Introduction
Sepsis is a common disease with a worldwide
incidence of 10% affecting patients in intensive care units that is
associated with the systemic inflammatory response syndrome caused
by infection (1), and is mainly
caused by pathogenic microorganisms or toxins that invade the
circulatory system (2,3). The clinical manifestations of sepsis
include fever, shortness of breath and peripheral leukocytosis, and
severe sepsis is accompanied by organ dysfunction and tissue
hypoperfusion (4). Without timely
treatment, it can result in septic shock, which causes acute
circulatory failure or death (5).
Sepsis is a dangerous condition with a mortality rate of >40%
between 1993-2003 in the United States of America (6). At present, the treatment for sepsis is
mainly based on the correction of pathophysiological changes, and
no effective cure is available (7).
The pathogenesis of sepsis is complicated. The
release of large amounts of inflammatory mediators caused by
infection initiates a cascade reaction in systemic tissues and
organs, forms a complex inflammatory network and ultimately causes
cell damage (8,9). In addition, the occurrence and
development of sepsis are closely associated with abnormal
coagulation, immune dysfunction, abnormal gene expression, tissue
damage and other pathophysiological changes (10). Vascular endothelial injury is one of
the pathological changes occurring in sepsis and it serves an
important role in mild and severe sepsis (3). When sepsis occurs, toxins and
inflammatory factors in the blood cause damage to vascular
endothelial cells. In addition to destruction of the vascular
endothelial barrier, the damaged endothelial cells may exhibit
secretory dysfunction (11).
Furthermore, damaged endothelial cells continue to secrete
inflammatory factors to promote an inflammatory response, leading
to dysfunctional vasoconstriction and the aggravation of tissue-
and microcirculation hypoperfusion (12,13).
Therefore, the status of vascular endothelial cells is important in
the maintenance of normal vascular functions, and is an important
target in the clinical treatment of sepsis.
A tight junction is a key cell structure for
sustaining the vascular endothelial barrier. It is closely
associated with the exchange of substances among cells, the
secretion of cytokines and the regulation of signaling pathways
(14). Claudin-2 (CLDN2) is a tight
junction-associated protein (15).
The claudin family currently has 24 identified members that are
involved in the formation and maintenance of intercellular tight
junctions (16). Abnormal expression
of claudin family members is closely associated with various
pathophysiological processes (17).
Claudin family proteins are involved in the regulation of the
occurrence and development of inflammation in tissues and organs
(18). In tumors, the abnormal
expression of claudin family members is associated with tumor cell
proliferation, drug resistance, apoptosis and distant metastasis,
and these proteins are targets in diagnosis and treatment (19). A study showed that the CLDN2 gene is
associated with chronic pancreatitis, colitis and cholangitis
(20). However, to date, the role
and regulation of CLDN2 in vascular endothelial injury are not
clear.
MicroRNA (miRNA/miR) is a class of non-coding small
RNAs (18-22 nucleotides) that bind to the 3'-untranslated region
(UTR) of target genes and regulate their expression (21). At present, it is not clear whether
miRNAs are involved in the regulation of CLDN2 during vascular
injury. In a preliminary bioinformatics analysis conducted by the
present research team, it was found that CLDN2 mRNA is a potential
target of miR-331. Additionally, it has been reported that miR-331
is associated with the PM2.5-induced injury of respiratory
epithelial cells (22). In the
present study, the function and upstream regulation mechanism of
CLDN2 in vascular endothelial injury induced by sepsis and the
roles of tight junction proteins in sepsis were investigated.
Materials and methods
Patients
A total of 25 patients with sepsis that received
treatment at Linyi Central Hospital (Linyi, China) between December
2015 and December 2016 were included in the present study. The
patients comprised 18 males and 7 females with a mean age of
48.5±5.7 years and age range of 38-56 years. The included sepsis
patients did not have any one of the exclusion criteria: History of
autoimmune diseases, diabetes, hypertension and long-term
medication. In addition, 20 healthy subjects (14 males and 6
females) aged between 22-45 years (mean age, 38±4.3 years) who
undertook physical examination at the same hospital during the same
recruitment time with the patients were included as a control
group. Peripheral blood (5 ml) was collected from all patients and
healthy subjects. To determine CLDN2 gene and miR-331 expression,
250 µl blood was used. The remaining 4.75 ml was used for the
separation of serum by centrifugation at 600 x g and 4˚C for 2 min.
Serum samples were stored at -80˚C. All procedures were approved by
the Ethics Committee of Linyi Central Hospital. Written informed
consent was obtained from all patients.
Reverse transcription-quantitative PCR
(RT-qPCR)
Peripheral blood (250 µl) was mixed with 750 µl
TRIzol® reagent (Thermo Fisher Scientific, Inc.) for
lysis. Then, total RNA was extracted using the phenol-chloroform
method. The concentration and quality of RNA was measured using UV
spectrophotometry. cDNA was obtained by RT from 1 µg RNA using the
TIANScript II cDNA First Strand Synthesis kit (Tiangen Biotech Co.,
Ltd.) and RT of miRNA was performed using miRcute miRNA cDNA First
Strand Synthesis kit (Tiangen Biotech Co., Ltd.) according to the
manufacturers' protocols. BeyoFast SYBR-Green qPCR mix kit
(Beyotime Institute of Biotechnology) was used to detect mRNA
expression of CLDN2, using GAPDH as internal reference. The primer
sequences were: CLDN2, forward, 5'-CCTTTATCACCTCAG CCCGT-3' and
reverse, 5'-GCTACCGCCACTCTGTCTTT-3'; and GAPDH, forward,
5'-CGGAGTCAACGGATTTGGTCG TAT-3' and reverse,
5'-AGCCTTCTCCATGGTGGTGAA GAC-3'. The thermocycling conditions were
as follows: 95˚C for 10 min, followed by 40 cycles of 95˚C for 1
min and 60˚C for 30 sec. The expression of miR-331 was determined
by miRcute miRNA RT-PCR kit (Tiangen Biotech Co., Ltd.), using U6
as internal reference. Primer sequences were: miR-331,
5'-GCCCCUGGGCCTATCCTAGAA-3' (single strand primer); and U6,
forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'. The thermocycling conditions were as
follows: 95˚C for 10 min, followed by 40 cycles of 95˚C for 1 min
and 60˚C for 30 sec. The 2-ΔΔCq method (23) was used to calculate relative
expression against the internal reference. Each sample was tested
in triplicate.
Cells
HUVECs (The Cell Bank of Type Culture Collection of
the Chinese Academy of Sciences) were cultured in RPMI-1640 medium
(BD Biosciences) supplemented with 10% FBS (BD Biosciences) at 37˚C
and 5% CO2. A total of 2x105 cells/well were seeded in
24-well plates and divided into negative control (NC) and sepsis
groups. When reaching 70-90% confluence, 250 µl serum from sepsis
patients and 250 µl RPMI-1640 with 10% FBS were added to the cells
in the sepsis group, and 250 µl serum from healthy subjects and 250
µl RPMI-1640 with 10% FBS were added to the cells in the NC group.
HUVECs were cultured for 24 h before subsequent tests were
performed as previously described (24,25).
To transfect HUVECs with miR-NC (cat. no. B04001;
Shanghai GenePharma Co., Ltd.) or miR-331 mimics
(5'-CTAGGTATGGTCCCAGGGATCC-3'; Shanghai GenePharma Co., Ltd.) cells
(2x105) were seeded in 24-well plates and cultured in
RPMI-1640 with 10% FBS until 70% confluence was reached. In the
first vial, 1.5 µl miR-331 mimic (20 pmol/µl; Hanbio Biotechnology
Co., Ltd.) was mixed with 50 µl Opti-MEM (Thermo Fisher Scientific,
Inc.). In the second vial, 1 µl Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) was mixed with 50 µl Opti-MEM. After incubation
at room temperature for 5 min, the two vials were combined and
incubated at room temperature for 20 min. Then, the mixtures were
added to cells in the respective groups. Six hours later, the
medium was replaced with RPMI-1640 with 10% FBS. After cultivation
for 48 h, cells were collected for further assays.
For infection with Lv-puro-CLDN2 overexpression
vector, HUVECs (1x105) were seeded in 24-well plates and
cultured until 70% confluence was reached. Then, Lv-puro-NC and
Lv-puro-CLDN2 lentiviral vectors were added to the cells
(multiplicity of infection, 20; Hanbio Biotechnology Co., Ltd.).
After incubation at 37˚C with 5% CO2 for 12 h, the
medium was replaced with RPMI-1640 containing 1 µg/ml puromycin
(cat. no. A1113803; Gibco; Thermo Fisher Scientific, Inc.) prior to
cultivation for a further 72 h.
For rescue experiments, HUVECs (2x105)
from the NC and CLDN2 overexpression groups were seeded in 24-well
plates containing RPMI-1640 with 10% FBS. When 60% confluence was
reached, HUVECs were transfected with miR-NC and miR-331 mimics as
described above. After cultivation for 48 h, cells were collected
for further analysis.
CCK-8 assay
HUVECs (2x103 cells/well) in the NC,
CLDN2 mimics and rescue (miR-331 upregulation) groups were seeded
in 96-well plates. At 0, 24, 48 and 72 h, 20 µl CCK-8 (5 g/l) was
added to the cells, which were then incubated at 37˚C for 30 min.
Then, the absorbance was measured at 490 nm and the results were
used to plot cell proliferation curves. Each group was tested in
three replicate wells and the mean values were determined.
Flow cytometry
At 24 h after transfection, cells (1x106)
from each group were washed with pre-cooled PBS (2X) and subjected
to flow cytometry using the Cell Cycle Assay kit (BD Biosciences)
to detect the cell cycle distribution according to the
manufacturer's instructions. The data were analyzed using ModFit
software (v4.1; Verity Software House, Inc.).
After treatment with serum from healthy subjects or
sepsis patients for 24 h, HUVECs (1x106) in each group
were washed with pre-cooled PBS (2X) and subjected to flow
cytometry using the ANXN V-FITC Apoptosis Detection kit I (BD
Biosciences) to detect apoptosis according to the manufacturer's
instructions. Annexin V-positive cells were early apoptotic,
PI-positive cells were necrotic and double-positive cells were late
apoptotic (CellQuest v5.1; BD Biosciences).
Transwell assay
In the upper chamber of 24-well Transwell chambers
(pore diameter, 8 µm; Corning Inc.), 200 µl serum-free RPMI-1640
containing 2x105 HUVECs from each group was added. In
addition, 600 µl RPMI-1640 with 10% FBS was added to the lower
chamber. After 24 h, the Transwell insert was removed and the cells
in the upper chamber were wiped off. After fixing with 4%
formaldehyde at room temperature for 10 min, the membrane was
stained using Giemsa staining at room temperature for 2 min for
light microscopic observation of five random fields (magnification,
x100). The number of migrated cells was calculated.
Western blotting
Cells in each group were trypsinized and collected.
Precooled RIPA lysis buffer (600 µl; Beyotime Institute of
Biotechnology) was added to the samples. After lysis for 30 min on
ice, the mixture was centrifuged at 14,000 x g for 10 min at 4˚C.
The protein concentration in the supernatant was determined by
bicinchoninic acid assay. Protein samples (20 µg) were then mixed
with 5X SDS loading buffer before denaturation in a boiling water
bath for 10 min. Then, samples (15 µg) were separated on 10%
SDS-PAGE gels and transferred to polyvinylidene difluoride
membranes on ice. Membranes were blocked with 50 g/l skimmed milk
at room temperature for 1 h followed by incubation with rabbit
anti-human CLDN2 polyclonal primary antibody (1:1,000; cat. no.
ab53032; Abcam) and mouse anti-human GAPDH primary antibody
(1:4,000; cat. no. AF0006; Beyotime Institute of Biotechnology) at
4˚C overnight. After washing with PBS containing Tween-20 (1%) five
times at room temperature for 5 min, the membranes were incubated
with goat anti-rabbit (cat. no. sc-2004) and goat anti-mouse (cat.
no. sc-2005) immunoglobulin G horseradish peroxidase-conjugated
secondary antibodies (1:3,000; Santa Cruz Biotechnology, Inc.) for
1 h at room temperature before repeating the washing step.
Membranes were developed using an enhanced chemiluminescence
detection kit (Sigma-Aldrich; Merck KGaA) for imaging. Image lab
v3.0 (Bio-Rad Laboratories, Inc.) was used to analyze the signals
and the CLDN2/GAPDH ratio was determined.
Bioinformatics
To understand the regulatory mechanism of CLDN2,
TargetScan (http://www.targetscan.org) was used
to predict miRNA molecules that may regulate CLDN2.
Dual luciferase reporter assay
Based on the bioinformatics results, wild-type (WT)
and mutant seed regions of the 3'-UTR of CLDN2 that is predicted to
interact with miR-331 were synthesized in vitro utilizing
the SpeI and HindIII restriction sites and cloned
into the pMIR-REPORT luciferase reporter plasmid (cat. no. D2106;
Beyotime Institute of Biotechnology). Plasmids (0.5 µg) containing
the negative control (NC) for the WT, the WT, the NC for mutant or
the mutant 3'-UTR sequences were co-transfected with miR-331 mimics
(100 nM; 5'-CTAGGTATGGTCCCAGGGATCC-3'; Sangon Biotech Co., Ltd.)
into 293 cells (The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences) using Lipofectamine™ 2000 according to
the manufacturer's instructions (Invitrogen; Thermo Fisher
Scientific, Inc.). After cultivation for 24 h, cells were lysed and
analyzed using the dual luciferase reporter assay kit (Promega
Corporation) according to the manufacturer's instructions and the
fluorescence intensity was measured using a GloMax 20/20
luminometer (Promega Corporation). Renilla was used as an
internal reference.
Statistical analysis
The results were analyzed using SPSS 17.0 (IBM
Corp.). The data are expressed as the mean ± standard deviation
(n≥3). Comparisons between two groups were performed using
Student's t-test. Comparisons of >2 groups were performed by
one-way ANOVA followed by Student-Newman-Keuls tests. Spearman's
correlation analysis was performed to assess correlation. P<0.05
was considered to indicate a statistically significant
difference.
Results
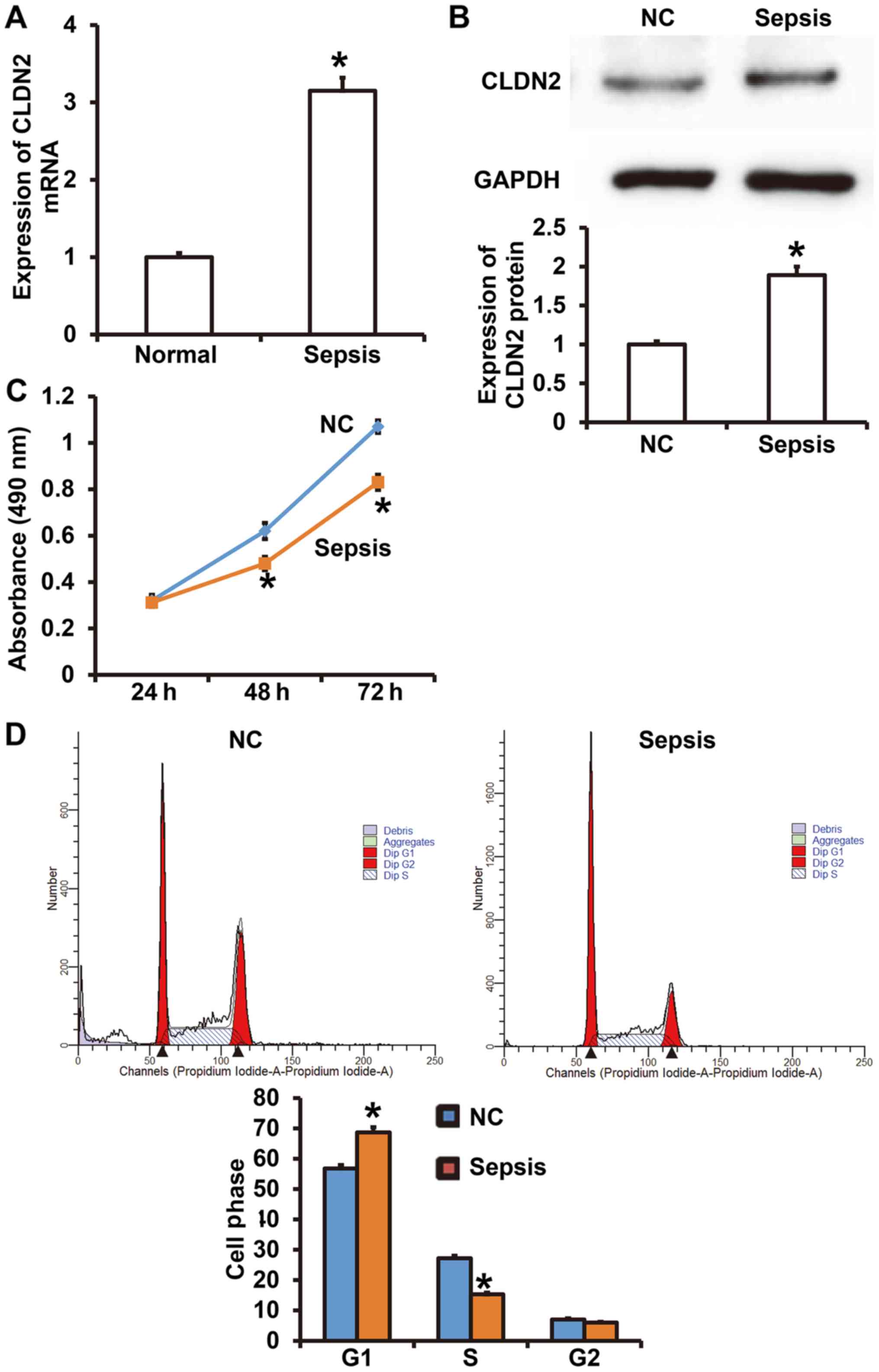
Elevated CLDN2 expression alters the
proliferation and cell cycle of peripheral vascular endothelial
cells
To determine the expression of CLDN2 and understand
how it affects the cellular function of HUVECs, RT-qPCR, Western
blotting, CCK-8 and flow cytometry assays were performed. The data
showed that CLDN2 mRNA levels in the peripheral blood from patients
with sepsis were significantly higher than those in healthy
subjects (P<0.05; Fig. 1A).
Similarly, CLDN2 protein expression in HUVECs treated with serum
from patients with sepsis was significantly increased compared with
that in HUVECs treated with serum from healthy subjects (P<0.05;
Fig. 1B). The CCK-8 assay showed
that the proliferation of HUVECs in the sepsis group was
significantly reduced compared with that in the negative control
group after 48 and 72 h (P<0.05; Fig.
1C). Flow cytometry demonstrated that the treatment of HUVECs
with serum from patients with sepsis decreased transition from the
G1 phase to the S phase of the cell cycle compared with that in
HUVECS treated with serum from healthy controls (P<0.05;
Fig. 1D). The results suggest that
elevated CLDN2 expression altered the proliferation and cell cycle
of peripheral vascular endothelial cells.
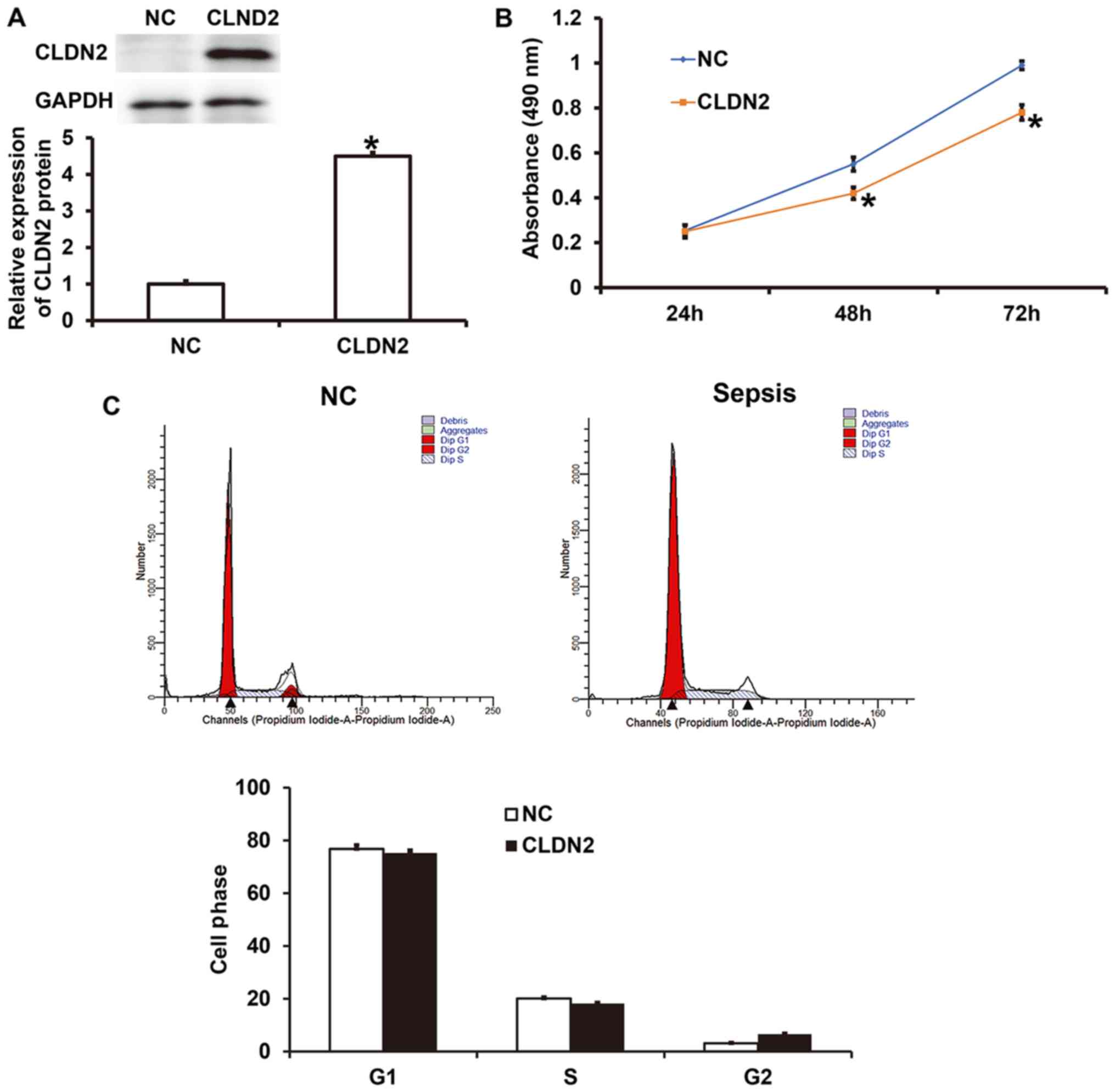
CLDN2 overexpression inhibits the
proliferation of HUVECs via mechanisms other than those affecting
the cell cycle
To test how CLDN2 overexpression affected the
proliferation of HUVECs, an analysis, including CCK-8 assays was
performed. Western blotting demonstrated that the expression of
CLDN2 in HUVECs transfected with a CLDN2 overexpression vector was
significantly higher than that in HUVECs transfected with the NC
vector (P<0.05; Fig. 2A). The
results of the CCK-8 assay showed that the proliferation of HUVECs
overexpressing CLDN2 was significantly lower compared with that of
the NC group after 48 and 72 h (P<0.05; Fig. 2B). In addition, flow cytometric
analysis showed that the overexpression of CLDN2 did not
significantly change the ratio of cells in the G1, S and G2/M
phases of the cell cycle compared with those in the NC group
(P>0.05; Fig. 2C). These results
indicate that CLDN2 overexpression may inhibit the proliferation of
HUVECs via mechanisms other than via affecting the cell cycle.
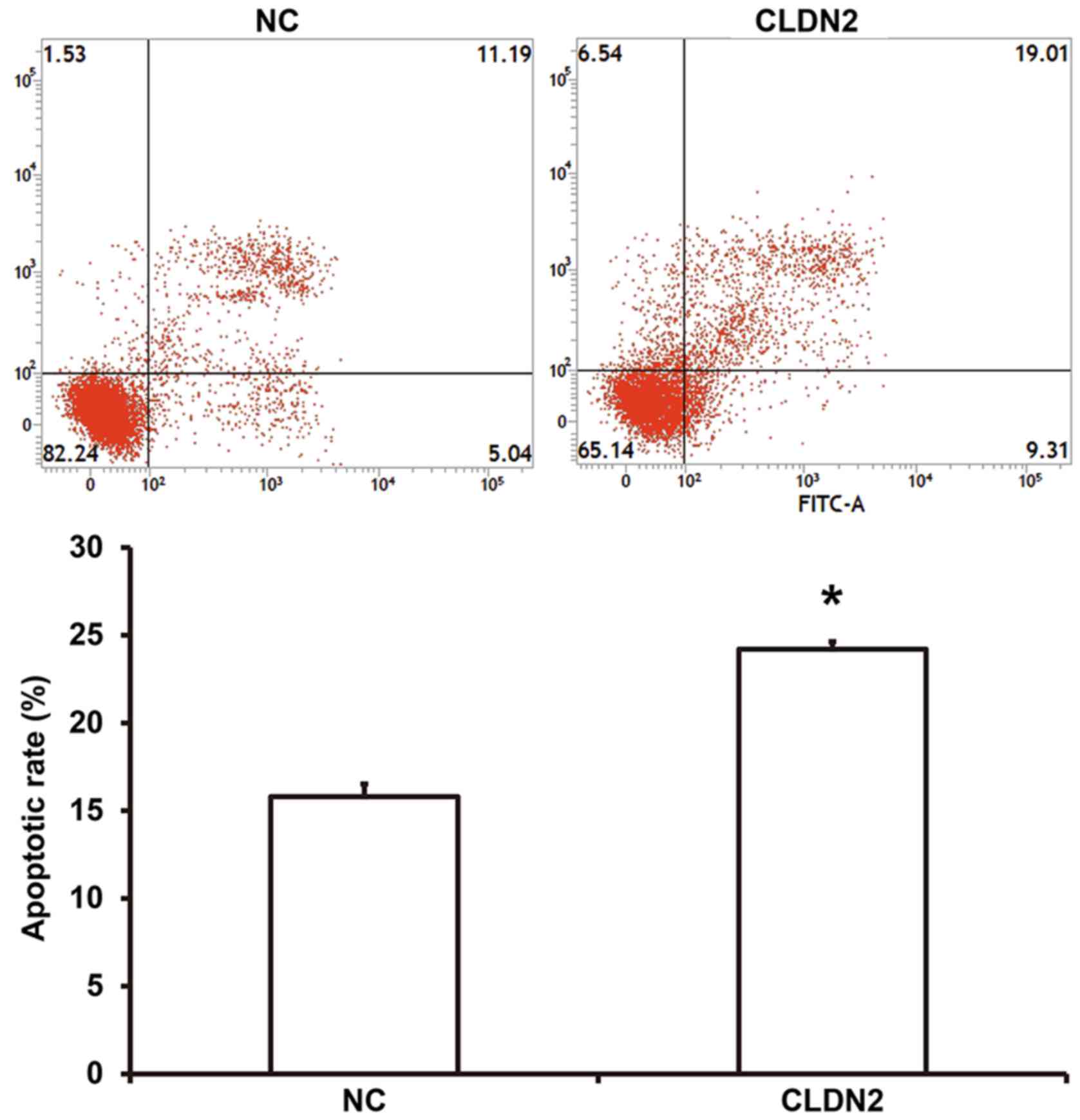
CLDN2 overexpression promotes the
apoptosis of HUVECs
To examine whether CLDN2 overexpression affected the
apoptosis of HUVECs, flow cytometry was performed. The data showed
that number of apoptotic cells was significantly higher in HUVECS
overexpressing CLDN2 compared with the NC group (P<0.05;
Fig. 3). The result suggest that
CLDN2 overexpression promotes the apoptosis of HUVECs.
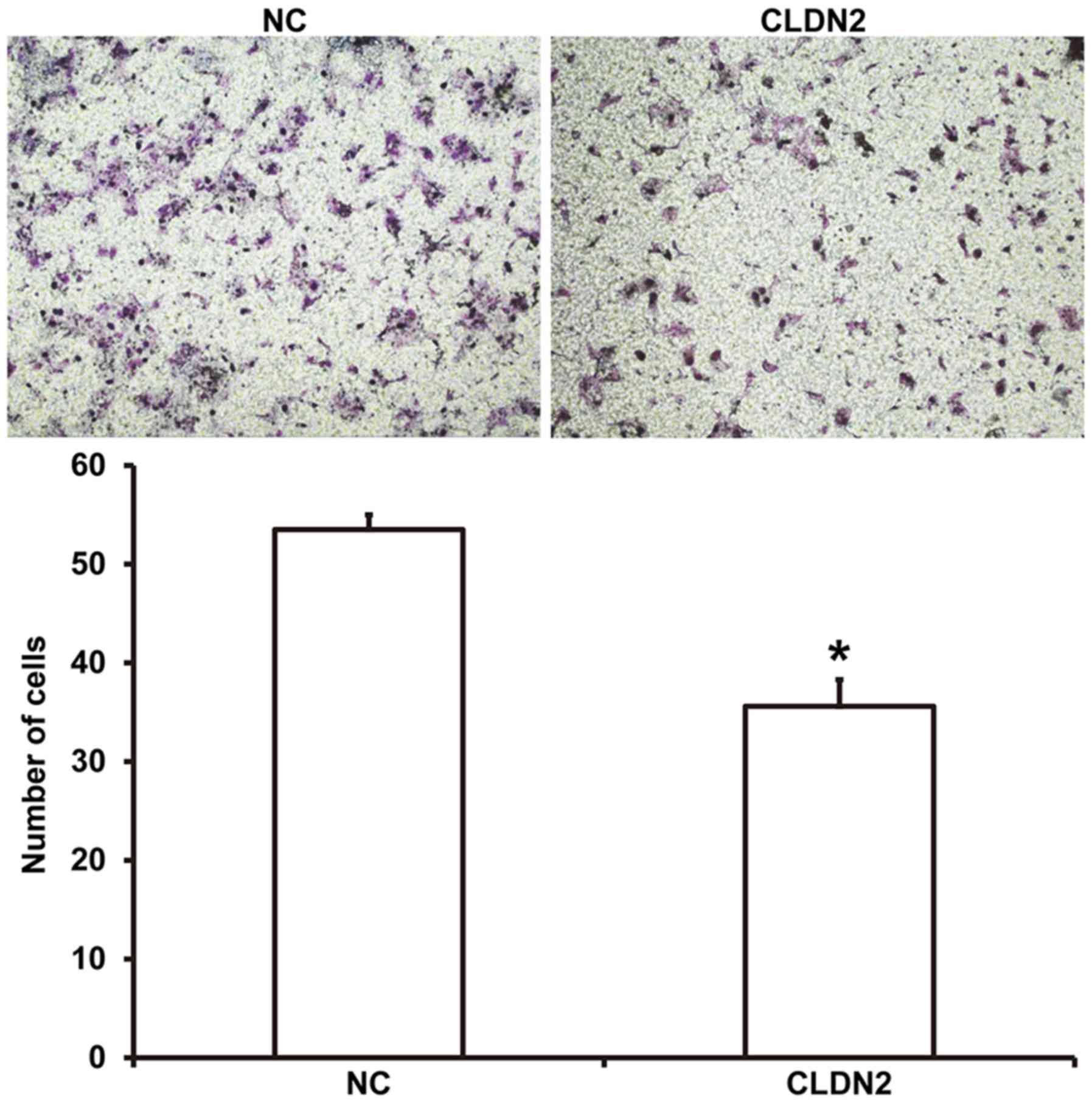
CLDN2 overexpression inhibits HUVEC
migration
To examine the migration ability of HUVECs,
Transwell assays were employed. The data showed that the number of
migrated cells was significantly reduced in CLDN2 overexpressing
cells compared with the NC group (P<0.05; Fig. 4). The result indicates that the
overexpression of CLDN2 inhibits HUVEC migration.
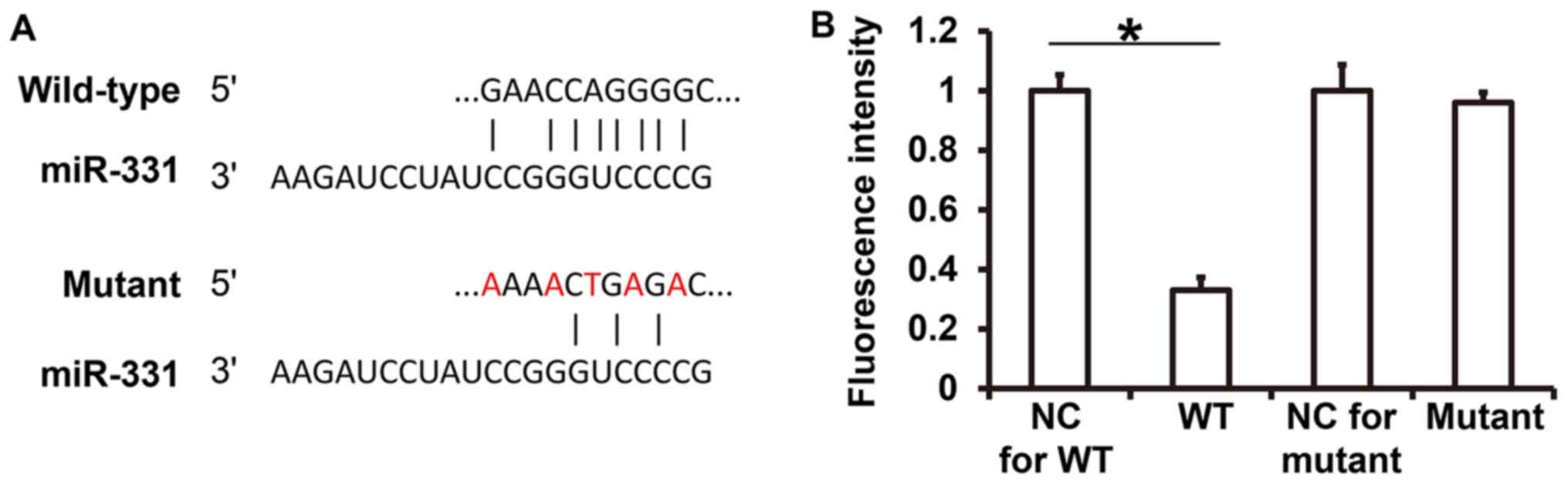
miR-331 binds to the 3'-UTR of CLDN2
and regulates its expression
TargetScan analysis indicated that miR-133 may bind
to the 3'-UTR of CLDN2 (Fig. 5A). To
detect the interaction between miR-331 and CLDN2, a dual luciferase
reporter assay was performed. Renilla activity in cells
co-transfected with agomiR-331 and pMIR-REPORT-WT luciferase
reporter plasmids was significantly lower than in the NC group
(P<0.05; Fig. 5B). By contrast,
the activity of cells co-transfected with agomiR-331 and the
pMIR-REPORT-mutant luciferase reporter plasmids was not
significantly different from that in the NC group (P>0.05;
Fig. 5B). The results suggest that
miR-331 can bind to the 3'-UTR of CLDN2 mRNA and regulate its
expression.
Upregulation of miR-331 expression
inhibits the expression of CLDN2 and restores the proliferation,
apoptosis and migration of HUVECs
To study the association of miR-331 with CLDN2
expression in the peripheral blood of patients with sepsis, a
correlation analysis was performed. The data indicate that miR-331
expression was negatively correlated with CLDN2 mRNA expression in
15 patients with sepsis (Fig. 6A).
RT-qPCR analysis confirmed that the expression level of miR-331 in
HUVECs transfected with miR-331 mimics was significantly higher
than that in HUVECs transfected with miR-NC (P<0.05; Fig. 6B). At the cellular level, the CLDN2
protein expression in HUVECs transfected with miR-331 mimics was
significantly lower than that in the miR-NC group (P<0.05;
Fig. 6C). In the CCK-8 assay, rescue
experiments showed that the upregulation of miR-331 significantly
increased the absorbance at 48 or 72 h in HUVECs overexpressing
CLDN2 (rescue group) compared with that in HUVECS overexpressing
CLDN2 and transfected with miR-NC (CLDN2 group) (P<0.05), and
restored the absorbance to levels similar to those in the NC group
(P>0.05; Fig. 6D). Flow cytometry
showed that the upregulation of miR-331 significantly reduced the
apoptotic rate in the rescue group compared with that in the CLDN2
group (P<0.05), and restored apoptosis to a level similar to
that in the NC group (P>0.05; Fig.
6E). In addition, the Transwell assay showed that the
upregulation of miR-331 significantly elevated the migration
ability of the rescue group compared with that in the CLDN2 group
(P<0.05), and raised it to a level comparable with that of the
NC group (P>0.05; Fig. 6F). The
results indicate that upregulation of miR-331 expression inhibits
the expression of CLDN2 and restores nearly normal proliferation,
apoptosis and migration to HUVECs.
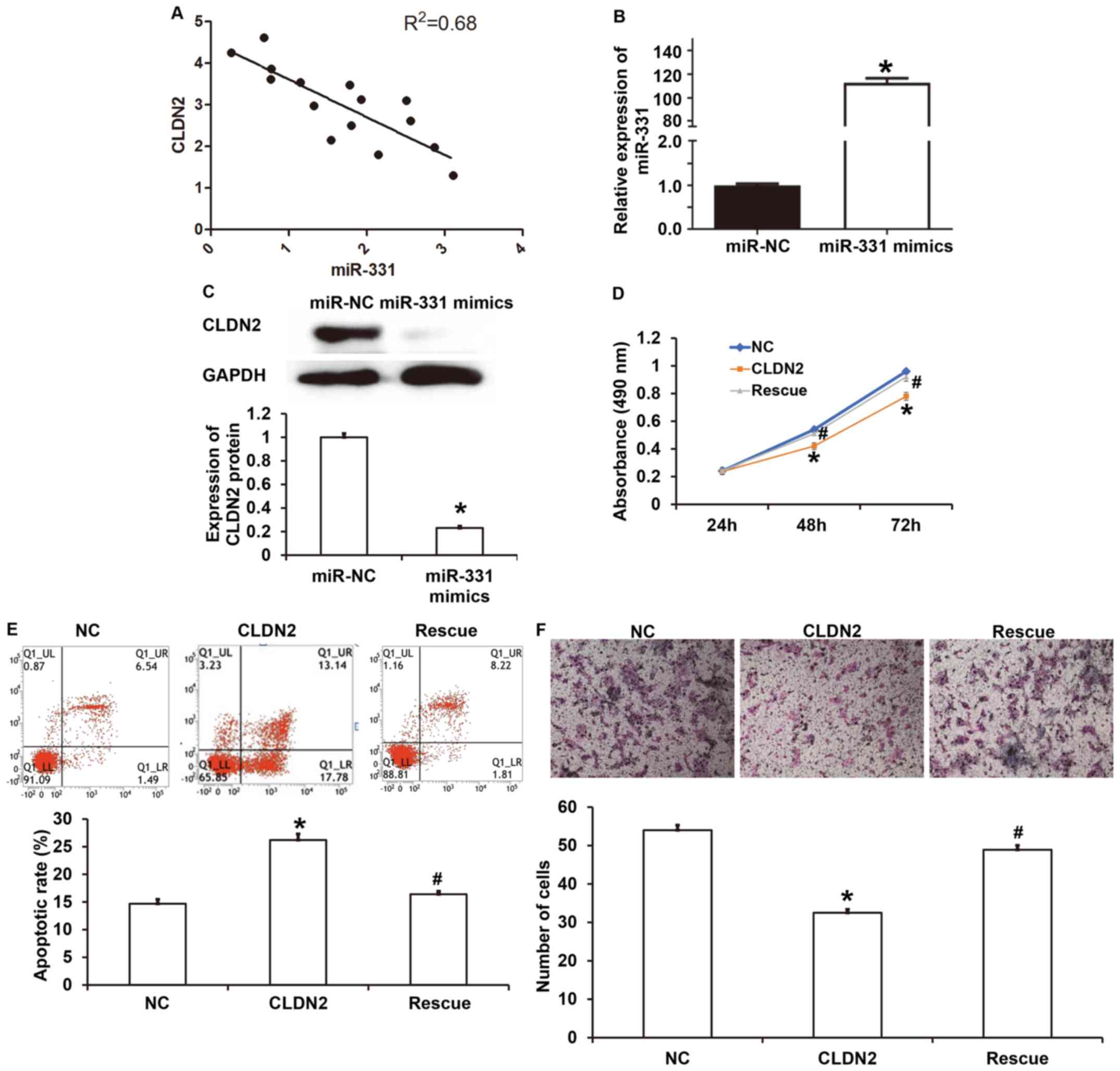 | Figure 6Correlation between miR-331 and CLDN2
and their effect on cellular functions. (A) Correlation between
miR-331 expression and CLDN2 expression in peripheral blood from
patients with sepsis analyzed using Spearman's correlation
analysis. (B) Expression level of miR-331 in HUVECs transfected
with miR-NC or miR-331 mimics. Reverse transcription-quantitative
PCR was used to determine the expression of miR-331. Student's
t-test was used for statistical analysis. *P<0.05 vs.
miR-NC group. (C) Expression of CLDN2 protein in HUVECs transfected
with miR-NC or miR-331 mimics as determined using Western blotting.
Student's t-test was used for statistical analysis.
*P<0.05 vs. miR-NC group. (D) Proliferation of HUVECs
in the NC, CLDN2 and rescue groups. Cells in the NC group were not
transfected, those in CLDN2 group stably expressed CLDN2, and those
in the rescue group stably expressed CLDN2 and were transfected
with miR-331. A Cell Counting Kit-8 assay was used to determine the
proliferation. One-way ANOVA followed by Student-Newman-Keuls tests
were used for statistical analysis. *P<0.05 vs. NC
and #P<0.05 vs. CLDN2 at the same time point. No
statistically significant difference was observed between the NC
and rescue groups. (E) Apoptotic rate of HUVECs in the negative
control, CLDN2 and rescue groups as determined by flow cytometry.
One-way ANOVA followed by Student-Newman-Keuls tests were used for
statistical analysis. *P<0.05 vs. NC and
#P<0.05 vs. CLDN2. No statistically significant
difference was observed between the NC and rescue groups. (F)
Migration ability of HUVECs in the NC, CLDN2 and rescue groups as
determined by Transwell assay. Magnification, x100.
*P<0.05 vs. NC and #P<0.05 vs. CLDN2.
One-way ANOVA followed by Student-Newman-Keuls tests were used for
statistical analysis. No statistically significant difference was
observed between the NC and rescue groups. CLDN2, claudin-2; WT,
wild-type; UTR, untranslated region; NC, negative control. |
Discussion
Sepsis is a clinically common complication. Without
timely treatment, it often induces systemic inflammatory responses,
circulatory failure, systemic organ damage or even death (26). Vascular endothelial cells are natural
barriers in the walls of blood vessels, and tight junction proteins
can crosslink endothelial cells mechanically, regulate the
transport of intracellular and extracellular substances, and
maintain the vascular endothelial barrier (27). In the present study, it was
discovered that the mRNA of the tight junction protein CLDN2 is
upregulated in the peripheral blood of patients with sepsis. In
vitro experiments revealed that serum from patients with sepsis
promoted the expression of CLDN2 in HUVECs, which inhibited the
proliferation and migration of HUVECs, and promoted apoptosis.
CLDN2 is a member of the claudin family. Claudin
proteins play important roles in the formation and maintenance of
tight junctions among cells (28).
Tight junctions divide the epithelial plasma membrane into apical
and basal sides, resulting in cell polarity, but have selective
permeability to different types of molecules and ions (29). It has been discovered that the
abnormal expression of CLDN2 protein is closely associated with
cell proliferation, migration, apoptosis and inflammation. For
example, the abnormal expression of CLDN2 in proximal renal tubular
epithelial cells often leads to apoptosis, necrosis and detachment,
which induces the occurrence of acute tubular necrosis (30). The infection of endothelial cells
with KSHV downregulates the expression of CLDN2, resulting in
impairment of the endothelial cell barrier (31). Disordered CLDN2 expression also
serves an important role in tumor cells. For example, Du et
al (32) found that
Spi-B-induced silencing of the CLDN2 gene promoted the distal
metastasis of lung cancer cells in mice. In addition, CLDN2 can be
used as an indicator for the prognosis of breast cancer, and higher
expression of CLDN2 usually corresponds to worse prognosis
(33). In the present study, it was
found that the expression of CLDN2 mRNA in the peripheral blood of
patients with sepsis was significantly higher than in healthy
subjects, and it was hypothesized that CLDN2 may be associated with
sepsis and vascular endothelial injury. For HUVECs cultured in the
presence of serum from patients with sepsis, the expression of
CLDN2 was significantly elevated, the proliferation and migration
of the HUVECs were reduced, and apoptosis was increased.
Furthermore, upregulation of CLDN2 expression inhibited the
proliferation and migration of HUVECs, and promoted their
apoptosis. These results also suggest that CLDN2 promotes the
damage of HUVECs and may serve an important role in vascular
endothelial injury in sepsis.
miR-331 is a type of miRNA that is located at
12q22(34). In an immortalized
lymphoblastoid cell line, miR-331 was shown to be associated with
the expression of a variety of cell cycle-associated mRNAs,
suggesting that miR-331 may be associated with cell proliferation
(35). Also, a study revealed that
p53 deficiency induces the expression of miR-331 in mouse and
embryo development (36).
Furthermore, it has been reported that miR-331-3p is abnormally
expressed in a number of tumors, such as gastric and prostate
cancer, and is closely associated with the proliferation and
migration of tumor cells (37). In
the present study, bioinformatics analysis indicated that the
3'-UTR of CLDN2 contains binding sites for miR-331, suggesting that
miR-331 may be involved the regulation of CLDN2 expression. A dual
luciferase reporter assay confirmed that miR-331 directly binds
with the 3'-UTR of CLDN2 in vitro, suggesting that CLDN2 is
a direct target gene of miR-331. Moreover, in the present study,
upregulation of miR-331 decreased the expression of CLDN2 in
HUVECs, and miR-331 overexpression reversed the effect of CLDN2 on
the cellular functions of HUVECs. These results indicate that CLDN2
expression in HUVECs is regulated by miR-331. However, more
evidence is required to confirm the direct regulatory relationship
between miR-331 and CLDN2. At present, the most convincing
experiment the present authors were able to perform to demonstrate
the direct interaction between miR-331 and CLDN2 is dual luciferase
report assay. Future studies are planned to obtain improved
experimental data, such as by the use of in situ
hybridization or RT-qPCR. A further limitation of the present study
is that the biological functions of CLDN2 were only investigated
in vitro. The functions of CLDN2 in endothelial cell injury
in vivo remain to be determined in future studies.
In conclusion, the present study demonstrated that
the expression of CLDN2 is upregulated in the peripheral blood of
patients with sepsis. In addition, CLDN2 upregulation is directly
associated with miR-331 downregulation, leading to the inhibition
of proliferation and migration, and the promotion of apoptosis of
vascular endothelial cells.
Acknowledgements
The authors would like to thank Dr Zhigang Zhang,
the Director of Linyi Central Hospital, for his supervision of the
study, and Dr Wenhong Peng, the Director of Department of Critical
Care Medicine, Linyi Central Hospital, for advice regarding the
writing up of the article.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LK and JL contributed to the design of the study. LK
and PW performed the experiments. LK and JL analysed the data,
interpreted the results and prepared the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Ethics Committee
of Linyi Central Hospital. Written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rhee C and Klompas M: Sepsis trends:
Increasing incidence and decreasing mortality, or changing
denominator? J Thorac Dis. 12 (Suppl 1):S89–S100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kuchler L, Sha LK, Giegerich AK, Knape T,
Angioni C, Ferreirós N, Schmidt MV, Weigert A, Brüne B and von
Knethen A: Elevated intrathymic sphingosine-1-phosphate promotes
thymus involution during sepsis. Mol Immunol. 90:255–263.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chao CH, Chen HR, Chuang YC and Yeh TM:
Macrophage migration inhibitory factor-induced autophagy
contributes to thrombin-triggered endothelial hyperpermeability in
sepsis. Shock. 50:103–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bobelytė O, Gailiūtė I, Zubka V and
Žilinskaitė V: Sepsis epidemiology and outcome in the paediatric
intensive care unit of Vilnius University Children's Hospital. Acta
Med Litu. 24:113–120. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Malik IA, Cardenas-Turanzas M, Gaeta S,
Borthakur G, Price K, Cortes J and Nates JL: Sepsis and acute
myeloid leukemia: A population-level study of comparative outcomes
of patients discharged from Texas hospitals. Clin Lymphoma Myeloma
Leuk. 17:e27–e32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dombrovskiy VY, Martin AA, Sunderram J and
Paz HL: Rapid increase in hospitalization and mortality rates for
severe sepsis in the United States: A trend analysis from 1993 to
2003. Crit Care Med. 35:1244–1250. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Raju R: Immune and metabolic alterations
following trauma and sepsis - An overview. Biochim Biophys Acta Mol
Basis Dis. 1863:2523–2525. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Katayama S, Nunomiya S, Koyama K, Wada M,
Koinuma T, Goto Y, Tonai K and Shima J: Markers of acute kidney
injury in patients with sepsis: The role of soluble thrombomodulin.
Crit Care. 21(229)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sergi C, Shen F, Lim DW, Liu W, Zhang M,
Chiu B, Anand V and Sun Z: Cardiovascular dysfunction in sepsis at
the dawn of emerging mediators. Biomed Pharmacother. 95:153–160.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schaalan M and Mohamed W: Predictive
ability of circulating osteoprotegerin as a novel biomarker for
early detection of acute kidney injury induced by sepsis. Eur
Cytokine Netw. 28:52–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rieg S, Bechet L, Naujoks K, Hromek J,
Lange B, Juzek-Küpper MF, Stete K, Müller MC, Jost I, Kern WV, et
al: A single-center prospective cohort study on postsplenectomy
sepsis and its prevention. Open Forum Infect Dis.
7(ofaa050)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pluskota E, Bledzka KM, Bialkowska K,
Szpak D, Soloviev DA, Jones SV, Verbovetskiy D, Plow EF, et al:
Kindlin-2 interacts with endothelial adherens junctions to support
vascular barrier integrity. J Physiol. 595:6443–6462.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Sun K, Lei Y, Wang R, Wu Z and Wu G:
Cinnamicaldehyde regulates the expression of tight junction
proteins and amino acid transporters in intestinal porcine
epithelial cells. J Anim Sci Biotechnol. 8(66)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Thuringer D, Solary E and Garrido C: The
microvascular gap junction channel: A route to deliver MicroRNAs
for neurological disease treatment. Front Mol Neurosci.
10(246)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li X, Song G, Zhao Y, Zhao F, Liu C, Liu
D, Li Q and Cui Z: Claudin7b is required for the formation and
function of inner ear in zebrafish. J Cell Physiol. 233:3195–3206.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kolosov D, Bui P, Wilkie MP and Kelly SP:
Claudins of sea lamprey (Petromyzon marinus) -
organ-specific expression and transcriptional responses to water of
varying ion content. J Fish Biol. 96:768–781. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Taylor NA, Vick SC, Iglesia MD, Brickey
WJ, Midkiff BR, McKinnon KP, Reisdorf S, Anders CK, Carey LA,
Parker JS, et al: Treg depletion potentiates checkpoint inhibition
in claudin-low breast cancer. J Clin Invest. 127:3472–3483.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Chung YH, Li SC, Kao YH, Luo HL, Cheng YT,
Lin PR, Tai MH and Chiang PH: MiR-30a-5p inhibits
epithelial-to-mesenchymal transition and upregulates expression of
tight junction protein claudin-5 in human upper tract urothelial
carcinoma cells. Int J Mol Sci. 18(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahmad R, Kumar B, Chen Z, Chen X, Müller
D, Lele SM, Washington MK, Batra SK, Dhawan P and Singh AB: Loss of
claudin-3 expression induces IL6/gp130/Stat3 signaling to promote
colon cancer malignancy by hyperactivating Wnt/β-catenin signaling.
Oncogene. 36:6592–6604. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hashimoto Y, Shirakura K, Okada Y, Takeda
H, Endo K, Tamura M, Watari A, Sadamura Y, Sawasaki T, Doi T, et
al: Claudin-5-binders enhance permeation of solutes across the
blood-brain barrier in a mammalian model. J Pharmacol Exp Ther.
363:275–283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tesfaye D, Gebremedhn S, Salilew-Wondim D,
Hailay T, Hoelker M, Grosse-Brinkhaus C and Schellander K:
MicroRNAs: tiny molecules with significant role in mammalian
follicular and oocyte development. Reproduction. 155:R121–R135.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Song L, Li D, Li X, Ma L, Bai X, Wen Z,
Zhang X, Chen D and Peng L: Exposure to PM2.5 induces aberrant
activation of NF-κB in human airway epithelial cells by
downregulating miR-331 expression. Environ Toxicol Pharmacol.
50:192–199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) μethod. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mao FY, Kong H, Zhao YL, Peng LS, Chen W,
Zhang JY, Cheng P, Wang TT, Lv YP, Teng YS, et al: Increased
tumor-infiltrating CD45RA-CCR7- regulatory T-cell subset with
immunosuppressive properties foster gastric cancer progress. Cell
Death Dis. 8(e3002)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Peng LS, Zhang JY, Teng YS, Zhao YL, Wang
TT, Mao FY, Lv YP, Cheng P, Li WH, Chen N, et al: Tumor-associated
monocytes/macrophages impair NK-cell function via TGFβ1 in human
gastric cancer. Cancer Immunol Res. 5:248–256. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kam HJ and Kim HY: Learning
representations for the early detection of sepsis with deep neural
networks. Comput Biol Med. 89:248–255. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tham CL, Hazeera Harith H, Wai Lam K,
Joong Chong Y, Singh Cheema M, Roslan Sulaiman M, Hj Lajis N and
Ahmad Israf D: The synthetic curcuminoid BHMC restores
endotoxin-stimulated HUVEC dysfunction: Specific disruption on
enzymatic activity of p38 MAPK. Eur J Pharmacol. 749:1–11.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sumitomo T: Streptococcus pyogenes
translocates across an epithelial barrier. Nippon Saikingaku
Zasshi. 72:213–218. 2017.PubMed/NCBI View Article : Google Scholar : (Ιn Japanese).
|
|
29
|
Rosas-Hernandez H, Cuevas E,
Escudero-Lourdes C, Lantz SM, Gomez-Crisostomo NP, Sturdivant NM,
Balachandran K, Imam SZ, Slikker W Jr, Paule MG, et al:
Characterization of Biaxial stretch as an in vitro model of
traumatic brain injury to the blood-brain barrier. Mol Neurobiol.
55:258–266. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fujii N, Matsuo Y, Matsunaga T, Endo S,
Sakai H, Yamaguchi M, Yamazaki Y, Sugatani J and Ikari A: Hypotonic
stress-induced down-regulation of claudin-1 and -2 mediated by
dephosphorylation and clathrin-dependent endocytosis in renal
tubular epithelial cells. J Biol Chem. 291:24787–24799. 2016.
|
|
31
|
Tan X, Li D, Wang X, Zeng Y, Yan Y and
Yang L: Claudin-2 downregulation by KSHV infection is involved in
the regulation of endothelial barrier function. J Cutan Pathol.
41:630–639. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Du W, Xu X, Niu Q, Zhang X, Wei Y, Wang Z,
Zhang W, Yan J, Ru Y, Fu Z, et al: Spi-B-mediated silencing of
claudin-2 promotes early dissemination of lung cancer cells from
primary tumors. Cancer Res. 77:4809–4822. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ma F, Ding X, Fan Y, Ying J, Zheng S, Lu N
and Xu B: A CLDN1-negative phenotype predicts poor prognosis in
triple-negative breast cancer. PLoS One. 9(e112765)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sierzega M, Kaczor M, Kolodziejczyk P,
Kulig J, Sanak M and Richter P: Evaluation of serum microRNA
biomarkers for gastric cancer based on blood and tissue pools
profiling: The importance of miR-21 and miR-331. Br J Cancer.
117:266–273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Butrym A, Rybka J, Baczyńska D, Tukiendorf
A, Kuliczkowski K and Mazur G: Expression of microRNA-331 can be
used as a predictor for response to therapy and survival in acute
myeloid leukemia patients. Biomarkers Med. 9:453–460.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hosako H, Martin GS, Barrier M, Chen YA,
Ivanov IV and Mirkes PE: Gene and microRNA expression in
p53-deficient day 8.5 mouse embryos. Birth Defects Res A Clin Mol
Teratol. 85:546–555. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cao Y, Chen J, Wang D, Peng H, Tan X,
Xiong D, Huang A and Tang H: Upregulated in hepatitis B
virus-associated hepatocellular carcinoma cells, miR-331-3p
promotes proliferation of hepatocellular carcinoma cells by
targeting ING5. Oncotarget. 6:38093–38106. 2015.PubMed/NCBI View Article : Google Scholar
|