Introduction
Cardiovascular disease is the leading cause of death
in western countries (1). According
to data from 2013, >17.3 million (or 31.5%) of all deaths
worldwide every year (2-4).
The American Heart Association estimated that >25 million people
will die of cardiovascular disease every year by 2020(5). Coronary atherosclerotic heart disease
is a common cardiovascular disease, therefore, preventing the
development of atherosclerosis (AS) will be a major task to help
with the prevention of cardiovascular diseases.
AS is a chronic inflammatory disease (6). It has been confirmed by numerous
studies that various cytokines play important roles in the
progression of AS and plaque instability (7-9).
Interleukin (IL)-6 is a cytokine that regulates the inflammatory
response produced by leukocytes and other cells, and is also
considered to be a biomarker of inflammation (8). Tumor necrosis factor (TNF)-α is
considered to be an effective pro-inflammatory mediator, which
promotes the expression of other inflammatory cytokines and
adhesion molecules as well as increasing the apoptosis of vascular
smooth muscle cells, thus promoting AS and plaque instability
(9). Chemotactic factors are small
molecule proteins that recruit leukocytes from circulation in the
blood to inflammatory injury sites (10). In chronic inflammatory diseases such
as AS, the binding of monocyte chemotactic protein (MCP)-1 and its
receptor, C-C motif chemokine receptor-2, induces monocyte
chemotaxis to the inflammatory site, leading to aggravation of the
inflammatory response (11).
Toll-like receptors (TLRs) are pattern recognition
receptors present on macrophage surfaces, which are extensively
expressed on macrophages in lipid-rich atherosclerotic plaques in
humans and mice and play an essential role in the host defense
response (12). It has been shown by
Zhou et al (13) that TLR2
and TLR4 are highly expressed in human umbilical vein endothelial
cells and in the human acute monocytic leukemia cell line, THP-1
epidermal cells. The expression of TLR2 and TLR4 is induced by
oxidized (ox)-low-density lipoprotein (LDL), and in TLR2 or TLR4
deficient cells, the formation of foam cells decreases
significantly (14). Thus, TLR4 has
the potential to enhance ox-LDL intake and/or impair the reverse
transportation of cholesterol.
MicroRNAs (miRNAs), are single-stranded, non-coding
nucleotides, 21-24 base pairs (bp) in length, which were first
found in nematodes (15). miRNAs are
involved in genomic expression and regulation by binding to the
target site of the mRNA 3'-untranslated region (3'-UTR), leading to
the suppression of transcription and/or affecting mRNA instability
(16). miRNA (miR)33 is localized in
the sterol-regulatory element–binding factor (SREBP) intron
(17). A previous study (16) reported that there are 3 highly
conserved miRNA binding sites in the 3'-UTR of ATP binding cassette
transporter (ABC)A1. Therefore, the role of miR33 in the regulation
of cholesterol efflux and the biosynthesis of high-density
lipoprotein (HDL) may be through the downregulation ABCA1 and
ABCG1.
Curcumin is a polyphenolic compound found primarily
in the rhizomes of the ginger plant, and is believed to be one of
the most biologically active natural products. It has been shown
that curcumin has pharmacological effects in a wide variety of
chronic diseases (18,19). Dong et al (20) speculated that curcumin or food rich
in curcumin, have the potential to be a novel therapy for
decreasing the risk of AS by increasing the expression levels of
ABCA1 and increasing the cholesterol efflux in mouse adipocytes by
the peroxisome proliferator activated receptor γ/liver X receptor α
signaling pathway.
Lin et al (18) found that curcumin can inhibit
ox-ldl-induced MCP-1 expression of VSMCs via the mitogen activated
protein kinases (MAPK) and nuclear transcription factor κB (NF-κB)
signaling pathway. Although a number of studies investigated the
mechanisms behind the pharmacological activity of curcumin
(18-20),
the exact mechanism behind its pharmacological effects still
remains to be elucidated.
Overall, the mechanism of action for curcumin, TLR4,
NF-κB and miR33a in the transfer of cholesterol and the secretion
of TNF-α, MCP-1 and IL-6 is still remains unclear. It has been
hypothesized that curcumin promotes cholesterol efflux and reduces
the secretion of TNF-α, IL-6 and MCP-1 through the
TLR4/NF-κB/miR33a signaling pathway.
Materials and methods
Reagents
THP-1 cells were purchased from the American Type
Culture Collection. FBS, v1640 medium, myllicin and trypsin were
purchased from Gibco; Thermo Fisher Scientific, Inc. Human ox-LDL
was purchased from Anhui Yiyuan Biotechnology Co., Ltd. Cell
Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies, Inc. Free cholesterol, CE and Triglycerides assay
kits were purchased from Nanjing Jiancheng Biotechnology Co., Ltd.
Curcumin, phorbol-12-myristate-13-acetate (PMA) and Ammonium
pyrrolidinedithiocarbamate (PDTC) were purchased from
Sigma-Aldrich; Merck KGaA. Antibodies targeting TLT4 (mouse
monoclonal antibody raised against TLR4 of human origin; cat. nos.
14358), TLR4 non-related isotype (cat. no. 2985) (isotype Ab),
NF-κB p65 (cat. no. 8242), NF-κB inhibitor α (cat. no. 4814)
(IκBα), phosphorylated (p)-IκBα (cat. no. 2859), GAPDH (cat. no.
5174), ABCA1 (cat. no. 96292) and histone H1 (cat. no. 41318) were
purchased from Cell Signaling Technology, Inc. Horseradish
perioxidase (HRP) goat anti-mouse IgG (cat. no. 074-1506) used as
secondary antibodies and was purchased from Kirkegaard & Perry
Laboratories, Inc. Chemiluminescence (ECL) test kit and RIPA lysis
buffer were purchased from ASPEN Biotechnology Co., Ltd. Image
Laboratory Software 4.0 was used (Bio-Rad Laboratories, Inc.).
Lipofectamine™ 2000 and M-MLV Reverse Transcriptase (cat. no.
28025013) was purchased from Invitrogen; Thermo Fisher Scientific,
Inc. PrimeScript™ RT reagent kit with gDNA Eraser (cat. no. RR047Q)
and SYBR® Premix Ex Taq™ (cat. no. DRR041A) were
purchased from Takara Biomedical Technology Co., Ltd. The EpiQuik
Nuclear Extraction kit was purchased from AmyJet Scientific Inc.
The Cholesterol Efflux Fluorometric assay kit was purchased from
BioVision, Inc. The IL-6 (cat. no. E-EL-H0102)/TNF-α (cat. no.
E-EL-M0049)/MCP-1 (cat. no. E-EL-H6005) ELISA kit was purchased
from Elabscience Biotechnology Co., Ltd. All were used according to
the manufacturers protocol.
THP-1 cell culture and foam cell model
establishment
THP-1 cells were cultured in RPMI-1640 medium which
contained 10% FBS and 1% penicillin/streptomycin at 37˚C in a 5%
CO2 incubator. Culture medium was replaced every 2 days,
cells were subcultured for a period of 5 days. To obtain THP-1
macrophages, medium containing 160 nmol/l PMA was used to induce
culture for 48 h in an incubator at 37˚C and 5% CO2. The
cell culture medium was replaced with serum-free RPMI-1640 culture
medium containing 50 µg/ml ox-LDL. Cells were then incubated in the
incubator for another 48 h in an incubator at 37˚C and 5%
CO2. An intracellular cholesterol ester (CE)/total
cholesterol (TC) ratio >50% was used as the standard for
successful replication of foam cell models (14).
Cell viability assay
THP-1 cells at the logarithmic growth phase were
prepared using a complete medium containing 160 nmol/l of PMA.
THP-1 cells (1x104 cells) were then seeded into 96-well
plates and cultured for 48 h in an incubator at 37˚C and 5%
CO2. The cells were treated with various concentrations
(0, 5, 10, 20, 40 and 80 mol/l) of curcumin for 24 h in an
incubator at 37˚C and 5% CO2, and subjected to the CCK-8
assay. The absorbance was measured at a wavelength of 490 nm and
was used as an indicator of cell viability.
Oil Red O staining and foam cell
formation rate
According to the aforementioned method, THP-1
macrophages were adjusted to a cell density of 4x105
cells/ml and 1x106 cells/well were seeded into 6-well
plates containing pre-implanted sterile coverslips in an incubator
at 37˚C and 5% CO2. Subsequently, cells on the
coverslips were washed with PBS 3 times, 5 min each time, and then
fixed with 4% paraformaldehyde for 30 min at room temperature. Oil
red O staining was performed for 10 min and the cells were
counterstained for 5 min with hematoxylin at room temperature.
Under the optical microscope, the number of larger red dye
particles in the cell >5 can be used as the standard for foam
cell formation. Ten fields were randomly observed to calculate the
number of foam cells and the total number of cells at
magnification, x200. The ratio of foam cells to total cells was
deemed the foam cell formation rate.
Measurement of intracellular TC, free
cholesterol (FC), CE and triglycerides (TGs)
THP-1 cell suspension stimulated by ox-ldl was
centrifuged at 200 x g at room temperature for 10 min. Cell samples
were collected and cell homogenates were obtained using ultrasonic
disruption at a low temperature (-4˚C). TC and FC were detected by
FC, CE assay kit. The CE levels were calculated by subtracting the
FC from the TC. Cells were treated as aforementioned, and the TG
content was determined using the TG assay kit.
Western blot assays
The cells were collected and the total protein was
extracted using RIPA buffer at a low temperature (-4˚C) NF-κB p65
is located in the nucleus of the cell; therefore, the EpiQuik
Nuclear Extraction kit was used to extract the nuclear protein.
Protein concentrations were determined using BCA assays. A 10%
separation gel and a 5% spacer gel were prepared based on
instructions provided in the SDS-polyacrylamide gel electrophoresis
preparation kit, which were then transferred to PVDF membranes.
After being blocked with 5% skim milk at room temperature for 1 h,
the membranes were incubated with primary antibodies against GAPDH
(1:1,000), histone H1 (1:500), ABCA1 (1:500), NF-κB p65 (1:2,000),
IκBα (1:1,000), p-IκBα (1:1,000), TLR4 and TLR4 non-related isotype
Ab (1:1,000) overnight at 4˚C, followed by an incubation with
HRP-Goat anti-mouse IgG secondary antibodies for 90 min. The
optical densities of bands were detected using an ECL test kit and
quantified using Image Lab software 4.0 (National Institutes of
Health). GAPDH was used as the endogenous control.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using TRIzol™
reagent (Thermo Fisher Scientific, Inc.) and reverse transcribed
into cDNA using M-MLV Reverse Transcriptase kit at 4˚C. Primers
were designed and synthesized by Invitrogen; Thermo Fisher
Scientific, Inc. (Table I). The
thermocycling conditions for qPCR were as follows: Pre-denaturation
at 95˚C for 1 min followed by 3 steps of 40 cycles. Each cycle
consisted of denaturation at 95˚C for 15 sec, annealing at 58˚C for
20 sec and extension 72˚C for 20 sec. miRNA molecules are a class
of nucleotides with a short length of ~21-24 bp, which cannot be
amplified using conventional RT-qPCR. miR33a pre-treatment was
performed with Stem-loop RT. U6 and GAPDH served as the endogenous
controls for miRNA and mRNA. Results were presented as the level of
mRNA relative to endogenous and calculated using the
2-ΔΔCq method (21).
 | Table IPrimer sequences used for
quantitative PCR. |
Table I
Primer sequences used for
quantitative PCR.
| Gene | Primer
sequence |
|---|
| H-GAPDH | Forward |
5'-GGTCGGAGTCAACGGATTTG-3' |
| Reverse |
5'-GGAAGATGGTGATGGGATTTC-3' |
| H-ABCA1 | Forward |
5'-AGGAAACCCAATCCCAGATACCC-3' |
| Reverse |
5'-GCTCGGAGGAAGTGCTTGAGAAT-3' |
| H-TLR4 | Forward |
5'-GGATGAGGACTGGGTAAGGAAT-3' |
| Reverse |
5'-AATGAAGATGATACCAGCACGAC-3' |
| U6 | Looped RT
primer |
5'-AACGCTTCACGAATTTGCGT-3' |
| Forward |
5'-AACGCTTCACGAATTTGCGT-3' |
| Reverse | 5'-AACGCTTCA
CGAATTTGCGT-3' |
| miR-33a-5p | Looped RT
primer | 5'-CTCAACTGGTGT
CGTGGAGTCGGCAATT CAGTTGAGGCAATGCA-3' |
| Forward | 5'-
GGTGCATTGTAGTTGCATTGC-3' |
| Reverse |
5'-GCGACGAGCAAAAAGCTTGT-3' |
miR33a inhibitor and control sequence
transfection
Cells were transfected with 60 nM miR33a inhibitor
or an equal concentration of miR-control using Lipofectamine™ 2000.
In order to ensure successful transfections were achieved, RT-qPCR
was used to detect the expression levels of miR33a mRNA after 6 h
in an incubator at 37˚C and 5% CO2. Cells were then used
for further experimentation.
ELISA
Cells were seeded in 6-well plates at a density of
4x105/ml, and treated according to the different
experimental conditions. The cells were collected by centrifugation
at 1,000 x g for 20 min and the supernatant were stored at -20˚C
prior to further experiments. The content of IL-6, TNFα and MCP-1
was measured by ELISA assays according to the manufacturers
protocol. Standard concentration curves were drawn using standard
diluents and the actual content of IL-6, TNF-α and MCP-1 was
calculated.
Cholesterol efflux assays
Cholesterol efflux was measured based on
instructions provided in Cholesterol Efflux Fluorometric assay kit
(cell-Based). Macrophages derived from THP-1 cells were inoculated
into the 96-well plates at a density of 1x105/ml.
Cholesterol was pre-labeled with 50 µl fluorescent labeling agent
and balanced buffer and cultured in the incubator overnight (>16
h) in an incubator at 37˚C and 5% CO2. After different
experimental treatments, the fluorescence levels (Ex/Em=482/515) of
the supernatant, as well as supernatant containing dissolved cell
and cellular debris, obtained using 100 µl RIPA lysis buffer were
measured. The ratio of fluorescence intensity of the media to the
total fluorescence intensity of the cell lysate and media, x100 was
the percentage cholesterol efflux.
Statistical analysis
All values are presented as the means ± SD of three
independent experiments. Differences between the groups were
analyzed using the one-way ANOVA followed by Dunnett's or Tukey's
post hoc tests, as applicable, with SPSS software (version 17.0;
SPPS, Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
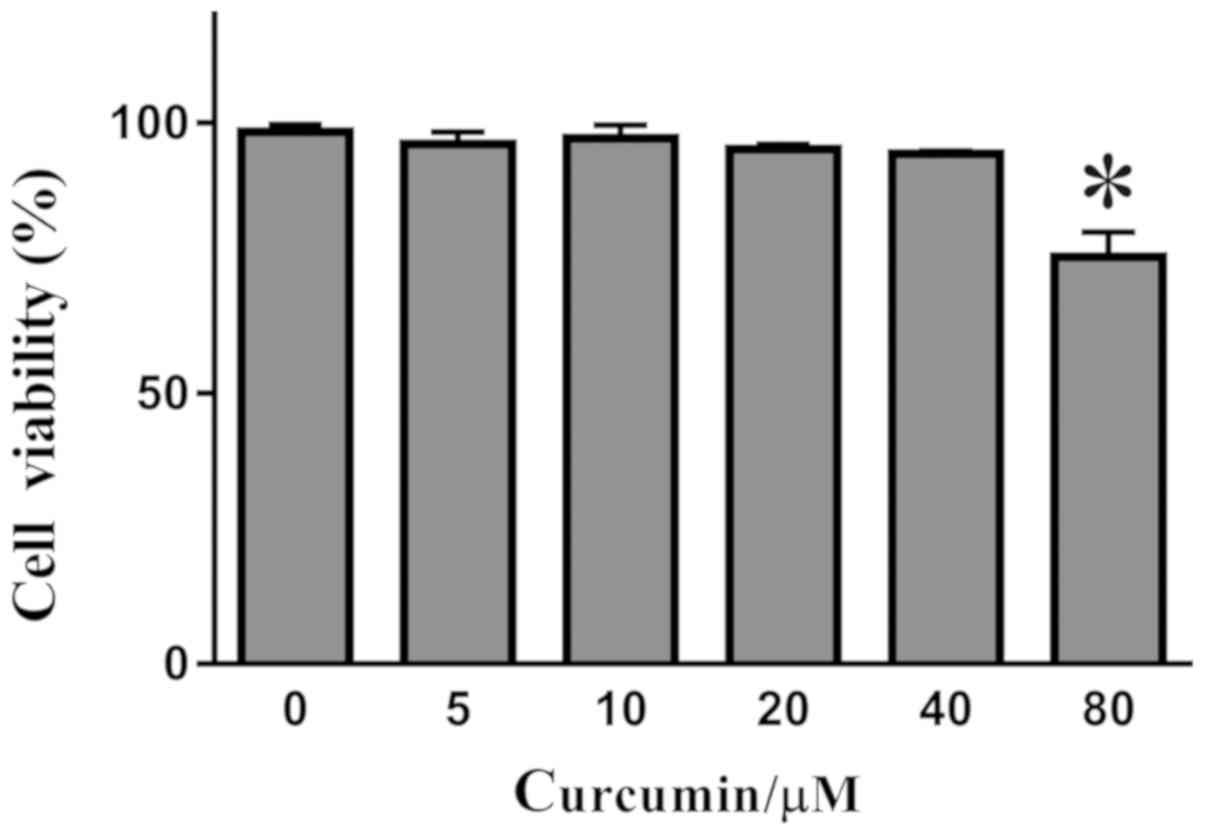
Detection of curcumin toxicity by
CCK-8
Curcumin of various concentrations (0, 5, 10, 20, 40
and 80 µM) was incubated with THP-1 derived macrophages for 24 h.
The toxicity of curcumin was detected using CCK-8 assays. Curcumin
of various concentrations was found to have an effect on cell
viability (Fig. 1). Further analysis
found that there was no significant difference between the effects
of curcumin at the concentrations of 0-40 µM. However, when the
concentration of curcumin was increased to 80 µM, the viability of
THP-1 derived macrophages decreased as a result of curcumin
toxicity.
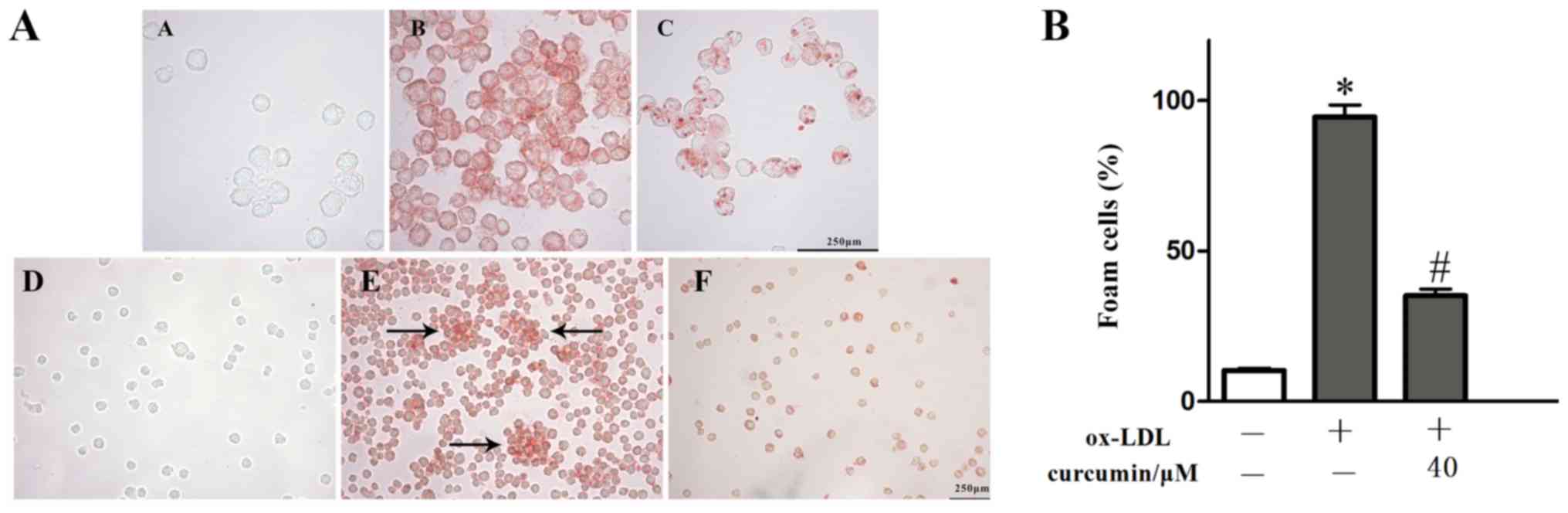
Effect of curcumin on the
intracellular lipid content and foam cell formation of THP-1 cells
induced by ox-LDL
THP-1 macrophages were stimulated with 50 μg /ml
ox-LDL, and then treated with curcumin at a safe concentration (40
μM) for 24 h. When observed at x200 and x400 using light
microscopy, it was found that numerous intracellular red stained
particles were assembled following ox-LDL treatment, while in the
safe concentration curcumin group, the number of intracellular red
stained particles and the clustering of foam cells (Fig. 2A b,e) was less
than that of the model group (Fig.
2A a,d). Detection of TC, FC, CE and TG content
using the TC, FC and TG kits showed that the intracellular lipid
content in THP-1 macrophages increased due to ox-LDL induction. Due
to the accumulation of lipids in cells, the ratio of CE/TC
increased up to 65.09%, the model met the criterion (22) to be considered a macrophage model
(Table II). Curcumin significantly
reduced the accumulation of intracelluar cholesterol and
triglyceride, consistent with the previous research in the current
report. It was demonstrated that ox-LDL significantly promoted the
formation of foam cells, while curcumin reduced this formation
(Fig. 2B). However, the specific
mechanism of action needs further investigation.
 | Table IIEffect of curcumin on the contents of
TC, FC and CE in cells. (n=3 per group, mean ± SD). |
Table II
Effect of curcumin on the contents of
TC, FC and CE in cells. (n=3 per group, mean ± SD).
| Treatment
groups | TC,
mmol·g-1 | FC,
mmol·g-1 | CE,
mmol·g-1 | TG,
mmol·g-1 | CE:TC, % |
|---|
| Control | 5.64±0.15 | 3.56±0.16 | 2.08±0.06 | 3.83±0.15 | 36.83±1.41 |
| Foam cell
model |
18.78±0.18a |
6.55±0.18a |
12.22±0.36a |
20.29±0.47a |
65.09±1.30a |
| Curcumin (40
µM) |
11.97±0.07b |
5.94±0.17b |
6.02±0.21b |
13.34±0.41b |
50.33±1.53b |
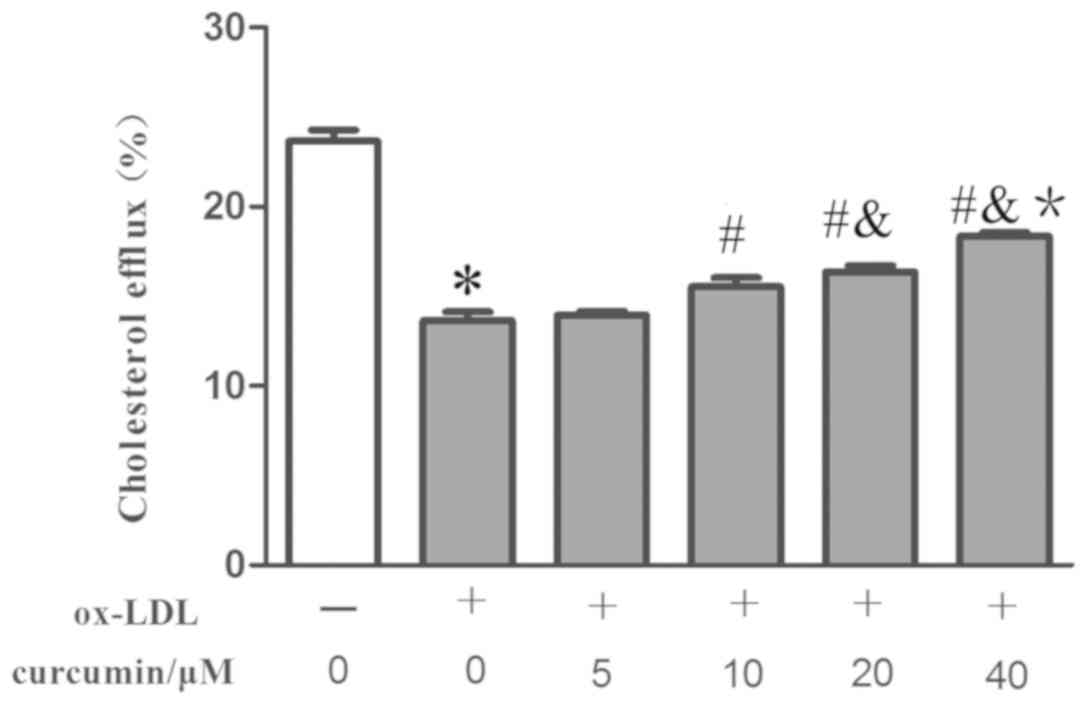
Effect of curcumin on the cholesterol
efflux rate of THP-1 induced by ox-LDL
Ox-ldl promoted lipid inflow, and the effect of
intervention conditions on cholesterol outflow rate was
subsequently examined (20). It was
discovered that curcumin of various concentration (10-40 µM)
increased the cholesterol efflux rate compared with the foam cell
model group (Fig. 3). The effect was
associated with curcumin concentration, but the curcumin with the
lowest analyzed concentration (5 µM) was unable to significantly
affect the cholesterol efflux rate. This observation helps explain
how curcumin reduced the intracelluar lipid content and formation
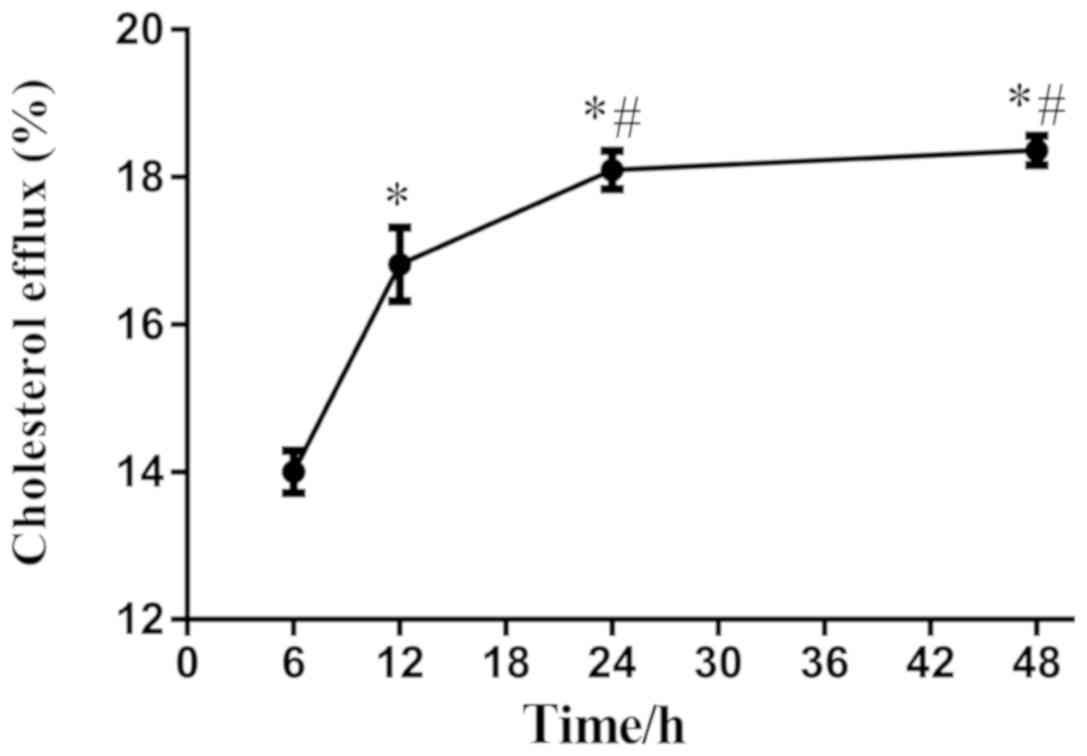
of foam cells by promoting cholesterol efflux in THP-1 macrophages.
Curcumin was then used at 40 µM to stimulate THP-1 macrophages for
various time periods, and it was found that the cholesterol efflux
was increased between 6-24 h. However, when the stimulation of
curcumin lasted 48 h, the cholesterol efflux did not increase
significantly compared with that at 24 h. Thus, curcumin
stimulation for 24 h resulted in peak cholesterol efflux (Fig. 4). To ensure the validity of the
experiment and to exclude inaccuracy, THP-1 macrophages were
incubated with 40 µM curcumin for 24 h for subsequent
experiments.
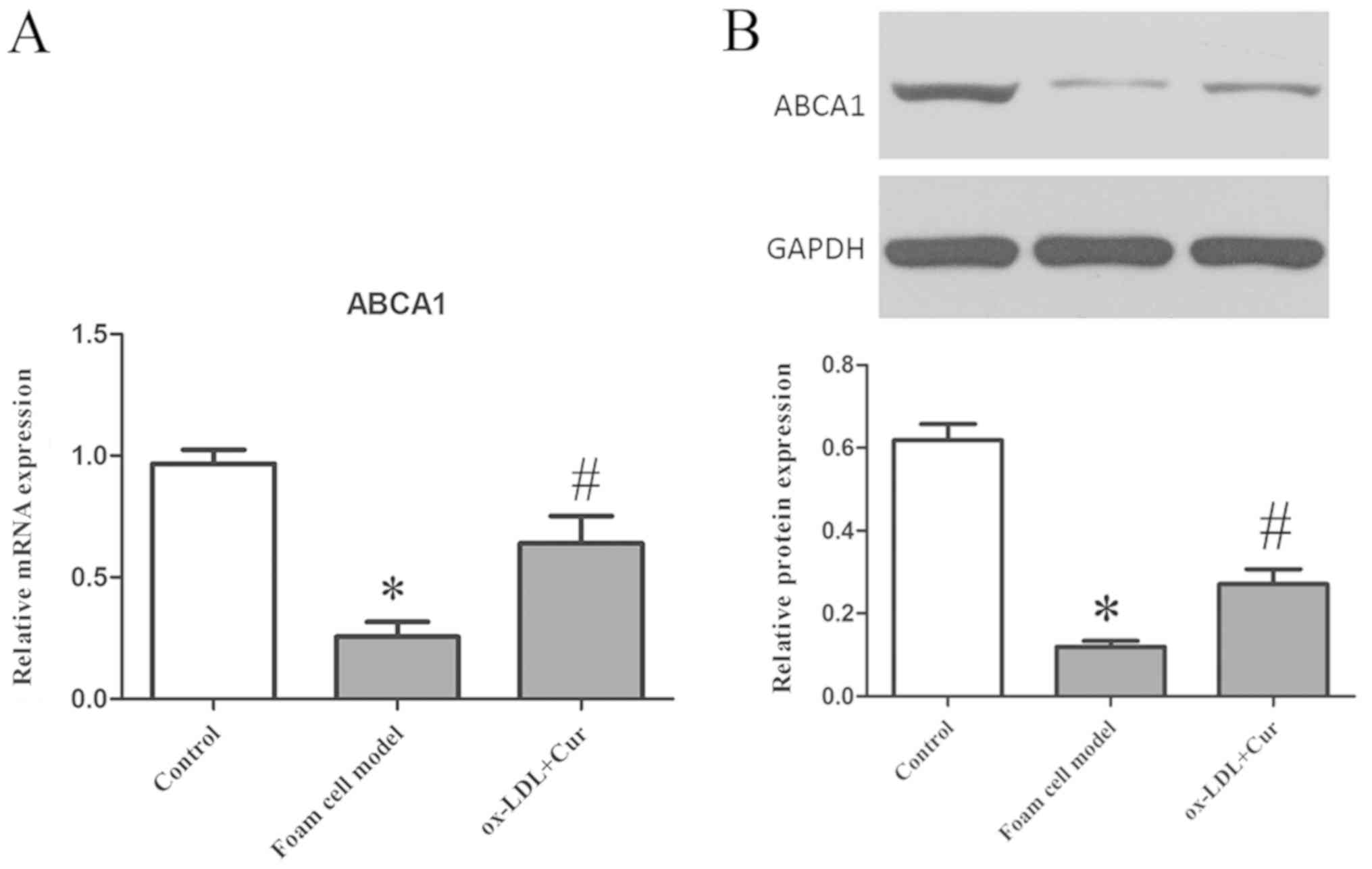
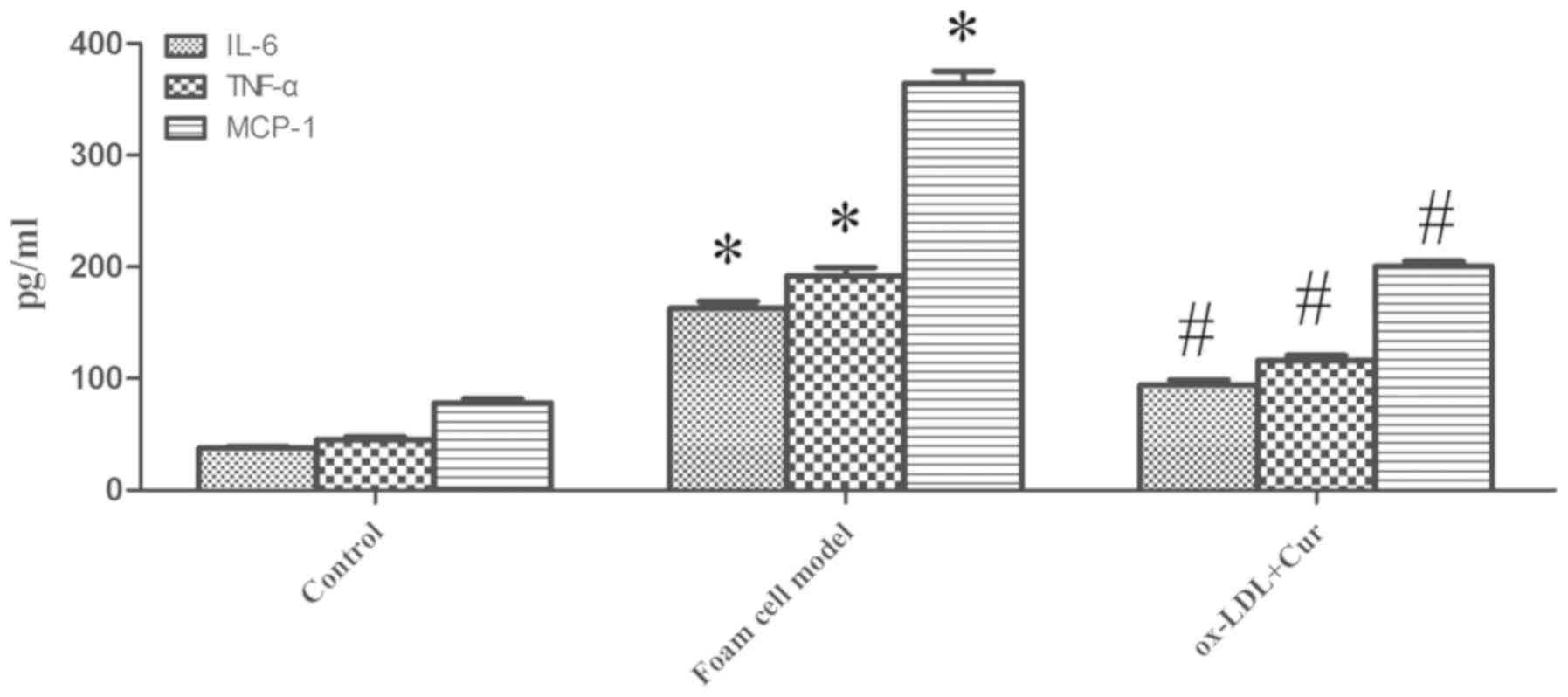
Curcumin promotes the expression of
ABCA1 and reduce the secretion of IL-6, TNF-α and MCP-1
ox-LDL (50 µg/ml) was used to stimulate cells, which
significantly reduced the expression levels of ABCA1 mRNA and
protein, while pre-treatment with 40 µM curcumin significantly
aggravated the expression of ABCA1 mRNA and protein (Fig. 5). A previous study (9,11)
confirmed that ox-LDL can promote the expression of IL-6, TNF-α and
MCP-1, which has also been verified by the current experiments.
Secretion of IL-6, TNF-α and MCP-1 were increased by ox-LDL
(Fig. 6), which were decreased by
curcumin. Thus, curcumin had the ability to promote the expression
of ABCA1 mRNA and protein in ox-LDL stimulated macrophages in
vitro, and curcumin also reduced the secretion of IL-6, TNF-α
and MCP-1.
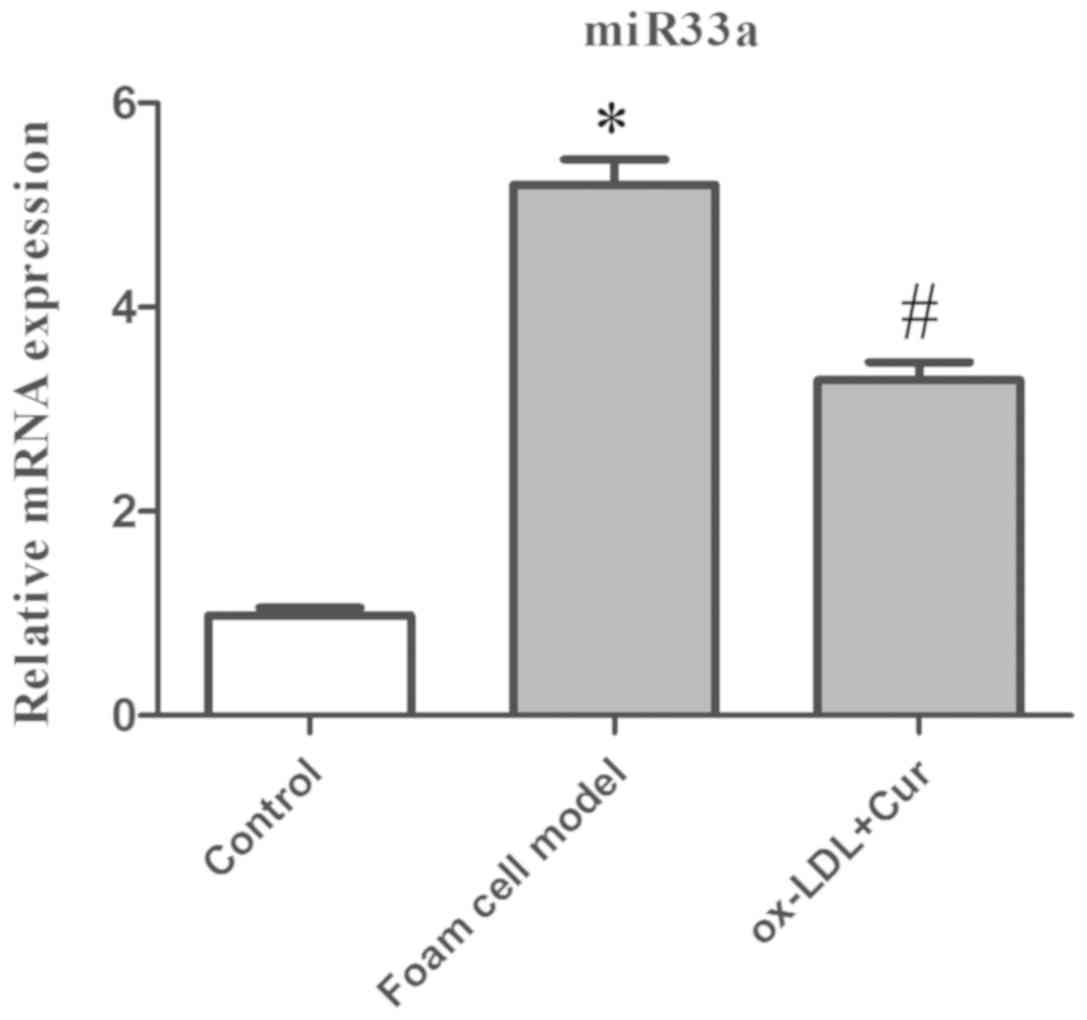
Curcumin and miR33a
Successful transfections of miR33a inhibitors were
firstly confirmed (Fig. S1). Using
50 g/ml ox-LDL-stimulated cells, detection of miR33a expression
levels showed that ox-LDL significantly promoted the expression of
miR33a, while the expression of miR33a mRNA was significantly
decreased in curcumin pre-treated cells (Fig. 7). Therefore, it was concluded that
curcumin had the capacity to regulate the expression of miR33a.
Further detection demonstrated that ox-LDL had the ability to
significantly reduce the expression levels of ABCA1 at the mRNA and
protein level, as well as promote the secretion of IL-6, TNF-α and
MCP-1. Both curcumin and the miR33a inhibitor increased the
expression of ABCA1, as well as the cholesterol efflux rate
(Figs.
8-10). However, there was no significant difference between the
foam cell model group and the miRNA control consequence group.
Moreover, it was discovered that the use of both curcumin and
miR33a inhibitors further significantly increased the expression of
ABCA1 at the mRNA and protein levels as well as further increasing
the cholesterol efflux rate. The combined treatment also further
significantly reduced the secretion of IL-6, TNF-α and MCP-1. While
no difference was indicated between the curcumin-only group and the
curcumin combined with the control consequence co-group. Therefore,
curcumin had the capacity to promote the expression of ABCA1 and
increase the cholesterol efflux rate in THP-1 derived macrophages.
Curcumin also reduced the secretion of IL-6, TNF-α and MCP-1
through regulating miR44.
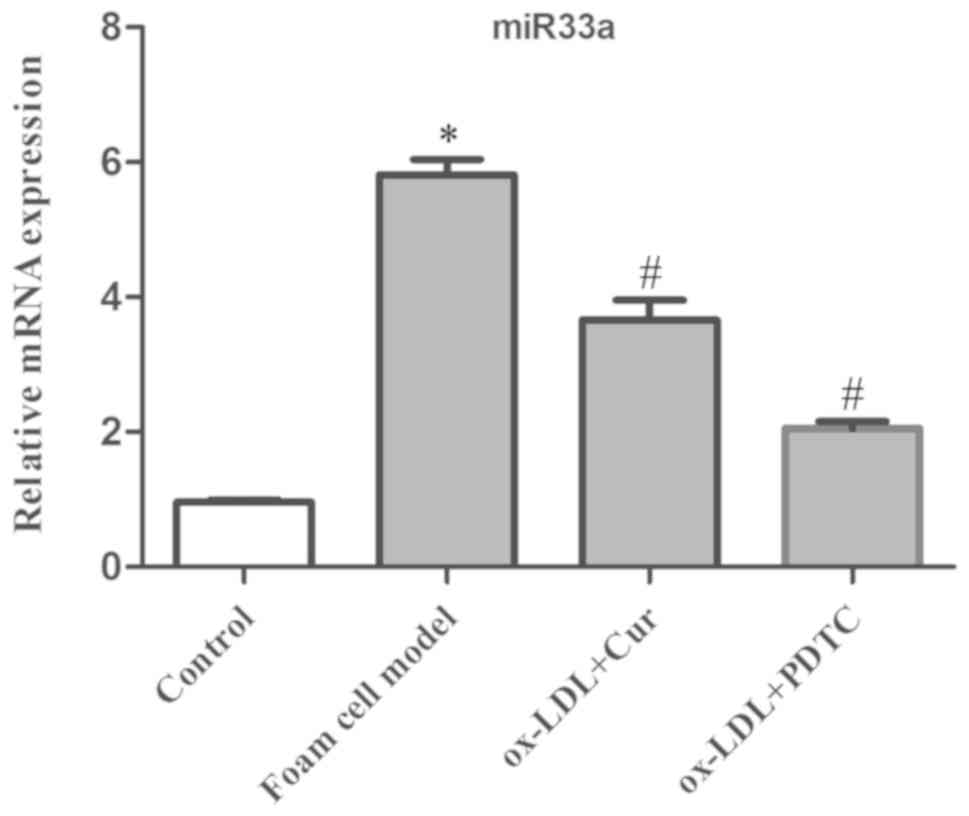
Relationship between curcumin and
NF-κ/miR33a
An inhibitor of the NF-κB signaling pathway, PDTC
(50 µM), was utilized in an incubator at 37˚C and 5% CO2
for 1 h, and was used to block the activation of NF-κB and detect
the expression of miR33a, nuclear NF-κB p65 and cytoplasmic IκBα
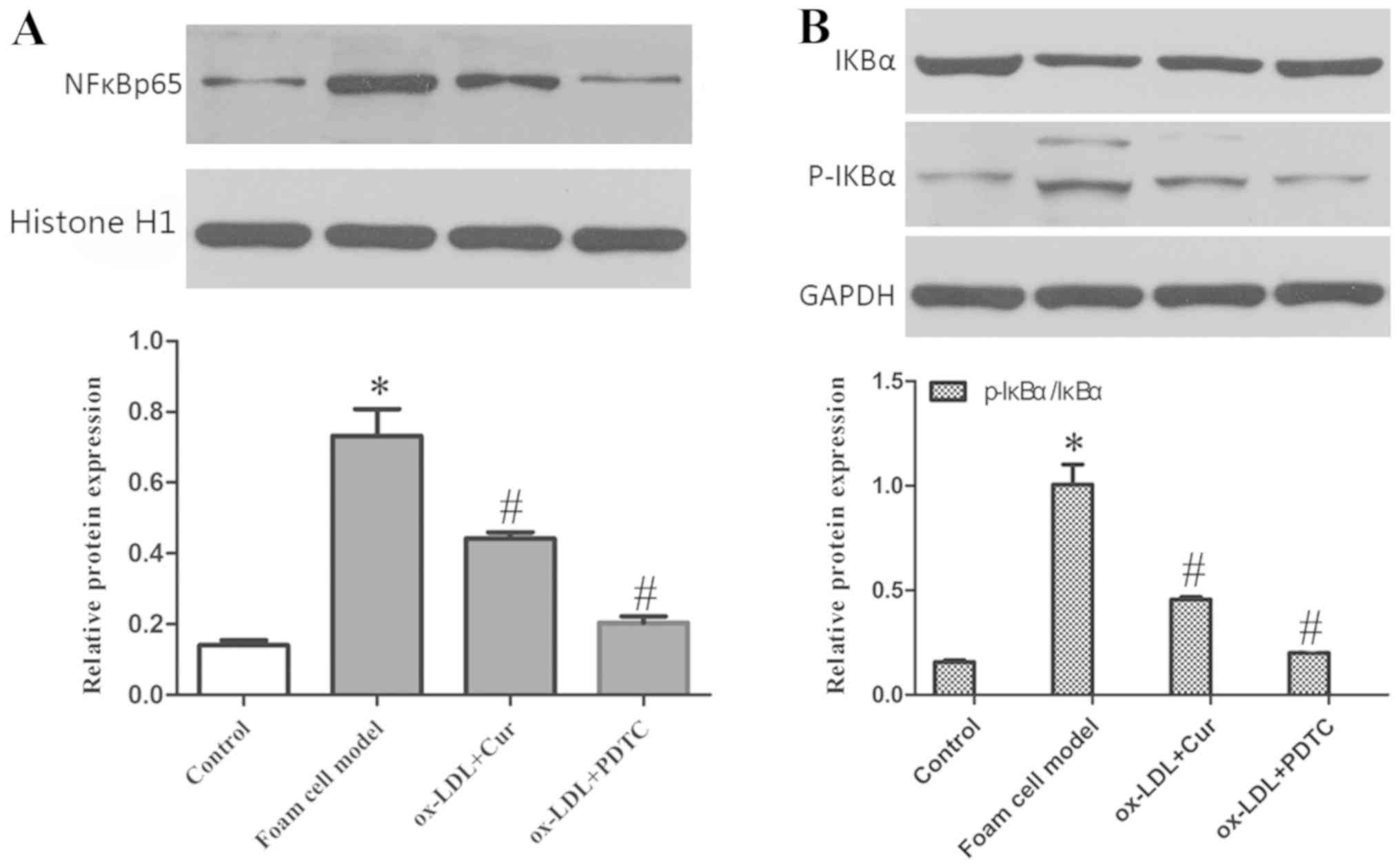
and p-IκBα. The data showed that ox-LDL significantly increased the
expression of miR33a and the phosphorylation of IκBα, and also led
to an increase in nuclear NF-κB p65 and cytoplasmic p-IκBα and a
decrease in cytoplasmic IκBα (Figs.
11 and 12). Expression of
miR33a in cells pre-treated with curcumin and PDTC was
significantly decreased. Cytoplasmic p-IκBα and nuclear NF-κB p65
expression levels were also reduced significantly, while the
cytoplasmic IκBα levels increased.
Relation of NF-κ/miR33a with ABCA1
expression, the cholesterol efflux rate and secretion of IL-6,
TNF-α and MCP-1 in THP-1 macrophages
To study the mechanism of action behind the effect
of miR33a on the development of AS and pharmacological activity of
curcumin, miR33a inhibitors or control sequences were transfected
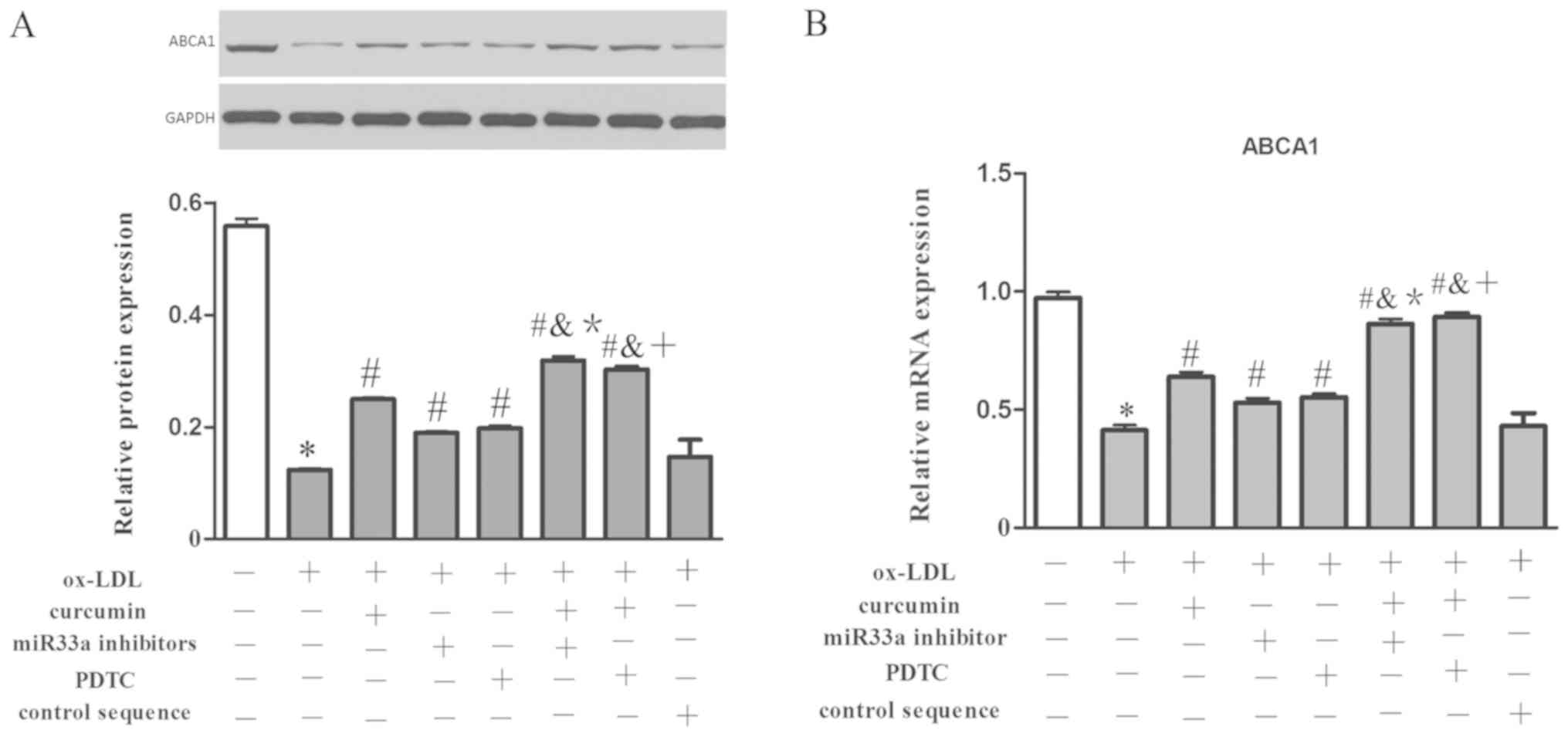
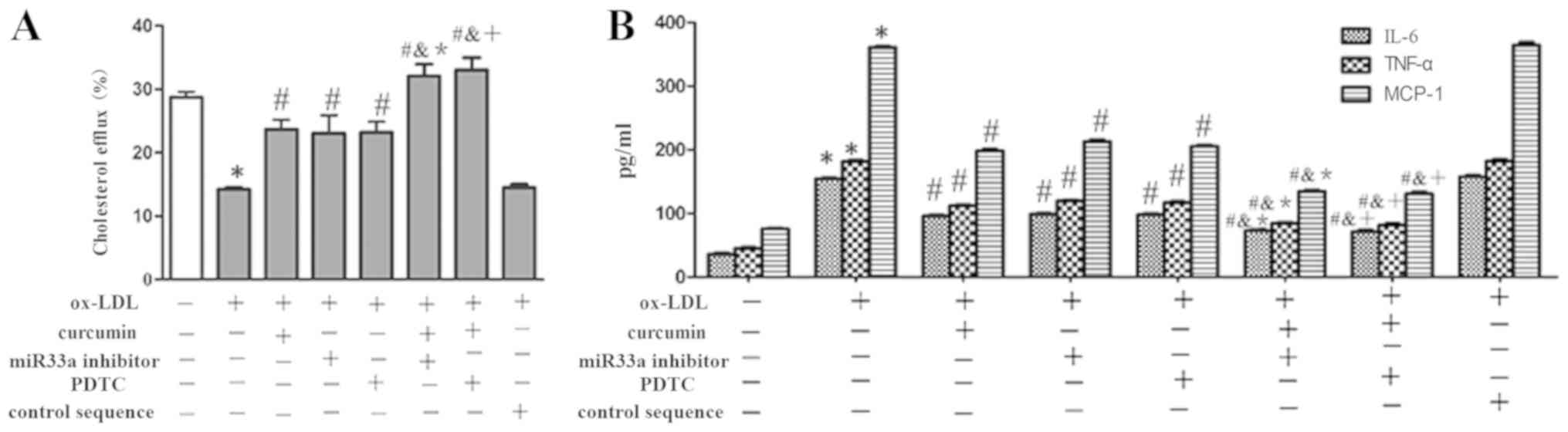
into THP-1 macrophages. The results indicated that ox-LDL
dramatically inhibited the expression of ABCA1 both at the mRNA and
protein levels, and boosted the secretion of IL-6, TNF-α and MCP-1
(Figs. 13 and 14). While curcumin, NF-κB blocker and
miR33a inhibitor promoted the expression of ABCA1. Secretions of
IL-6, TNF-α and MCP-1 were also decreased by these treatments.
However, the miRNA control sequence had no significant effect.
Relation of curcumin with TLR4
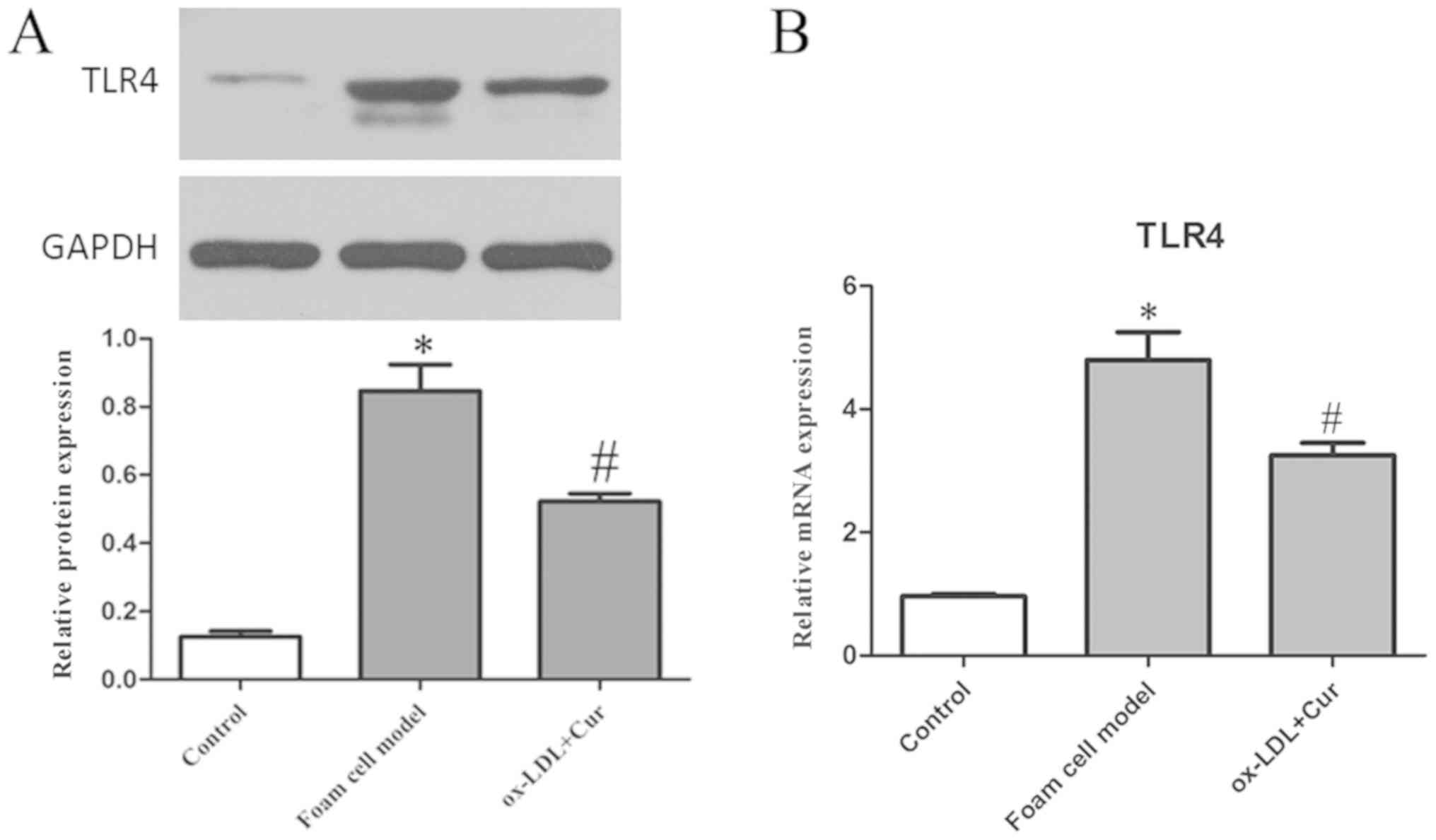
Cells were stimulated with 50 µg/ml ox-LDL and the
expression of TLR4 was detected. ox-LDL significantly increased the
expression levels of TLR4 both at the mRNA and protein level, which
was reduced by curcumin (Fig. 15).
As such, it was concluded that curcumin inhibited the expression of
TLR4 and the TLR4 signaling pathway.
Relation of curcumin with
TLR4/NF-κ/miR33a
To further study the relationship between TLR4 and
NF-κB/miR33a, THP-1 macrophages were pretreated with TLR4
antibodies or TLR4 isotype Ab antibodies at a concentration of 10
µg/ml in an incubator at 37˚C and 5% CO2 for 1 h. The
foam cell model was established following the same protocol. The
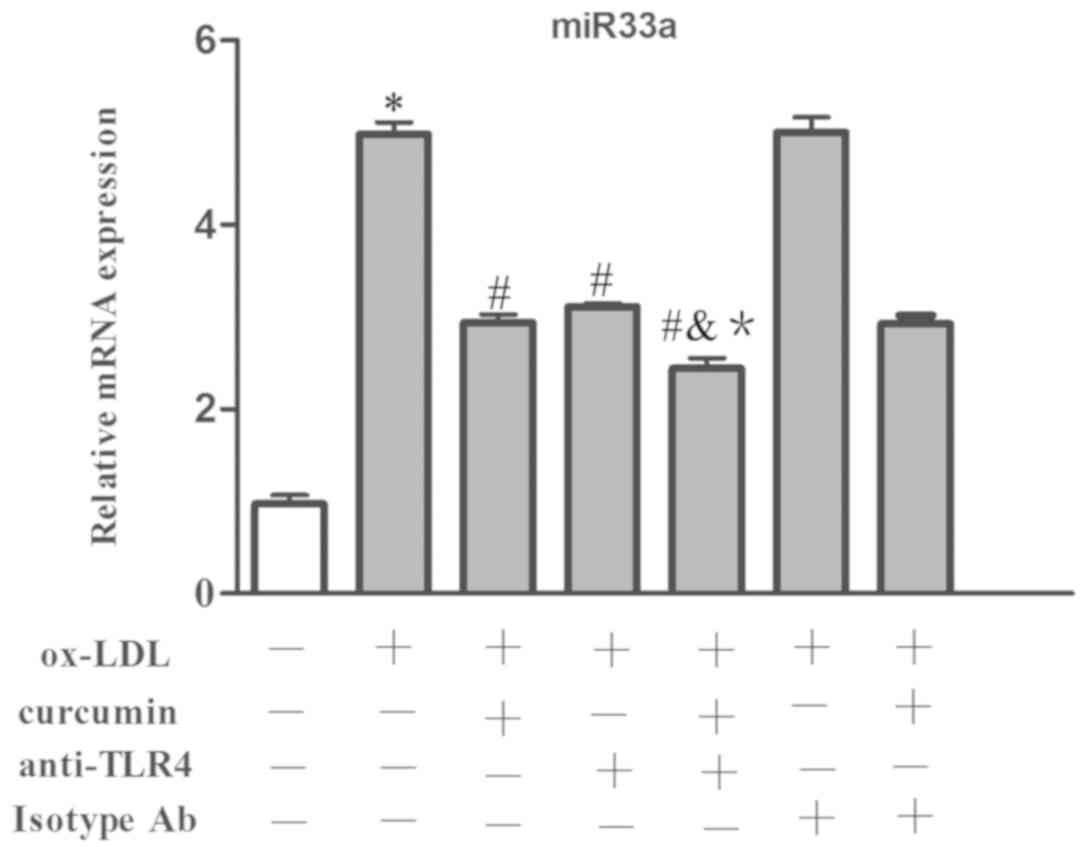
data showed that ox-LDL made the expression of miR33a increase
significantly at the mRNA level (Fig.
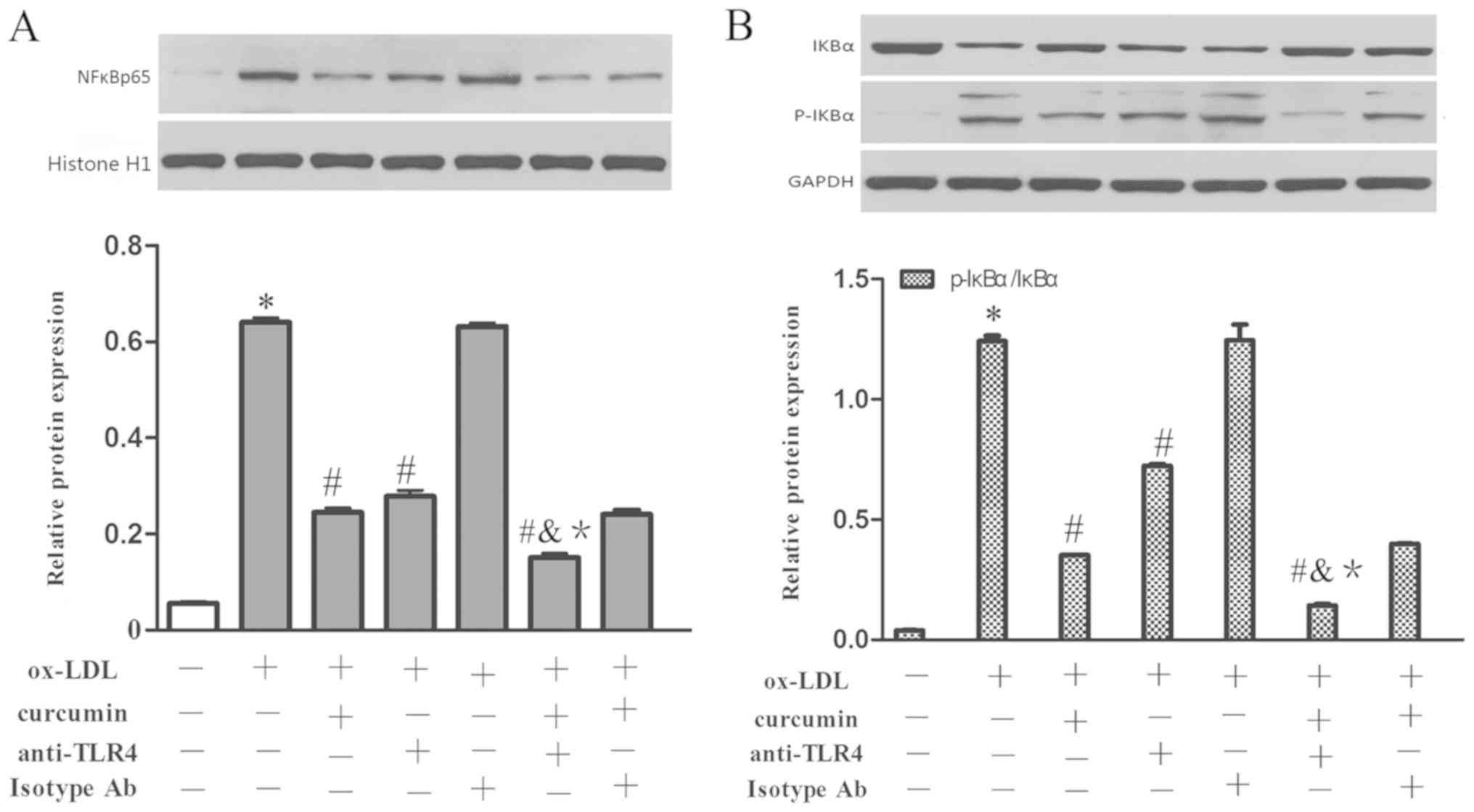
16). Simultaneously, nuclear NF-κB p65 and cytoplasmic p-IκBα
were increased as well, while the protein content of IκBα was
reduced (Fig. 17). Curcumin and
TLR4 antibodies reduced the expression levels of miR33a at the mRNA
level as well as the content of nuclear NF-κB p65 and cytoplasmic
p-IκBα. The levels of IκBα were also increased. The TLR4 isotype Ab
group had no obvious difference with that of the foam cell model
group. Additionally, it was discovered that there was a synergistic
effect gained from curcumin and the TLR4 antibodies. However, there
was no significant difference between the effects when combining
TLR4 homologous isotype Ab and curcumin with that of curcumin used
alone. Therefore, it is believed that both the TLR4 antibodies and
curcumin have the capacity to suppress the expression of TLR4 at
the mRNA and protein levels. The expression levels of NF-κB and
miR33a were downregulated by inhibiting TLR4. Therefore, TLR4 may
be involved in regulating the NF-κB/miR33a signaling pathway and
curcumin may block the NF-κB/miR33a signaling pathway by blocking
the expression of TLR4.
Relation of TLR4/NF-κ/miR33a with the
expression of ABCA1, cholesterol efflux rate and secretion of
IL-6,TNF-α and MCP-1 in THP-1 macrophages
To validate the effect of curcumin and the
TLR4/NF-κB/miR33a signaling pathway on the expression of ABCA1;
cholesterol efflux rate; and secretion of IL-6, TNF-α and MCP-1,
these parameters were investigated using the aforementioned
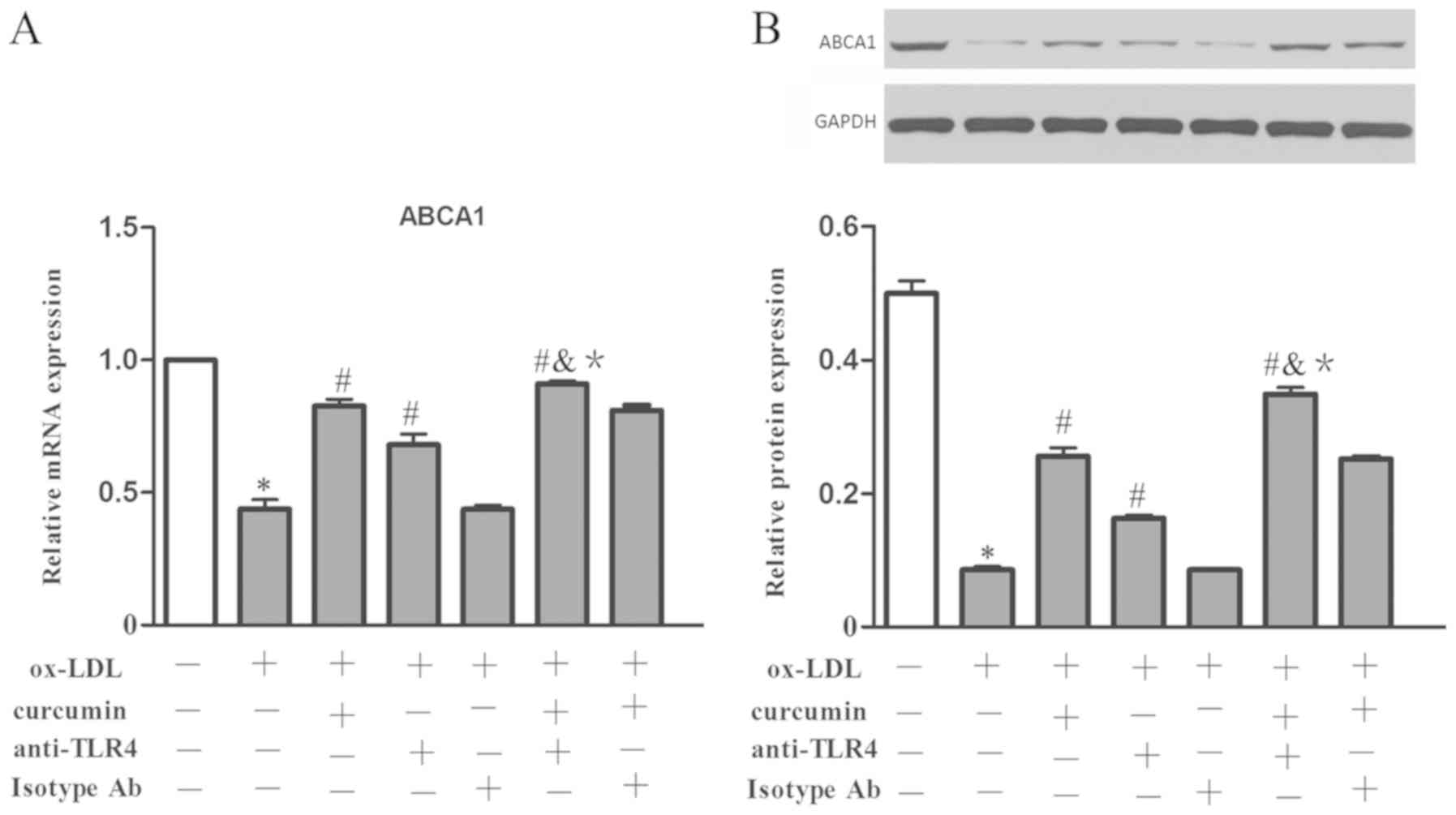
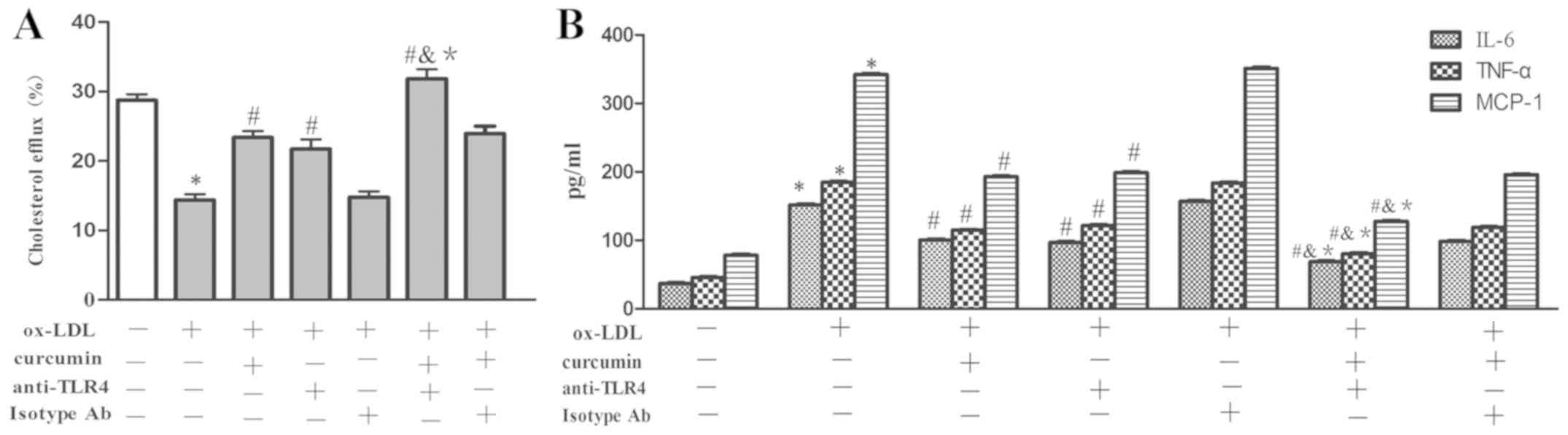
treatment groups. The data showed that the expression of ABCA1 at
the mRNA and protein levels was decreased by ox-LDL treatment,
which also promoted the secretion of IL-6, TNF-α and MCP-1
(Figs. 18 and 19). Curcumin and the TLR4 antibodies were
able to boost the expression of ABCA1 at the mRNA and protein level
and boost the cholesterol efflux rate, as well as reduce the
secretion of IL-6, TNF-α and MCP-1. While there was no difference
between the TLR4 isotype Ab group and the foam cell model group. In
addition, a synergistic affect was gained from curcumin and TLR4
antibodies, which was enhanced compared to that of curcumin or TLR4
antibodies alone. There was no difference between the effect of the
combination of TLR4 isotype Ab and curcumin with that of curcumin
used alone. Therefore, the TLR4/NF-κB/miR33a pathway may be
involved in the regulation of ABCA1 expression; cholesterol efflux;
and secretion of IL-6, TNF-α and MCP-1. Curcumin promoted the
expression of ABCA1 in THP-1 macrophages and reduced secretions of
IL-6, TNF-α and MCP-1 through the TLR4/NF-κB/miR33a signaling
pathway.
Discussion
A large number of cardiovascular diseases begin with
the development of AS, which can be characterized by formation of
foam cells, accumulation of lipids and inflammation (23). Most natural products are active
ingredients in plants. It has been shown that lipid metabolic
disorders can be regulated by natural products (active ingredients
within plants), which may also affect the intake and efflux of
cholesterol, stabilize the atherosclerotic plaques, reduce the
cholesterol content in the blood, inhibit or downregulate the
activity of pro-inflammatory cytokines, as well as their related
mediators, and reduce the inflammatory response (24,25).
Thus, natural products have the potential to provide a therapy for
preventing and treating AS (26,27).
Curcumin is a natural polyphenol active substance in
turmeric, which has been widely used in the food industry as a
spice and food pigment (20).
Studies have shown that curcumin has pharmacological effects such
as anti-inflammation, antioxidation, antitumor and cardiovascular
protection (28,29). The effect may be attributed to the
ability of curcumin to regulate various molecular targets. Zhong
et al (30) reported that
curcumin inhibited the overexpression of vascular smooth muscle
cell (VSMC) pro-inflammatory factor induced by ox-LDL and
suppressed the activation of inflammatory signaling pathways.
Moreover, in vivo experiments demonstrated that 20 mg/kg
curcumin consumed orally, daily could alleviate the development of
AS and systemic inflammatory responses in
apoE-/- mice, as well as reduce serum
cholesterol and triglyceride levels and increase HDL levels. The
results indicated that curcumin regulates esters and has an
anti-inflammatory effect, which can inhibit the formation of foam
cells and prevent AS. However, the specific effect of curcumin
requires further investigation.
LDLs are regarded as a marker of AS pathogenesis,
and high concentrations of LDLs in serum are usually considered a
major risk factor for Coronary atherosclerotic disease (CAD).
Steinberg (31) revealed that the
main mechanism behind macrophage formation stems from disorders of
ox-LDL intake and lipid efflux. Additionally, ox-LDL is toxic to
cells and induces inflammatory gene expression, thus promoting the
formation of foam cells. The present results indicated that ox-LDL
boosted the accumulation of intracellular lipids and increased the
amount of large Oil Red O stained lipid particles deposited within
cells. Further examination also showed that the amount of
intracellular TC, CE and TG increased significantly and the rate of
foam cell formation increased as well. Compared with the control
group, the CE/TC ratio was up to 65.09±1.30, demonstrating the
formation of foam cells and that ox-LDL had successfully induced
the establishment of the foam cell model.
Determination of the rate of cholesterol efflux
usually requires the advanced employment of ox-LDL to promote the
intake of lipid in macrophages, then various interventions are used
for further study (14,26). By adopting this method, the present
study discovered that curcumin could increase the cholesterol
efflux rate, and in the safe concentration range (10-40 µM), the
potency of curcumin acted in a dose-dependent manner. The
cholesterol efflux rate was positively associated with the
concentration of curcumin, while the lower concentration (5 µM) of
curcumin did not significantly increase cholesterol efflux.
Moreover, it was also found that the effects of curcumin increased
with time. When the treatment time lasted 24 h, the effect of
curcumin on cholesterol efflux rate reached its peak. Therefore, 40
µM for 24 h was chosen as a suitable treatment condition for
further experiments.
ABCA1 plays a vital role in the metabolism of
cholesterol, which mediates the release of cellular FC and
phospholipids to extracellular receptor apolipoprotein I and
finally forms the nascent HDL (20,26).
Data from the present study revealed that ox-LDL reduced the
expression of ABCA1 at the mRNA and protein levels, while curcumin
promoted its expression, thus providing a pathway for cholesterol
efflux. The rate of cholesterol efflux was indeed increased in
these experiments. From these results, it was speculated that
curcumin may promote cholesterol efflux by regulating the
expression of ABCA1 at both the mRNA and protein levels.
Curcumin has been reported to have the capacity of
boosting secretions of TNF-α in human umbilical vein endothelial
cells and slow down the development of AS (25). According to the present study, ox-LDL
significantly aggravated the secretion of TNF-α, while curcumin
could remarkably reduce the secretion of TNF-α, IL-6 and MCP-1 in
THP-1 macrophages induced by ox-LDL. As a limiting factor for HDL
assembly, ABCA1 is also regulated post-transcriptionally (32,33). It
has been previously shown decreases in ABCA1 expression are
attributed to the stimulation of inflammation, for example, through
the activation of IL-1β, TNF-α, interferon γ and NF-κB (34). Hence, inhibition of inflammatory
pathways could alleviate the effect of inflammation through the
expression of ABCA1. Therefore, curcumin exhibits anti-inflammatory
effect by increasing the expression of ABCA1 and inhibiting the
secretion of IL-6, TNF-α and MCP-1.
There are two subtypes of miR33 in humans, miR33a
and miR33b. miR33a is located at the 16th intron of the SREBP-2
gene on chromosome 22 and is related to cholesterol efflux
(35), while miR33b is located on
intron 17 of the SREBP-1 gene of chromosome 17 and may be
associated with the synthesis of fatty acids and TGs (36). There has been a focus on how miR33
regulates its possible target genes; however, little is known of
the mechanism of action behind how miR33 regulates other targets
within cells (17,26). It has been previously found that when
the cholesterol load in mouse peritoneal macrophages was increased,
the expression levels of miR33a and SREBP-2 are downregulated,
indicating that molecules that regulate SREBP-2 may also regulate
the production of miR33a (34).
According to Rakcheev et al (37), curcumin suppresses mRNA and protein
expression of SREBP-2 and Niemann-Pick C1-Like 1 in mice fed with a
high-fat diet, which plays a key part in preventing the formation
of gallstones and reducing blood lipid and bile acid cholesterol
content. Hence, it was hypothesized that miR33a, as an intron of
the SREBP-2 gene, was involved in the regulation of curcumin in
cholesterol homeostasis. Therefore, the miR33a inhibitor was
transfected into THP-1 macrophages in the present study, aiming to
bind with miR33a and blocking its expression. In addition, miR33a
control sequences were simultaneously transfected into cells using
the same protocol, acting as a control and excluding non-sequence
specific interactions. ox-LDL significantly promoted the expression
of miR33a at the mRNA level, which was significantly inhibited by
curcumin.
A previous study reported that 3'-UTR of ABCA1
contains 3 highly conserved binding sites for miR33a (38). By binding with these sites, miR33a
suppresses the expression of ABCA1. According to the present data,
compared with the foam cell model group, curcumin or the miR33a
inhibitor increased the expression of ABCA1 at both the mRNA and
protein levels, and promoted the cholesterol efflux, while the
control sequence of miR33a had no obvious effects. Additionally,
the combination of curcumin and miR33a inhibitor had a stronger
effect than that of curcumin or miR33a inhibitor used
independently. The combinatory utilization of curcumin and miR33a
control sequence had no remarkable difference with that of curcumin
used alone. Thus, it was conjectured that miR33a participated in
regulating the expression of ABCA1 at the mRNA and protein levels
and has an impact on the cholesterol efflux rate. Curcumin may
affect the expression of ABCA1 by regulating miR33a.
Disorders of lipid metabolism are often accompanied
by inflammation. It has been demonstrated that inflammation can
boost the accumulation of lipids by promoting the intake and
synthesis of cholesterol; however, the mechanisms remain to be
discovered (34). A previous study
has shown that the inflammatory factors IL-6 and TNF-α promote the
expression of miR-33a-5p and SREBP2 and also to downregulate
cholesterol transfer of ABCA1/G1, contributing to accumulation of
intracellular lipids (39).
Additionally, Rayner et al (40) showed that a high fat diet could boost
the generation of receptor-interacting protein 140 (RIP140), TNF-α
and IL-1β and reduce the content of miR33a simultaneously.
Therefore, miR33a may act as the bridge between inflammation and
lipid metabolism. The current data showed that curcumin or miR33a
inhibitor could significantly reduce the expression of IL-6, TNF-α
and MCP-1, while there was no remarkable difference between the
effect of control sequence of miR33a and that of the foam cell
group. Furthermore, it was also discovered that the effects of
combining of miR33 and curcumin was stronger than that of curcumin
or miR33a utilized independently. There was no clear difference
between the effect of combining treatments of curcumin and the
control sequence group and that of curcumin applied alone. Hence,
curcumin may be involved in regulating the secretion of IL-6, TNF-α
and MCP-1 and alleviating the inflammatory response by suppressing
miR33a expression. Recently, Rayner et al (40) reported that the anti-inflammatory
cytokines released from injured macrophages were increased in
anti-miR33 treated mice, and pro-inflammatory cytokines also
decreased. There was also a reduction in the concentration of
inflammatory macrophages. The results of the present study were
consistent with the results from the experiments of Rayner et
al (40). Thus, miR33 may be a
pro-inflammatory miRNA. Interestingly, Ho et al (41) reported that agonists of miR33 mimic
could markedly reduce the content of RIP140, the NF-κB coactivator,
both at the mRNA and protein levels, leading to inhibition of TNF-α
and IL-1β in macrophages. This indicated that miR33 may be an
anti-inflammatory miRNA in macrophages, opposing the current
results. de Beer et al (42)
demonstrated that ABCA1/G1 affected the expression of TNF-α, IL-1β
and IL-6 through the Janus activated kinase signal transducer
2/activator of transcription 3 signaling pathway. From the previous
results, it was hypothesized that the effect of miR33 is related to
the state of macrophages; however, miRNA33 downregulated the
expression of ABCA1, which affects the expression of inflammatory
cytokines. The contradictory results may be a result of the complex
interaction between the two molecules. To summarize, it is
hypothesized that miR33 may exert a pro-inflammatory role in the
early stage of AS; therefore, inhibition of miR33a may be effective
in preventing the inflammatory response in the early phase of
AS.
NF-κB binds with IκBα in the cytoplasm, forming a
complex. Once IκBα is phosphorylated and degraded by kinase
dependent phosphorylation, the free NF-κB will translocate into the
nucleus and bind to the DNA sequences of pro-inflammatory factors,
promoting the expression of their DNA sequences. Curcumin may
inhibit various inflammatory responses that are induced by these
pro-inflammatory factors, such as ox-LDL. Its inhibitory potency is
associated with the inhibition of ox-LDL induced phosphorylation
and degradation of IκBα; phosphorylation and nuclear translocation
of P65; and transcription of the target gene, NF-κB (43). The present data showed that ox-LDL
could boost the levels of nuclear NF-κB p65 and cytoplasmic p-IκBα
as well as reduce the content of cytoplasmic IκBα. This provided a
strong case for the hypothesis that ox-LDL induces the degradation
of IκBα and nuclear translocation of p65. Curcumin significantly
reduced the expression levels of nuclear NF-κB p65 and cytoplasmic
p-IκBα, while the content in cytoplasm was raised by curcumin
compared to that of the foam cell model group. The results revealed
that curcumin may suppress the activation of the NF-κB pathway
effectively and that curcumin can reliably inhibit NF-κB.
Li et al (44)
discovered that ox-LDL could upregulate mir146a expression by
activating the NF-κB pathway, while over expressed mir146a can
reverse regulate macrophage maturation by inhibiting the production
of CD86 and CD80. As such, there may be a relationship between
miRNA and NF-κB. According to the present data, ox-LDL may promote
the expression of miR33a, while curcumin reduces its expression.
The results showed that curcumin is indirectly involved in
regulating the expression of miR33a by blocking the activation of
the NF-κB signaling pathway. A previous study (39) confirmed that there is a connection
between miR33a and the cholesterol efflux. Accordingly, it was
speculated that there existed an association between NF-κB and
miR33a with proteins involved in the transfer of cholesterol and in
the cholesterol efflux. The present results revealed that the NF-κB
inhibitor, PDTC, and miR33a inhibitors promoted cholesterol efflux
and expression of ABCA1 at both the mRNA and protein levels.
Similarly, the effect of the combination of curcumin with NF-κB
inhibitor or miR33a inhibitor, was more significant than that of
curcumin, NF-κB inhibitor or miR33a inhibitor used alone. ELISAs
demonstrated that PDTC and miR33a inhibitor cold both reduce the
content of IL-6, TNF-α and MCP-1, and the synergistic effect of
curcumin with PDTC or miR33a inhibitor is more stronger than that
of curcumin, NF-κB inhibitor or miR33a inhibitor used
independently. The results indicated that the NF-κB/miR33a pathway
participated in the expression of IL-6, TNF-α and MCP-1. Curcumin
may inhibit the secretion of IL-6, TNF-α and MCP-1 through the
NF-κB/miR33a pathway.
TLR4 is a transmembrane, non-catalytic protein,
which plays a key role in the initiation and progression of
inflammation (45). It was
discovered by Edfeldt et al (46) that there is a significantly raised
expression of TLR4 in atherosclerotic plaques in human arteries.
Michelsen et al (47)
reported that knockdown of TLR4 can effectively reduce the area of
atherosclerotic plaques and promote the stability of the plaques.
Accordingly, inhibition of TLR4 favors prevention and reversal of
AS. The present study showed that ox-LDL significantly promoted the
expression of TLR4 at both the mRNA and protein levels, while
curcumin significantly reduced its expression. There was no evident
difference between the TLR4 isotype Ab group and the model group,
thus non-specific interference was excluded. Curcumin suppressed
the TLR4 pathway and inhibited the signal transduction in cells.
According to Baker et al (48), the NF-κB signaling pathway is
downstream of the TLR4-mediated signaling pathway and various
external stimuli can regulate the NF-κB pathway through the TLR4
pathway. This leads to the upregulation of a variety of
pro-inflammatory cytokines and enhancing the inflammatory response.
The current study indicated that curcumin regulates the
NF-κB/miR33a signaling pathway, therefore it was speculated that
curcumin may increase the expression of ABCA1 and enhance
cholesterol efflux, thereby inhibiting the expression of
inflammatory factors and reducing the secretion of IL-6, TNF-α and
MCP-1 through the TLR4/NF-κB/miR33a signaling pathway. The present
data revealed that curcumin or TLR4 specific antibodies
significantly reduced the content of miR33a mRNA, nuclear NF-κB p65
and cytoplasmic p-IκBα that was stimulated by ox-LDL. Meanwhile,
the content of cytoplasmic IκBα was increased, as well as the
expression of ABCA1 and cholesterol efflux. Secretion of IL-6,
TNF-α and MCP-1 was decreased. It was also discovered that the
effect of combining curcumin and TLR4 was stronger than that of
curcumin or TLR4-specific antibodies used independently. Finally,
the results confirmed that the TLR4/NF-κB/miR33a signaling pathway
is related to the expression of IL-6, TNF-α and MCP-1 and that
curcumin increases the expression of ABCA1 and the cholesterol
efflux rate, whilst also reducing secretion of IL-6, TNF-α and
MCP-1.
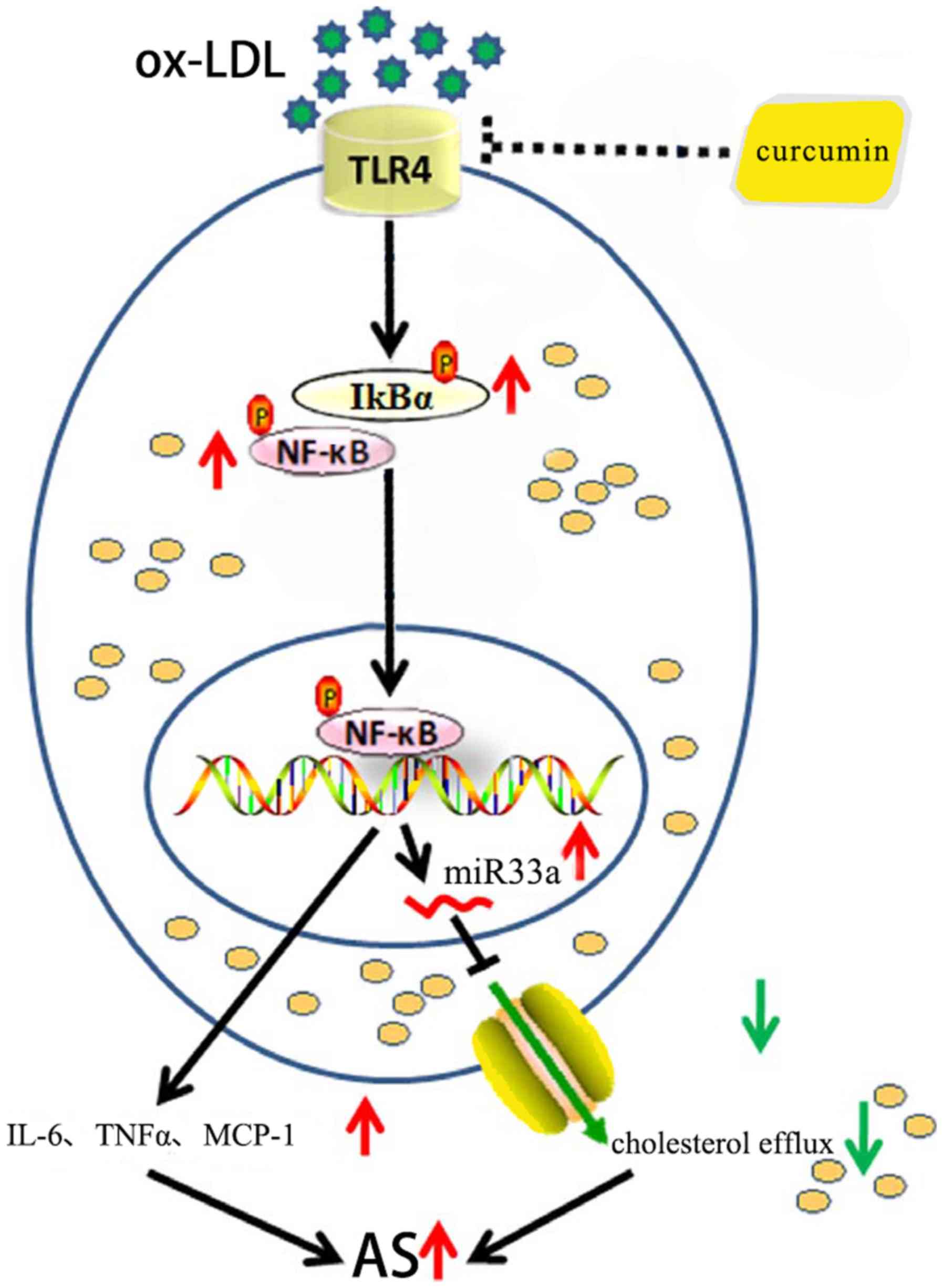
In conclusion, it was confirmed that curcumin could
increase the expression of ABCA1, a protein associated with
cholesterol transfer, and promote cholesterol efflux, as well as
reduce secretion of IL-6, TNF-α and MCP-1. The mechanism behind
these responses is associated with the TLR4/NF-κB/miR33a signaling
pathway (Fig. 20). Curcumin was
shown to be an effective regulator of lipid metabolism and an
anti-inflammatory agent, hence curcumin may be a promising new drug
for the treatment of various chronic reactions, including AS.
Supplementary Material
Effect of miR33a inhibitor and the
control sequence on the expression levels of miR33ain THP-1
macrophages (n=3 per group). *P<0.05 vs. the control
group. #P<0.05 vs. the foam cell model group. miR, microRNA;
ox-LDL, oxidized low-density lipoprotein.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant. no. 31300946),
Luzhou-Southwest Medical University National Science Foundation
Cultivation Project (grant. no. 2018LZXNYD ZK48) and Science and
Technology Department of Sichuan Province (grant. no. 2016TD0017
and 2017TD0015).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and CL designed all the experiments and carried
out the experiments. JF performed the statistical analysis, created
the figures, and wrote the manuscript. JFL and ZCF helped with
designing the experiments and assisted in manuscript writing. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lancellotti P, Ancion A and Piérard L:
Cardiac rehabilitation, state of the art 2017. Rev Med Liege.
72:481–487. 2017.PubMed/NCBI(In French).
|
|
2
|
Rosamond W, Flegal K, Furie K, Go A,
Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, et
al: American Heart Association Statistics Committee and Stroke
Statistics Subcommittee. Heart disease and stroke statistics - 2008
update: A report from the American Heart Association Statistics
Committee and Stroke Statistics Subcommittee. Circulation.
117(e25-e146)2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roger VL, Go AS, Lloyd-Jones DM, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al:
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee. Executive summary: heart disease and
stroke statistics--2012 update: a report from the American Heart
Association. Circulation. 125:188–197. 2012. View Article : Google Scholar
|
|
4
|
Casas R, Castro-Barquero S, Estruch R and
Sacanella E: Nutrition and Cardiovascular Health. Int J Mol Sci.
19(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Veitenhansl M, Stegner K, Hierl FX,
Dieterle C, Feldmeier H, et al: 40th EASD Annual Meeting of the
European Association for the Study of Diabetes: Munich, Germany,
5-9 September 2004. Diabetologia. 47 (Suppl
1)(A1-A464)2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Z and Nakayama T: Inflammation, a
link between obesity and cardiovascular disease. Mediators Inflamm.
2010(535918)2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Levi M, van der Poll T and Schultz M:
Infection and inflammation as risk factors for thrombosis and
atherosclerosis. Semin Thromb Hemost. 38:506–514. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nelken NA, Coughlin SR, Gordon D and
Wilcox JN: Monocyte chemoattractant protein-1 in human atheromatous
plaques. J Clin Invest. 88:1121–1127. 1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Boyle JJ, Weissberg PL and Bennett MR:
Tumor necrosis factor-alpha promotes macrophage-induced vascular
smooth muscle cell apoptosis by direct and autocrine mechanisms.
Arterioscler Thromb Vasc Biol. 23:1553–1558. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jafarzadeh A, Nemati M and Jafarzadeh S:
The important role played by chemokines influence the clinical
outcome of Helicobacter pylori infection. Life Sci.
231(116688)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rull A, Beltrán-Debón R, Aragonès G,
Rodríguez-Sanabria F, Alonso-Villaverde C, Camps J and Joven J:
Expression of cytokine genes in the aorta is altered by the
deficiency in MCP-1: Effect of a high-fat, high-cholesterol diet.
Cytokine. 50:121–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen S, Sorrentino R, Shimada K, Bulut Y,
Doherty TM, Crother TR and Arditi M: Chlamydia
pneumoniae-induced foam cell formation requires MyD88-dependent
and -independent signaling and is reciprocally modulated by liver X
receptor activation. J Immunol. 181:7186–7193. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou Z, Wu Y, Chen L, Liu L, Chen H, Li Z
and Chen C: Heat shock protein 10 of Chlamydophila
pneumoniae induces proinflammatory cytokines through Toll-like
receptor (TLR) 2 and TLR4 in human monocytes THP-1. In Vitro Cell
Dev Biol Anim. 47:541–549. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Paolillo R, Iovene MR, Romano Carratelli C
and Rizzo A: Induction of VEGF and MMP-9 expression by toll-like
receptor 2/4 in human endothelial cells infected with Chlamydia
pneumoniae. Int J Immunopathol Pharmacol. 25:377–386.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854.
1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nakazeki F, Tsuge I, Horie T, Imamura K,
Tsukita K, Hotta A, Baba O, Kuwabara Y, Nishino T, Nakao T, et al:
miR-33a is a therapeutic target in SPG4-related hereditary spastic
paraplegia human neurons. Clin Sci (Lond). 133:583–595.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lin K, Chen H, Chen X, Qian J, Huang S and
Huang W: Efficacy of Curcumin on Aortic Atherosclerosis: A
Systematic Review and Meta-Analysis in Mouse Studies and Insights
into Possible Mechanisms. Oxid Med Cell Longev.
2020(1520747)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mohammadian Haftcheshmeh S, Karimzadeh MR,
Azhdari S, Vahedi P, Abdollahi E and Momtazi-Borojeni AA:
Modulatory effects of curcumin on the atherogenic activities of
inflammatory monocytes: Evidence from in vitro and animal models of
human atherosclerosis. Biofactors. Dec 24. 2019.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print). View Article : Google Scholar
|
|
20
|
Dong SZ, Zhao SP, Wu ZH, Yang J, Xie XZ,
Yu BL and Nie S: Curcumin promotes cholesterol efflux from
adipocytes related to PPARgamma-LXRalpha-ABCA1 passway. Mol Cell
Biochem. 358:281–285. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fogelman AM, Shechter I, Seager J, Hokom
M, Child JS and Edwards PA: Malondialdehyde alteration of low
density lipoproteins leads to cholesteryl ester accumulation in
human monocyte-macrophages. Proc Natl Acad Sci USA. 77:2214–2218.
1980.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chiang YM, Lo CP, Chen YP, Wang SY, Yang
NS, Kuo YH and Shyur LF: Ethyl caffeate suppresses NF-kappaB
activation and its downstream inflammatory mediators, iNOS, COX-2,
and PGE2 in vitro or in mouse skin. Br J Pharmacol. 146:352–363.
2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Coban D, Milenkovic D, Chanet A,
Khallou-Laschet J, Sabbe L, Palagani A, Vanden Berghe W, Mazur A
and Morand C: Dietary curcumin inhibits atherosclerosis by
affecting the expression of genes involved in leukocyte adhesion
and transendothelial migration. Mol Nutr Food Res. 56:1270–1281.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao GJ, Tang SL, Lv YC, Ouyang XP, He PP,
Yao F, Chen WJ, Lu Q, Tang YY, Zhang M, et al: Antagonism of
betulinic acid on LPS-mediated inhibition of ABCA1 and cholesterol
efflux through inhibiting nuclear factor-kappaB signaling pathway
and miR-33 expression. PLoS One. 8(e74782)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Janeesh PA, Sasikala V, Dhanya CR and
Abraham A: Robinin modulates TLR/NF-κB signaling pathway in
oxidized LDL induced human peripheral blood mononuclear cells. Int
Immunopharmacol. 18:191–197. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sikora E, Scapagnini G and Barbagallo M:
Curcumin, inflammation, ageing and age-related diseases. Immun
Ageing. 7(1)2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Olszanecki R, Jawień J, Gajda M, Mateuszuk
L, Gebska A, Korabiowska M, Chłopicki S and Korbut R: Effect of
curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J
Physiol Pharmacol. 56:627–635. 2005.PubMed/NCBI
|
|
30
|
Zhong Y, Liu T and Guo Z: Curcumin
inhibits ox-LDL-induced MCP-1 expression by suppressing the p38MAPK
and NF-κB pathways in rat vascular smooth muscle cells. Inflamm
Res. 61:61–67. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Steinberg D: Low density lipoprotein
oxidation and its pathobiological significance. J Biol Chem.
272:20963–20966. 1997. View Article : Google Scholar
|
|
32
|
Zhao GJ, Yin K, Fu YC and Tang CK: The
interaction of ApoA-I and ABCA1 triggers signal transduction
pathways to mediate efflux of cellular lipids. Mol Med. 18:149–158.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee J, Park Y and Koo SI: ATP-binding
cassette transporter A1 and HDL metabolism: Effects of fatty acids.
J Nutr Biochem. 23:1–7. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yin K, Liao DF and Tang CK: ATP-binding
membrane cassette transporter A1 (ABCA1): A possible link between
inflammation and reverse cholesterol transport. Mol Med.
16:438–449. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Moore KJ, Rayner KJ, Suárez Y and
Fernández-Hernando C: The role of microRNAs in cholesterol efflux
and hepatic lipid metabolism. Annu Rev Nutr. 31:49–63.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Moore KJ: microRNAs: Small regulators with
a big impact on lipid metabolism. J Lipid Res. 54:1159–1160.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rakcheev AP, Chistiakova IA and Kamennykh
PV: [The successful treatment of cutaneous leishmaniasis with an
argon laser]. Vestn Dermatol Venerol. 12:53–55. 1989.PubMed/NCBI(In Russian).
|
|
38
|
Rayner KJ, Suárez Y, Dávalos A, Parathath
S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ and
Fernández-Hernando C: miR-33 contributes to the regulation of
cholesterol homeostasis. Science. 328:1570–1573. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhou C, Lei H, Chen Y, Liu Q, Li LC,
Moorhead JF, Varghese Z and Ruan XZ: Enhanced SCAP glycosylation by
inflammation induces macrophage foam cell formation. PLoS One.
8(e75650)2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rayner KJ, Sheedy FJ, Esau CC, Hussain FN,
Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y,
et al: Antagonism of miR-33 in mice promotes reverse cholesterol
transport and regression of atherosclerosis. J Clin Invest.
121:2921–2931. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ho PC, Chang KC, Chuang YS and Wei LN:
Cholesterol regulation of receptor-interacting protein 140 via
microRNA-33 in inflammatory cytokine production. FASEB J.
25:1758–1766. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
de Beer MC, Ji A, Jahangiri A, Vaughan AM,
de Beer FC, van der Westhuyzen DR and Webb NR: ATP binding cassette
G1-dependent cholesterol efflux during inflammation. J Lipid Res.
52:345–353. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shih VF, Tsui R, Caldwell A and Hoffmann
A: A single NFκB system for both canonical and non-canonical
signaling. Cell Res. 21:86–102. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li Z, Wang S, Zhao W, Sun Z, Yan H and Zhu
J: Oxidized low-density lipoprotein upregulates microRNA-146a via
JNK and NF-κB signaling. Mol Med Rep. 13:1709–1716. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
den Dekker WK, Cheng C, Pasterkamp G and
Duckers HJ: Toll like receptor 4 in atherosclerosis and plaque
destabilization. Atherosclerosis. 209:314–320. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Edfeldt K, Swedenborg J, Hansson GK and
Yan ZQ: Expression of toll-like receptors in human atherosclerotic
lesions: A possible pathway for plaque activation. Circulation.
105:1158–1161. 2002.PubMed/NCBI
|
|
47
|
Michelsen KS, Wong MH, Shah PK, Zhang W,
Yano J, Doherty TM, Akira S, Rajavashisth TB and Arditi M: Lack of
Toll-like receptor 4 or myeloid differentiation factor 88 reduces
atherosclerosis and alters plaque phenotype in mice deficient in
apolipoprotein E. Proc Natl Acad Sci USA. 101:10679–10684.
2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22.
2011.PubMed/NCBI View Article : Google Scholar
|