Introduction
Approximately one-fifth of patients with ulcerative
colitis (UC) develop acute severe ulcerative colitis (ASC) during
disease progression (1,2). In patients with ASC, intravenous
glucocorticoids remain the basic treatment. When corticosteroid
cannot control the disease, the treatment strategy should be
adjusted in time to salvage treatment or surgical treatment
(3). Delay in treatment increases
the risk of treatment and surgical complications in ASC patients,
and increases the financial burden of patients (4).
The determination of disease activity is an
important basis for the evaluation of glucocorticoids or the
adjustment of treatment regimens (5). Common methods for determining disease
activity include clinical manifestations (body temperature, heart
rate, times of stool blood), inflammatory indicators [C-reactive
protein (CRP), erythrocyte sedimentation rate (ESR)], and
endoscopic evaluation (6-8). At
present, colonoscopy and, if necessary, colonoscopy specimen is
still the gold standard for UC activity determination, and UC
endoscopic severity score (UCEIS), as an objective and effective
reflection and assessment of endoscopic intestinal mucosal injury,
has largely reduced the subjective factors of clinicians (9-11).
However, colonoscopy at ASC has the risk of causing intestinal
perforation and aggravating toxic enteritis, and repeated
colonoscopy increases patient suffering and risk when evaluating
treatment outcomes (12). Therefore,
it is necessary to implement a non-invasive, safe and accurate
surrogate index reflecting the severity of the lesion under the
endoscope.
Fecal calprotectin (FC) is derived from neutrophils
and macrophages and is released into the feces along with
degranulation of inflammatory cells during intestinal mucosal
inflammation. It is stable in nature and can be stored at room
temperature for 7 days without being decomposed by various bacteria
and enzymes. It has a significant correlation with the degree of
mucosal lesions and the therapeutic effect of the disease, and is a
sensitive marker for the evaluation of inflammatory activity and
therapeutic effects (13-15).
As a non-invasive method, FC has some advantages that are not
available in colonoscopy and hematologic inflammation indicators.
However, in ASC patients, there are few related studies regarding
the correlation between FC level and endoscopic intestinal mucosal
inflammatory activity index and clinical outcomes. This study was
designed to determine the association between FC and UCEIS and ASC
clinical outcomes by measuring FC in patients with ASC of different
endoscopy severity.
Patients and methods
Case selection
A retrospective analysis of UC patients who met our
ASC criteria from January 2014 to April 2016 was performed. The
diagnosis of UC is based on clinical signs and symptoms, laboratory
tests, and pathology; the diagnosis of ASC is based on the
Truelove-Witts criteria (16), which
is bloody purulent stool >6 times/days, accompanied by any one
or more of the following: heart rate >90 beats/min, body
temperature >37.8˚C, hemoglobin (Hb) <10.5 g/dl, CRP >30
mg/dl or ESR >30 mm/h. All the patients underwent rectal
sigmoidoscopy within one week of venous corticosteroid therapy and
FC examination within 3 days of colonoscopy. All patients required
a CT scan of the abdomen. The exclusion criteria were: age <18
years; ASC diagnosis was not clear; treatment data was incomplete,
such as no colonoscopy, no FC results; venous corticosteroid or
biological agents were used within 3 months. The study was approved
by the Ethics Committee of Jining No. 1 People's Hospital (Jining,
China). Signed informed consents were obtained from the patients
and/or guardians.
UCEIS
The UCEIS scoring system consists of 3 parts, and
the cumulative score is the final evaluation, including: vascular
network (score 0-2), bleeding (score 0-3), erosion and ulceration
(score 0-3), total score was 0-8 points (Table I). Two endoscopists independently
performed UCEIS assessments based on colonoscopy images, and the
entire assessment process followed the blinded principle. When the
two results were inconsistent, a senior doctor participated in the
final judgment. According to the UCEIS cumulative score, the
disease activity was divided into three levels: mild (UCEIS=2-4);
moderate (UCEIS=5-6) and severe (UCEIS=7-8).
 | Table IDefinition and composition of
ulcerative colitis endoscopic severity score (UCEIS). |
Table I
Definition and composition of
ulcerative colitis endoscopic severity score (UCEIS).
| Parameters (the most
severe part of the lesion) | Score | Description |
|---|
| Vascular network | Normal (0) | Normal vascular
network, clear branches of capillaries, or blurred or partially
missing edges of capillary networks. |
| | Incomplete occlusion
(1) | The vascular network
is incompletely occluded. |
| | Complete occlusion
(2) | The vascular network
is completely occluded. |
| Bleeding | None (0) | No visible
bleeding. |
| | Mucosal bleeding
(1) | The mucosal surface
has coagulated bleeding spots that can be washed away. |
| | Mild bleeding in the
lumen (2) | A small amount of
active bleeding was seen in the lumen. |
| | Intracavitary
moderate to severe bleeding (3) | Obvious bleeding
observed in the lumen, or mucosal oozing seen after flushing the
lumen, or the mucosa of active bleeding was observed. |
| Erosion and
ulcer | None (0) | Normal mucosa, no
erosion or ulceration. |
| | Erosion (1) | Small (≤5 mm) damaged
mucosa with neat edge, white or yellow. |
| | Superficial ulcer
(2) | The larger (>5 mm)
mucosa is damaged, showing discontinuous fibrin-covered ulcers, but
still superficial. |
| | Deep ulcer (3) | The deep, transparent
mucosa is damaged and the edges are slightly raised. |
Collection and inspection of stool
specimens
Stool samples (5-10 g) were collected within 3 days
of colonoscopy for FC testing. The sample and the extractant were
mixed according to weight: Volume (g/ml)=1:49 and thoroughly
stirred. After centrifugation at high speed (10,000 x g at 4˚C for
5 min), the supernatant was collected for detection of FC, and 1 ml
was reserved for use. Double antibody sandwich enzyme-linked
immunosorbent assay (ELISA) was used to analyze the FC level, and
the microplate reader detection wavelength was 450 nm. If
calprotectin was not detected at a ratio of 1:49, the dilution
ratio was increased until detected.
Treatment strategy and clinical
outcome evaluation
The patient's treatment process and related clinical
decision-making after admission were based on the consensus opinion
on the diagnosis and treatment of inflammatory bowel disease in
China (17), and the patient's
lesion range was based on Montréal classification (E1/E2/E3).
Patients with ASC were routinely given
methylprednisolone 60 mg/day or hydrocortisone 400 mg/day after
admission. The corticosteroid treatment effect was judged after 3-5
days of continuous administration. For patients with ineffective or
partially effective outcomes, prolonged treatment time or salvage
therapy (infliximab or cyclosporine) was given. The rescue
treatment was intravenous infliximab (0, 2, 6 weeks; dose, 5
mg/kg). For patients with persistent signs and symptoms, surgery
was performed immediately after surgical indications such as
gastrointestinal bleeding, intestinal perforation, or toxic
megacolon. Patients with severe malnutrition were given enteral or
parenteral nutritional support depending on their intestinal
condition. For patients with hypoproteinemia [albumin (ALB) <25
g/l], intravenous infusion of ALB was given. Low molecular weight
heparin was routinely administered subcutaneously to prevent
thrombosis.
Main outcome indicators include: i) Ineffective
treatment of glucocorticoid, which indicates that after 3-5 days of
continuous treatment of glucocorticoid, the symptoms did not
improve significantly, and ultimately need to receive salvage
treatment or even surgical treatment. ii) Surgery. After
glucocorticoid or salvage treatment, some patients had continued
deterioration of their symptoms, presenting surgical indications
and eventually requiring surgery. Follow-up time: The median
follow-up time was 14 days from the admission of the patient for
acute severe ulcerative colitis to the discharge at the end of
treatment.
Statistical methods
The data was statistically analyzed using SPSS 20.0
statistical software. A single sample Kolmogorov-Smirnov (K-S) test
was used to test whether the data was normally distributed, and the
median and quartile distance of FC were calculated. Mann-Whitney U
test was used for comparison between non-normal distribution data
sets; other measurement data were expressed as mean ± standard
deviation (mean ± SD), and t-test was performed. Correlation tests
were performed using Spearman's rank correlation, and correlation
coefficients were calculated. Sample size estimation was performed
using MEDCAL 19.0 statistical software was used to calculate the
sample size. The specific parameters were set as follows: Type I
error (α, significance) = 0.05; type II error (β, 1-Power) = 0.10;
area under receiver operating characteristics (ROC) curve = 0.75;
null hypothesis value = 0.5; ratio of sample sizes in
negative/positive groups = 2. After calculation, the minimum sample
size is n=60. The ROC curve was also plotted, and the area under
the curve (AUC) was calculated for analysis. The Youden's index was
used to calculate the diagnostic value, and the sensitivity and
specificity of the diagnostic value were calculated. P<0.05 was
considered statistically significant.
Results
Patient data
In total, 71 patients with ASC were enrolled, and 32
patients achieved remission within 5 days of intravenous steroid
therapy. Of the remaining 39 patients, 16 had surgery due to
worsening symptoms. Eleven patients were treated with prolonged
venous corticosteroid treatment (7-10 days), and the symptoms
improved. Twelve patients were given salvage therapy; 8 patients
were induced to remission, and 4 patients were treated with surgery
after ineffective treatment. A total of 28 patients failed venous
corticosteroid therapy, and 20 patients underwent surgical
treatment during admission. General information of patients is
shown in Table II.
 | Table IIPatient data. |
Table II
Patient data.
| Variable | mean ± SD, n (%) |
|---|
| Sex (% female) | 39 (54.9) |
| Age (years) | 42.1±14.8 |
| Comorbidity | 18 (17.8) |
| Course of disease
(month) | 46.9±64.1 |
| History of smoking
(within one year) | 12 (16.9) |
| Lesion and extent
E2/E3 | 25 (35.2)/46
(64.8) |
| Body temperature
(˚C) | 37.0±0.6 |
| Heart rate
(/min) | 82.5±13.3 |
| Defecation frequency
(/day) | 8.8±4.1 |
| White blood cells
(x107/mm3) | 9.6±6.6 |
| CRP (mg/l) | 37.3±28.6 |
| ESR (mm/h) | 31.9±20.4 |
| Hemoglobin
(g/dl) | 101.3±22.7 |
Differences in FC and hematological
parameters between different UCEIS score groups
FC was statistically different between UCEIS mild
[2-4] and moderate [5-6] (P<0.001) and moderate and severe [7-8]
(P=0.016). There was a significant difference in CRP, Hb and ALB
between UCEIS mild and moderate groups (P=0.023, P=0.011 and
P=0.016, respectively), but there was no difference between
moderate and severe groups (P=0.418, P=0.211 and P=0.071,
respectively). There was no difference in ESR between different
groups (P=0.228 and P=0.401) (Table
III).
 | Table IIIFecal calprotectin, C-reactive
protein, erythrocyte sedimentation rate and hemoglobin. |
Table III
Fecal calprotectin, C-reactive
protein, erythrocyte sedimentation rate and hemoglobin.
| | Mild (2-4) (34) | Moderate (5-6)
(47) | Severe (7-8)
(20) | P1 | P2 |
|---|
| FC (µg/g) | 690.5
(57.7-1827.5) | 1393.1
(603.5-2800.0) | 1872.6
(900.3-2933.7) | <0.001 | 0.016 |
| CRP (mg/l) | 29.1±26.8 | 40.2±26.1 | 47.1±35.4 | 0.023 | 0.418 |
| ESR (mm/h) | 29.1±19.7 | 34.9±21.2 | 29.7±20.1 | 0.228 | 0.401 |
| Hb (g/l) | 111.0±22.4 | 97.7±21.8 | 89.9±18.2 | 0.011 | 0.211 |
| ALB (g/l) | 39.6±11.7 | 31.5±13.7 | 28.2±9.9 | 0.016 | 0.071 |
Correlation between UCEIS and FC and
CRP, ESR and Hb
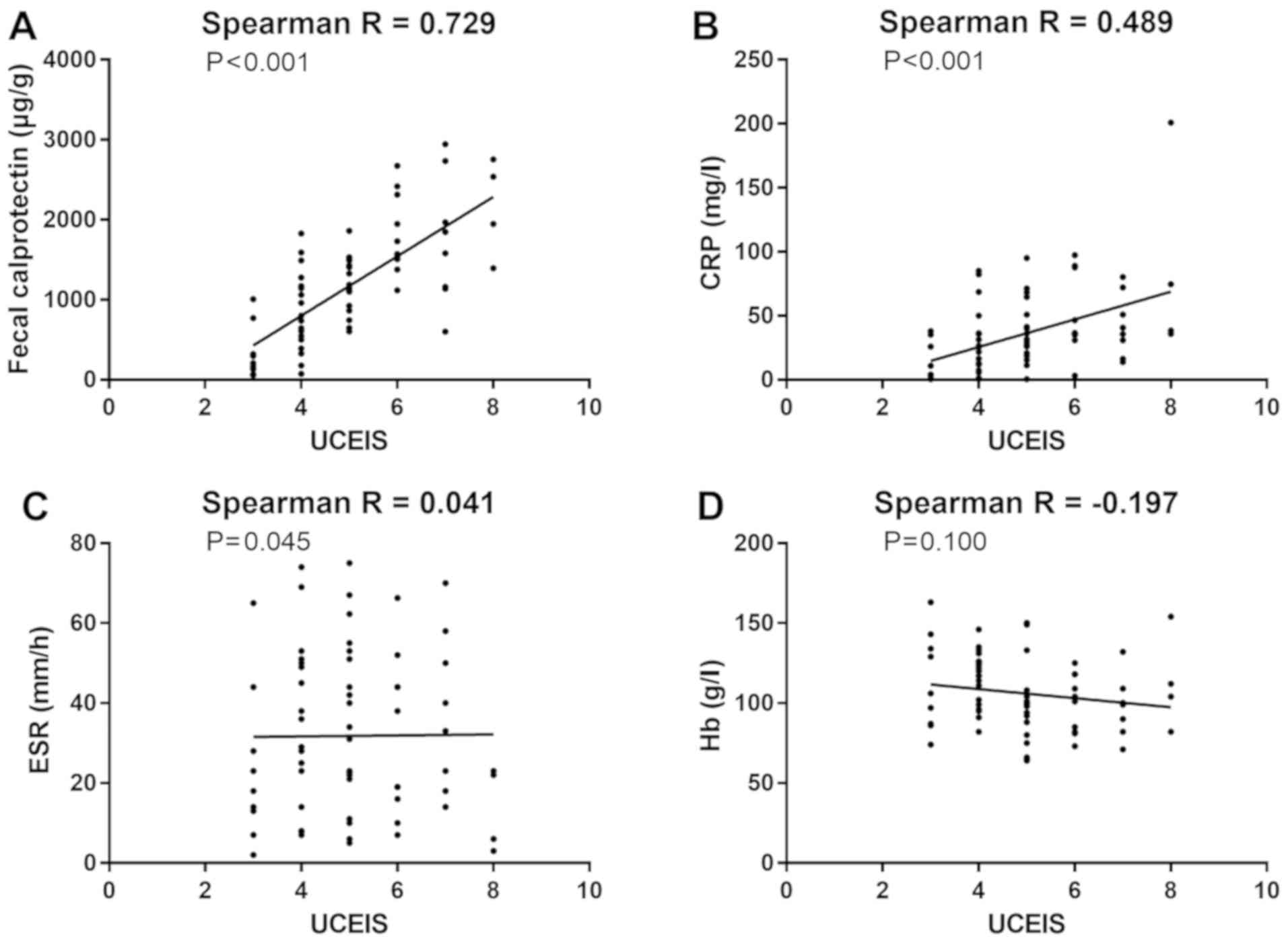
UCEIS was significantly associated with FC level
(r=0.7290, P<0.001), significantly associated with CRP level
(r=0.4889, P<0.001), and had no significant correlation with ESR
(r=0.0405, P=0.736) and Hb (r=-0.1965, P=0.100) (Fig. 1).
FC and clinical outcome in patients
with ASC
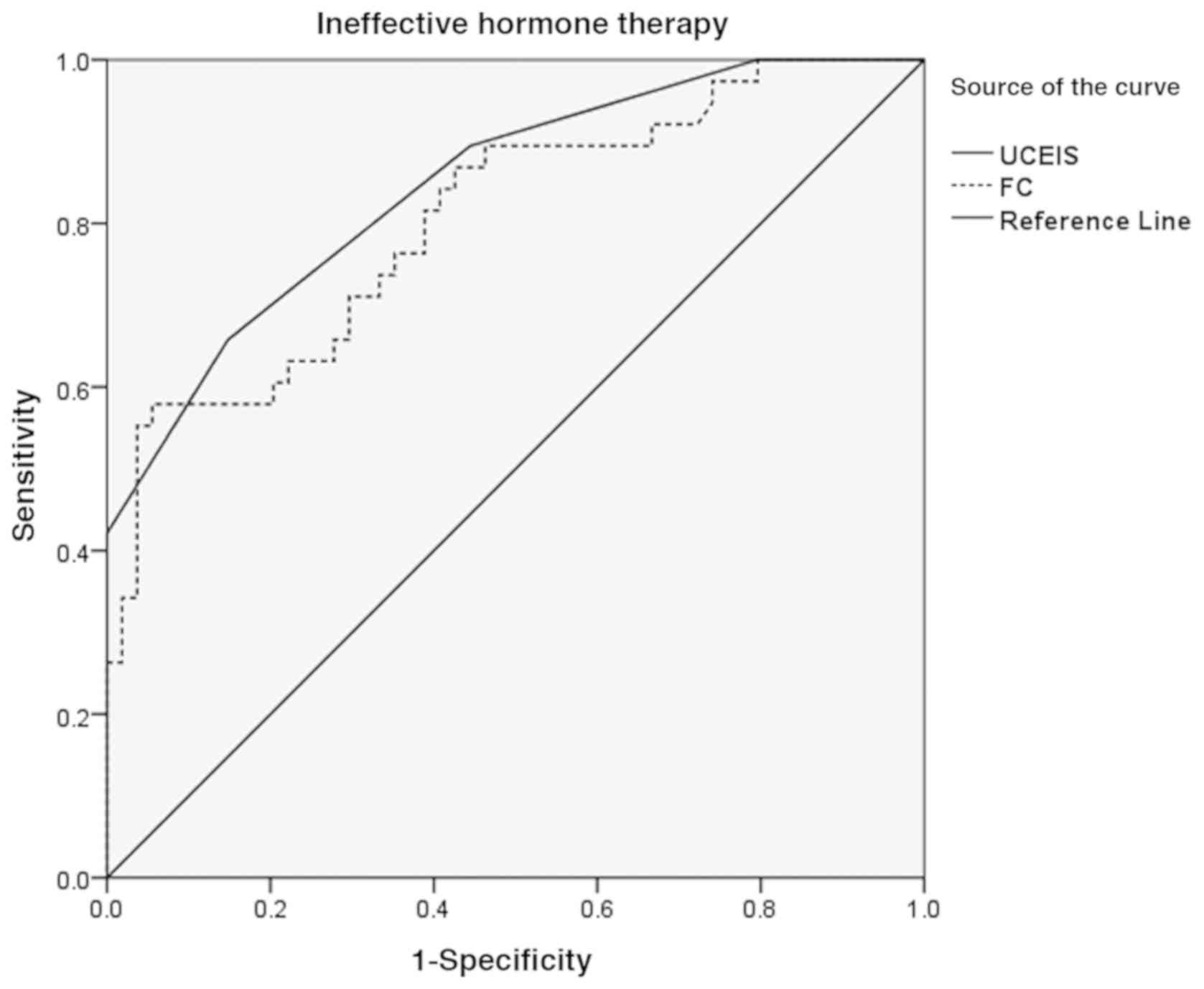
Diagnostic performance of FC on the failure rate
of corticosteroid therapy in patients with ASC. When FC
concentration was >1327 µg/g, UCEIS >5.5 and the
concentration of ALB <29.6 g, Youdon's index was the highest,
which was the best threshold for diagnosing venous corticosteroid
induction failure. Correlation specificity, sensitivity, AUC and
its 95% confidence interval are shown in Table IV. The ROC curve is shown in
Fig. 2.
 | Table IVDiagnostic efficacy of different
cut-off points of UCEIS and fecal calprotectin on clinical outcomes
of ASC. |
Table IV
Diagnostic efficacy of different
cut-off points of UCEIS and fecal calprotectin on clinical outcomes
of ASC.
| Clinical outcome | Indicator | Sensitivity (%) | Specificity (%) | AUC | AUC 95% CI | P-value |
|---|
| Ineffective hormone
therapy | UCEIS >5.5 | 65.8 | 85.2 | 0.847 | 0.768-0.926 | <0.001 |
| | FC >1327
µg/g | 60.5 | 76.4 | 0.805 | 0.692-0.875 | <0.001 |
| | ALB <29.6
g/l | 60.1 | 73.4 | 0.747 | 0.616-0.792 | 0.017 |
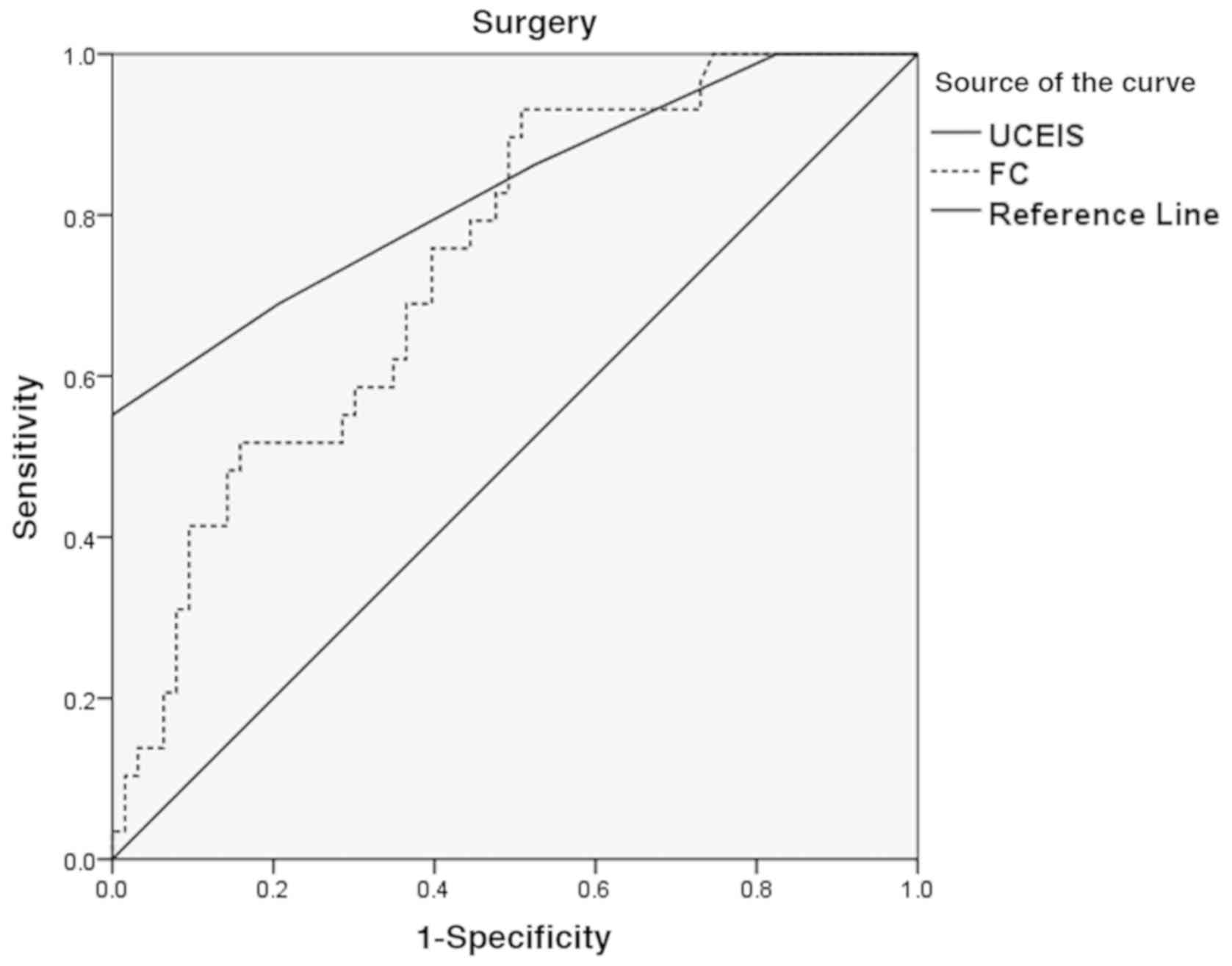
| Surgery | UCEIS >6.5 | 69.0 | 79.4 | 0.831 | 0.733-0.927 | 0.010 |
| | FC >1672
µg/g | 80.2 | 66.7 | 0.740 | 0.638-0.845 | 0.006 |
| | ALB <25.8
g/l | 77.1 | 70.4 | 0.692 | 0.603-0.747 | 0.036 |
Diagnostic performance of FC in patients with
ASC. According to the diagnostic efficacy of different cut-off
points of FC and UCEIS scores in ASC patients, it was found that
when the concentration of FC was >1681 µg/g, UCEIS >6.5 and
the concentration of ALB <25.8 g, Youdon's index was the
highest, which was the best threshold value for predicting the need
for surgery in patients with ASC. The correlation specificity,
sensitivity, AUC and its 95% confidence interval are shown in
Table IV. The ROC curve is shown in
Fig. 3.
Discussion
Approximately 30-40% of ASC patients are unable to
achieve clinical remission even with standard venous corticosteroid
therapy (18). Selecting appropriate
objective indicators to monitor changes in the patient's condition,
together with timely, effective conversion of treatment strategies
can reduce the risk of death in ASC patients and improve the
success rate of salvage treatment or surgery (3,19,20). For
ASC, clinical symptoms cannot fully reflect the activity of the
disease. Blood indicators such as CRP and erythrocyte sedimentation
rate can only be used as indicators of systemic inflammatory
response, but cannot directly reflect endoscopic intestinal mucosal
damage.
Colonoscopy is still the gold standard for
reflecting ASC activity. As an objective evaluation index for
endoscopic intestinal mucosal injury, UCEIS score is receiving
increasing attention. UCEIS is based on intestinal mucosal vascular
network injury, erosive and ulcer status and bleeding, by assessing
the most severe part of mucosal damage, maximizing the
objectiveness of the results, eliminating 86-88% inter-observer
heterogeneity, and significantly correlating with patients'
clinical outcomes. Travis et al (10) found that, when the UCEIS score was
7-8, 13/14 of the symptoms in patients could not be relieved by
intravenous corticosteroid therapy. However, colonoscopy, as an
invasive examination, has a large burden on the patient. It is not
well-accepted by the patients, and cannot continuously monitor the
changes in patient's condition, often restricted in clinical
practice. In patients with ASC, colonoscopy increases the risk of
intestinal mucosal damage, and even results in toxic colitis and
intestinal perforation.
FC detection is a convenient, non-invasive method
for assessing intestinal mucosal damage. As a monitoring and
evaluation tool, it can replace colonoscopy to some extent. FC was
first isolated from neutrophils and monocytes by Fagerhol et
al (15), and released into the
intestinal lumen during the degerming process of inflammatory cells
in the intestinal inflammation site. In patients with IBD, FC is an
important intestinal inflammatory reactive protein, and FC plays a
more important role in UC than Crohn's disease in determining
disease activity. This study found that there was a statistically
significant difference in FC concentrations between different UCEIS
grades, and there was a correlation between the two. By analyzing
CRP, ESR and Hb, it was found that CRP and Hb can distinguish mild
and moderate UCEIS, but lack sensitivity to endoscopic
differentiation of moderate to severe mucosal lesions. After
analyzing the correlation between UCEIS and CRP, ESR and Hb, it was
found that the correlation between UCEIS and the above three
indicators was not good. The results indicated that CRP, as an
inflammatory protein synthesized in the liver, lacks specificity
for local inflammation in the intestine. As an indicator of acute
and chronic infection, ESR is also less sensitive to ASC with
different levels of activity. Patients with ASC are often
accompanied by frequent bloody stools, and the progression of the
disease may lead to decreased hemoglobin levels, but Hb does not
distinguish endoscopic lesions.
The severity of ASC is directly related to the
possibility of surgery. After 3 days of regular adequate steroid
therapy, 85% of patients with hemorrhage more than 8 times/days or
CRP greater than 45 mg/l will need surgery (21). Once a sufficient amount of steroid
therapy for ASC patients for 305 days cannot achieve clinical
remission, the treatment strategy should be switched immediately,
and salvage treatment or surgery should be selected. There are a
variety of clinical indicators for predicting surgery, in which the
ones with good clinical operability and the comprehensive index
composed of reliable and objective basis is applied in clinical
practice, such as Oxford index, Swedish index and Ho index
(21-23).
The Oxford index and the Swedish index both consider CRP and the
number of patients' bowel movements as an important part. The Ho
index lists the expansion of the colon and hypoalbuminemia as an
important part on the basis of the number of bowel movements. All
three indicators have certain predictive value in clinical
practice. For example, when the Swedish index is >8, the
positive predictive value of surgery within 90 days is 69%
(24). However, there are two main
problems with the above clinical indicators. First, the
classification is too simple, and the severity of inflammation in
patients of the same level will be very different. Second, the
indicators are mostly indirect, such as CRP, ALB level or the
number of bowel movement, lack of direct attention to intestinal
mucosal injury. Therefore, in the mid-1960s, Baron et al
(25) introduced the degree of
inflammation of the intestinal mucosa under colonoscopy into the
Baron standard, which further increased the reliability of the
standard.
This study confirmed that venous corticosteroid
therapy had a higher failure rate (AUC=0.805) when the FC
concentration value was >1327 µg/g, and the patient has a higher
degree of surgical potential (AUC=0.740) when the FC concentration
was >1681, as a simple indicator of intestinal mucosal damage.
For the assessment of the condition, FC is relatively convenient to
detect, without damage to the patient, it is easily accepted, and
can be repeatedly tested for real-time monitoring and guidance.
Accurate assessment of the patient's condition and the likelihood
of failure of the patient's corticosteroid therapy can help the
clinician make timely clinical decisions. Insignificant
corticosteroid therapy will reduce the success rate of salvage
treatment and increase the risk of surgery. The delay of treatment
time will also further worsen the patient's general condition, and
increase the complications of surgery.
In conclusion, FC has a good correlation with UCEIS
in ASC patients, and the correlation is better than CRP, ESR and
Hb. Fc is an ideal non-invasive procedure and is significantly
associated with clinical outcomes in ASC patients. Dynamic
monitoring of FC level in ASC patients can help clinicians
accurately assess the likelihood of corticosteroid therapy and
salvage treatment success, and change treatment strategy in a
timely manner.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RMa and RMe conceived and designed the study, and
drafted the manuscript. RMa, XZ, ZS and YL collected, analyzed and
interpreted the experimental data. RMe revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jining No. 1 People's Hospital (Jining, China). Signed informed
consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dinesen LC, Walsh AJ, Protic MN, Heap G,
Cummings F, Warren BF, George B, Mortensen NJ and Travis SP: The
pattern and outcome of acute severe colitis. J Crohn's Colitis.
4:431–437. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Edwards FC and Truelove SC: The course and
prognosis of ulcerative colitis. Gut. 4:299–315. 1963.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Macken L and Blaker PA: Management of
acute severe ulcerative colitis (NICE CG 166). Clin Med (Lond).
15:473–476. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Park KT, Tsai R, Perez F, Cipriano LE,
Bass D and Garber AM: Cost-effectiveness of early colectomy with
ileal pouch-anal anastamosis versus standard medical therapy in
severe ulcerative colitis. Ann Surg. 256:117–124. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Han W, Xu JM, Hu NZ, Mei Q and Liu MW:
Early predictors of responses and clinical outcomes of
corticosteroid treatment for severe ulcerative colitis. Scand J
Gastroenterol. 49:424–433. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Travis S, Satsangi J and Lémann M:
Predicting the need for colectomy in severe ulcerative colitis: A
critical appraisal of clinical parameters and currently available
biomarkers. Gut. 60:3–9. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Walsh AJ, Ghosh A, Brain AO, Buchel O,
Burger D, Thomas S, White L, Collins GS, Keshav S and Travis SP:
Comparing disease activity indices in ulcerative colitis. J Crohn's
Colitis. 8:318–325. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
D'Haens G, Sandborn WJ, Feagan BG, Geboes
K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P,
Schölmerich J, et al: A review of activity indices and efficacy end
points for clinical trials of medical therapy in adults with
ulcerative colitis. Gastroenterology. 132:763–786. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Travis SPL, Schnell D, Krzeski P, Abreu
MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lémann M,
Lichtenstein GR, et al: Developing an instrument to assess the
endoscopic severity of ulcerative colitis: The Ulcerative Colitis
Endoscopic Index of Severity (UCEIS). Gut. 61:535–542.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Travis S, Corte C and Keshav S: Does
disease extent matter when scoring the UCEIS? J Crohn's Colitis.
9(694)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Travis SPL, Schnell D, Feagan BG, Abreu
MT, Altman DG, Hanauer SB, Krzeski P, Lichtenstein GR, Marteau PR,
Mary JY, et al: The impact of clinical information on the
assessment of endoscopic activity: Characteristics of the
ulcerative colitis endoscopic index of severity. J Crohn's Colitis.
9:607–616. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fefferman DS and Farrell RJ: Endoscopy in
inflammatory bowel disease: Indications, surveillance, and use in
clinical practice. Clin Gastroenterol Hepatol. 3:11–24.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Røseth AG, Aadland E, Jahnsen J and
Raknerud N: Assessment of disease activity in ulcerative colitis by
faecal calprotectin, a novel granulocyte marker protein. Digestion.
58:176–180. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lobatón T, Rodríguez-Moranta F, Lopez A,
Sánchez E, Rodríguez-Alonso L and Guardiola J: A new rapid
quantitative test for fecal calprotectin predicts endoscopic
activity in ulcerative colitis. Inflamm Bowel Dis. 19:1034–1042.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fagerhol MK: Calprotectin, a faecal marker
of organic gastrointestinal abnormality. Lancet. 356:1783–1784.
2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goenka MK, Nag S, Kumar A and Pai CG:
Diagnosis of acute severe colitis. Trop Gastroenterol. 35 (Suppl
1):S1–S8. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Chinese Medical Association Digestive
Diseases Branch Inflammatory Bowel Disease Cooperative Group.
Consensus on the diagnosis and treatment of inflammatory bowel
disease in China. Chin J Integr Med. 47:73–79. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bewtra M, Newcomb CW, Wu Q, Chen L, Xie F,
Roy JA, Aarons CB, Osterman MT, Forde KA, Curtis JR, et al:
Mortality associated with medical therapy versus elective colectomy
in ulcerative colitis: A cohort study. Ann Intern Med. 163:262–270.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Andersson P, Söderholm JD and Derholm JD:
Surgery in ulcerative colitis: Indication and timing. Dig Dis.
27:335–340. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gulliford SR and Limdi JK: Acute severe
ulcerative colitis: Timing is everything. Postgrad Med J.
87:215–222. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Travis SP, Farrant JM, Ricketts C, Nolan
DJ, Mortensen NM, Kettlewell MG and Jewell DP: Predicting outcome
in severe ulcerative colitis. Gut. 38:905–910. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lindgren SC, Flood LM, Kilander AF,
Löfberg R, Persson TB and Sjödahl RI: Early predictors of
glucocorticosteroid treatment failure in severe and moderately
severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol.
10:831–835. 1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ho GT, Mowat C, Goddard CJ, Fennell JM,
Shah NB, Prescott RJ and Satsangi J: Predicting the outcome of
severe ulcerative colitis: Development of a novel risk score to aid
early selection of patients for second-line medical therapy or
surgery. Aliment Pharmacol Ther. 19:1079–1087. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Järnerot G, Hertervig E, Friis-Liby I,
Blomquist L, Karlén P, Grännö C, Vilien M, Ström M, Danielsson A,
Verbaan H, et al: Infliximab as rescue therapy in severe to
moderately severe ulcerative colitis: A randomized,
placebo-controlled study. Gastroenterology. 128:1805–1811.
2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Baron JH, Connell AM and Lennard-Jones JE:
Variation between observers in describing mucosal appearances in
proctocolitis. BMJ. 1:89–92. 1964.PubMed/NCBI View Article : Google Scholar
|