Introduction
As social environment and living habits change, the
incidence of diabetes has increased (1). Heart disease is a major complication of
this condition. Diabetic patients have reduced cardiac function
with disease progression, and eventually experience heart failure.
Thus, cardiovascular functional disorder is also a major cause of
death (2,3). Cardiovascular diseases in diabetic
patients begin with vascular endothelium, and hyperglycemia and
hyperlipidemia in the body fluids are the leading causes of
endothelial cell injury (4).
Vascular endothelial cell injury has already occurred in patients
with early diabetes, so intervention in the injury is significant
for the patients (5).
MicroRNA, a research hotspot, is a non-coding single
stranded small molecule RNA and a marker for cell damage.
miR-223-3p is also a recent hotspot in cardiovascular diseases
(6,7). A study on a mouse model of sepsis
revealed that the deletion of miR-223-3p promotes myocardial
dysfunction in septic mice and protects vascular endothelial cells
from injury (8). Nod-like receptor
protein 3 (NLRP3), which is a macromolecular complex protein and an
inflammasome that can be activated by the hyperglycemia of islet
cells and the deposition of amyloid polypeptides, plays an
important role during the progression of diabetes (9,10).
Another study showed that NLRP3 in cardiac microvascular
endothelial cells (CMECs) is activated when the body is in a state
of myocardial ischemia reperfusion, which leads to myocardial cell
injury (11).
According to the targeted prediction by TargetScan,
miR-223-3p and NLRP3 have target binding sites. However, the role
of miR-223-3p in the endothelial cell injury of diabetic patients
has rarely been studied. Therefore, in order to confirm the
conjecture that miR-223-3p can inhibit the injury of CMECs in
diabetic patients by regulating the expression of NLRP3, a mouse
model of diabetes was studied.
Materials and methods
Materials and reagents
Twenty C57BL/6J female mice (purchased from the
Experimental Animal Center of Sun Yat-sen University, Guangzhou,
China), 4 weeks of age, with a body mass of ~30 g, were selected
and randomized into the control and model groups (n=10 each). The
mice were fed at 20-25˚C with free access to food and water, with
relative humidity of 40-75% and normal circadian rhythm. Mouse
cardiac microvascular endothelial cells (MCMECs; cat. no. CP-M129)
were purchased from Procell Life Science & Technology Co., Ltd.
and were frozen in liquid nitrogen. DMEM (high glucose) was
purchased from Gibco (Thermo Fisher Scientific, Inc.). Fetal bovine
serum (FBS) and trypsin were purchased from HyClone (GE Healthcare
Life Sciences). TRIzol and reverse transcription kits were
purchased from Takara Bio, Inc. RT-qPCR kit was purchased from
Beijing Transgen Biotech Co., Ltd. qPCR fluorophore SYBR-Green
(SY1020) was purchased from Beijing Solarbio Science &
Technology Co., Ltd. NLRP3 primary antibody (ab214185) was
purchased from Abcam. Primary antibodies (β-actin, Bax, caspase-3
and Bcl-2) and secondary antibody (goat anti-rabbit) were purchased
from Shanghai Universal Biotech Co., Ltd. (cat. nos. 4970S, 2772S,
9662S, 3498S and A25012, respectively). Radioimmunoprecipitation
assay buffer (SS0892) was purchased from Beijing Xinhua Lvyuan
Technology Co., Ltd. ECL developing solution (GOY-C3194) was
purchased from Shanghai Guyan Industry Co., Ltd. Annexin V-FITC/PI
apoptosis detection kit was purchased from Jiangsu KeyGEN Biotech
Co., Ltd. Lipofectamine™ 2000 was purchased from Invitrogen (Thermo
Fisher Scientific, Inc.). A dual luciferase reporter gene assay kit
(D0010) was purchased from Beijing Solarbio Science &
Technology Co., Ltd. RT-qPCR primers were designed and synthesized
by Shanghai GenePharma Co., Ltd. The study was approved by the
Ethics Committee of The Third Affiliated Hospital of Nanchang
University (Nanchang, China).
Modeling
Diabetic mice were modeled through high glucose and
high fat (HGHF) diet. Sucrose, cream, premix, and water were mixed
at 2:4:1:3 and heated to prepare a suspended HGHF emulsion.
Streptozotocin powder injection (purchased from Sigma-Aldrich;
Merck KGaA) was dissolved with 1% citrate buffer solution and
degermed by a bacteria-proof filter, so as to prepare a
streptozotocin solution. Before modeling, the mice in the three
groups fasted overnight. Then, mice in the model and miR-223-3p
groups were injected with streptozotocin solution (35 mg/kg) each
time for 5 consecutive days, and fed with HGHF emulsion (3 ml) each
time for 6 consecutive weeks at the same time. At 48 h after the
injection, the blood was drawn from the mouse tail vein for
detecting blood glucose. The modeling was successful if the blood
glucose was >16.5 mmol/l. After the blood glucose was elevated,
the mice were injected through the tail vein with the drug delivery
system (1 mg/kg) that was synthetized by neutral fat emulsion and
miR-223-3p-mimics every 3 days, until the experiment was concluded.
Mice in the control group were injected with normal saline (the
same dose as that of streptozotocin solution) for 5 consecutive
days. Six weeks later, the mice in both groups were sacrificed by
cervical dislocation, to obtain the left ventricular tissues for
detecting miR-223-3p and NLRP3 mRNA.
Cell modeling
MCMECs stored in liquid nitrogen were taken out and
resuscitated in an incubator at 37˚C, then placed in a medium
containing 10% FBS and cultured in an incubator at 37˚C with 5%
CO2. After the adherent growth reached 80%, the cells
were washed with PBS, digested with 25% trypsin, and then cultured
in the environment containing 10% culture fluid at 37˚C with 5%
CO2 for passage. After the passage, cells in logarithmic
growth phase were selected for grouping and transfection. They were
divided into normal, model, blank carrier, miR-223-3p-mimics, and
miR-223-3p-inhibitor groups. Cells in the normal and model groups
were not transfected. However, the cells in the blank carrier group
were transfected with miR-NC, the cells in the miR-223-3p-mimics
group were transfected with miR-223-3p-mimics, and the cells in the
miR-223-3p-inhibitor group were transfected with
miR-223-3p-inhibitor. The specific steps were as follows: The cells
were inoculated in a 6-well plate at 3x105 cells/well,
and then Lipofectamine™ 2000 was diluted and mixed with DNA
according to the manufacturer's instructions of Lipofectamine 2000
transfection kit. The mixture was allowed to stand at room
temperature for 5 min, evenly mixed with cells, and then
transfected for 48 h at 37˚C with 5% CO2. After
transfection, the expression levels of miR-223-3p and NLRP3 in each
group were detected, and cell models were established. Cells in the
normal group continued to be cultured in the environment containing
10% culture fluid at 37˚C with 5% CO2. Cells in each
group were placed in a high-glucose medium containing 10% FBS and
glucose at 25 mmol/l, added with palmitic acid at 300 µmol/l to
simulate a high-fat environment, and then cultured in an incubator
at 37˚C with 5% CO2. All the cells were continuously
cultured for 24 h, and subsequent experiments were carried out.
RT-qPCR detection of miR-223-3p and
NLRP3 mRNA in tissues and cells
The left ventricular tissues of the mice in the two
groups were ground and prepared into a suspension. TRIzol reagent
was used to extract total RNA from miR-223-3p and NLRP3 mRNA in the
suspension and cells. An ultraviolet spectrophotometer was used to
detect its purity and concentration. Next, 5 µg of total RNA were
reversely transcribed into cDNA according to the manufacturer's
instructions of the reverse transcription kit, and the parameters
were 37˚C for 15 min, 42˚C for 42 min, and 70˚C for 5 min. The
transcribed cDNA was used for PCR amplification, with β-actin as an
internal reference for NLRP3 mRNA and U6 as an internal reference
for miR-223-3p. qPCR was performed using the SYBR-Green fluorophore
(Beijing Solarbio Science & Technology Co., Ltd.). Primer
sequences are shown in Table Ⅰ. PCR conditions for miR-223-3p were
as follows: Pre-denaturation at 95˚C for 15 min, then at 94˚C for
15 sec and at 55˚C for 40 sec for 40 cycles, finally extension at
70˚C for 30 sec. PCR conditions for NLRP3 mRNA were as follows:
Pre-denaturation at 95˚C for 2 min, then at 95˚C for 10 sec and at
60˚C for 40 sec for 40 cycles, final extension at 72˚C for 90 sec.
The 2-∆Cq method (12)
was used to express the relative expression levels of genes, and a
PCR instrument was used for fluorescence quantitative PCR. The
experiment was carried out 3 times.
Western blot analysis of expression
levels of NLRP3 and apoptosis-related proteins
The cells and tissues were homogenized in a
radioimmunoprecipitation assay buffer. BCA protein assay kit was
used to determine the concentration of proteins. Approximately 100
µg of protein were loaded per lane, and 5% concentrated SDS-PAGE
gel and 15% separating gel were used for electrophoresis. Then, the
gels were transferred to a 0.45-µm PVDF membrane that was sealed
with 5% skimmed milk powder at room temperature for 2 h. The cells
were added with mouse monoclonal primary antibodies NLRP3 (1:500),
Bax (1:500), caspase-3 (1:500), Bcl-2 (1:500), and β-actin
(1:1,000), and then incubated overnight at 4˚C. Next, the cells
were added with HRP-labeled goat anti-rabbit antibody (1:1,000),
incubated at 37˚C for 1 h, and rinsed with PBS solution. Finally,
the cells were developed with ECL developing solution. The protein
bands were scanned and their gray values were analyzed using
Quantity One software (Bio-Rad Laboratories, Inc.). The relative
expression level of the protein = (the gray value of the target
protein band)/(the gray value of β-actin protein band).
Flow cytometry detection of apoptosis
of MCMECs
Annexin V-FITC/PI double staining combined with flow
cytometry was used to detect apoptosis. After cell modeling, MCMECs
in each group were inoculated in a 6-well plate at 3x105
cells/well and then incubated for 24 h. Then, they were rinsed
twice with PBS and added with Annexin V-FITC (5 µl). After 10-min
reaction at room temperature, the cells were added with PI (10 µl)
and incubated at room temperature in the dark for 20 min. Finally,
the flow cytometer was used to detect apoptosis. The experiment was
carried out 3 times. FlowJo v.10 software (FlowJo LLC) was used for
analysis.
Dual-luciferase reporter gene
assay
The downstream target genes of miR-223-3p were
predicted using TargetScan 7.1 (http://www.targetscan.org/vert_71/). Oligonucleotides
containing NLRP3 target sequence were amplified and cloned into
wild type (WT) pmirGLO plasmids (Biovector Co., Ltd;
Biovector105805). pmirGLO-NLRP3 3' UTR-WT and pmirGLO-NLRP3 3'
UTR-mutant (Mut) were constructed and then transferred to the
downstream of the luciferase reporter genes, so as to sequence and
identify the constructed plasmids. NLRP3 3' UTR-WT, NLRP3 3'
UTR-Mut, miR-223-3p-mimics, miR-223-3p-inhibitor, and miR-NC were
transferred into the MCMECs using Lipofectamine 2000 kit. At 48 h
after the transfection, a dual-luciferase reporter gene assay kit
(Promega Corp.) was used to determine luciferase activity, with
Renilla luciferase activity considered as the standard.
Statistical analysis
SPSS 18.0 [Bizinsight (Beijing) Information
Technology Co., Ltd.] was used to statistically analyze the data.
GraphPad Prism 6 (GraphPad Software, Inc.) was used to plot the
figures. Measurement data were expressed as the mean ± standard
deviation, and independent samples t-test was used for comparisons
between two groups, whereas analysis of variance with LSD post hoc
test were used for comparisons between multiple groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
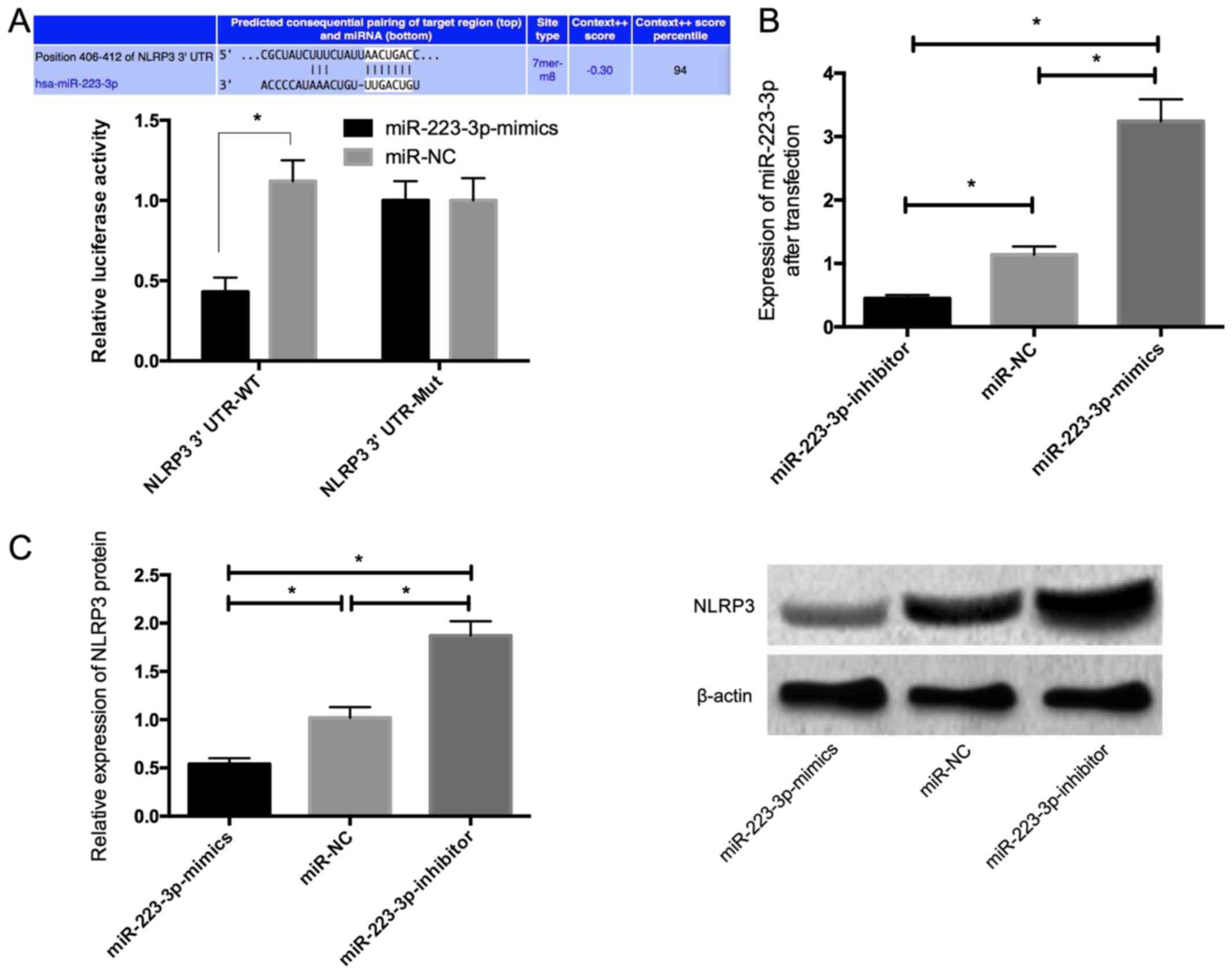
Target relationship between miR-223-3p
and NLRP3
According to the prediction by TargetScan, bases
406-412 of NLRP3 3' UTR were binding sites of miR-223-3p.
Dual-luciferase reporter gene assay was conducted to confirm the
direct interaction between miR-223-3p and NLRP3. Co-transfection
with miR-223-3p reduced the luciferase activity of plasmids
containing fragments of NLRP3 3' UTR-WT (Fig. 1A). These results indicated that
miR-223-3p directly interacted with NLRP3 3' UTR. The results of
RT-qPCR after transfection revealed that the expression of
miR-223-3p in the miR-223-3p-mimics group was significantly higher
than that in the miR-NC group, while the expression in the
miR-223-3p-inhibitor group was significantly lower than that in the
miR-NC group (Fig. 1B). The results
of western blot analysis revealed that the expression of NLRP3
significantly decreased in the MCMECs transfected with
miR-223-3p-mimics, while NLRP3 expression significantly increased
in the MCMECs transfected with miR-223-3p-inhibitor (P<0.05)
(Fig. 1C).
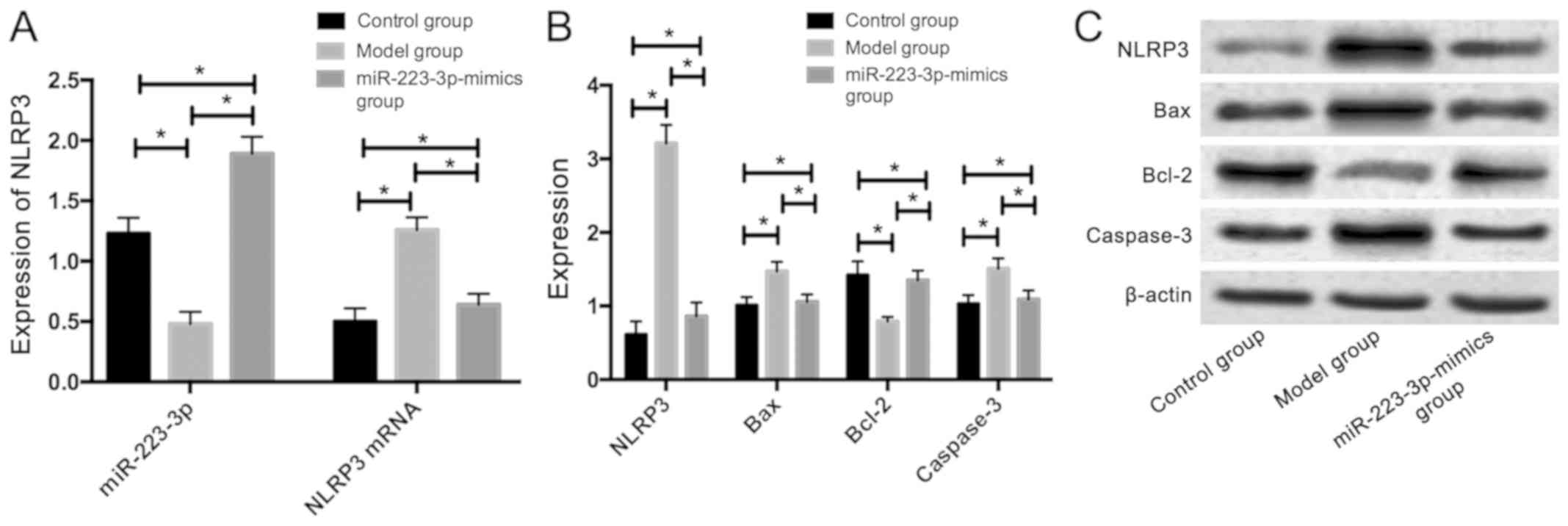
Expression levels of miR-223-3p,
NLRP3, and apoptosis-related proteins in heart tissue
The mice in the miR-223-3p-mimics group were
injected through the tail vein with the drug delivery system that
was synthetized by neutral fat emulsion and miR-223-3p-mimics. The
results revealed that compared with those in the model group, the
mice in the control and miR-223-3p-mimics groups had significantly
higher expression of miR-223-3p. However, significantly lower mRNA
and protein expression levels of NLRP3 were observed. Additionally,
they had significantly lower protein expression levels of Bax and
caspase-3; however,a significantly higher protein expression of
Bcl-2 was observed (P<0.05) (Fig.
2).
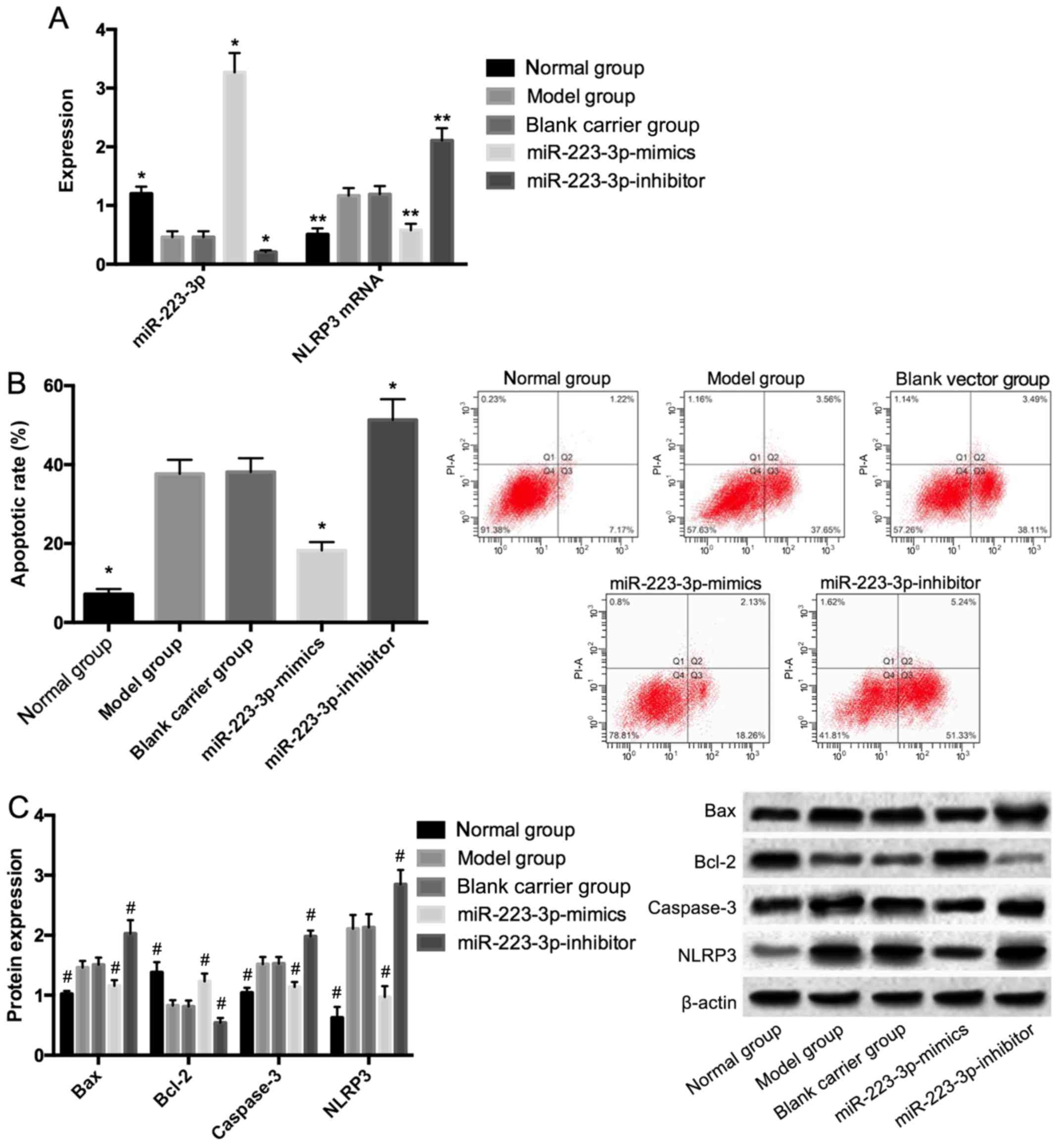
Effects of miR-233-3p on NLRP3
expression and endothelial cell apoptosis
After cell modeling and culture in the HGHF
environment, the cells in the model and blank carrier groups had
significantly lower expression of miR-223-3p, compared with those
in the normal group; however, significantly higher mRNA and protein
expression levels of NLRP3 (P<0.05) were observed. Additionally,
compared with those in the model and blank carrier groups, the
cells in the miR-223-3p-mimics group had significantly higher
expression of miR-223-3p, but significantly lower mRNA and protein
expression levels of NLRP3 (P<0.05). Also, the cells in the
miR-223-3p-inhibitor group had significantly higher mRNA and
protein expression levels of NLRP3 (P<0.05), compared with those
in the model and blank carrier groups. Compared with those in the
normal group, the cells in the model and blank carrier groups had a
significantly higher apoptotic rate, significantly higher protein
expression levels of Bax and caspase-3, but significantly lower
protein expression of Bcl-2 (P<0.05). Compared with those in the
model and blank carrier groups, the cells in miR-223-3p-mimics
group had a significantly lower apoptotic rate, and significantly
lower protein expression levels of Bax and caspase-3. However, a
significantly higher protein expression of Bcl-2 was observed
(P<0.05). In addition, compared with those in the model and
blank carrier groups, the cells in the miR-223-3p-inhibitor group
had a significantly higher apoptotic rate, significantly higher
protein expression levels of Bax and caspase-3; whereas a
significantly lower protein expression of Bcl-2 was observed
(P<0.05) (Fig. 3).
Discussion
Diabetes is a metabolic disease caused by multiple
factors which is clinicopathologically characterized by
hyperglycemia (13). Diabetes leads
to metabolic disorders and a series of complications, which not
only seriously affect the patients' quality of life, but also bring
heavy burdens to the family of the patients and society (14,15).
Diabetic cardiomyopathy is a common complication of diabetes and is
mainly caused by the dysfunction of CMECs (16).
NLRP3 is currently the most widely studied
inflammasome. According to previous studies, during the
pathogenesis of diabetes, the increase of inflammatory responses
over-activates NLRP3 and the over-activation induces the production
of a large amount of IL-1β, which further causes damage to islet
cells and results in insulin resistance. This shows that NLRP3 is
closely related to diabetes (17,18). A
previous study has shown that diabetic cardiovascular diseases have
the characteristics of inflammatory diseases, and the
over-activation of NLRP3 may also lead to vascular dysfunction in
diabetic patients (19). The results
of the targeted prediction by TargetScan revealed that miR-223-3p
and NLRP3 have target binding sites. Another study has reported
that miR-223-3p is a new biomarker and plays a protective role in
cardiovascular and related fields (20). Therefore, it is speculated that
miR-223-3p can reduce the diabetes-induced injury of CMECs through
regulating NLRP3 in a targeted manner.
In the present study, mice were modeled for diabetes
through HGHF diet, and miR-223-3p and NLRP3 expression levels in
the heart tissue of diabetic and normal mice were detected. The
results revealed that compared with those in the model group, the
mice in the control group had significantly higher expression of
miR-223-3p, and significantly lower mRNA and protein expression
levels of NLRP3, suggesting that NLRP3 may cause diabetic vascular
injury. Mice in the miR-223-3p-mimics group were injected through
the tail vein with the drug delivery system that was synthetized by
neutral fat emulsion and miR-223-3p-mimics. The results revealed
that compared with those in the model group, mice in the
miR-223-3p-mimics group had significantly higher expression of
miR-223-3p; however, significantly lower mRNA and protein
expression levels of NLRP3 were observed. Additionally, they had
significantly lower protein expression levels of Bax and caspase-3;
whereas a significantly higher protein expression of Bcl-2 was
observed. These findings reveal that miR-223-3p may regulate NLRP3
and apoptosis-related proteins. A previous study found a
significant increase in the serum NLRP3 of patients with diabetic
cardiomyopathy (21), which is
consistent with our conclusions.
In a study of miR-223-3p on patients with rheumatic
heart disease, it was shown that miR-223-3p inhibits the activation
of T cells and reduces myocardial damage and myocardial cell
apoptosis by regulating inflammatory cytokines that are secreted by
inflammatory cells (22). This
indicates that miR-223-3p can regulate inflammatory cytokines and
thereby protect angiocarpy. As known, glycolipid metabolic
abnormalities are of great significance for the development and
progression of diabetes (23,24), but
whether NLRP3 could be regulated by miR-223-3p has rarely been
explored. Moreover, in vivo experiments alone cannot prove
that miR-223-3p can reduce the injury of CMECs and regulate NLRP3.
Therefore, an endothelial cell injury model was established in
vitro through HGHF diet. The results revealed that after cell
modeling and culture in the HGHF environment, the cells in the
miR-223-3p overexpression group had significantly higher expression
of miR-223-3p, compared with those in the model group, while cells
in the low miR-223-3p expression group had significantly lower
expression of miR-223-3p. Compared with those in the model and
blank carrier groups, the cells in the miR-223-3p overexpression
group had significantly lower mRNA expression of NLRP3, while cells
in the low miR-223-3p expression group had significantly higher
mRNA expression of NLRP3. Apoptosis in each group was detected and
compared. The results revealed that after modeling, the protein
expression levels of Bax and NLRP3 in the normal group were
significantly lower than those in the model, blank carrier, and
miR-223-3p overexpression groups, while the protein expression
levels of Bax, caspase-3, and NLRP3 in the miR-223-3p
overexpression group were significantly lower than those in the
model and blank carrier groups. The protein expression of Bcl-2 in
the normal group was significantly higher than that in the model,
blank carrier, and miR-223-3p overexpression groups, while the
protein expression in the miR-223-3p overexpression group was
significantly higher than that in the model and blank carrier
groups. These findings suggest that the overexpression of
miR-223-3p in injured endothelial cells can inhibit the expression
of NLRP3, thus protecting endothelial cells and reducing apoptosis
of CMECs. In addition, NLRP3 may inhibit endothelial cell apoptosis
by affecting apoptosis-related proteins Bax and caspase-3, as well
as anti-apoptotic protein Bcl-2. A previous study has shown that
long-term hyperglycemia leads to massive generation of oxygen-free
radicals, which causes damage to DNA in cells and results in cell
damage with apoptosis as the main manifestation (25). Although NLRP3 is a target gene of
miR-223-3p, the regulatory mechanism of miR-223-3p on NLRP3 remains
unclear, which is also a deficiency of this study.
In conclusion, miR-223-3p can inhibit the
diabetes-induced apoptosis of CMECs by regulating the expression of
NLRP3, and protect CMECs from injury. However, the regulatory
mechanism of miR-223-3p on NLRP3 and the regulatory mechanism of
NLRP3 on apoptosis-related proteins were not explored. These
aspects need further study to provide more theoretical data for the
treatment and prevention of diabetic patients with cardiovascular
injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BD and YiH conceived and designed the study. BD, XS,
HZ and YaH were responsible for the collection and analysis of the
experimental data. YiH and XS interpreted the data and drafted the
manuscript. BD and YaH revised the manuscript critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Third Affiliated Hospital of Nanchang University (Nanchang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chamberlain JJ, Rhinehart AS, Shaefer CF
Jr and Neuman A: Diagnosis and management of diabetes: Synopsis of
the 2016 American Diabetes Association Standards of Medical Care in
Diabetes. Ann Intern Med. 164:542–552. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Micha R, Peñalvo JL, Cudhea F, Imamura F,
Rehm CD and Mozaffarian D: Association between dietary factors and
mortality from heart disease, stroke, and type 2 diabetes in the
United States. JAMA. 317:912–924. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao W, Rasheed A, Tikkanen E, Lee JJ,
Butterworth AS, Howson JMM, Assimes TL, Chowdhury R, Orho-Melander
M, Damrauer S, et al: CHD Exome+ Consortium; EPIC-CVD Consortium;
EPIC-Interact Consortium; Michigan Biobank: Identification of new
susceptibility loci for type 2 diabetes and shared etiological
pathways with coronary heart disease. Nat Genet. 49:1450–1457.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Parenti A, Pala L, Paccosi S and Rotella
CM: Potential role for dendritic cells in endothelial dysfunction,
diabetes and cardiovascular disease. Curr Pharm Des. 23:1435–1444.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rawal S, Munasinghe PE, Shindikar A,
Paulin J, Cameron V, Manning P, Williams MJ, Jones GT, Bunton R,
Galvin I, et al: Down-regulation of proangiogenic microRNA-126 and
microRNA-132 are early modulators of diabetic cardiac
microangiopathy. Cardiovasc Res. 113:90–101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dai GH, Ma PZ, Song XB, Liu N, Zhang T and
Wu B: MicroRNA-223-3p inhibits the angiogenesis of ischemic cardiac
microvascular endothelial cells via affecting RPS6KB1/hif-1a signal
pathway. PLoS One. 9(e108468)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu X, Zhang Y, Du W, Liang H, He H, Zhang
L, Pan Z, Li X, Xu C, Zhou Y, et al: MiR-223-3p as a novel microRNA
regulator of expression of voltage-gated K+ channel
Kv4.2 in acute myocardial infarction. Cell Physiol Biochem.
39:102–114. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang X, Huang W, Yang Y, Wang Y, Peng T,
Chang J, Caldwell CC, Zingarelli B and Fan GC: Loss of duplex
miR-223 (5p and 3p) aggravates myocardial depression and mortality
in polymicrobial sepsis. Biochim Biophys Acta. 1842:701–711.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yi H, Peng R, Zhang LY, Sun Y, Peng HM,
Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH, et al: LincRNA-Gm4419
knockdown ameliorates NF-κB/NLRP3 inflammasome-mediated
inflammation in diabetic nephropathy. Cell Death Dis.
8(e2583)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stienstra R, van Diepen JA, Tack CJ, Zaki
MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A,
Vroegrijk I, et al: Inflammasome is a central player in the
induction of obesity and insulin resistance. Proc Natl Acad Sci
USA. 108:15324–15329. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang L, Chen Y, Li X and Zhang Y, Gulbins
E and Zhang Y: Enhancement of endothelial permeability by free
fatty acid through lysosomal cathepsin B-mediated Nlrp3
inflammasome activation. Oncotarget. 7:73229–73241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou W, Wang G, Zhao X, Xiong F, Zhou S,
Peng J, Cheng Y, Xu S and Xu X: A multiplex qPCR gene dosage assay
for rapid genotyping and large-scale population screening for
deletional α-thalassemia. J Mol Diagn. 5:642–651. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Soliman EZ, Backlund JC, Bebu I, Orchard
TJ, Zinman B and Lachin JM: DCCT/EDIC Research Group.
Electrocardiographic abnormalities and cardiovascular disease risk
in type 1 diabetes: The Epidemiology of Diabetes Interventions and
Complications (EDIC) Study. Diabetes Care. 40:793–799.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Trikkalinou A, Papazafiropoulou AK and
Melidonis A: Type 2 diabetes and quality of life. World J Diabetes.
8:120–129. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cai H, Li G, Zhang P, Xu D and Chen L:
Effect of exercise on the quality of life in type 2 diabetes
mellitus: A systematic review. Qual Life Res. 26:515–530.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guo R and Nair S: Role of microRNA in
diabetic cardiomyopathy: From mechanism to intervention. Biochim
Biophys Acta Mol Basis Dis. 1863:2070–2077. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ye Y, Bajaj M, Yang HC, Perez-Polo JR and
Birnbaum Y: SGLT-2 inhibition with dapagliflozin reduces the
activation of the Nlrp3/ASC inflammasome and attenuates the
development of diabetic cardiomyopathy in mice with type 2
diabetes. Further augmentation of the effects with saxagliptin, a
DPP4 inhibitor. Cardiovasc Drugs Ther. 31:119–132. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang X, Lu F, Li L, Li J, Luo J, Zhang S,
Liu X and Chen G: Wu-Mei-wan protects pancreatic β cells by
inhibiting NLRP3 inflammasome activation in diabetic mice. BMC
Complement Altern Med. 19(35)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao
C, Xin C, Zhu D, Li Y, Yan W, et al: TXNIP mediates NLRP3
inflammasome activation in cardiac microvascular endothelial cells
as a novel mechanism in myocardial ischemia/reperfusion injury.
Basic Res Cardiol. 109(415)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chu M, Wu R, Qin S, Hua W, Shan Z, Rong X,
Zeng J, Hong L, Sun Y, Liu Y, et al: Bone marrow-derived
microRNA-223 works as an endocrine genetic signal in vascular
endothelial cells and participates in vascular injury from Kawasaki
disease. J Am Heart Assoc. 6(e004878)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang J, Xia L, Zhang F, Zhu D, Xin C,
Wang H, Zhang F, Guo X, Lee Y, Zhang L, et al: A novel mechanism of
diabetic vascular endothelial dysfunction:
Hypoadiponectinemia-induced NLRP3 inflammasome activation. Biochim
Biophys Acta Mol Basis Dis. 1863:1556–1567. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Castro-Villegas C, Pérez-Sánchez C,
Escudero A, Filipescu I, Verdu M, Ruiz-Limón P, Aguirre MA,
Jiménez-Gomez Y, Font P, Rodriguez-Ariza A, et al: Circulating
miRNAs as biomarkers of therapy effectiveness in rheumatoid
arthritis patients treated with anti-TNFα. Arthritis Res Ther.
17(49)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Akushevich I, Yashkin AP, Kravchenko J,
Fang F, Arbeev K, Sloan F and Yashin AI: Identifying the causes of
the changes in the prevalence patterns of diabetes in older U.S.
adults: A new trend partitioning approach. J Diabetes
Complications. 32:362–367. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Patterson R, McNamara E, Tainio M, de Sá
TH, Smith AD, Sharp SJ, Edwards P, Woodcock J, Brage S and
Wijndaele K: Sedentary behaviour and risk of all-cause,
cardiovascular and cancer mortality, and incident type 2 diabetes:
A systematic review and dose response meta-analysis. Eur J
Epidemiol. 33:811–829. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kowluru RA, Kowluru V, Xiong Y and Ho YS:
Overexpression of mitochondrial superoxide dismutase in mice
protects the retina from diabetes-induced oxidative stress. Free
Radic Biol Med. 41:1191–1196. 2006.PubMed/NCBI View Article : Google Scholar
|