Introduction
Ischemic heart disease (IHD) is an important global
public health concern, which is the most common cause of mortality
and disability worldwide (1-3). An
estimated 110.55 million prevalent cases of IHD were present
globally in 2015(4). The World
Health Organization estimates further increases in IHD-associated
mortality by 2030 in all country income strata (5). Therefore, IHD remains a huge burden on
healthcare systems.
Early diagnosis may efficiently improve the survival
rate and prognosis of patients with IHD, which relies on medical
history, electrocardiogram and cardiac biomarkers (6). As clinical history and physical
examination of patients are limited and 12-lead electrocardiogram
is only used for diagnosis in a small proportion of cases, the use
of specific biomarkers as diagnostic indicators has added value for
rapidly diagnosis of IHD in populations (7-11).
Myoglobin (Myo), cardiac troponin I (cTnI) and creatine kinase-MB
isoenzyme (CK-MB) are common biomarkers frequently used for IHD
diagnosis in hospital settings (8,12-14).
The concentration of these cardiac biomarkers may be determined by
radioimmunoassay, ELISA and chemiluminescence immunoassay (CLIA)
with excellent specificity and sensitivity (15,16). At
present, CLIA is commonly used in hospitals. However, the complex
procedures take a large amount of time and rely on bulky
instruments, which may delay the rescue of patients and are
inconvenient to perform. Therefore, the rapid detection of these
biomarkers with a portable device may be an efficient approach for
better prognostic prediction.
Disposable strips based on a fluorescent immunoassay
are generally used for the quantitative determination of specific
markers (17). This method allows
for a one-step, rapid and inexpensive analysis, which is easy to
perform at community health centers and in under-developed regions.
In the present study, the strips for the quantification of Myo,
cTnI and CK-MB were employed for a substantial reduction in the
time of IHD diagnosis.
Materials and methods
Materials
Myo, cTnI and CK-MB disposable strips and a UNICELL
immunofluorescence reader were obtained from Shenzhen YHLO
Biotech.
UNICELL-cTnI/CK-MB/Myo employs an
immuno-chromatography sandwich assay that generates a fluorescence
signal, which is proportional to the level of cTnI/CK-MB/Myo in the
blood specimen. Corresponding mouse anti-cTnI/CK-MB/Myo antibody is
coated on the test line (T line) of the testing strip and
anti-avidin antibody is coated on the control line (C line) of the
testing strip. Fluorescence particles labeled anti-cTnI/CK-MB/Myo
antibody and avidin are coated on the binding pad (Shenzhen YHLO
Biotech Co. Ltd.).
Whole blood samples were obtained from patients at
Shenzhen Second People's Hospital (Shenzhen, China). Testing was
performed by adding 100 µl whole blood, serum or plasma into the
sample well. If cTnI/CK-MB/Myo was present in the specimen, an
immune complex is formed by binding cTnI/CK-MB/Myo with
fluorescence particle-labelled anti-cTnI/CK-MB/Myo antibody.
Subsequently, through the capillary effect, this immune complex
moves forward to the T line and is captured by another
anti-cTnI/CK-MB/Myo antibody on it. At the same time, the
fluorescence particle-labeled avidin on the binding pad passes
through the C line and binds with the anti-avidin antibody coated
on the C line.
The UNICELL immunofluorescence reader was used to
determine the signal intensity of fluorescence of the T line and
the C line. The concentration of cTnI/CK-MB/Myo in the specimen was
automatically calculated according to the master curve that was
stored in the ID card and the result was displayed in the units of
ng/ml.
Patient recruitment and data
collection
For the present clinical validation study,
consecutive patients who had been subjected to assessment of Myo,
cTnI and CK-MB by CLIA at the Emergency Department of Shenzhen
Second People's Hospital (Shenzhen, China) between December 2017
and March 2018 were subjected to screening with the test strips.
Patients were excluded if they had received medical treatment prior
to blood collection or were without complete data. A total of 391
randomized patients were enrolled in the study, ranging from 15 to
96 years. A total of 62.40% of the patients were male. Baseline
data were collected by trained research staff according to a
standard operating procedure. The data included gender, age, the
concentrations of Myo, cTnI and CK-MB quantified by CLIA and
disposable strips. The study was performed in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
Shenzhen Second People's Hospital (Shenzhen, China; approval no.
20170811015). During data collection, all patients were informed to
fully understand the study and signed the consent documents
referenced from the Chinese Clinical Trial registry (http://www.chictr.org.cn/, no. ChiCTR2000032549). All
of the experiments were performed in accordance with relevant
guidelines and regulations.
Quantification methods
The concentrations of Myo/cTnI/CK-MB in the blood
samples were determined with the disposable strips and
quantitatively evaluated with the UNICELL immunofluorescence
reader. The procedure was as follows: All of the blood samples were
stored at 4˚C for 24 h. The samples were left to reach
room temperature for 10 min prior to further testing. Each sample
(100 µl) was dispensed into the sample well and the strips were
then inserted into the reader. The fluorescent signal intensities
of the T line and the C line were recorded 15 min later, which were
used to calculate the concentrations of Myo/cTnI/CK-MB in the
samples according to the master curve that is stored in the ID
Card. The values (ng/ml) were displayed automatically on the screen
of the device. The Myo/cTnI/CK-MB quantification results by the
CLIA method were obtained from the department of laboratory
medicine of Shenzhen Second People's Hospital (Shenzhen,
China).
Statistical analysis
In the setting of the present study, a total of 57
patients diagnosed with IHD would provide a statistical power of
99% to validate the use of test strips for cardiac biomarkers.
Baseline characteristics, including gender, age and the
concentrations of Myo, cTnI and CK-MB quantified by CLIA and
disposable strips, were analysed to construct the receiver
operating characteristic (ROC) curves, which were used to determine
the optimal cut-off concentrations and compare the sensitivity and
specificity of different cardiac biomarkers and their predictive
value to diagnose IHD. The ROC curves with or without bootstrap
resampling (time=500) and a nomogram were established with
EmpowerStats software (www.empowerstats.com, X&Y solutions, Inc.; updated
on 28/02/2020).
Results
Patients
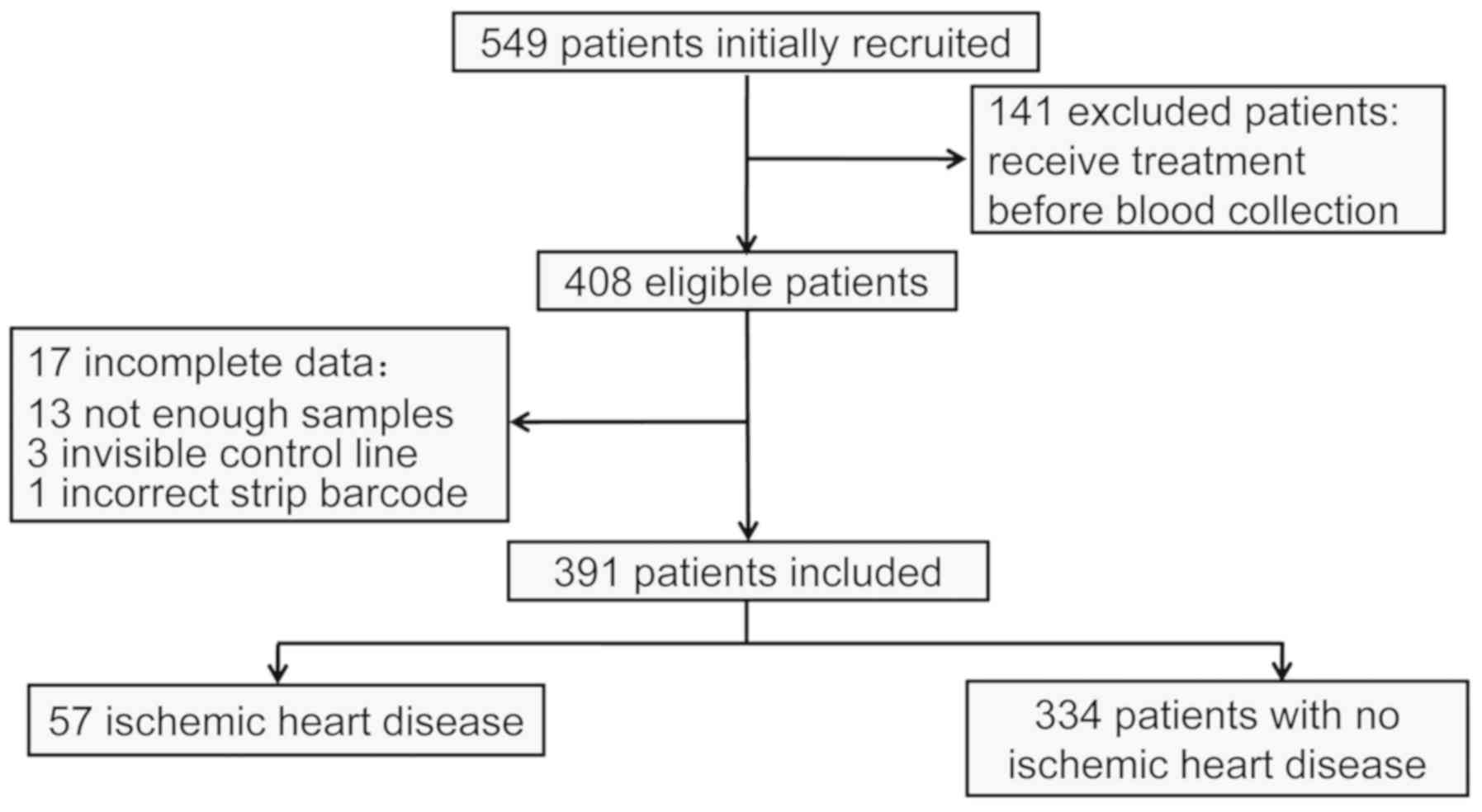
A total of 549 participants from the Emergency
Department received Myo/cTnI/CK-MB tests during the study period
and 141 of them were excluded due to having received treatment
prior to blood collection. Another 17 participants were excluded
due to an insufficient blood volume for completing the test,
invisible control line or incorrect barcode. The final study
population comprised 391 patients. The median age was 62.9±17.21
years and 62.40% of the patients were male (Table I). At baseline, 57 patients were
diagnosed with IHD, including 23 cases of acute myocardial
infarction, 21 of acute coronary syndrome, 4 cases of acute heart
failure, 1 of acute myopericarditis, 1 of cardiogenic shock and 1
case of cardiopulmonary arrest. The remaining 334 patients were
diagnosed with other diseases (Fig.
1; Table I).
 | Table IBaseline characteristics of the
patients (n=391). |
Table I
Baseline characteristics of the
patients (n=391).
| Clinical
characteristic | Value |
|---|
| Age (years) | 62.9 (49.5-77) |
| Male sex | 244 (62.40) |
| Diagnosed
diseases | |
|
Ischemic
heart disease | 57 (14.58) |
|
Acute
myocardial infarction | 23 (5.88) |
|
Acute
coronary syndrome | 21 (5.37) |
|
Acute
heart failure | 4 (1.02) |
|
Acute
myopericarditis | 1 (0.26) |
|
Cardiogenic
shock | 1 (0.26) |
|
Cardiopulmonary
arrest | 1 (0.26) |
|
Other
diseases | 334 (85.42) |
Comparison of the CLIA model and
disposable strip model
A total of 2 different multivariable logistic
regression models were constructed. Model 1 was constructed with
baseline predictors, including gender, age and concentration of
Myo/cTnI/CK-MB assessed by CLIA method. Model 2 included gender,
age and concentration of Myo/cTnI/CK-MB quantified by disposable
strip. The regression coefficients from the multivariate logistic
model were used to construct the model for predicting the presence
of IHD. The scoring model 1 was as follows: -1.22037+0.17693 x
gender -0.01179 x age + 0.46488 x cTnI -0.00207 x Myo + 0.00102 x
CK-MB. The scoring model 2 was as follows: -0.95456+0.16130 x
gender -0.00966 x age + 0.19297 x cTnI -0.01119 x Myo + 0.02895 x
CK-MB (sex: ‘0’ for male and ‘1’ for female; ‘years’ for age;
‘ng/ml’ for the laboratory parameters). ROC analysis was used to
identify the area under the ROC curve (AUC) of each model (Fig. 2A). The AUC of model 1 was 0.787 (95%
CI, 0.709-0.865) with a specificity of 76.7% and a sensitivity of
71.9% at a cutoff value of -1.867. The AUC of model 2 was 0.792
(95% CI, 0.728-0.855). The specificity was 66.8% and the
sensitivity was 79.0%. The optimal cutoff value was determined to
be -1.820. There was no significant difference between model 1 and
model 2 in terms of IHD diagnosis (P=0.858). Parameters regarding
the predictive value of the disposable strip test for IHD are
presented in Table II. The negative
predictive value at the cutoff value of -1.820 or below for the
scoring model 2 was 94.9% and the positive predictive value was
28.9%. The results indicated that with a cutoff value of -7.008,
the sensitivity and negative predictive value for the diagnosis of
IHD were 98.3 and 98.8%, respectively.
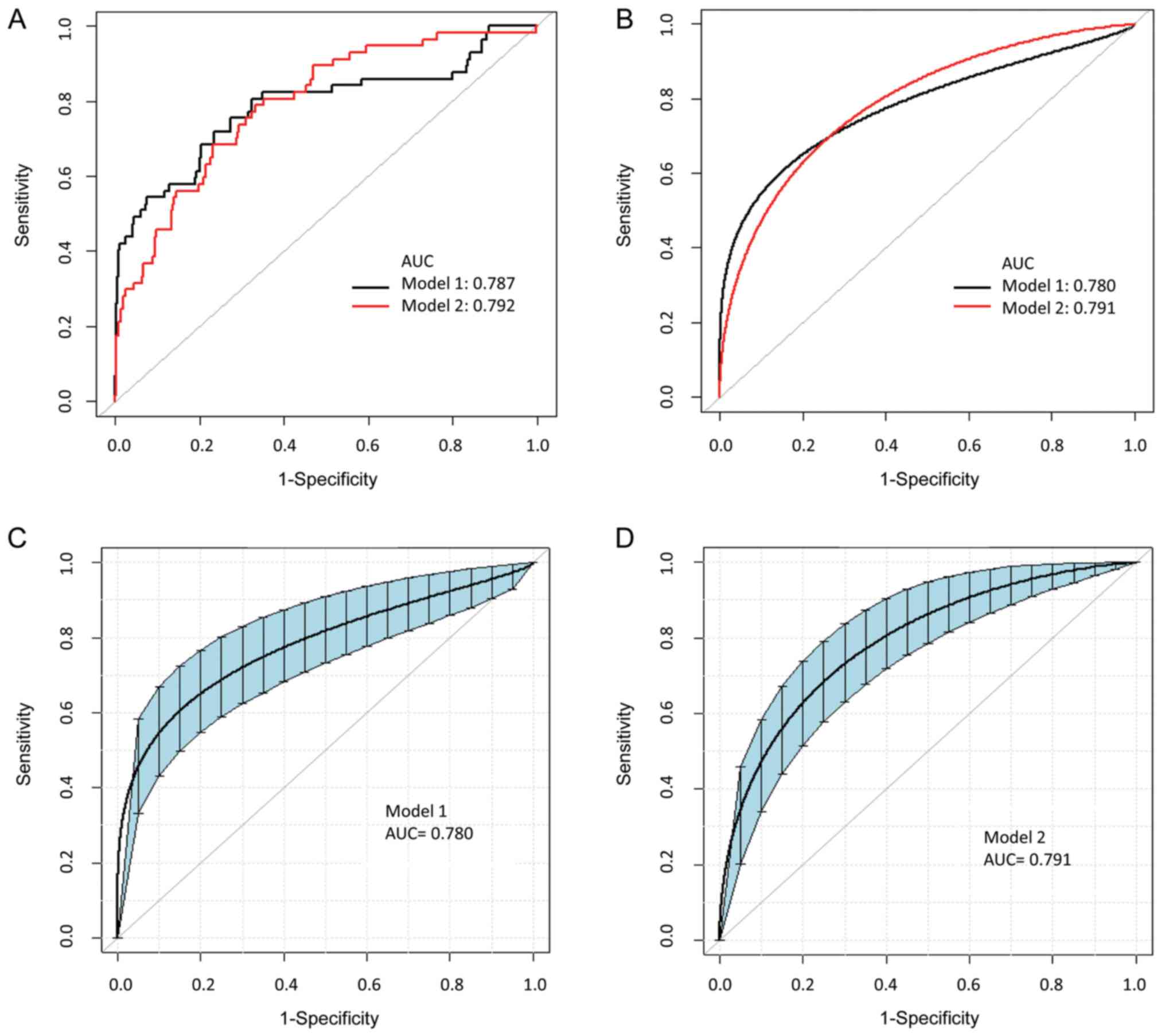 | Figure 2ROC curve for diagnosis of IHD. (A)
Model 1 (black) is for the CLIA model including gender, age,
concentration of Myo/cTnI/CK-MB assessed by the CLIA method. Model
2 (red) is for the disposable strip model including gender, age and
concentration of Myo/cTnI/CK-MB quantified by disposable strips.
The AUCs for model 1 and model 2 for predicting IHD were 0.787 and
0.792. There was no significant difference between the two models.
The optimal cutoff values of modle 1 and modle 2 were -1.867 and
-1.820. (B) ROC curves for the diagnosis of IHD in each model using
Bootstrap resampling (times=500). The AUCs for model 1 (black) and
model 2 (red) for predicting IHD were 0.780 and 0.791. (C) ROC
curves for the diagnosis of IHD in model 1 using Bootstrap
resampling (times=500). The optimal cutoff value was -1.867. The
blue area represented 95% CI (0.710-0.854) of the AUC. (D) ROC
curves for the diagnosis of IHD in model 2 using Bootstrap
resampling (times=500). The optimal cutoff value was -1.820. The
blue area represented 95% CI (0.729-0.845) of the AUC. ROC,
receiver operating characteristic; AUC, area under the ROC curve;
CLIA, chemiluminescence immunoassay; CK-MB, creatine kinase-MB
isoenzyme; cTnI, cardiac troponin I; IHD, ischemic heart disease;
Myo, myoglobin. |
 | Table IIProportions of patients with positive
samples, sensitivities, specificities, positive and negative
predictive values and likelihood ratios for the disposable strip to
identify patients with ischemic heart disease. |
Table II
Proportions of patients with positive
samples, sensitivities, specificities, positive and negative
predictive values and likelihood ratios for the disposable strip to
identify patients with ischemic heart disease.
| Cut-off value | Specificity (%) | Sensitivity (%) | PPV (%) | NPV (%) | + likelihood
ratio | - likelihood
ratio |
|---|
| -7.008 | 23.7 | 98.3 | 18.0 | 98.8 | 1.293 | 0.072 |
| -2.119 | 48.2 | 89.5 | 22.8 | 96.4 | 1.733 | 0.217 |
| -1.820 | 66.8 | 79.0 | 28.9 | 94.9 | 2.382 | 0.324 |
| -1.641 | 74.3 | 68.4 | 31.2 | 93.2 | 2.658 | 0.432 |
| -1.601 | 78.4 | 59.7 | 32.1 | 92.9 | 2.766 | 0.514 |
ROC curves for the diagnosis of IHD in each model
using Bootstrap resampling (times=500) indicated that the AUC of
model 1 was 0.78 (95% CI, 0.6899-0.8519; specificity, 76,65%;
sensitivity, 71,93%). The AUC of model 2 was slightly higher than
that of model 1 (AUC=0.79; 95% CI, 0.6899-0.8519; specificity,
66.77%; sensitivity, 78.95%). The P-value was 0.7465 (P>0.05),
indicating that the disposable strip model shared comparable
accuracy in IHD diagnosis with the CLIA model (Fig. 2B-D). ROC curves for the individual
biomarkers quantified by disposable strip are provided in Fig. 3. Compared with the combined use of
the biomarkers (AUC=0.792), the AUC for single use of Myo, cTnI and
CK-MB was 0.685 (P<0.001), 0.706 (P=0.0092) and 0.662
(P<0.001), respectively. The result suggested that the
predictive performance of Myo, cTnI and CK-MB, used separately, was
not superior to the predictive performance of the combination use.
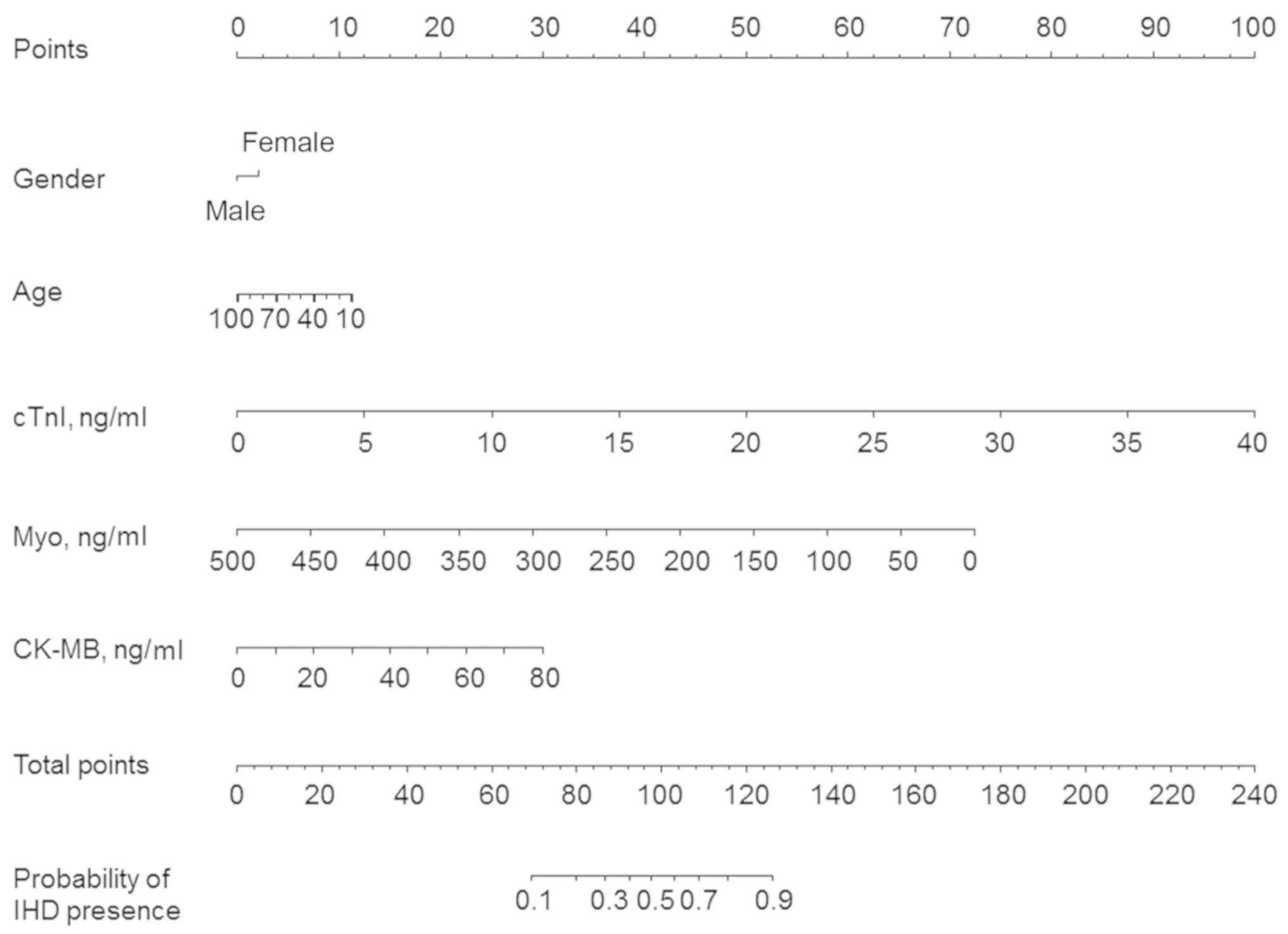
To predict the presence of IHD, a prognostic nomogram was
established through Cox regression model analysis according to all
significant independent indicators, including gender, age and the
concentration of Myo, cTnI and CK-MB quantified by the disposable
strip. Each factor in the nomogram was assigned a weighted number
of points and the sum of points for each patient reflected the
probability of the presence of IHD (Fig.
4).
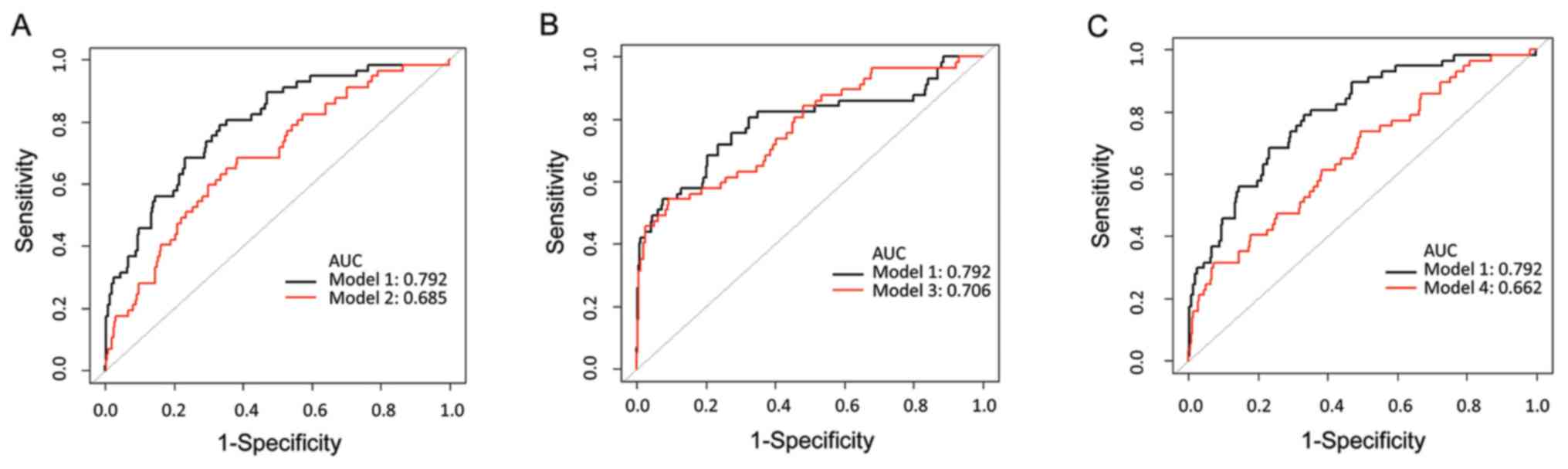 | Figure 3Comparison of the biomarkers applied
individually and collectively. Model 1 (black) is the combination
model including gender, age and concentration of Myo/cTnI/CK-MB
quantified by disposable strip. Model 2, model 3 and model 4 (red)
are single models including gender, age and concentration of (A)
Myo, (B) cTnI or (C) CK-MB quantified by a disposable strip,
separately. ROC, receiver operating characteristic; AUC, area under
the ROC curve; CK-MB, creatine kinase-MB isoenzyme; cTnI, cardiac
troponin I; Myo, myoglobin. |
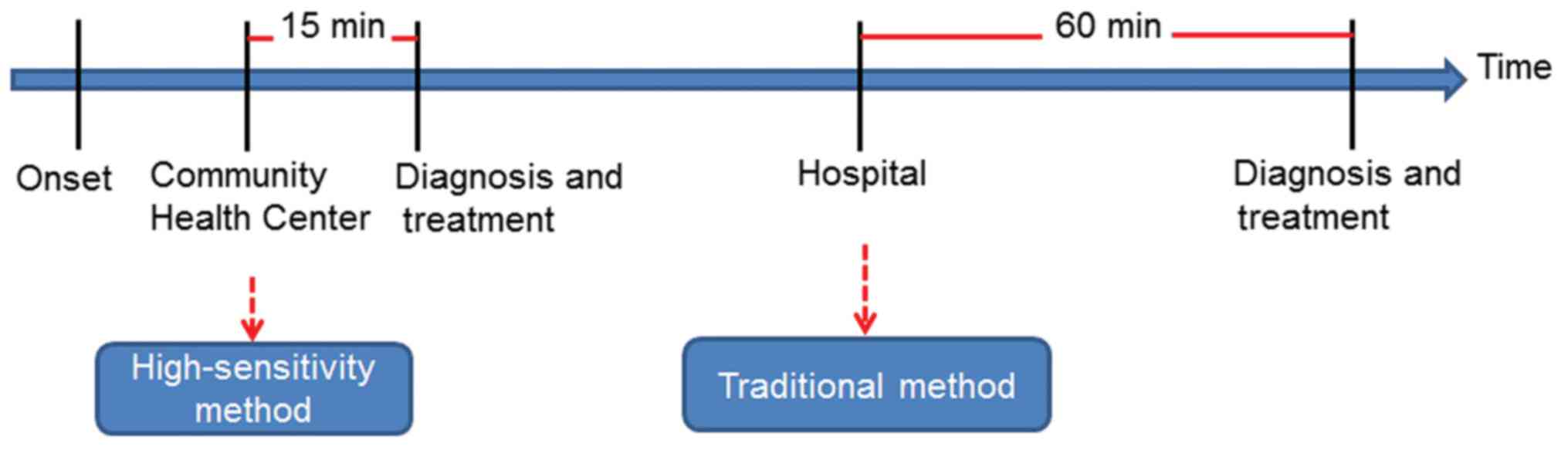
The disposable strip is a cost-saving
and rapid method for IHD diagnosis
The cost of the two methods was compared. The
patients paid 47.18 US$ for the quantification of Myo/cTnI/CK-MB by
CLIA, while the disposable strip method only cost 21.91 US$, saving
>50% compared to the CLIA method. The time spent on each method
was also compared in the present study. The doctors of the
emergency department usually waited for >1 h to get the results
of the Myo/cTnI/CK-MB tests by CLIA, while it only took 15 min for
the disposable strip method, which saved 75% of the time to
diagnose and treat the patients with IHD earlier (Fig. 5).
Discussion
Disposable strips based on a fluorescent immunoassay
have received considerable attention due to their portability,
rapid performance, cost effectiveness and ease of use (16,17).
This qualitative assay is easy to perform and evaluate based on the
fluorescence intensity of the band on the test zone, and the
quantitative data may be obtained by recording the optical
responses with a hand-held ‘strip reader’. It has been derived to
recognize a diverse range of targets, including metal ions
(18), protein (19), cells (20) and toxicants (21), with high specificity and sensitivity.
In the present study, a method to rapidly diagnose IHD was
established using patient gender, age and the concentration of
Myo/cTnI/CK-MB assessed by disposable strips. It exhibited a
similar diagnostic capability for IHD compared with the traditional
CLIA method frequently used in hospitals. Furthermore, the method
of the present study had the following advantages: First, since
cardiac myocytes may withstand ischemia for only 30 min prior to
the occurrence of permanent injury, reliable and rapid tests for
myocardial damage urgently require inclusion for rapid treatment.
According to clinical guidelines, the test information of cardiac
biomarkers is required within 1 h to diagnose patients and manage
their treatment (22). Furthermore,
the National Academy of Clinical Biochemistry Laboratory Medicine
Practice guidelines have also suggested that laboratory testing for
cardiac markers should be performed with a turnaround time of 1 h,
optimally 30 min or less (23,24). In
institutions unable to meet the 1-h turnaround time requirement,
quantitative point-of-care testing (POCT) should be implemented
(23,24). As the conventional approach for IHD
diagnosis takes >1 h (25), the
15-min rapid method provided by the present study is time-saving,
which greatly facilitates faster decision-making and interventional
therapy. This assay is likely to improve the survival rate and
prognosis of patients with IHD. Furthermore, the CLIA method
requires bulky instruments, which is in conflict with the
bedside-testing purpose of portable devices, while the system of
the present study perfectly meets the requirement for POCT and
demonstrated a reliable analytical performance. To this end, the
disposable strip-based method of the present study may be widely
applied in under-developed regions and community health service
centers and at marathon races. In addition, disposable strip has
the advantage of easy manufacture and low cost, making their use an
inexpensive analysis compared with the traditional method. Finally,
the performance of complex instruments applied in traditional CLIA
methods depends on skilled and properly trained technicians, while
the ease of performing the assessment with the present disposable
strip-based method makes it a suitable system for POCT that does
not require prior training.
The present analysis only took factors associated
with technical success into account, without considering any risk
factors, including hypertension, smoking and family history of
coronary artery disease (26). It is
necessary to validate the results with the inclusion of these risk
factors in further investigations. Although the present study only
included limited factors, it had similar accuracy in the diagnosis
of IHD to that of the traditional CLIA method.
Acknowledgements
The authors thank Dr Chen Xinglin (Department of
Epidemiology and Biostatistics, X&Y solutions Inc. in Boston)
for guiding the use of EmpowerStats software.
Funding
This project was supported by the National Natural
Science Foundation of China (grant no. 21602138), the Natural
Science Foundation of Guangdong (grant nos. 2016A030313029,
2016A030310032 and 2017A030313668), Sanming Project of Medicine in
Shenzhen (grant nos. SZSM201612031 and SZXJ2017076), Health and
Family Planning Commission of Shenzhen Municipality (grant no.
201605009) and the Shenzhen Municipal Government of China (grant
nos. GJHZ20160301163138685, JCYJ20170817171808368 and
JCYJ20170818085657917).
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH and NX conceived and designed the study. LQ made
the draft and SH revised the manuscript. SH, LQ and DZ performed
the experiments. SH and XH analysed the data. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The study was performed in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
Shenzhen Second People's Hospital (Shenzhen, China). During data
collection, all patients were informed to fully understand the
study and signed the consent documents referenced from the Chinese
Clinical Trial registry (http://www.chictr.org.cn/, no. ChiCTR2000032549). All
of the experiments were performed in accordance with relevant
guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joseph P, Leong D, McKee M, Anand SS,
Schwalm JD, Teo K, Mente A and Yusuf S: Reducing the global burden
of cardiovascular disease, part 1: The epidemiology and risk
factors. Circ Res. 121:677–694. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lamelas PM, Yusuf S and Schwalm JD:
Effective approaches to address the global cardiovascular disease
burden. Curr Opin Cardiol. 32:557–566. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP,
Fullerton HJ, et al: Executive summary: Heart disease and stroke
statistics-2016 update: A report from the American heart
association. Circulation. 133:447–454. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Roth GA, Johnson C, Abajobir A, Abd-Allah
F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al:
Global, regional, and national burden of cardiovascular diseases
for 10 causes, 1990 to 2015. J Am Coll Cardiol. 70:1–25.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
World Health Organization (WHO): Global
health estimates: Deaths by cause, age, sex and country, 2000-2012.
WHO, Geneva, 2014.
|
|
6
|
Lippi G, Franchini M and Cervellin G:
Diagnosis and management of ischemic heart disease. Semin Thromb
Hemost. 39:202–213. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Safdar B, Nagurney JT, Anise A, DeVon HA,
D'Onofrio G, Hess EP, Hollander JE, Legato MJ, McGregor AJ, Scott
J, et al: Gender-specific research for emergency diagnosis and
management of ischemic heart disease: Proceedings from the 2014
academic emergency medicine consensus conference cardiovascular
research workgroup. Acad Emerg Med. 21:1350–1360. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
de Winter RJ, Koster RW, Sturk A and
Sanders GT: Value of myoglobin, troponin T, and CK-MBmass in ruling
out an acute myocardial infarction in the emergency room.
Circulation. 92:3401–3407. 1995.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Scebba F, Papale M, Rocchiccioli S,
Ucciferri N, Bigazzi F, Sampietro T, Carpeggiani C, L'Abbate A,
Coceani F and Angeloni D: Differential proteome profile in ischemic
heart disease: Prognostic value in chronic angina versus myocardial
infarction. A proof of concept. Clin Chim Acta. 471:68–75.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hachey BJ, Kontos MC, Newby LK,
Christenson RH, Peacock WF, Brewer KC and Mccord J: Trends in use
of biomarker protocols for the evaluation of possible myocardial
infarction. J Am Heart Assoc. 6(e005852)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Haeck JD, Verouden NJ, Kuijt WJ, Koch KT,
Van Straalen JP, Fischer J, Groenink M, Bilodeau L, Tijssen JG,
Krucoff MW and De Winter RJ: Comparison of usefulness of N-terminal
pro-brain natriuretic peptide as an independent predictor of
cardiac function among admission cardiac serum biomarkers in
patients with anterior wall versus nonanterior wall ST-segment
elevation myocardial infarction. Am J Cardiol. 105:1065–1069.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hetland O and Dickstein K: Cardiac markers
in the early hours of acute myocardial infarction: Clinical
performance of creatine kinase, creatine kinase MB isoenzyme
(activity and mass concentration), creatine kinase MM and MB
subform ratios, myoglobin and cardiac troponin T. Scand J Clin Lab
Invest. 56:701–713. 1996.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jolly SS, Shenkman H, Brieger D, Fox KA,
Yan AT, Eagle KA, Steg PG, Lim KD, Quill A and Goodman SG: GRACE
Investigators. Quantitative troponin and death, cardiogenic shock,
cardiac arrest and new heart failure in patients with
non-ST-segment elevation acute coronary syndromes (NSTE ACS):
Insights from the global registry of acute coronary events. Heart.
97:197–202. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zeller T, Ojeda F, Brunner FJ, Peitsmeyer
P, Munzel T, Binder H, Pfeiffer N, Michal M, Wild PS, Blankenberg S
and Lackner KJ: High-sensitivity cardiac troponin I in the general
population-defining reference populations for the determination of
the 99th percentile in the gutenberg health study. Clin Chem Lab
Med. 53:699–706. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Penttila I, Penttila K and Rantanen T:
Laboratory diagnosis of patients with acute chest pain. Clin Chem
Lab Med. 38:187–197. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Weigl B, Domingo G, Labarre P and Gerlach
J: Towards non- and minimally instrumented, microfluidics-based
diagnostic devices. Lab Chip. 8:1999–2014. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Chen A and Yang S: Replacing antibodies
with aptamers in lateral flow immunoassay. Biosens Bioelectron.
71:230–242. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen J, Zhou S and Wen J: Disposable strip
biosensor for visual detection of Hg(2+) based on Hg(2+)-triggered
toehold binding and exonuclease III-assisted signal amplification.
Anal Chem. 86:3108–3114. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yu L, Shi Z, Fang C, Zhang Y, Liu Y and Li
C: Disposable lateral flow-through strip for smartphone-camera to
quantitatively detect alkaline phosphatase activity in milk.
Biosens Bioelectron. 69:307–315. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Weng CW, Hsieh BC, Hou YT and Cheng TJ:
Determination of hematocrit by voltage-induced hemolysis on a
disposable electrochemical sensing strip. Analyst. 140:6619–6624.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li X, Wang X, Fang T, Zhang L and Gong J:
Disposable photoelectrochemical sensing strip for highly sensitive
determination of perfluorooctane sulfonyl fluoride on
functionalized screen-printed carbon electrode. Talanta.
181:147–153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Braunwald E, Antman EM, Beasley JW, Califf
RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J,
Levin TN, et al: ACC/AHA 2002 guideline update for the management
of patients with unstable angina and non-ST-segment elevation
myocardial infarction-summary article: A report of the American
college of cardiology/American heart association task force on
practice guidelines (committee on the management of patients with
unstable angina). J Am Coll Cardiol. 40:1366–1374. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Christenson RH and Azzazy HM: Cardiac
point of care testing: A focused review of current national academy
of clinical biochemistry guidelines and measurement platforms. Clin
Biochem. 42:150–157. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Storrow AB, Apple FS, Wu AHB, Jesse RL,
Francis GS, Christenson RH, Cannon CP, Morrow DA, Newby LK,
Ravkilde J, et al: National academy of clinical biochemistry
laboratory medicine practice guidelines: Point of care testing,
oversight, and administration of cardiac biomarkers for acute
coronary syndromes. Point Care. 6:215–222. 2007.
|
|
25
|
McRae AD, Innes G, Graham M, Lang E,
Andruchow JE, Yang H, Ji Y, Vatanpour S, Southern DA, Wang D, et
al: Comparative evaluation of 2-hour rapid diagnostic algorithms
for acute myocardial infarction using high-sensitivity cardiac
troponin T. Can J Cardiol. 33:1006–1012. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mancini GB, Gosselin G, Chow B, Kostuk W,
Stone J, Yvorchuk KJ, Abramson BL, Cartier R, Huckell V, Tardif JC,
et al: Canadian cardiovascular society guidelines for the diagnosis
and management of stable ischemic heart disease. Can J Cardiol.
30:837–849. 2014.PubMed/NCBI View Article : Google Scholar
|