Introduction
The term ‘sialic acid’ (SA) originates from the
Greek word ‘sialon’, which means saliva, and it was introduced for
the first time in 1952 by Gunnar Blix, a Swedish biochemist who
found this new acidic compound in saliva, 15 years earlier, in
1937(1).
The main chemical compound of SA is
N-acetylneuraminic acid (NANA), a monocarboxylic acid with the
formula C11H19O9N (1). It has been found that there is a whole
family of related compounds that originate from NANA and SA became
the generic name for this family of >50 members of acid
neuraminic derivatives (sugar chains or glycans) (1). Other members of the SA family can be
obtained from the molecule of neuraminic acid by changing the amino
group with a hydroxyl substituent, or methyl, lactyl, acetyl or
phosphate group.
Chemically, members of this family are considered
condensation products of N-acetylhexosamine with pyruvic acid.
Gunnar Blix agreed that this unacetylated primary compound should
be called neuraminic acid, and the term SA should be used as a
collective term for acetylated and glycosylated derivatives of
neuraminic acid (1).
SA serves an important role in the interaction,
communication and signaling between cells, cell aggregation, immune
response, reproduction, tissue development and regeneration, and
also in human diseases, including in the infection process, tumor
growth and metastasis (2). At the
surface of animal cells and microorganisms there is a dense and
complex array of glycans, mostly attached to proteins and lipids,
which mediate specific roles in health and disease (3,4).
Given their location and ubiquitous distribution,
SAs can mediate or modulate a wide variety of physiological and
pathological processes, however, further understanding of SAs is
required and many hypotheses are awaiting clarification (5,6). The aim
of the present study was to evaluate the age-related changes of
serum SA in elderly individuals, including men and postmenopausal
women.
Patients and methods
The present study was a cross-sectional single
center study comprising 97 subjects, including 77 women and 20 men,
aged ≥55 years. All subjects were admitted to ‘Ana Aslan’ National
Institute of Gerontology and Geriatrics (Bucharest, Romania), and
selected for the study between 2014 and 2019. The Ethics Committee
of the ‘Ana Aslan’ National Institute of Gerontology and Geriatrics
approved the study protocol. Each subject signed an informed
consent form prior to the study. All procedures performed in the
study were in accordance with the ethical standards of the National
Research Committee, and the Helsinki Declaration and its later
amendments.
Eligible patients included men and postmenopausal
women, who were able to give informed consent, and who were able to
communicate and cooperate, for example they did not have
Alzheimer's disease at an advanced stage, a cognitive disease nor
advanced disabilities. The exclusion criteria were: i) age <55
years; ii) women that did not have a postmenopausal status; and
iii) subjects with a severe or disabling pathology, including
dementia, severe physical disabilities, neoplasia, severe or acute
inflammatory syndrome, stroke, cardiac failure, kidney failure and
cirrhosis. The age range of the women was 55-87 years, with a mean
age of 67.74±8.64 years, and the age range of the men was 55-87
years, with a mean age of 69.05±10.43 years.
The selected subjects were first divided into two
groups: i) group 1, men (n=20); and ii) group 2, women (n=77). The
group of women was further divided into three subgroups depending
on age: i) subgroup 1 (n=32); age range, 55-64 years; mean age,
59.43±3.30 years; ii) subgroup 2 (n=28); age range, 65-74 years;
mean age, 69.71±2.73 years; and iii) subgroup 3 (n=17); age range,
75-87 years; mean age 80.11±3.67 years.
Venous blood samples were obtained following
overnight fasting, and serum was collected after 20 min of
centrifugation at 1,000 x g and refrigerated at -70˚C until
use.
Total SA in serum samples was determined by the
standard Ehrlich's method, a colorimetric assay, using standard
reagents and chemicals with an analytical grade, which were
purchased from Sigma-Aldrich; Merck KGaA. According to this method,
the supernatant of serum containing SA chemically reacted with the
Ehrlich's reagent and produced a colorimetric reaction that was
quantitatively measured using a spectrophotometer (2). This procedure is based on the release
of bound SA by heating with 5% perchloric acid. After cooling in
the ice bath at 0˚C and brief centrifugation for 5 min at 1,000 x g
and 22˚C, the supernatant was heated for 15 min with Ehrlich's
reagent at 100˚C. Subsequently, the absorbance of the color
developed in the sample was measured at 565 nm, and the serum
concentration of SA was expressed in mg/dl.
Statistical analysis
All data are presented as mean ± standard deviation
(SD). Statistical analysis was performed by Student's t-test using
Excel 7.0 (Microsoft Corporation) and SPSS 8.0 (SPPS, Inc.)
software. Pearson's correlation coefficients (r) were calculated to
evaluate the correlations between serum SA and age. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
SAs are a class of sugars that belong to the larger
family of glycans. Current science includes a special field
‘glycobiology’, which refers to the physiological and pathological
interactions and processes of SAs. This class of glycans has four
main categories of functions (5): i)
Structural and modulator roles through their negative charge and
hydrophilicity; ii) components of binding sites for diverse
pathogens and toxins; iii) a role in binding proteins; and iv)
molecular mimicry; microbial pathogens decorated with SAs assist in
invasion of host immunity.
SA is very important in blood circulation, having a
major function in non-adherence of blood cells to the endovascular
epithelium (7). Total serum SA has
been reported in some studies as a cardiovascular risk factor, and
it is increased following menopause in females, however, the reason
is not clear (8).
A study published in 1998 by Crook et al
(9) stated that in females there is
a strong univariate correlation between total serum SA level and
plasma fasting insulin and glucose concentration, whereas in males
there is a weaker univariate correlation between total serum SA and
fasting plasma glucose and the insulin resistance index. In
addition, total serum SA is significantly correlated with fasting
serum cholesterol concentrations and body mass index in females,
but not in males.
Other studies have shown that SA represents a
cardiovascular risk factor, as it is associated with increased
cardiovascular mortality (10) and
cerebrovascular disease (8,11). In addition, it has been demonstrated
that SA levels are elevated in patients with type I and II diabetes
mellitus, therefore it is assumed that SA could be associated with
insulin resistance or hyperinsulinemia (12,13).
Furthermore, SA could play a role in tissue repair,
for example in regeneration of axons and remodeling of synapses
(14). The roles of SA in infections
are complex; on the one hand they provide protection, but on the
other hand, SA serves a role as a Trojan Horse when a germ mimics
the host structures (15,16). SA also plays a key role in the
fertility maturation and activation of sperm cells (17-19),
as well as development of the embryo (20,21).
Some studies have found a link between serum estrogen levels and SA
on the surface of endometrial cells or changes that determine the
pathological state in endometrial cancer or endometriosis (22,23).
Crook et al (9) had already studied the changes of serum
SA levels in adult age-matched women (pre-menopausal,
peri-menopausal and post-menopausal women), and their conclusion of
the study was that there is no significant difference between the
serum SA concentration in young women compared with pre-, peri- or
post-menopausal women (24).
However, in an age-related study Mehdi et al (25) in 2012 found that in elderly women and
men, the mean values of serum SA were elevated.
Therefore, there are no clear conclusions regarding
the correlations between serum concentrations of SA, age and
menopause.
By performing statistical analysis of the data
presented in Table I, it was
demonstrated there were no significant differences between men and
women for the determined clinical and biochemical parameters,
except for a slight increase in the mean value of glucose in men
compared with women (Table I).
 | Table IClinical and serum biochemical
parameters of subjects included in the study groups. |
Table I
Clinical and serum biochemical
parameters of subjects included in the study groups.
| Parameter | Women (n=77) | Men (n=20) |
|---|
| Age, years | 67.74±8.64 | 69.05±10.43 |
| Body mass index,
kg/m2 | 29.07±5.66 | 28.78±4.00 |
| Glucose, mg/dl | 127.51±49.26 | 132.45±45.98 |
| Total cholesterol,
mg/dl | 229.41±44.53 | 216.8±44.67 |
| HDL-cholesterol,
mg/dl | 48.82±15.08 | 44.53±16.38 |
| LDL-cholesterol,
mg/dl | 140.89±38.14 | 133.13±41.08 |
| Triglycerides,
mg/dl | 155.55±85.28 | 158.4±83.99 |
| Serum SA, mg/dl | 88.07±19.74 | 89.15±14.8 |
Differences were identified when analyzing the
correlations between the levels of SA and the individuals'
chronological age in the group of postmenopausal women compared
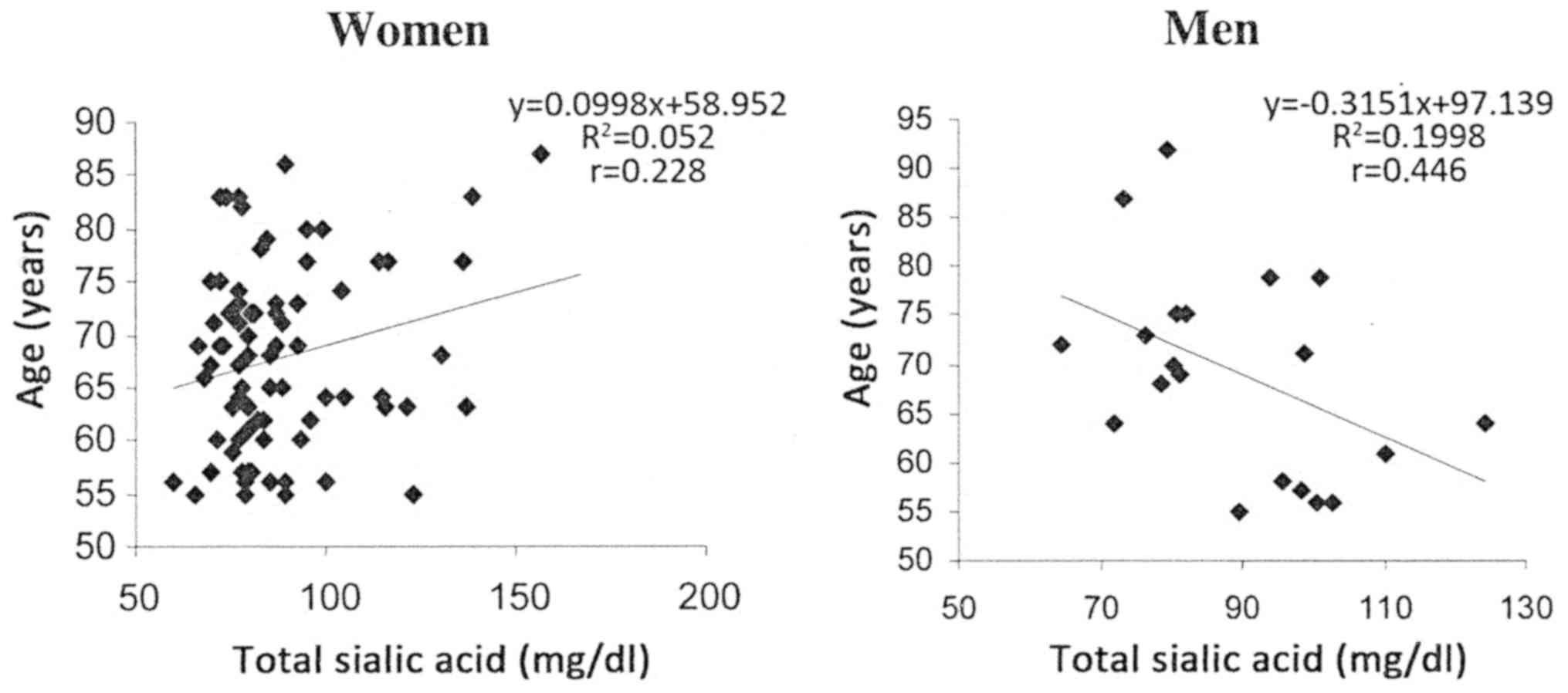
with the group of men. A significant positive correlation
(P=0.0461) was identified between individual values of total SA and
age in the group of postmenopausal women, and a significant
negative correlation (P=0.0482) was observed in the group of men
(Fig. 1).
The group of postmenopausal women was further
studied by dividing subjects by age into the following subgroups:
i) 55-64 years; ii) 65-74 years; and iii) 75-87 years. Statistical
analysis of the data presented in Table
II allowed us to compare the variation of the biochemical and
clinical parameters according to age, for these three subgroups of
women.
 | Table IIClinical and serum biochemical
parameters of women included in the study divided by age. |
Table II
Clinical and serum biochemical
parameters of women included in the study divided by age.
| Parameter | Subgroup I (55-64
years; n=32) | Subgroup II (65-74
years; n=28) | Subgroup III (75-87
years; n=17) |
|---|
| Age, years | 59.43±3.30 | 69.71±2.73 | 80.11±3.67 |
| Body mass index,
kg/m2 | 28.27±5.17 | 30.48±6.48 | 28.25±4.90 |
| Glucose, mg/dl | 121.65±40.06 | 121.60±31.54 | 148.29±78.42 |
| Total cholesterol,
mg/dl | 226.78±50.59 | 230.57±34.61 | 232.47±49.13 |
| HDL-cholesterol,
mg/dl | 49.25±15.74 | 54.55±14.68 | 38.56±8.14 |
| LDL-cholesterol,
mg/dl | 140.55±43.27 | 135.88±27.65 | 149.78±43.17 |
| Triglycerides,
mg/dl | 151.71±91.38 | 144.82±65.52 | 180.47±101.15 |
| Serum SA, mg/dl | 87.91±18.23 | 82.42±12.66 | 97.69±27.98 |
The mean values for glucose and total cholesterol
increased with age; the total cholesterol levels increased slowly
and steadily for each decade of age, while the glucose levels were
similar in the first two subgroups and increased after the age of
75 years. The mean values of HDL and BMI also increased between the
first two age subgroups, and in the third subgroup they markedly
decreased, in such a way that the mean values in the third group
were lower than those measured for individuals in the first age
group. For LDL and TG, the changes in the mean values according to
age were contradictory to those for HDL and BMI (Table II).
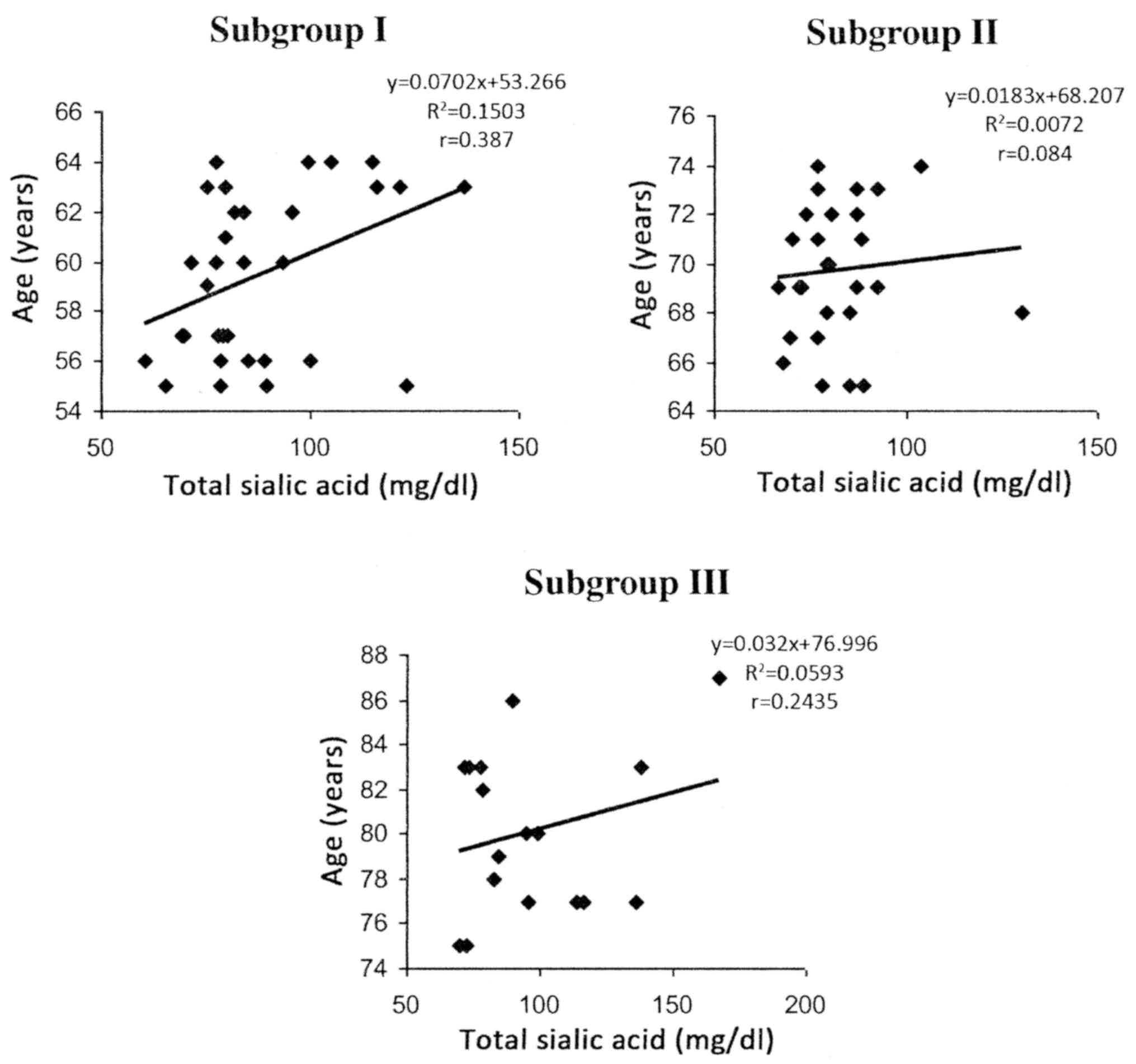
By analyzing the group of postmenopausal women to
see how the mean values of serum SA varied with age, a significant
decrease (P<0.01) between the first and second subgroups was
observed, followed by a significant increase (P<0.001) between
the second and third age groups. In addition, in postmenopausal
women, positive correlations were observed between the total serum
SA and age in all the decade subgroups; however, they were only
statistically significant in subgroup I (P=0.0283) (subgroup II,
P=0.667; subgroup III, P=0.346 (Fig.
2).
In conclusion, regarding the age-related changes in
serum concentrations of SA, the present study found differences
depending on sex; namely a significant increase in postmenopausal
women compared with a significant decrease in men.
Although overall an age-related increase in SA serum
levels was observed in postmenopausal women, for them this does not
evolve uniformly, a slowdown in growth was identified in the decade
following the postmenopausal period, followed by a significant
increase with age.
The sex-related significant differences identified
in the present study could be associated with the differences in
sex-morbidity in the postmenopausal period, after which uniformity
occur as the physiological differences tend to decrease at older
ages.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
OGO contributed to the establishment of the study
groups, the acquisition, analysis and interpretation of the patient
data, and the writing of the manuscript. GIC contributed to the
establishment of the study groups, the analysis and interpretation
of the patient data, and the writing of the manuscript. CMP
contributed to the establishment of the study groups, and the
analysis and interpretation of the patient data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of ‘Ana Aslan’ National
Institute of Gerontology and Geriatrics (Bucharest, Romania)
approved the study protocol. Each subject signed an informed
consent form prior to the study. All procedures were performed in
accordance with the ethical standards of the National Research
Committee, and the Helsinki Declaration and its later
amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Varki A and Schauer R: Sialic acids. In:
Essentials of Glycobiology. 2nd edition. Varki A, Cummings RD, Esko
JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW and Etzler ME (eds).
Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY,
2009.
|
|
2
|
Ghosh S: Sialic acid and biology of life:
an introduction. In: Sialic Acids and Sialoglycoconjugates in the
Biology of Life, Health and Disease. Ghosh S (ed). Academic Press,
pp1-61, 2020.
|
|
3
|
Pena CM and Constantin IG: Sialic acid
modifications in aging and pathology. Rom J Gerontol Geriatr.
6:21–26. 2017.
|
|
4
|
Sillanaukee P, Pönniö M and Jääskeläinen
IP: Occurrence of sialic acids in healthy humans and different
disorders. Eur J Clin Invest. 29:413–425. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Varki A: Sialic acids in human health and
disease. Trends Mol Med. 14:351–360. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Papacocea T, Buraga I, Papacocea R,
Badarau AI, Buraga M, Ciornei C, Mihai G, Stoian I and Adam D:
Antioxidant enzymes-potential targets in intracerebral haemorrhage.
Farmacia. 62:1118–1125. 2014.
|
|
7
|
Born GV and Palinski W: Unusually high
concentrations of sialic acids on the surface of vascular
endothelia. Br J Exp Pathol. 66:543–549. 1985.PubMed/NCBI
|
|
8
|
Lindberg G, Råstam L, Gullberg B and
Eklund GA: Serum sialic acid concentration predicts both coronary
heart disease and stroke mortality: Multivariate analysis including
54,385 men and women during 20.5 years follow-up. Int J Epidemiol.
21:253–257. 1992.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Crook M, Lumb P, Andrews V and Swaminathan
R: Serum total sialic acid, a reputed cardiovascular risk factor,
and its relationship to lipids, plasma fasting insulin, blood
pressure and body mass index in normal individuals. Clin Sci
(Lond). 95:53–57. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lindberg G, Eklund GA, Gullberg B and
Råstam L: Serum sialic acid concentration and cardiovascular
mortality. BMJ. 302:143–146. 1991.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Papacocea MT, Badarau AI, Radoi M and
Papacocea RI: Predictive role of biochemical plasma factors in
patients with severe traumatic brain injuries. Rev Chim.
70:1754–1757. 2019.
|
|
12
|
Rajappa M, Ikkruthi S, Nandeesha H,
Satheesh S, Sundar I, Ananthanarayanan PH and Harichandrakumar KT:
Relationship of raised serum total and protein bound sialic acid
levels with hyperinsulinemia and indices of insulin sensitivity and
insulin resistance in non-diabetic normotensive obese subjects.
Diabetes Metab Syndr. 7:17–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Khalili P, Sundström J, Jendle J, Lundin
F, Jungner I and Nilsson PM: Sialic acid and incidence of
hospitalization for diabetes and its complications during 40-years
of follow-up in a large cohort: The Värmland survey. Prim Care
Diabetes. 8:352–357. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
El Maarouf A, Petridis AK and Rutishauser
U: Use of polysialic acid in repair of the central nervous system.
Proc Natl Acad Sci USA. 103:16989–16994. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schauer R: Achievements and challenges of
sialic acid research. Glycoconj J. 17:485–499. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vimr ER, Kalivoda KA, Deszo EL and
Steenbergen SM: Diversity of microbial sialic acid metabolism.
Microbiol Mol Biol Rev. 68:132–153. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mengerink KJ and Vacquier VD: Glycobiology
of sperm-egg interactions in deuterostomes. Glycobiology.
11:R37–R43. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ma X, Pan Q, Feng Y, Choudhury BP, Ma Q,
Gagneux P and Ma F: Sialylation facilitates the maturation of
mammalian sperm and affects its survival in female uterus. Biol
Reprod. 94(123)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chatterji U, Sen AK, Schauer R and
Chowdhury M: Paracrine effects of a uterine agglutinin are mediated
via the sialic acids present in the rat uterine endometrium. Mol
Cell Biochem. 215:47–55. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schwarzkopf M, Knobeloch KP, Rohde E,
Hinderlich S, Wiechens N, Lucka L, Horak I, Reutter W and
Horstkorte R: Sialylation is essential for early development in
mice. Proc Natl Acad Sci USA. 99:5267–5270. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ioffe E and Stanley P: Mice lacking
N-acetylglucosaminyl transferase I activity die at mid-gestation,
revealing an essential role for complex or hybrid N-linked
carbohydrates. Proc Natl Acad Sci USA. 91:728–732. 1994.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Choi H, Chung T, Choi H, Han JH, Choi JH,
Kim CH and Ha KT: Increased α2-6 sialylation of endometrial cells
contributes to the development of endometriosis. Exp Mol Med.
50:1–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Paszkowska A, Cybulski M, Semczuk A,
Postawski K and Berbe H: Total sialic acid content in endometrial
cancer tissue in relation to normal and hyperplastic human
endometrium. Cancer Detect Prev. 24:459–463. 2000.PubMed/NCBI
|
|
24
|
Crook M, Collins D, Lumb P, Fogelman I and
Treloar A: The relationship between the female menopause and serum
sialic acid, a known cardiovascular risk factor. Eur J Obstet
Gynecol Reprod Biol. 76:185–187. 1998.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mehdi MM, Singh P and Rizvi SI:
Erythrocyte sialic acid content during aging in humans: Correlation
with markers of oxidative stress. Dis Markers. 32:179–186.
2012.PubMed/NCBI View Article : Google Scholar
|