), prostate-specific antigen (PSA),
adverse events (AEs), decreased libido and impotence. These results
were considered clinically significant as their impact on patients
was measurable. No ethical approval was required for this
study.
The primary outcomes were IPSS and PSA. High IPSS
indicated more severe symptoms. Data on secondary outcomes,
including PV, Qmax and PVRV were reported with acceptable
consistency among the studies to allow for analysis. In addition,
the number of any AEs, decreased libido and impotence were also
analyzed between the two groups of patients receiving different
treatments.
Statistical and meta-analysis
The analysis of the study was performed using RevMan
version 5.3.0 (Cochrane Collaboration) (16). Fixed or random-effects models were
used to evaluate the publications. The mean difference (MD) was
used to analyze continuous data and the odds ratio (OR) was
calculated for dichotomous results with the corresponding 95% CI
(17). The results of analysis
showed that the P-value >0.05 for the I2 statistic,
the study was considered to be homogeneous and the fixed-effects
model was used for the analysis. Inconsistency was analyzed by the
I2 statistic, which reflected the proportion of
heterogeneity across trials. A random-effects model was used for
studies with an I2 value >50%, suggesting significant
heterogeneity. P<0.05 was considered to indicate a statistically
significant difference.
Results
Study selection process, search
results and characteristics of the trials
A total of 240 publications were initially retrieved
from the databases. Scrutinizing their abstracts and titles
resulted in the exclusion of 204 publications. Among the remaining
36 studies, 29 were excluded due to a lack of effective data. In
addition, two publications described the same data and one of these
publications was excluded. Finally, six publications describing six
RCTs (18-23)
were included in the present study to compare the effects of
dutasteride and finasteride in treating BPH during a treatment
period of ≥6 months. The basic features of the six studies are
presented in Table I.
 | Table IStudy and patient characteristics. |
Table I
Study and patient characteristics.
| | | | Sample size | | | | |
|---|
| First author
(year) | Therapy in
experimental groupa | Therapy in control
groupa | Experimental | Control | Treatment
Administration method | Duration
(months) | Main inclusion
population | (Refs.) |
|---|
| Clark (2004) | Dutasteride | Finasteride | 57 | 55 | Oral | 6 | Patients were aged
≥50 years with a prior diagnosis of BPH according to medical
history and physical examination and a baseline prostate volume of
≥30 cc. | (18) |
| Mohanty (2006) | Dutasteride | Finasteride | 52 | 53 | Oral | 6 | Patients with
symptomatic BPH but no absolute indication for surgery; age, 40-80
years. | (19) |
| Jeong (2009) | Dutasteride | Finasteride | 40 | 37 | Oral | 12 | Males aged ≥50
years with moderate to severe BPH symptoms as determined by the
IPSS without previous 5ARI treatment or surgical or experimental
interventions for BPH. | (20) |
| Nickel (2011) | Dutasteride | Finasteride | 813 | 817 | Oral | 12 | Males aged ≥50
years with a clinical diagnosis of BPH according to medical history
and physical examination. | (21) |
| Li (2013) | Dutasteride | Finasteride | 36 | 36 | Oral | 6 | Patients with BPH,
IPSS >13, Qmax <15 ml/sec, PSA <4 µg/l, urine volume
<150 ml per urination. | (22) |
| Qian (2015) | Dutasteride | Finasteride | 16 | 29 | Oral | 6 | Patients ≥60 years
of age with BPH, PV >80 ml, IPSS ≥13, QoL ≥3, PVRV ≥200 ml, Qmax
<15 ml/sec. | (23) |
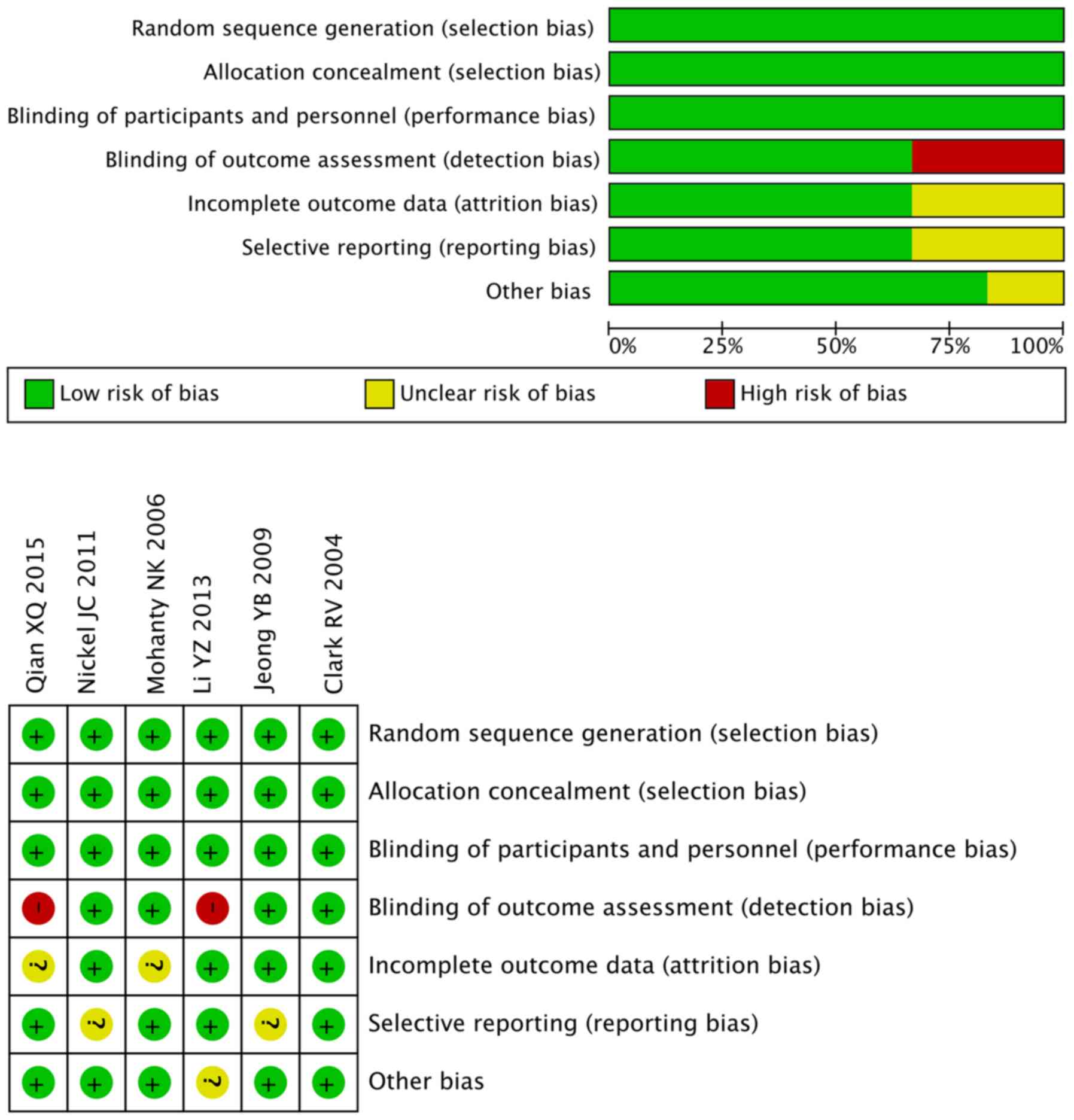
Risk of bias in the studies
All studies included in the meta-analysis were RCTs;
however, not all of the studies specified the protocol of
randomization. All studies included an appropriate number of
participants and one study included an intention-to-treat analysis
(21) (Table II). In addition, two studies used a
combined medication regimen with α-blockers (19,20) and
one study described a post-operative medication regimen (23). However, in these studies, the
specific methods of blinding were not explicitly explained and
their grade were rated as ‘B’ based on the Cochrane handbook. The
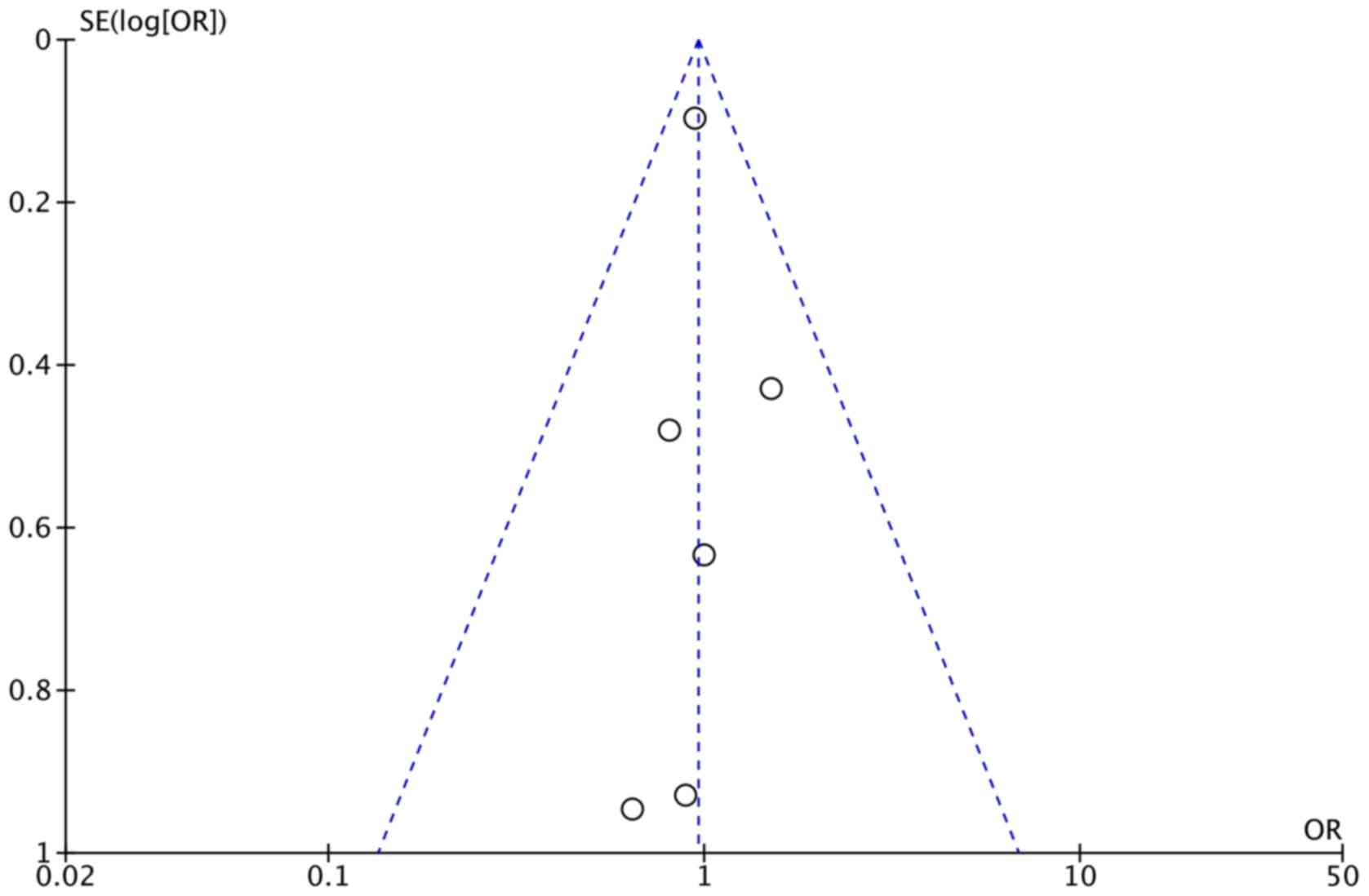
plot was highly symmetrical and six squares were contained in the
accepted region of the funnel plot, with no evidence of bias being
found (Fig. 2). The bias of quality
assessment are presented in Fig.
3.
 | Table IIQuality assessment of individual
studies. |
Table II
Quality assessment of individual
studies.
| First author
(year) | Allocation sequence
generation | Allocation
concealment | Blinding | Loss to
follow-up | Calculation of
sample size | Statistical
analysis | Level of
quality | ITT analysis | (Refs.) |
|---|
| Clark (2004) | A | A | A | 2 | Yes | Fisher's exact
test; Student's t-test | A | No | (18) |
| Mohanty (2006) | A | A | B | 5 | Yes | ANCOVA; Student's
t-test | A | No | (19) |
| Jeong (2009) | A | A | B | 0 | Yes | ANCOVA; Student's
t-test | A | No | (20) |
| Nickel (2011) | A | A | A | 72 | Yes | Log-transformed
linear model | A | Yes | (21) |
| Li (2013) | A | A | B | 0 | Yes | Student's t-test;
χ2 test | A | No | (22) |
| Qian (2015) | A | A | B | 8 | Yes | Student's t-test;
Fisher's exact test; χ2 test | A | No | (23) |
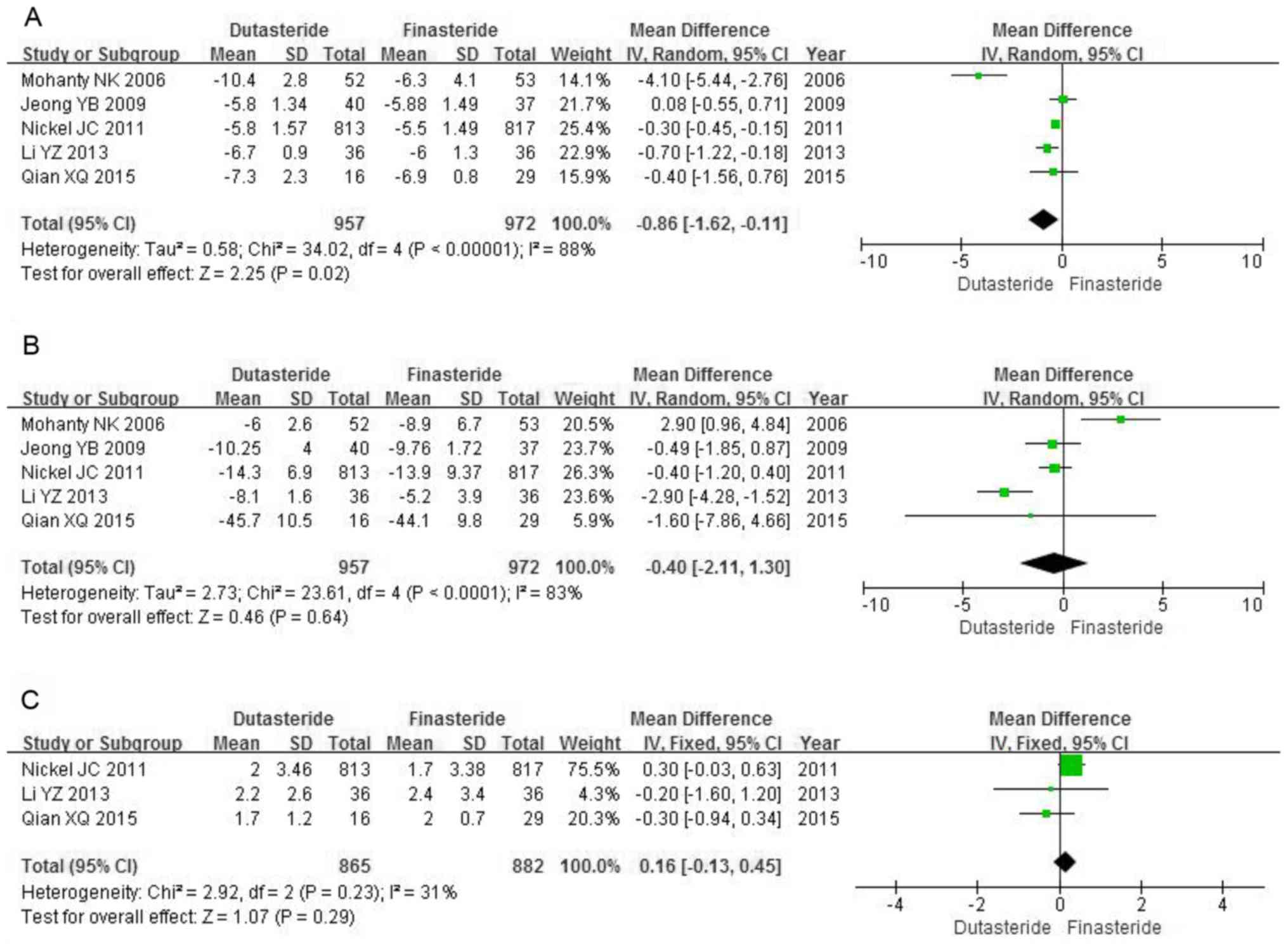
Efficacy IPSS
A total of five RCTs including 1,929 patients were
used for the IPSS analysis. High heterogeneity among the trials was
identified (P<0.00001; I2=88%). The forest plots
indicated a significantly greater decrease in IPSS in the
dutasteride group compared with that in the finasteride group (MD,
-0.86; 95% CI, -1.62 to -0.11; P=0.02; Fig. 4A).
PV
A total of five RCTs including 1,929 patients were
used for the analysis of the change of PV. High heterogeneity was
identified among the studies in the forest plots (P<0.0001;
I2=83%). Dutasteride was not significantly more
effective compared with finasteride in reducing the PV (MD, -0.40;
95% CI, -2.11 to 1.30; P=0.64; Fig.
4B).
Qmax
A total of three RCTs including 1,747 patients
contained data on Qmax. Low risk of heterogeneity was identified
among the studies (P=0.23; I2=31%). The fixed-effects
model demonstrated no significant differences between dutasteride
and finasteride in improving the Qmax (MD, 0.16; 95% CI, -0.13 to
0.45; P=0.29; Fig. 4C).
PVRV
A total of three RCTs including 222 patients were
used for the analysis of PVRV. The results of the heterogeneity
test were P=0.58 and I2=0%. The fixed-effects model did
not identify any statistically significant differences between
dutasteride and finasteride in reducing PVRV (MD, -1.92; 95% CI,
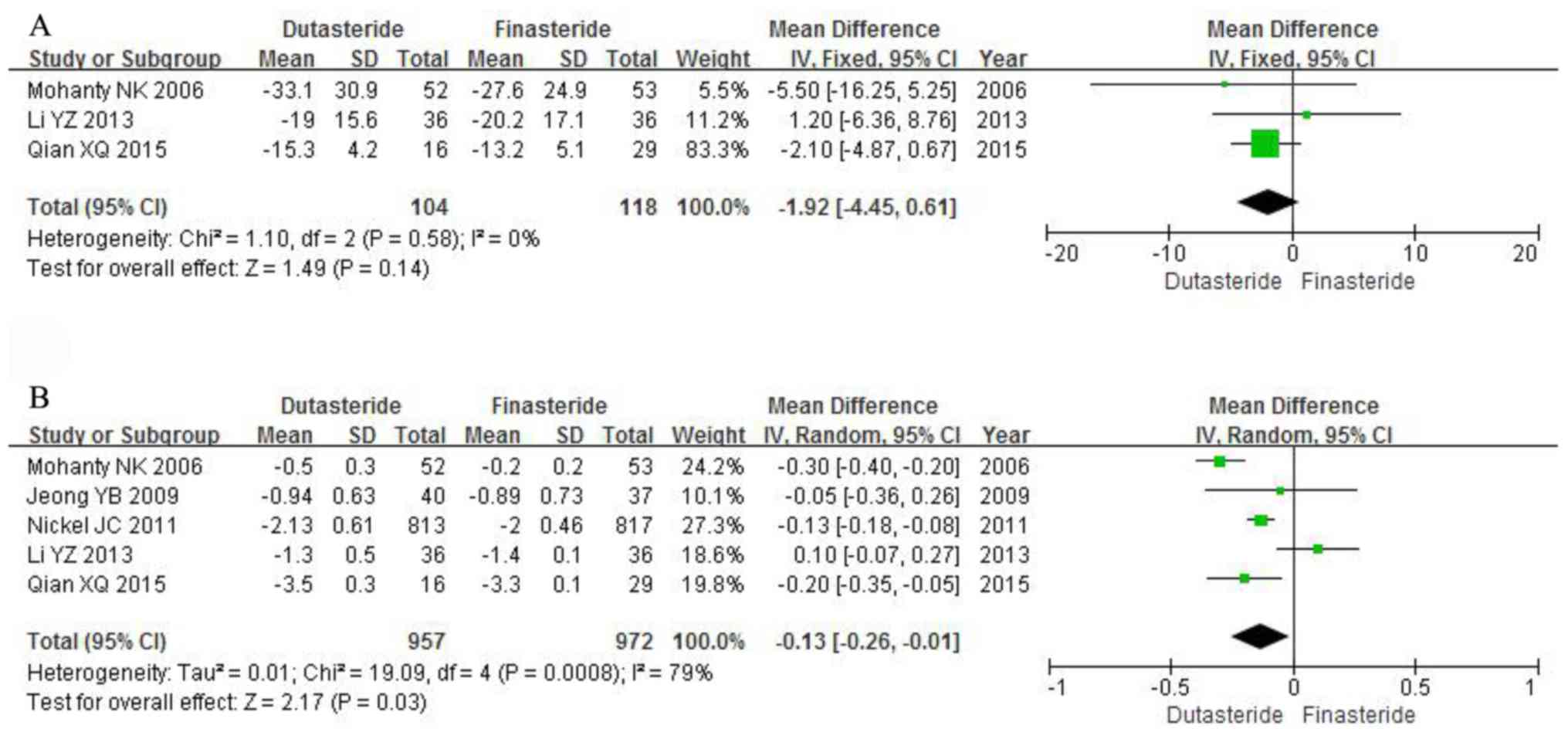
-4.45 to 0.61; P=0.14; Fig. 5A).
PSA
A total of five RCTs including 1,929 patients
contained data on PSA. Heterogeneity was identified among the
studies (P=0.0008; I2=79%). Dutasteride was
significantly more effective compared with finasteride in lowering
PSA (MD, -0.13; 95% CI, -0.26 to -0.01; P=0.03; Fig. 5B).
Safety Any AE
A total of five RCTs with a sample of 1,964
participants evaluated the severity of any AE. The results of the
heterogeneity test were P=0.84 and I2=0%. The
meta-analysis identified no significant differences between the
dutasteride and finasteride groups in the severity of any AE across
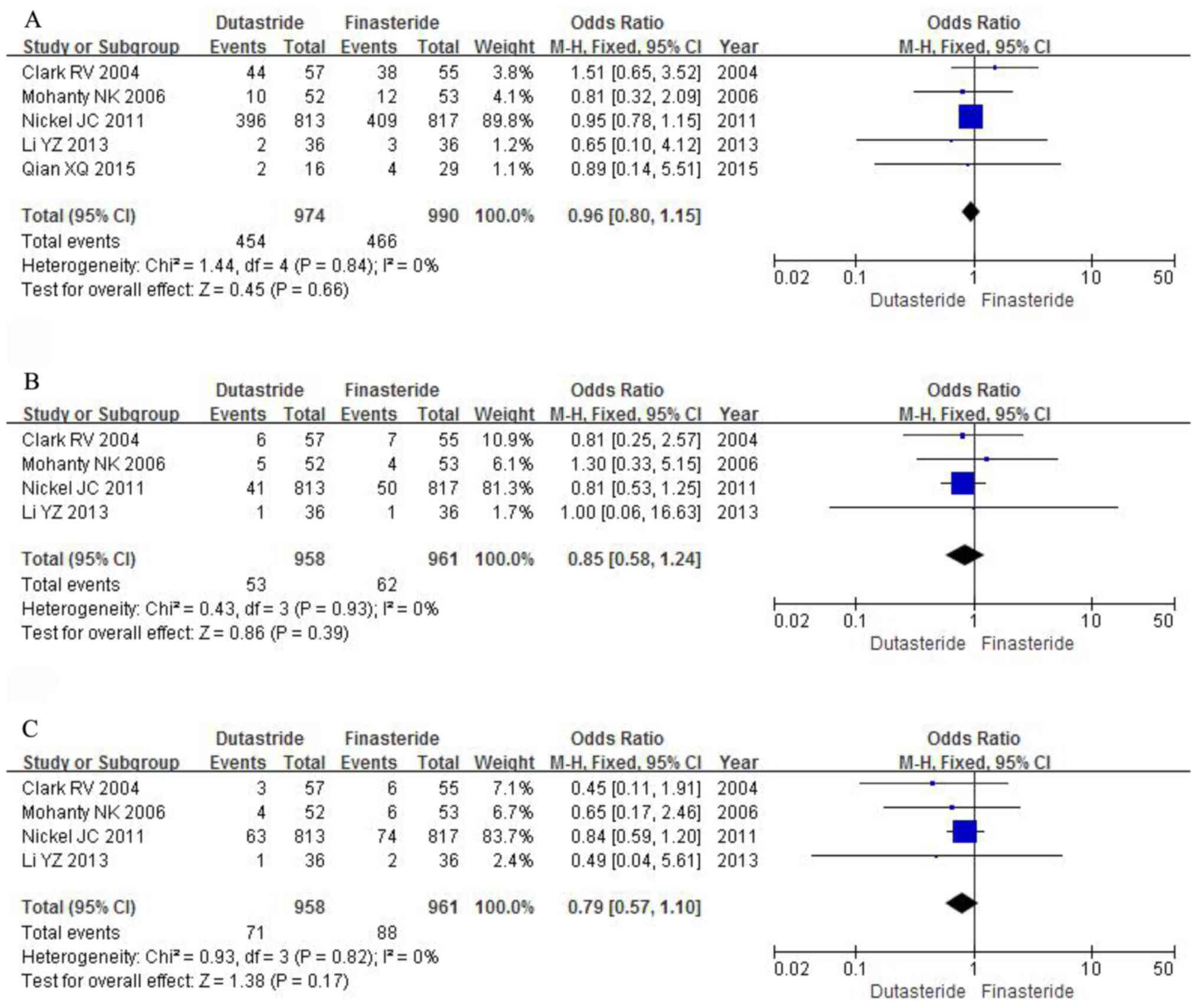
the five studies (OR, 0.96; 95% CI, 0.80 to 1.15; P=0.66; Fig. 6A).
Decreased libido
A total of four RCTs with a sample of 1,919
participants assessed the severity of decreased libido. The results
of the heterogeneity test were P=0.93 and I2=0%. The
fixed-effects model identified no significant differences between
the dutasteride and finasteride groups in the severity of decreased
libido among the four studies (OR, 0.85; 95% CI, 0.58 to 1.24;
P=0.39; Fig. 6B).
Impotence
A total of four RCTs with a suitable sample of 1,919
participants analyzed the severity of impotence. The results of the
heterogeneity test were P=0.82 and I2=0%. The
fixed-effects model identified no statistically significant
differences between the dutasteride and finasteride groups in the
severity of impotence among the four studies (OR, 0.79; 95% CI,
0.57 to 1.10; P=0.17; Fig. 6C).
Discussion
BPH is the most common benign disease among males
aged >50 years and its occurrence rate increases with age. BPH
manifests as lower urinary tract symptoms (LUTS), increased total
PV, decreased peak urinary flow and increased IPSS (24,25).
Previously identified pathogenetic mechanisms suggest that
androgenic disorders are the vital factors for the progress of BPH,
resulting in abnormal prostate gland enlargement, compression of
the prostatic part of the urethra and changes in the urinary tract
(5,26). Without treatment, the quality of life
and sexual function of the patients severely declines (27).
Pharmacological treatments are generally reserved
for patients with moderate or severe BPH, as it helps to alleviate
the symptoms of bladder outlet obstruction and reduces
pre-operative-to-post-operative risk of acute urinary retention.
Finasteride and dutasteride, which are types of 5ARIs, are
currently most frequently prescribed to improve the unpleasant
symptoms (10,28). The therapeutic effect of monotherapy
using the two drugs was confirmed in previous clinical studies and
a systematic review; however, growing concern has arisen regarding
the AEs of the drugs, particularly on sexual function (mainly
decreased libido and impotence) (29). To address the limitations of previous
analyses, the present study re-searched the literature, extracted
and analyzed the data, systematically explained the advantages and
disadvantages of the two drugs and provided novel results.
The present updated meta-analysis was performed
using six studies including 2,041 participants to compare the
efficacy and safety of dutasteride (0.5 mg/day) and finasteride (5
mg/day) in treating BPH for ≥6 months. The analysis demonstrated a
significantly greater decrease in IPSS and PSA in the dutasteride
group compared with that in the finasteride group, whereas no
significant differences were identified in PV, Qmax and PVRV. Of
note, five RCTs evaluating the changes of IPSS as a subjective
measurement, which required the patients to assess their symptoms
themselves, demonstrated that dutasteride was more effective
compared with finasteride for improving the patients' subjective
wellbeing and BPH symptoms.
A previous study demonstrated that the normal
development of the prostate and the progression of BPH are
inseparable from the role of DHT, which has a high affinity for the
androgen receptor and an inhibitory effect on testosterone
(6). The catalytic enzyme that
converts testosterone to DHT is 5AR, and 5ARIs inhibit the increase
of the PV by suppressing the generation of DHT (30). A previous study found that
dutasteride was 45-fold more effective in inhibiting type 1 5AR and
2.5-fold more effective in inhibiting type 2 5AR compared with
finasteride (31). In addition, one
RCT (18) demonstrated that the
percent changes in DHT from baseline achieved with 0.5 mg
dutasteride (94.7%) were significantly greater compared with those
obtained with 5 mg finasteride (70.8%) after a 24-week treatment.
Therefore, dutasteride offered a significant improvement in
patients with LUTS compared with finasteride. However, measurement
of IPSS requires the subjective perception of the participants and
the values are easily affected by subjective factors. If patients
are aware of undergoing experimental treatment, they may be more
vigorous regarding their symptoms; further randomized controlled
double-blinded studies are required to clarify the changes in
IPSS.
A total of five RCTs containing PSA data
demonstrated that dutasteride was more effective compared with
finasteride in reducing the serum level of PSA. 5ARI may induce the
degradation of prostate tissue, which is a source of serum PSA, and
the inhibition of DHT may indirectly decrease the level of serum
PSA (32). PSA is a commonly used
screening indicator for the diagnosis of prostate cancer (PC)
(33). Long-term use of 5ARI may
lead to low PSA levels, which may reduce the detection rate of PC
and increase the rate of misdiagnosis (34). The results of the present study
indicated that compared with finasteride, dutasteride may reduce
the diagnostic efficacy of PSA in PC to a greater extent. If PSA
changes in patients being screened for PC during treatment with
dutasterid, further examination may be required for patients being
screened for PC. Andriole et al (35) reported that patients exhibited a
>40% decrease of PSA after 6-month treatment with dutasteride,
which may indicate a low risk for PC and re-biopsy may be
required.
The safety assessment of the studies included in the
present analysis suggested that dutasteride and finasteride were
well-tolerated. Regarding the adverse reactions assessed, including
any AE, decreased libido and impotence, the dutasteride group
exhibited no significant differences compared with the finasteride
group. Traish et al (36)
suggested that long-term dutasteride therapy led to deterioration
of erectile dysfunction, reduced testosterone levels and increased
glucose and glycated hemoglobin and changed lipid profiles,
suggesting an imbalance of metabolic function and deterioration of
gonadal function. It is strongly recommended that the physician
explains the potential serious side effects of long-term 5ARI
treatment to the patient prior to adopting this treatment.
The limitations of the present meta-analysis require
to be acknowledged. The quality of the selected studies was flawed,
primarily in terms of study design, patient selection, blinding and
outcome data. Therefore, the results of the present meta-analysis
should be interpreted with caution. However, the publications
included in the present study were all RCTs, which reinforced the
results. Bias regarding selection and subjective factors may also
affect the final results of the present study. More high-quality
RCTs with sufficient sample sizes and statistics are required to
confirm the efficacy of dutasteride and finasteride in treating
BPH.
The present meta-analysis demonstrated that
dutasteride exhibited a greater decrease in IPSS and PSA in the
treatment of BPH compared with finasteride, whereas no significant
differences were observed in PV, Qmax and PVRV. The two drugs
appeared to exhibit similar rates of adverse reactions. In contrast
to finasteride, dutasteride offered a significant improvement in
patients with LUTS; however, long-term use of dutasteride may
result in low PSA levels, which may lead to a significant reduction
in the relevance ratio of PSA regarding PC.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Nature
Science Foundation of China (grant nos. 81572835, 81801429 and
81870525); Shandong Key Research and Development Program (grant no.
2018GSF118118); and the Natural Science Foundation of Shandong
Province (grant no. ZR2017LH016).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJ made substantial contributions to research
concept, screening process, identification of eligible studies and
manuscript preparation. ZZ and YC performed data analysis and
prepared the manuscript. JW screened, identified eligible studies
and analyzed the data. ZZ, YC and JW performed the data extraction
and quality evaluation. HJ conceived the research study and
supervised the other authors to ensure integrity of the analysis.
All authors reviewed, read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guess HA, Arrighi HM, Metter EJ and Fozard
JL: Cumulative prevalence of prostatism matches the autopsy
prevalence of benign prostatic hyperplasia. Prostate. 17:241–246.
1990.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lepor H, Williford W, Barry M, Haakenson C
and Jones K: The impact of medical therapy on bother due to
symptoms, quality of life and global outcome, and factors
predicting response. J Urol. 160:1358–1367. 1998.PubMed/NCBI
|
|
3
|
AUA Practice Guidelines Committee. AUA
guideline on management of benign prostatic hyperplasia (2003).
Chapter 1: Diagnosis and treatment recommendations. J Urol.
170:530–547. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Anderson JB, Roehrborn CG, Schalken JA and
Emberton M: The progression of benign prostatic hyperplasia:
Examining the evidence and determining the risk. Eur Urol.
39:390–399. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Roehrborn CG, McConnell JD, Lieber M,
Kaplan S, Geller J, Malek GH, Castellanos R, Coffield S, Saltzman
B, Resnick M, et al: Serum prostate-specific antigen concentration
is a powerful predictor of acute urinary retention and need for
surgery in men with clinical benign prostatic hyperplasia. PLESS
Study Group Urology. 53:473–480. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Deslypere JP, Young M, Wilson JD and
McPhaul MJ: Testosteronem and 5 alpha-dihydrotestosterone interact
differently with the androgen receptor to enhance transcription of
the MMTV-CAT reporter gene. Mol Cell Endocrinol. 88:15–22.
1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Welén K and Damber JE: Prostate
diseases-role of sex steroids and their inhibitors. Best Pract Res
Clin Endocrinol Metab. 25:355–367. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Griffiths K, Eaton CL, Harper ME, Peeling
B and Davies P: Steroid hormones and the pathogenesis of benign
prostatic hyperplasia. Eur Urol. 20 (Suppl 1):S68–S77.
1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Naslund M, Regan TS, Ong C and Hogue SL:
5-Alpha reductase inhibitors in men with an enlarged prostate: An
evaluation of outcomes and therapeutic alternatives. Am J Manag
Care. 14 (5 Suppl 2):S148–S153. 2008.PubMed/NCBI
|
|
10
|
McConnell JD, Bruskewitz R, Walsh P,
Andriole G, Lieber M, Holtgrewe HL, Albertsen P, Roehrborn CG,
Nickel JC, Wang DZ, et al: The effect of finasteride on the risk of
acute urinary retention and the need for surgical treatment among
men with benign prostatic hyperplasia Finasteride Long-Term
Efficacy and Safety Study Group. N Engl J Med. 338:557–563.
1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Roehrborn CG, Boyle P, Nickel JC, Hoefner
K and Andriole G: ARIA3001 ARIA3002 and ARIA3003 Study
Investigators. Efficacy and safety of a dual inhibitor of
5-alpha-reductase types 1 and 2 (dutasteride) in men with benign
prostatic hyperplasia. Urology. 60:434–441. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yin T, Qiao Z, Li Y, Li D, Jiang M, An C,
Wang F, Zuo M, Hu K and Li Q: Comparisons of the efficacy and
safety of finasteride and dutasteride for benign prostatic
hyperplasia: A network meta-analysis. Am J Ther. 24:e517–e523.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jun JEJ, Kinkade A, Tung ACH and Tejani
AM: 5α-reductase inhibitors for treatment of benign prostatic
hyperplasia: A systematic review and meta-analysis. Can J Hosp
Pharm. 70:113–119. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. Ann Intern Med.
151:264–269. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jadad AR: Randomised controlled trials: A
user's guide. BMJ Publishing Group, London, 1998.
|
|
16
|
Higgins JP and Green S (eds): Cochrane
handbook for systematic reviews of interventions. Version 5.1.0.
The Cochrane Collaboration, 2011. http://www.cochrane-handbook.orgsimplewww.cochrane-handbook.org.
Updated March 2011.
|
|
17
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Clark RV, Hermann DJ, Cunningham GR,
Wilson TH, Morrill BB and Hobbs S: Marked suppression of
dihydrotestosterone in men with benign prostatic hyperplasia by
dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol
Metab. 89:2179–2184. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mohanty NK, Singh UP, Sharma NK, Arora RP
and Amitabh V: A comparative study of fixed dose of tamsulosin with
finasteride vs tamsulosin with dutasteride in the management of
benign prostatic hyperplasia. Indian J Urol. 22:130–134. 2006.
|
|
20
|
Jeong YB, Kwon KS, Kim SD and Kim HJ:
Effect of discontinuation of 5alpha-reductase inhibitors on
prostate volume and symptoms in men with BPH: A prospective study.
Urology. 73:802–806. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nickel JC, Gilling P, Tammela TL, Morrill
B, Wilson TH and Rittmaster RS: Comparison of dutasteride and
finasteride for treating benign prostatic hyperplasia: The Enlarged
Prostate International Comparator Study (EPICS). BJU Int.
108:388–394. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Y and Wang J: Clinical efficacy and
safety analysis of dutasteride in treatment of benign prostatic
hyperplasia. Chinese J Androl. 27:49–55. 2013.
|
|
23
|
Qian X, Yu G, Qian Y, Xu D, Liu H, Kong X,
Zhu Y, Wang Z, Zheng J and Qi J: Efficacy of 5α-reductase
inhibitors for patients with large benign prostatic hyperplasia
(>80 ml) after transurethral resection of the prostate. Aging
Male. 18:238–243. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Girman CJ: Population-based studies of the
epidemiology of benign prostatic hyperplasia. Br J Urol. 82 (Suppl
1):S34–S43. 1998.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Emberton M, Andriole GL, de la Rosette J,
Djavan B, Hoefner K, Vela Navarrete R, Nordling J, Roehrborn C,
Schulman C, Teillac P, et al: Benign prostatic hyperplasia: A
progressive disease of aging men. Urology. 61:267–273.
2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jacobsen SJ, Jacobson DJ, Girman CJ,
Roberts RO, Rhodes T, Guess HA and Lieber MM: Natural history of
prostatism: Risk factors for acute urinary retention. J Urol.
158:481–487. 1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Corona G, Vignozzi L, Rastrelli G, Lotti
F, Cipriani S and Maggi M: Benign prostatic hyperplasia: A new
metabolic disease of the aging male and its correlation with sexual
dysfunctions. Int J Endocrinol. 2014(329456)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
O'Leary MP, Roehrborn CG and Black L:
Dutasteride significantly improves quality of life measures in
patients with enlarged prostate. Prostate Cancer Prostatic Dis.
11:129–133. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Corona G, Tirabassi G, Santi D, Maseroli
E, Gacci M, Dicuio M, Sforza A, Mannucci E and Maggi M: Sexual
dysfunction in subjects treated with inhibitors of 5α-reductase for
benign prostatic hyperplasia: A comprehensive review and
meta-analysis. Andrology. 5:671–678. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shibata Y, Arai S, Miyazawa Y, Shuto T,
Nomura M, Sekine Y, Koike H, Matsui H, Ito K and Suzuki K: Effects
of steroidal antiandrogen or 5-alpha-reductase inhibitor on
prostate tissue hormone content. Prostate. 77:672–680.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Evans HC and Goa KL: Dutasteride. Drugs
Aging. 20:905–918. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guess HA, Gromley GJ, Stoner E and
Oesterling JE: The effect of finasteride on prostate specific
antigen: Review of available data. J Urol. 155:3–9. 1996.PubMed/NCBI
|
|
33
|
Schröder FH: Review of diagnostic markers
for prostate cancer. Recent Results Cancer Res. 181:173–182.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Morgia G, Urzì D and Russo GI: 5ARI and
PSA: Evidences. Urologia. 81 (Suppl 24):S4–S11. 2014.PubMed/NCBI View Article : Google Scholar : (In Italian).
|
|
35
|
Andriole GL, Bostwick D, Brawley OW,
Gomella L, Marberger M, Montorsi F, Pettaway C, Tammela TL, Teloken
C, Tindall D, et al: The effect of dutasteride on the usefulness of
prostate specific antigen for the diagnosis of high grade and
clinically relevant prostate cancer in men with a previous negative
biopsy: Results from the REDUCE study. J Urol. 185:126–131.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Traish A, Haider KS, Doros G and Haider A:
Long-term dutasteride therapy in men with benign prostatic
hyperplasia alters glucose and lipid profiles and increases
severity of erectile dysfunction. Horm Mol Biol Clin Investig 30,
2017.
|