Introduction
Chronic obstructive pulmonary disease (COPD)
accounts for a major proportion of global morbidity and mortality
(1). An estimated 384 million people
aged ≥30 years are believed to be affected by COPD [global
prevalence: 11.7% (8.4-15.0%)] (1).
Hospitalization in the intensive care unit (ICU) is required for
~12-18% of patients with COPD due to acute exacerbations (2), with the mortality rate of these
patients approaching 15% (3).
Invasive mechanical ventilation (IMV) is the primary choice of
treatment for 5.9-26.0% of patients with acute exacerbation of COPD
(AECOPD), especially comatosed patients (4,5). Despite
the development of protective lung ventilation strategies and the
optimization of treatment for respiratory failure, the reported
incidence of AECOPD-induced respiratory failure is >24.5%
(6). Prolonged IMV may lead to
ventilator-associated pneumonia (VAP), ventilator-associated lung
injury and difficulty in weaning from mechanical ventilation, which
can exacerbate respiratory distress and necessitate the
continuation of invasive ventilation (7,8).
Prolonged endotracheal intubation can also lead to airway injury,
tracheoesophageal fistula and other potentially serious
complications (7). Furthermore,
long-term endotracheal intubation reduces the quality of life of
patients and leads to a poor prognosis; therefore, timely weaning
from mechanical ventilation is an important factor for the use of
IMV (8).
Sequential invasive-noninvasive ventilation (NIV) is
a widely used strategy to decrease the duration of IMV. However,
there is no clear consensus on the optimal switching point
(5). Excellent results have been
reported with the use of a pulmonary infection control (PIC) window
as the switching point for the implementation of a sequential
ventilation strategy (9). However,
the PIC approach is mostly based on x-ray imaging findings and does
not take into account the lag time between the appearance of x-ray
findings and clinical manifestations (9).
Indeed, patients with AECOPD and respiratory failure
who exhibit a high level of consciousness and cooperation,
typically benefit from NIV, while impaired consciousness has been
identified as a risk factor for extubation failure (10,11).
Previous studies have investigated the use of a modified Glasgow
Coma Scale (GCS) score for evaluating the level of consciousness of
intubated patients (12,13). the modified GCS score was found to be
a more objective, quantitative measure of the overall clinical
condition. The present study aimed to compare two weaning
strategies entailing the use of different levels of modified GCS
score (13 vs. 10 points) as the switching point for the sequential
invasive-NIV in patients with AECOPD.
Materials and methods
Study participants
In this prospective, randomized, controlled study,
consecutive patients with AECOPD who received intubation for
respiratory failure at the ICU of 3 hospitals (Wenling Hospital
Affiliated to Wenzhou Medical University, the First Affiliated
Hospital of Wenzhou Medical University and Changsha Central
Hospital) were recruited between January 1st 2016 and December 31st
2018. The Ethics Committee of the Wenling Hospital Affiliated to
the Wenzhou Medical University approved the research protocol for
this study. Written informed consent was obtained from all patients
prior to their enrolment.
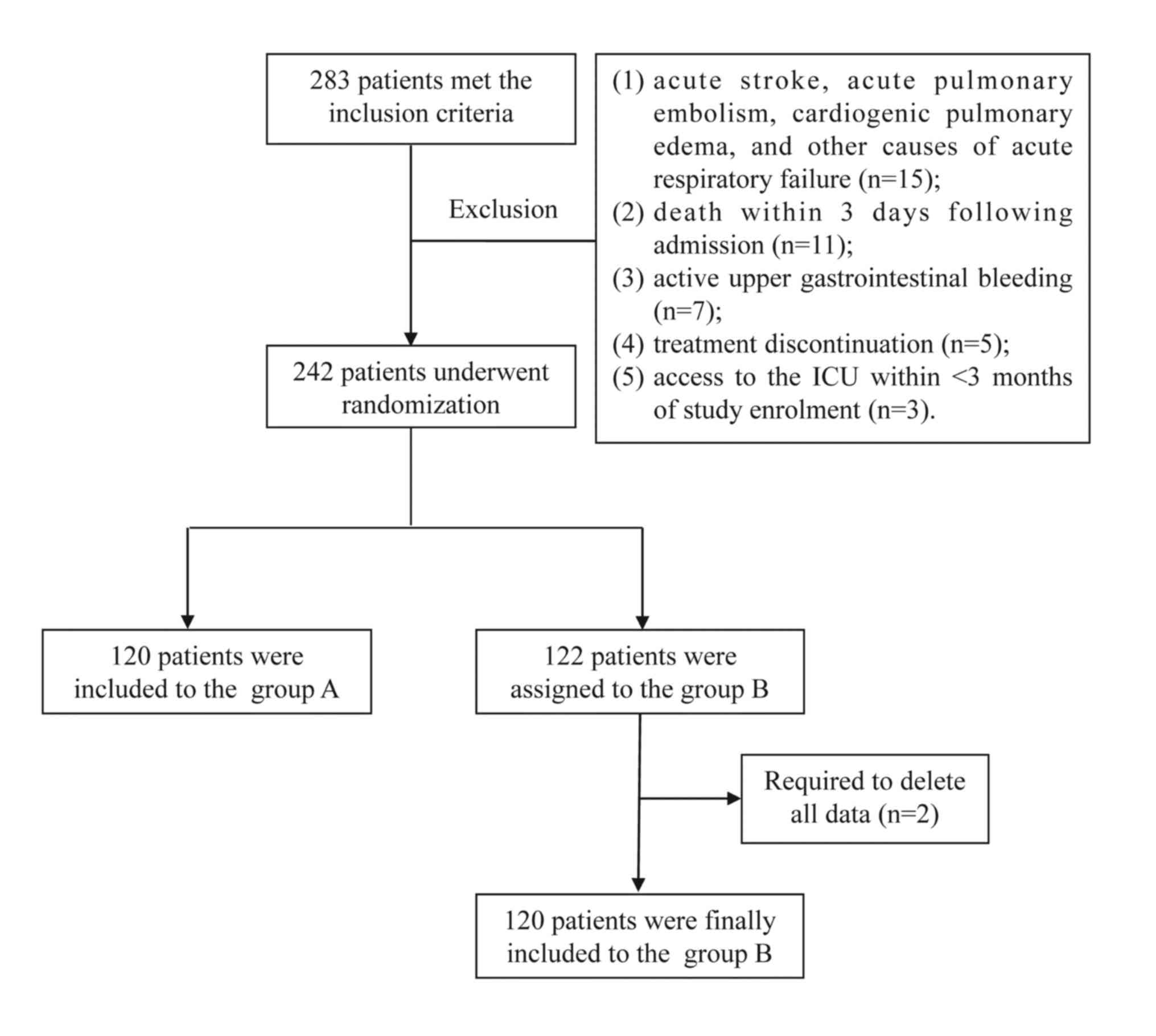
The inclusion criteria were as follows: i) Age ≥18
years; ii) patients who received IMV for respiratory failure; iii)
patients who met the COPD diagnostic criteria in the 2017
guidelines of the Global Initiative for Chronic Obstructive Lung
Disease (14); iv) partial pressure
of arterial oxygen (PaO2) and CO2
(PaCO2) in arterial blood gas (ABG) analysis <60
mmHg; and v) no absolute contraindications to NIV (15). The exclusion criteria were as
follows: i) Acute stroke, acute pulmonary embolism, cardiogenic
pulmonary edema or other causes of acute respiratory failure; ii)
death within 3 days of admission; iii) active upper
gastrointestinal bleeding; iv) treatment discontinuation; or v)
readmission to the ICU <3 months following enrolment with the
study. A total of 283 patients qualified for the inclusion
criteria, with 240 patients finally included after screening. A
total of 141 men and 99 women were included in the current study
(mean age, 55.3±9.1 years; age range, 31-86 years). All the
enrolled patients were randomly assigned to groups A and B, and
there were no significant between-group differences with respect to
sex, age or body mass index (BMI). A schematic illustration of the
study design and patient-selection criteria is presented in
Fig. 1.
Groups and definitions of the modified
GCS score
Using a random number table, subjects were randomly
assigned to group A and B, based on the use of modified GCS score
≥13 or 10 points, respectively, as the switching point for
sequential weaning strategy. The detailed definition of the
modified GCS score is shown in Table
I.
 | Table IModified GCS. |
Table I
Modified GCS.
| Score | Eye | Verbal | Motor |
|---|
| 1 | Does not open
eyes | No response to
speech | Makes no
movements |
| 2 | Opens eyes in
response to painful stimuli | Response to loudly
call | Extension to painful
stimuli |
| 3 | Opens eyes in
response to voice | Understanding
error | Abnormal flexion to
painful stimuli |
| 4 | Opens eyes
spontaneously | Slow understanding of
speech | Flexion/withdrawal
from painful stimuli |
| 5 | N/A | Correct understanding
of speech | Localizes painful
stimuli |
| 6 | N/A | N/A | Obeys commands |
Treatment protocols
All subjects received the following treatments:
Anti-infective agents, antispasmodics, glucocorticoids
(methylprednisolone, 40-80 mg/day), anti-inflammatory agents,
expectorants, nutritional support and sedatives, as well as
measures for maintaining internal homeostasis. Dexmedetomidine was
used as the first-line sedative, which was administered as an
intravenous bolus infusion over 10 min at a dose of 1 µg/kg,
followed by 0.2-0.8 µg/kg/h using a micro-pump. The infusion rate
was titrated to maintain a Richmond Agitation-Sedation score
between-2 and +1(16). The daily
neurological wake-up test consisted of 3 components: Eye-opening in
response to verbal command, eye tracking and shaking hands-on
instruction if the sedation was in the target range. If the above
criteria were not met, the sedative dose was adjusted until the
target was reached.
Weaning protocol
The parameters of mechanical ventilation were
calibrated based on the results of the ABG analysis and the disease
course. In Group A, patients were ventilated with synchronized
intermittent mandatory ventilation mode, with the addition of
pressure support ventilation or the assist/control mode. The
respiratory rate (RR) was set at 13-18 cycles/min and tidal volume
at 8-10 ml/kg to maintain the arterial partial pressure of
CO2 (PaCO2) at 35-50 mmHg. The inspired
oxygen fraction and positive end-expiratory pressure were adjusted
to maintain arterial oxygen saturation of ≥90%. IMV was switched to
NIV (Philips Medical Systems, Inc.) if the patients remained stable
for 3 h after achieving the target modified GCS score in their
respective groups (13 points in Group A and 10 points in Group B).
Subsequently, the spontaneous/timed mode was applied, with an
inspiratory positive airway pressure of 12-14 cmH2O and
expiratory positive airway pressure of 5 cmH2O, which
were gradually increased to the appropriate level within 5-20 min
of switching to NIV.
Data collection and outcomes
Data pertaining to the baseline characteristics and
indices at admission were collected, including the Acute Physiology
and Chronic Health Enquiry score, modified GCS score, mean arterial
blood pressure (MBP) and oxygenation index (OI). Indices such as
the MBP, OI, heart rate (HR), RR and results of the ABG analysis
were also measured before and 3 h after weaning to NIV. The primary
outcome was the duration of invasive ventilation. Other outcomes
included the incidence of VAP, re-intubation rate, in-hospital
mortality and the length of hospital stay.
Statistical analysis
All statistical analyses were conducted using SPSS
25.0 (for Windows; IBM Corp.). Continuous variables are expressed
as the mean ± (SD). Between-group differences with respect to
continuous variables were assessed using the unpaired Student's
t-test. Categorical variables are expressed as ratios and
between-group differences were assessed using the χ2
test. All statistical analyses were two-sided, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline characteristics
A total of 240 patients [141 men and 99 women; mean
age ± SD: 55.3±9.1 (range 31-86) years] qualified the study
selection criteria and were enrolled; of these 120 patients each
were randomly assigned to groups A and B. The baseline demographic
characteristics of patients in the two groups were comparable at
the time of randomization (Table
II). There were no significant between-group differences with
respect to sex, age or BMI. There were no significant differences
with respect to concomitant diseases such as cardiovascular
diseases, cerebrovascular diseases, diabetes and chronic kidney
disease. Likewise, there were no significant between-group
differences with respect to MBP, HR, respiratory rate, OI or
arterial blood analysis at admission.
 | Table IIBaseline characteristics of the study
population. |
Table II
Baseline characteristics of the study
population.
| Variables | Group A, n=120 | Group B, n=120 | t/χ2
value | P-value |
|---|
| Male | 75 (62.5%) | 66 (55.0%) | -0.464a | 0.496 |
| Age, years | 57±8 | 54±10 | 0.693b | 0.489 |
| BMI,
kg/m2 | 21.87±3.79 | 20.33±2.51 | 0.702b | 0.484 |
| Primary diseases |
|
Cardiovascular
disease | 19 (15.83%) | 22 (18.33%) | 0.265a | 0.607 |
|
Cerebrovascular
disease | 25 (20.83%) | 17 (14.17%) | 1.847a | 0.174 |
|
Diabetes | 16 (13.33%) | 22 (18.33%) | 1.126a | 0.289 |
|
Chronic
kidney disease | 9 (7.50%) | 6 (5.00%) | 0.640a | 0.424 |
| APACHE II | 23.89±4.32 | 22.90±3.79 | 0.768b | 0.452 |
| MBP, mmHg | 102.59±20.03 | 98.25±21.92 | 1.251b | 0.224 |
| OI, mmHg | 158.26±32.82 | 161.13±26.57 | 0.42b | 0.813 |
| Heart rate,
bpm | 87.23±15.81 | 85.62±17.19 | 0.853b | 0.392 |
| Respiratory rate,
bpm | 21.21±5.14 | 19.68±4.17 | 1.35b | 0.187 |
| pH value | 7.21±1.51 | 7.25±1.90 | 0.148b | 0.881 |
| PaO2, mmHg | 58.35±11.24 | 56.09±9.83 | 1.649b | 0.105 |
| PaCO2, mmHg | 85.19±17.53 | 82.39±18.07 | 1.276b | 0.207 |
Safety and efficiency of sequential
weaning as guided by two different modified GCS scores
Patients in groups A and B underwent extubation
followed by sequential NIV upon reaching modified GCS score of ≥13
points and 10, respectively. There were no significant
between-group differences with respect to OI, MBP, PaO2
and PaCO2 (P>0.05; Table
III) both before extubation and at 3 h after extubation.
 | Table IIIIndices before and 3 h after
extubation. |
Table III
Indices before and 3 h after
extubation.
| Time | Variables | Group A | Group B | Comparison between
Group A and B |
|---|
| t value | P-value |
|---|
| Before
extubation | MBP, mmHg | 101.50±19.53 | 105.37±17.63 | 0.672 | 0.506 |
| OI, mmHg | 222.16±40.83 | 225.25±43.09 | 1.298 | 0.192 |
| PaO2,
mmHg | 88.65±19.26 | 87.58±17.30 | 1.034 | 0.316 |
| PaCO2,
mmHg | 44.26± 12.64 | 43.60± 10.25 | 0.685 | 0.493 |
| 3 hours after
extubation and noninvasive ventialtion | MBP, mmHg | 102.86±22.69 | 102.56±20.35 | 0.857 | 0.389 |
| OI, mmHg | 210.29±37.47 | 213.06±37.31 | 0.831 | 0.408 |
| PaO2,
mmHg | 81.55±21.63 | 84.63±22.65 | 0.658 | 0.514 |
| PaCO2,
mmHg | 45.75±16.14 | 48.32±11.74 | 0.693 | 0.491 |
Primary and secondary outcomes
The duration of IMV in group A was significantly
shorter than that in Group B (Table
IV). However, there were no significant between-group
differences with respect to the incidence rate of re-intubation,
VAP, in-hospital mortality or the length of hospital stay.
 | Table IVPrimary and secondary outcomes. |
Table IV
Primary and secondary outcomes.
| Variables | Group A
(n=120) | Group B
(n=120) | t/χ2
value | P-value |
|---|
| Hospital mortality
(n, %) | 8 (6.67%) | 10 (8.33%) | 0.240a | 0.624 |
| Duration of IMV
(days) | 4.13±1.04 | 4.96±0.69 | 2.003b | 0.045 |
| Incidence of VAP
(n, %) | 3 (7.5) | 6 (15.0) | 1.127a | 0.288 |
| Re-intubation (n,
%) | 2 (5.0) | 5 (12.5) | 1.409a | 0.235 |
| Length of hospital
stay (days) | 15.85±3.87 | 17.16±5.02 | 1.485b | 0.152 |
Discussion
The present study was a randomized clinical trial
conducted at three ICUs in Eastern China. It was found that
sequential NIV, guided by a modified GCS score, was an effective
and safe weaning strategy in AECOPD patients undergoing IMV.
Moreover, the use of a modified GCS score ≥13 points as the
criterion to switch to sequential NIV, decreased the duration of
invasive ventilation in ICU patients with AECOPD-induced
respiratory failure, as compared to a modified GCS score ≥10
points. However, there were no significant differences with respect
to the incidence of re-intubation, VAP, hospital mortality or the
length of hospital stay.
The identification of a timely and optimal
‘switching point’ is the key to an effective sequential ventilation
strategy (9). Use of spontaneous
breathing trial (SBT) to predict the successful withdrawal of the
IMV is recommended by the European Respiratory Society, the
American Thoracic Society, the European Society of Intensive Care
Medicine, the Society of Critical Care Medicine and the Société de
Réanimation de langue Française (17). It is believed that the duration of
SBT should be between 30-120 min (18); however, it is not clear whether the
same duration is applicable to patients who require mechanical
ventilation because of different diseases. It has been found that
SBT may cause a delay in weaning and may also cause VAP, which in
turn may contribute to increased mortality (19). In China, the switching point is
mainly the PIC window, as proposed by the Beijing Institute of
Respiratory Diseases (20), while
other countries, such as England, have employed a 48-h window for
this purpose (21). However, both of
these strategies have certain limitations. The former ignores the
time-lag between the appearance of imaging findings and the onset
of clinical manifestations, and does not take account of
noninfective factors. The latter strategy overlooks
inter-individual variability among patients.
There are 3 prerequisites for the application of
sequential NIV: A high level of consciousness, a certain degree of
cooperation and appropriate compliance; therefore, the changes in
consciousness should be factored while considering the switch to
noninvasive respiratory support (22). The main causes of AECOPD-induced
respiratory failure are pulmonary infection, ventilatory
insufficiency or respiratory muscle fatigue (9). Therefore, the level of consciousness
tends to vary with the development, exacerbation or improvement of
AECOPD (22). The GCS score is
widely used for the assessment of awareness. It is an objective
measure for the dynamic assessment of the overall and physical
condition of patients with COPD with severe respiratory failure at
each stage of the disease (23).
Patients with endotracheal intubation cannot speak even if they are
conscious (24). Therefore, the GCS
scoring system has been improved by modifying the correct response
instead of using speech (23). A
previous study found that it was safe and feasible to initiate
sequential NIV if modified GCS score ≥13 points, and that it was
acceptable to stablely maintain for 3 h (25). The sequential ventilation strategy
was found to reduce the discomfort, restlessness and pain induced
by intubation, and increased patient trust and coordination. This
resulted in a higher rate of successful weaning, lower incidence of
re-intubation and shorter length of hospital stay. Chen et
al (26) found that the use of
modified GCS score ≥10 points was a more suitable criterion for
initiating the switch to sequential invasive-non-invasive weaning.
In the present study, the two weaning strategies, which were guided
respectively by ‘improved GCS ≥13 points’ and ‘improved GCS ≥10
points’, were compared. The use of the modified GCS score ≥13
points as the criterion to switch to sequential NIV was associated
with a shorter duration of invasive ventilation in ICU patients
with AECOPD-induced respiratory failure as compared to using the
modified GCS score ≥10 points.
In conclusion, using a improvement of the modified
GCS score ≥13 points as the switching point for sequential
invasive-NIV may significantly improve the prognosis of patients
with AECOPD with respiratory failure.
Acknowledgements
Not applicable.
Funding
This study was supported by the Wenling Science and
Technology Bureau Project (grant no. 2014C31BA0032).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JBZ and JQZ designed/performed most of the
investigation, data analysis and wrote the manuscript. LXC, XJ and
LLC provided statistics assistance. YS, SZ, JM, HF, JX, MD, LY, XW,
HW, JYZ and YW contributed to interpretation of the data and
analyses. All of the authors have read and approved the
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Wenling Hospital
Affiliated to the Wenzhou Medical University approved the research
protocol for this study. Written informed consent was obtained from
all patients prior to their enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adeloye D, Chua S, Lee C, Basquill C,
Papana A, Theodoratou E, Nair H, Gasevic D, Sridhar D, Campbell H,
et al: Global and regional estimates of COPD prevalence: Systematic
review and meta-analysis. J Glob Health. 5(020415)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rothberg MB, Pekow PS, Maureen L, Oren B,
Skiest DJ and Lindenauer PK: Antibiotic therapy and treatment
failure in patients hospitalized for acute exacerbations of chronic
obstructive pulmonary disease. JAMA. 303:2035–2042. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Afessa B, Morales I, Scanlon PD and Peters
SG: Prognostic factors, clinical course, and hospital outcome of
patients with chronic obstructive pulmonary disease admitted to an
intensive care unit for acute respiratory failure. Crit Care Med.
30:1610–1615. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Plant PK, Owen JL and Elliott MW: Early
use of non-invasive ventilation for acute exacerbations of chronic
obstructive pulmonary disease on general respiratory wards: A
multicentre randomised controlled trial. Lancet. 355:1931–1935.
2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stefan MS, Shieh MS, Pekow PS, Hill N,
Rothberg MB and Lindenauer PK: Trends in mechanical ventilation
among patients hospitalized with acute exacerbations of COPD in the
United States, 2001 to 2011. Chest. 147:959–968. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ai-Ping C, Lee KH and Lim TK: In-hospital
and 5-year mortality of patients treated in the ICU for acute
exacerbation of COPD: A retrospective study. Chest. 128:518–524.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Michelle B, Kashiouris MG and Ognjen G:
Ventilator-induced lung injury: Minimizing its impact in patients
with or at risk for ARDS. Respir Care. 58:927–934. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Forel JM, Voillet F, Pulina D, Gacouin A,
Perrin G, Barrau K, Jaber S, Arnal JM, Fathallah M and Auquier P:
Ventilator-associated pneumonia and ICU mortality in severe ARDS
patients ventilated according to a lung-protective strategy. Crit
Care. 16(R65)2012.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Lv Y, Lv Q, Lv Q and Lai T: Pulmonary
infection control window as a switching point for sequential
ventilation in the treatment of COPD patients: A meta-analysis. Int
J Chron Obstruct Pulmon Dis. 12:1255–1267. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Frutos-Vivar F, Esteban A, Apezteguia C,
González M, Arabi Y, Restrepo MI, Gordo F, Santos C, Alhashemi JA,
Pérez FJ, et al: Outcome of reintubated patients after scheduled
extubation. J Crit Care. 26:502–509. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang CT and Yu CJ: Conventional weaning
parameters do not predict extubation outcome in intubated subjects
requiring prolonged mechanical ventilation. Respir Care.
58:1307–1314. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hendra KP, Bonis PA and Joyce-Brady M:
Development and prospective validation of a model for predicting
weaning in chronic ventilator dependent patients. BMC Pulm Med.
3(3)2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu YK, Kao KC, Hsu KH, Hsieh MJ and Tsai
YH: Predictors of successful weaning from prolonged mechanical
ventilation in Taiwan. Respir Med. 103:1189–1195. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vogelmeier CF, Criner GJ, Martinez FJ,
Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M,
Fabbri LM, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive lung disease 2017 report.
GOLD Executive Summary. Am J Respir Crit Care Med. 195:557–582.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ornico SR, Lobo SM, Sanches HS,
Deberaldini M, Tófoli LT, Vidal AM, Schettino GP, Amato MB,
Carvalho CR and Barbas CS: Noninvasive ventilation immediately
after extubation improves weaning outcome after acute respiratory
failure: A randomized controlled trial. Crit Care.
17(R39-R39)2013.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Vasilevskis EE, Pandharipande PP, Graves
AJ, Shintani A, Tsuruta R, Ely EW and Girard TD: Validity of a
modified sequential organ failure assessment score using the
richmond agitation-sedation scale. Crit Care Med. 44:138–146.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Boles JM, Bion J, Connors A, Herridge M,
Marsh B, Melot C, Pearl R, Silverman H, Stanchina M,
Vieillard-Baron A and Welte T: Weaning from mechanical ventilation.
Eur Respir J. 29:1033–1056. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Burns KEA, Karim HMR and Esquinas AM:
Characteristic of Subjects Who Fail a 120-minute spontaneous
breathing trial: When minutes are taken into account. Respir Care.
64(114)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kallet RH: Ventilator bundles in
transition: From prevention of ventilator-associated pneumonia to
prevention of ventilator-associated events. Respir Care.
64:994–1006. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Luo Z, Zhan Q and Wang C: Noninvasive
positive pressure ventilation is required following extubation at
the pulmonary infection control window: A prospective observational
study. Clin Respir J. 8:338–349. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Perkins GD, Mistry D, Gates S, Gao F,
Snelson C, Hart N, Camporota L, Varley J, Carle C, Paramasivam E,
et al: Effect of protocolized weaning with early extubation to
noninvasive ventilation vs invasive weaning on time to liberation
from mechanical ventilation among patients with respiratory
failure: The breathe randomized clinical trial. JAMA.
320:1881–1888. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Briones Claudett KH, Briones Claudett M,
Chung Sang Wong M, Nuques Martinez A, Soto Espinoza R, Montalvo M,
Esquinas Rodriguez A, Gonzalez Diaz G and Grunauer Andrade M:
Noninvasive mechanical ventilation with average volume assured
pressure support (AVAPS) in patients with chronic obstructive
pulmonary disease and hypercapnic encephalopathy. BMC Pulm Med.
13(12)2013. View Article : Google Scholar
|
|
23
|
Ciftci F, Ciledag A, Erol S, Oz M, Acar D
and Kaya A: Evaluation of the feasibility of average volume-assured
pressure support ventilation in the treatment of acute hypercapnic
respiratory failure associated with chronic obstructive pulmonary
disease: A pilot study. J Crit Care. 39:232–237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Moskopp D, Stahle C and Wassmann H:
Problems of the Glasgow Coma Scale with early intubated patients.
Neurosurg Rev. 18:253–257. 1995.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang J, Zhu J, Zhou S, Ma J, Fu H, Song
Y, Xu J, Cao L, Dong M, Yan L, et al: Noninvasive mechanical
ventilation in the weaning of AECOPD patients with respiratory
failure: Modified glasgow coma scale score ≥13 as the switching
point. Int J Clinical Exp Med. 12:7808–7813. 2019.
|
|
26
|
Chen XLaW: Clincial investigation of
modified GCS ≥10 points as the switching point of sequantial
sequential invasive-noninvasive mechanical ventilation weaning in
AECOPD patients with respiratory failure. Chong Qing Yi Xue.
45:1381–1383. 2016.(In Chinese).
|