Introduction
Inflammatory bowel disease (IBD), a gastrointestinal
tract disease, is regulated by the inflammatory response and is
associated with symptoms such as gastrointestinal bleeding, colonic
shortening, abdominal pain, diarrhea and colonic chronic
inflammation (1,2). Ulcerative colitis (UC) and Crohn's
disease (CD) are the two main types of IBD (3). Recently, the inflammatory response has
been demonstrated to accelerate the pathogenesis of IBD (4). Although numerous efforts have been made
to elucidate the mechanisms underlying IBD, the exact pathogenesis
and mechanisms of IBD remain unclear. Therefore, anti-inflammatory
drugs and the recognition of specific IBD agents may serve as novel
therapeutic approaches for IBD.
MicroRNAs (miRNAs or miRs) are small non-coding RNA
molecules that can regulate target gene levels or cell behaviors by
degrading mRNAs or restricting translational levels (5). Multiple reports have confirmed that the
abnormal expression of miRNAs is highly associated with various
diseases, including IBD (6-8).
Moreover, certain miRNAs exhibit anti-inflammatory effects and can
be used as potential therapeutic targets. For instance, Huang et
al (9) reported that the
inhibition of miRNA-210 suppresses the pro-inflammatory response
and reduces acute brain injury due to ischemic stroke in mice. In
addition, Kumar et al (10)
demonstrated that miRNA-26a modulates the inflammatory response
induced by Toll-like receptor 4 stimulation in microglia. miR-4262,
a recently discovered miRNA, has been identified as a biomarker and
has been demonstrated to serve a promotive role in various
diseases, including breast cancer (11), colon cancer (12) and cutaneous malignant melanoma
(13). However, whether miR-4262
influences the development of IBD has not yet been reported, to the
best of our knowledge.
Sirtuin 1 (SIRT1) serves a role in a number of
biological functions, such as the inflammatory responses, cell
apoptosis and signaling pathway regulations (14,15).
However, whether SIRT1 serves a role in the progression of IBD and
the underlying molecular mechanisms requires further elucidation.
It has been indicated that dextran sodium sulfate (DSS) can be used
to establish an intestinal in vitro barrier model due to its
rapidity, simplicity and controllability (16,17).
The present study attempted to explore the role and
mechanisms of miR-4262 in IBD. The expression of miR-4262 in IBD
colonic mucosa tissues, normal tissues, DSS-treated Caco-2 cells
and normal Caco-2 cells was determined. The role of miR-4262 in
DSS-induced inflammation was investigated in the intestinal
epithelial cell line, Caco-2, and the possible mechanisms of action
of miR-4262 in IBD were analyzed.
Materials and methods
Clinical tissue samples
Between December 2016 and December 2018, colonic
mucosa tissues were collected from 30 children with IBD (15 males;
15 females; age range: 7-11 years; mean age: 9.4 years) and 30
children without IBD (15 males; 15 females; age range: 6-12 years;
mean age: 8.8 years) at Chengdu Women's and Children's Central
Hospital (Chengdu, China). Patients with infectious colitis and
colorectal cancer were excluded. Liquid nitrogen was employed to
preserve the specimens at -80˚C until use. All specimens were
obtained with written informed consent and the present study was
approved by the Ethics Committee of Chengdu Women's and Children's
Central Hospital.
Caco-2 cell culture and DSS
treatment
The normal intestinal epithelial cell line Caco-2
was purchased from American Type Culture Collection. The cells were
cultured in Dulbecco's modified Eagle medium (DMEM)/F12 (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% fetal calf serum
(FBS; HyClone; GE Healthcare Life Sciences) and 1%
penicillin-streptomycin and were maintained at 37˚C with 5%
CO2 in an incubator. Caco-2 cells were treated with 2%
DSS at 37˚C for 4 days to construct the inflammatory model
(18).
Reverse transcription-quantitative PCR
(RT-qPCR)
SIRT1, inflammatory cytokines [interleukin (IL)-1β,
IL-6 and tumor necrosis factor (TNF)-α] and miR-4262 levels were
measured via RT-qPCR. The isolation of RNA from colonic mucosa
tissues and Caco-2 cells was performed using the RNA-isolation kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. cDNA was obtained using a PrimeScript RT kit (Takara
Bio, Inc.) according to the manufacturer's protocol, followed by
PCR amplification on an ABI Prism 7500 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with SYBR Premix Ex-Taq (Takara
Bio, Inc.). miRNA and mRNA expression were normalized to U6 and
GAPDH, respectively. The reaction conditions were as follows:
Initial denaturation at 95˚C for 5 min; 30 cycles of 94˚C for 30
sec, 60˚C for 30 sec and 72˚C for 1 min; and a final extension at
72˚C for 5 min. Primers were produced by Sangon Biotech Co., Ltd.
and the sequences were as follows: miR-4262 forward,
5'-TGCGGGACATTCAGA-3' and reverse, 5'-CCAGTGCAGGGTCCGAGGT-3'; SIRT1
forward, 5'-AATCCAGTCATTAAAGGTCTACAA-3' and reverse,
5'-TAGGACCATTACTGCCAGAGG-3'; TNF-α forward,
5'-GAACTGGCAGAAGAGGCACT-3' and reverse 5'-GGTCTGGGCCATAGAACTGA-3';
IL-1β forward, 5'-TGTGAAATGCCACCTTTTGA-3' and reverse,
5'-TGAGTGATACTGCCTGCCTG-3'; IL-6 forward,
5'-CCGGAGAGGAGACTTCACAG-3' and reverse, 5'-CAGAATTGCCATTGCACA-3';
U6 forward, 5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; and GAPDH forward,
5'-CTTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'. The relative results were analyzed
using the 2-ΔΔCq method (19).
Cell transfection
The miR-4262 inhibitor and inhibitor control were
synthesized by Shanghai Genepharma Co. Ltd. Caco-2 cells were
cultured to 70-80% confluency and transiently transfected with 100
nM miR-4262 inhibitor (cat no. HSTUD1216; Sigma-Aldrich; Merck
KGaA), 100 nM inhibitor control (cat no. HMC0002; Sigma-Aldrich;
Merck KGaA), 0.2 µM control-siRNA (cat no. sc-36869; Santa Cruz
Biotechnology, Inc.) or 0.2 µM SIRT1-siRNA (cat no. sc-40986; Santa
Cruz Biotechnology, Inc.) using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) for 48 h, according to the manufacturer's
instructions. Following transfection, the cells were stimulated
with 2% DSS at 37˚C for 4 days for further analysis.
Dual-luciferase reporter assay
TargetScan 7.2 (http://www.targetscan.org/vert_72/) was adopted to
investigate the putative binding sites. The data suggested that
SIRT1 was a potential target of miR-4262. Subsequently, the
miR-4262 binding sites on SIRT1 3'-UTR, including wild-type or
mutant, were inserted into the pGL-control vector (Promega
Corporation) to construct a wild-type SIRT1 plasmid (SIRT1-WT) or
SIRT1 mutation plasmid (SIRT1-MUT), respectively. The mimic control
or miR-4262 mimic (Shanghai GenePharma Co., Ltd.) were then
co-transfected with luciferase reporter vectors into Caco-2 cells
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) for 48 h, according to the manufacturer's protocol. The
luciferase activity was assessed using the Dual-Luciferase Reporter
assay system (Promega Corporation) following the manufacturer's
protocol. All luciferase activities were normalized to
Renilla luciferase activity.
Enzyme-linked immunosorbent assay
(ELISA)
ELISAs (all Beyotime Institute of Biotechnology) was
adopted to measure the levels of TNF-α (cat. no. PT518), IL-6 (cat.
no. PI330) and IL-1β (cat. no. PI305) in the cell supernatant
following treatment. The optical density (OD) value of each well at
450 nm was detected, according to the manufacturer's
instructions.
MTT assay
Cell viability was evaluated via MTT assay. The
Caco-2 cells were cultured in 96-well plates at 37˚C, and the cells
were then transfected with miR-4262 inhibitor, inhibitor control,
control-siRNA and SIRT1-siRNA for 48 h and treated with 2% DSS
(18) at 37˚C for a further 4 days.
Following treatment, 10 µl MTT solution (Amresco LLC) was added to
the cells, followed by incubation at 37˚C for 4 h, and DMSO (Sangon
Biotech Co., Ltd.) was then used to dissolve the formazan crystals.
Finally, the OD values at 490 nm were detected using a
multifunctional plate reader (BD Biosciences).
Flow cytometric analysis
Caco-2 cell apoptosis was detected via flow
cytometric analysis. In the present study, the cells were
transfected with miR-4262 inhibitor, inhibitor control,
control-siRNA and SIRT1-siRNA for 48 h and treated with 2% DSS for
4 days. Subsequently, the cells were trypsinized at room
temperature for 1 min and stained with Annexin V-FITC/propidium
iodide (PI; Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. The results were analyzed using a
FACSCalibur flow cytometer (BD Biosciences) and FlowJo software
(version 7.6.1; Tree Star, Inc.).
Western blot analysis
Total proteins from Caco-2 cells were extracted
using RIPA buffer (Invitrogen; Thermo Fisher Scientific, Inc.) and
quantified using a BCA Protein assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.). The denatured proteins were loaded on 10%
SDS-PAGE, and transferred to PVDF membranes (Bio-Rad Laboratories,
Inc.). After blocking with 5% skim milk in PBST at room temperature
for 90 min, the membranes were cultured with primary antibodies
against GAPDH (cat no. G9545), SIRT1 (cat no. S5447),
phosphorylated (p)-p65 (cat no. SAB4504488) and p65 (cat no.
SAB4502609; all from Sigma-Aldrich; Merck KGaA; 1:1,000) overnight
at 4˚C. Horesradish peroxidase-conjugated oat anti-rabbit
immunoglobulin G secondary antibody (cat no. ab7090; Abcam;
1:2,000) was incubated with the membranes at room temperature for
90 min. Finally, the results were quantified using Enhanced
Chemiluminescence detection system reagents (EMD Millipore),
according to the manufacturer's instructions.
Statistical analysis
GraphPad Prism 6 software (GraphPad Software, Inc.)
was used for statistical analyses. All results are expressed as the
mean ± standard deviation from three independent experiments. Group
comparisons between multiple groups were performed by one-way ANOVA
followed by Tukey's post hoc test. Unpaired Student's t-test was
used to make comparisons between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
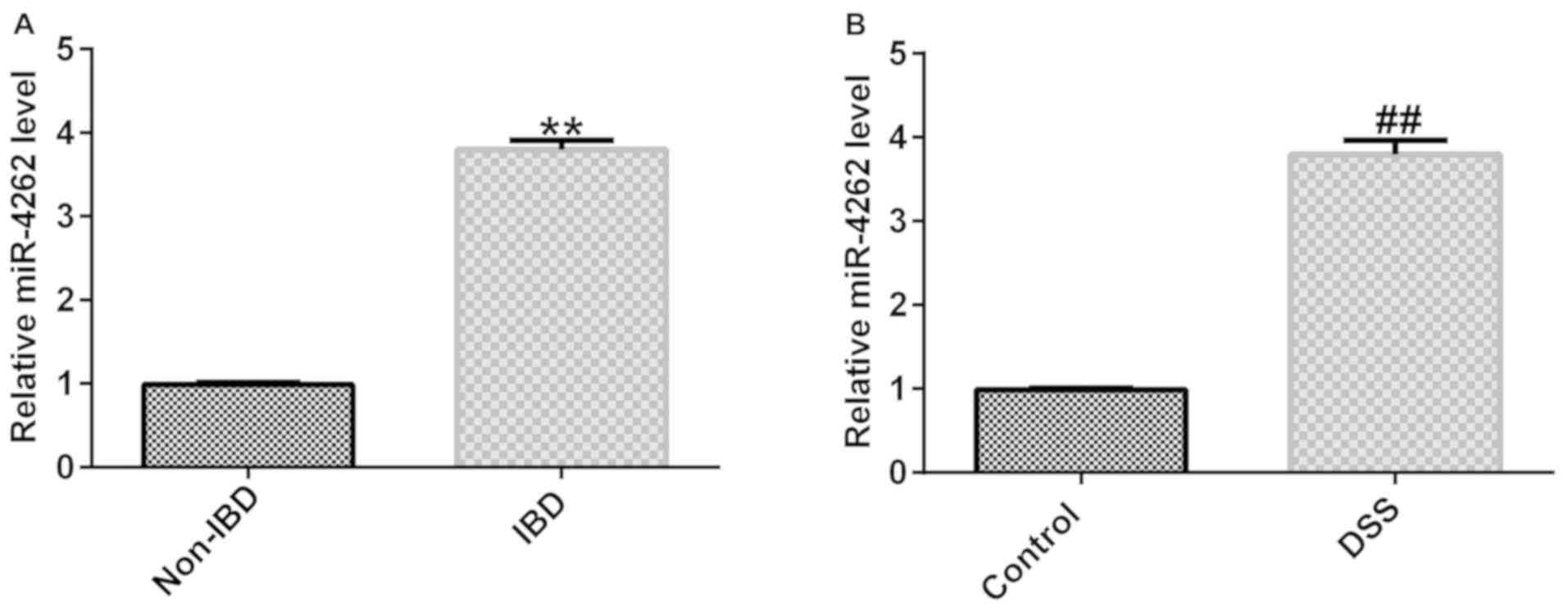
miR-4262 is upregulated in 2%
DSS-stimulated Caco-2 cells and IBD colonic mucosa tissues
To analyze the role of miR-4262 in IBD, the
expression of miR-4262 in 30 IBD colonic mucosa tissues and 30
normal colonic mucosa tissues was determined via RT-qPCR. As
presented in Fig. 1A, miR-4262
expression was significantly increased in the colonic mucosa
tissues from children with IBD compared with the normal tissues.
Furthermore, a higher miR-4262 expression was found in the 2%
DSS-induced Caco-2 cells compared with the control (Fig. 1B). These data demonstrated that
miR-4262 was upregulated in colonic mucosa tissues from patients
with IBD and in 2% DSS-stimulated Caco-2 cells and may thus be a
regulator of IBD.
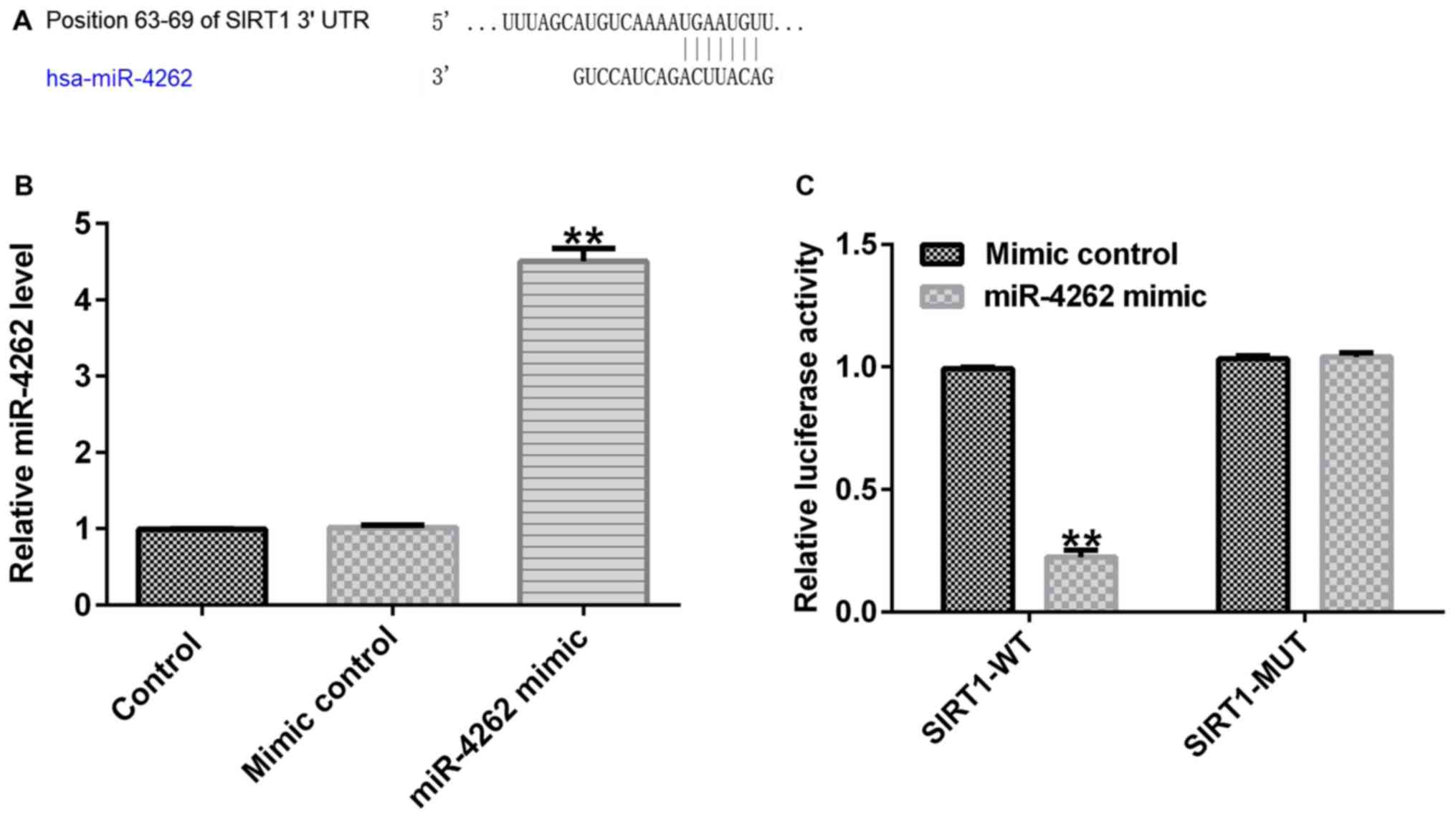
Prediction of SIRT1 as a direct target
of miR-4262
miRNAs perform biological functions by mediating
targets levels (5). Based on
TargetScan 7.2 analysis, the role of miR-4262 in IBD was
identified. As presented in Fig. 2A,
SIRT1 was identified as a potential target of miR-4262. To further
verify the hypothesis, a dual luciferase reporter system was
conducted to explore the relevance between miR-4262 and SIRT1.
Primarily, it was confirmed that miR-4262 mimic significantly
enhanced miR-4262 levels in Caco-2 cells (Fig. 2B). A significant suppression in SIRT1
3'-UTR wild-type luciferase activity was observed compared with the
mimic control, while SIRT1-MUT luciferase activity exhibited no
significant differences (Fig. 2C).
In summary, these data confirmed that SIRT1 represents a direct
target of miR-4262.
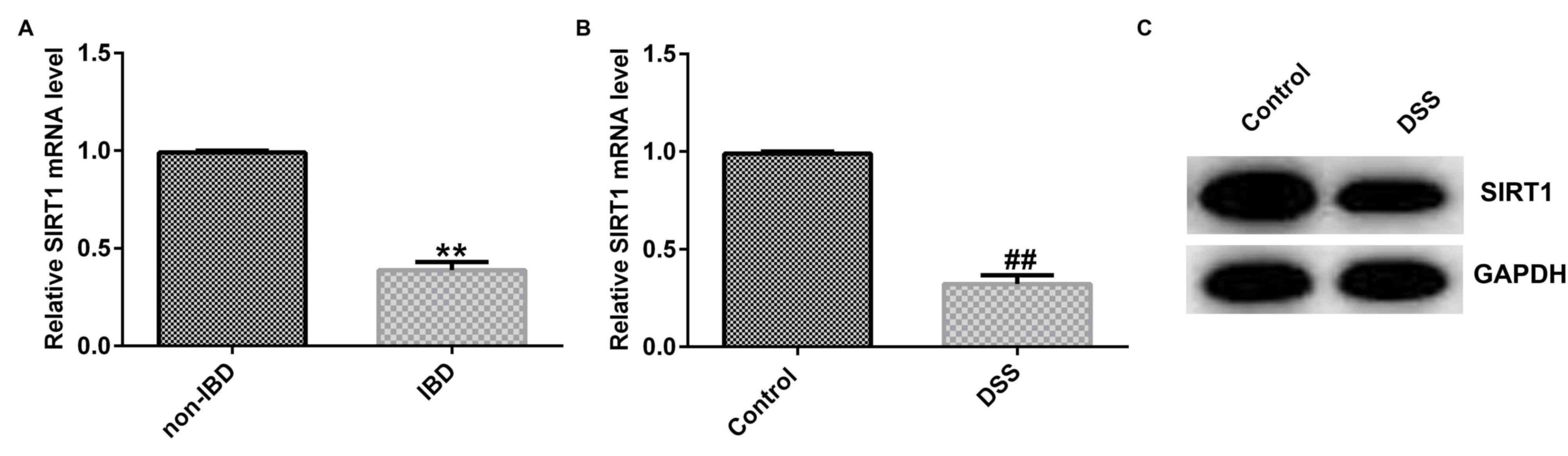
Expression of SIRT1 is suppressed in
2% DSS-stimulated Caco-2 cells and colonic mucosa tissues from
patients with IBD
According to the aforementioned resukts, it was
speculated that SIRT1 may participate in the progression of IBD. To
confirm this assumption, RT-qPCR analysis was performed to detect
the SIRT1 levels in colonic mucosa tissues from patients with IBD
and in normal colonic mucosa tissues. The results suggested that
SIRT1 mRNA expression was markedly decreased in tissues from
patients with IBD compared with normal tissues (Fig. 3A). Moreover, the results from RT-qPCR
and western blot analysis revealed that SIRT1 was downregulated in
the 2% DSS-treated Caco-2 cells compared with the control cells
(Fig. 3B and C). Thus, from these data, it can be
concluded that SIRT1 participates in the development of IBD.
SIRT1-siRNA reverses the effects of
miR-4262 inhibitor on SIRT1 expression in Caco-2 cells
Subsequently, it was determined whether miR-4262
affects SIRT1 expression in IBD. Inhibitor control, miR-4262
inhibitor, control-siRNA or SIRT1-siRNA were transfected into
Caco-2 cells for 48 h. RT-qPCR analysis suggested that compared
with the inhibitor control group, miR-4262 was downregulated in
Caco-2 cells following transfection with miR-4262 inhibitor
(Fig. 4A). Moreover, as presented in
Fig. 4B, compared with the
control-siRNA group, SIRT1-siRNA significantly decreased the SIRT1
levels. To further explore the association between miR-4262 and
SIRT1, RT-qPCR and western blot analysis were performed to measure
SIRT1 expression in the different groups following transfection.
Higher mRNA and protein expression levels of SIRT1 were identified
in the miR-4262 inhibitor group; however, these effects were
partially restored in the miR-4262 inhibitor + SIRT1-siRNA group
compared with the miR-4262 inhibitor + control-siRNA group
(Fig. 4C and D). These results further confirmed that
SIRT1 represents a target gene of miR-4262 and that miR-4262
negatively regulates SIRT1 in IBD cells.
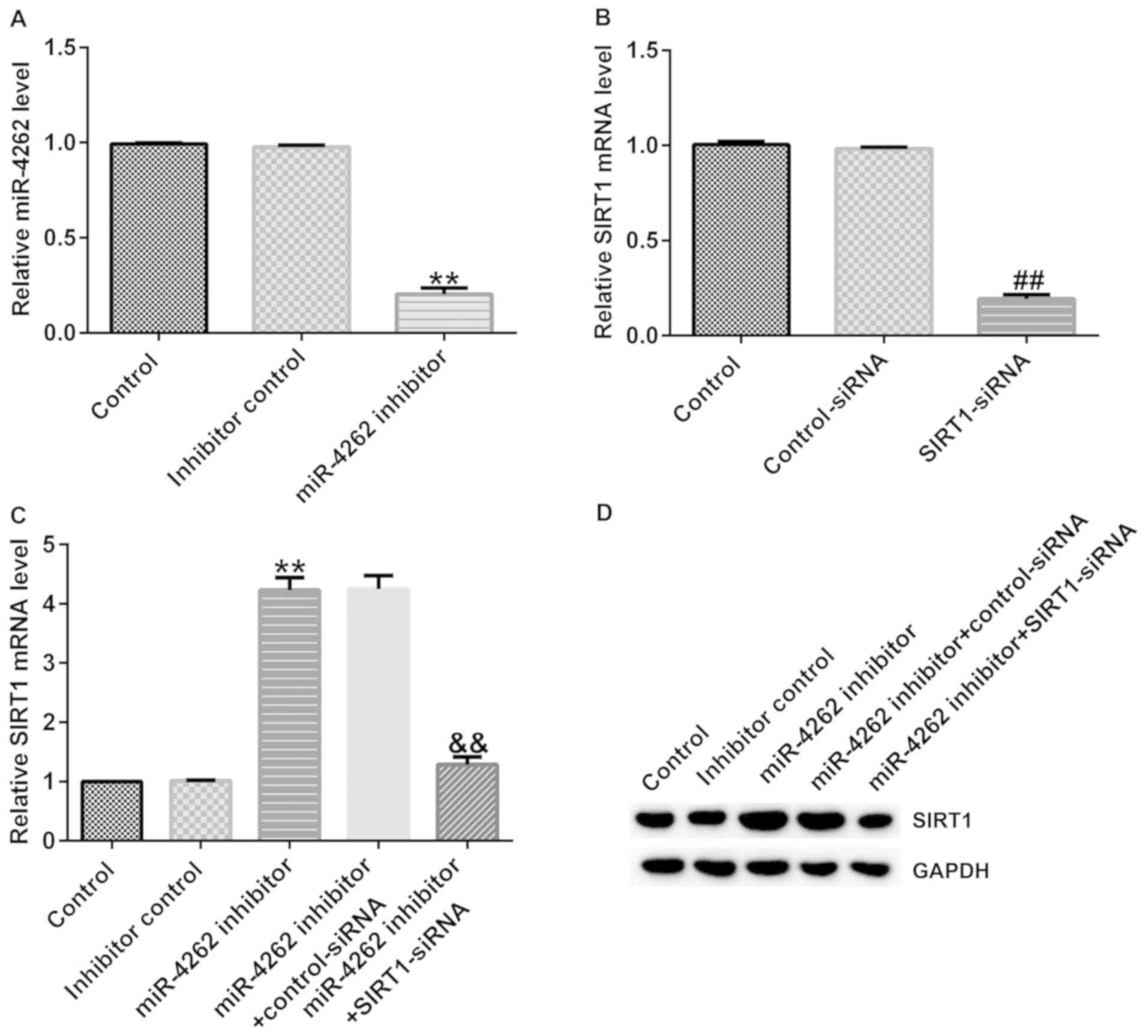 | Figure 4SIRT1-siRNA reverses the effects of
miR-4262 inhibitor on SIRT1 expression. Control-siRNA, SIRT1-siRNA,
inhibitor control or miR-4262 inhibitor were transfected into
Caco-2 cells for 48 h. Following transfection, RT-qPCR was
performed to verify the transfection efficiency. (A) mRNA levels of
miR-4262 following the transfection of miR-4262 inhibitor and
inhibitor control in Caco-2 cells. (B) mRNA level of SIRT1 in
Caco-2 cells following the transfection of control-siRNA and
SIRT1-siRNA. The (C) mRNA and (D) protein level of SIRT1 were
detected via RT-qPCR and western blot analysis, respectively, in
Caco-2 cells following transfection with miR-4262 inhibitor +
control-siRNA and miR-4262 inhibitor + SIRT1-siRNA. Three
independent experiment were conducted, and the data are presented
as the mean ± SD. **P<0.01 vs. inhibitor control;
##P<0.01 vs. control-siRNA;
&&P<0.01 vs. miR-4262 inhibitor +
control-siRNA. RT-qPCR, reverse transcription-quantitative PCR;
miR, microRNA; siRNA, small interfering RNA; DSS, dextran sulfate
sodium; SIRT1, sirtuin 1. |
miR-4262 inhibitor decreases the
secretion of inflammatory factors in DSS-stimulated Caco-2 cells
via regulating SIRT1
Multiple reports have confirmed that IBD is
associated with inflammatory factor secretion (20,21).
Thus, the levels of inflammatory cytokines were measured in
DSS-treated Caco-2 cells following transfection for 48 h. The
results were verified using ELISAs. It was revealed that DSS
promoted the release of TNF-α, IL-1β and IL-6 in Caco-2 cells
compared with the control group (Fig.
5A-C). In addition, cells cultured with DSS and miR-4262
inhibitor exhibited a lower inflammatory response, verified by the
decreased TNF-α, IL-1β and IL-6 expression levels compared with the
DSS + inhibitor control group. However, these effects were
partially reversed following transfection with SIRT1-siRNA, and it
was revealed that the levels of TNF-α, IL-1β and IL-6 were
significantly enhanced in the DSS + miR-4246 inhibitor +
SIRT1-siRNA group (Fig. 5A-C). The
same trends were observed at the mRNA level via RT-qPCR (Fig. 5D-F). The aforementioned results
demonstrated that SIRT1-siRNA reversed the inhibitory function of
the miR-4262 inhibitor on the expression of inflammatory factors in
DSS-treated Caco-2 cells, suggesting that the silencing of SIRT1
may prevent miR-4262 inhibitor from inhibiting the inflammatory
response in DSS-induced Caco-2 cells.
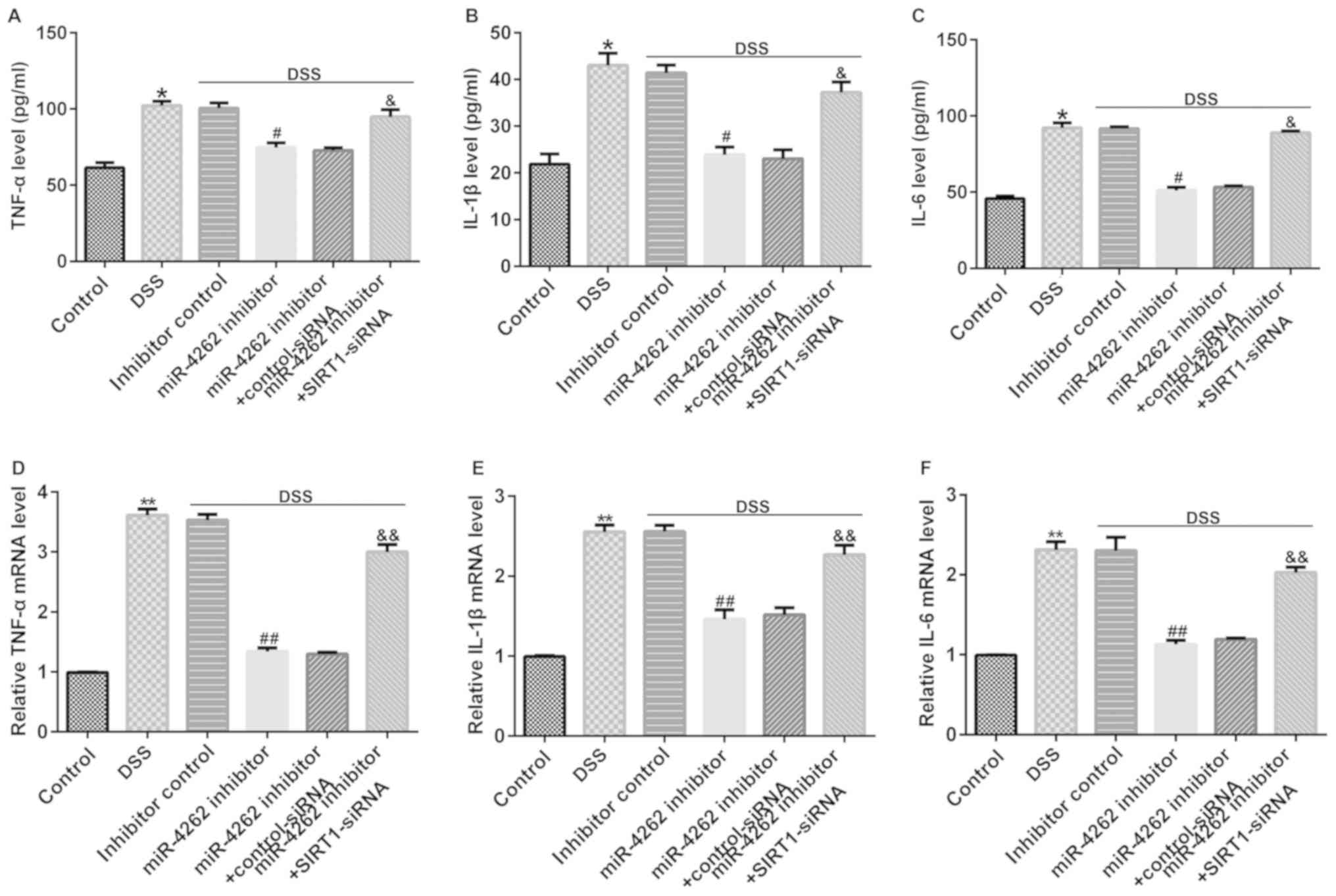 | Figure 5SIRT1-siRNA reverses the effects of
miR-4262 inhibitor on the secretion of inflammatory factors.
Inhibitor control, miR-4262 inhibitor, control-siRNA and
SIRT1-siRNA were transfected into Caco-2 cells for 48 h. Then, the
cells were stimulated with DSS for 4 days. Cells were divided into
6 groups: Control, DSS, DSS + inhibitor control, DSS + miR-4246
inhibitor, DSS + miR-4246 inhibitor + control-siRNA, DSS + miR-4246
inhibitor + SIRT1-siRNA. (The release of the inflammatory factors
(A) TNF-α, (B) IL-1β and (C) IL-6 were detected via ELISAs. The (D)
TNF-α, (E) IL-1β and (F) IL-6 mRNA levels were evaluated via
reverse transcription-quantitative PCR in 6 groups. Three
independent experiment were conducted, and the data are presented
as the mean ± SD. *P<0.05, **P<0.01 vs.
control; #P<0.05, ##P<0.01 vs. DSS +
inhibitor control; &P<0.05,
&&P<0.01 vs. DSS + miR-4246 inhibitor +
control-siRNA. miR, microRNA; siRNA, small interfering RNA; DSS,
dextran sulfate sodium; SIRT1, sirtuin 1; IL, interleukin; TNF,
tumor necrosis factor. |
miR-4262 inhibitor promotes cell
viability and decreases cell apoptosis in DSS-stimulated Caco-2 by
regulating SIRT1
To intensively explore the functional mechanism of
miR-4262 in IBD, Caco-2 cells were transfected with inhibitor
control, miR-4262 inhibitor, control-siRNA or SIRT1-siRNA for 48 h,
followed by 2% DSS treatment for 4 days. MTT assays and flow
cytometric analysis were conducted to evaluate the Caco-2 cell
viability and apoptosis, respectively. As presented in Fig. 6A, DSS treatment markedly inhibited
Caco-2 cell viability compared with the control. Moreover, in the
DSS-induced cells, miR-4262 inhibitor stimulated cell viability
compared to the inhibitor control. However, in the DSS + miR-4246
inhibitor + SIRT1-siRNA group, cell viability was significantly
suppressed. As indicated in Fig. 6B
and C, it was demonstrated that
compared with the control group, DSS significantly induced Caco-2
cell apoptosis, while compared with the DSS + inhibitor control
group, transfection with a miR-4246 inhibitor markedly inhibited
cell apoptosis; however, this effect was reversed in the DSS +
miR-4246 inhibitor + SIRT1-siRNA group compared with the DSS +
miR-4246 inhibitor + control-siRNA group.
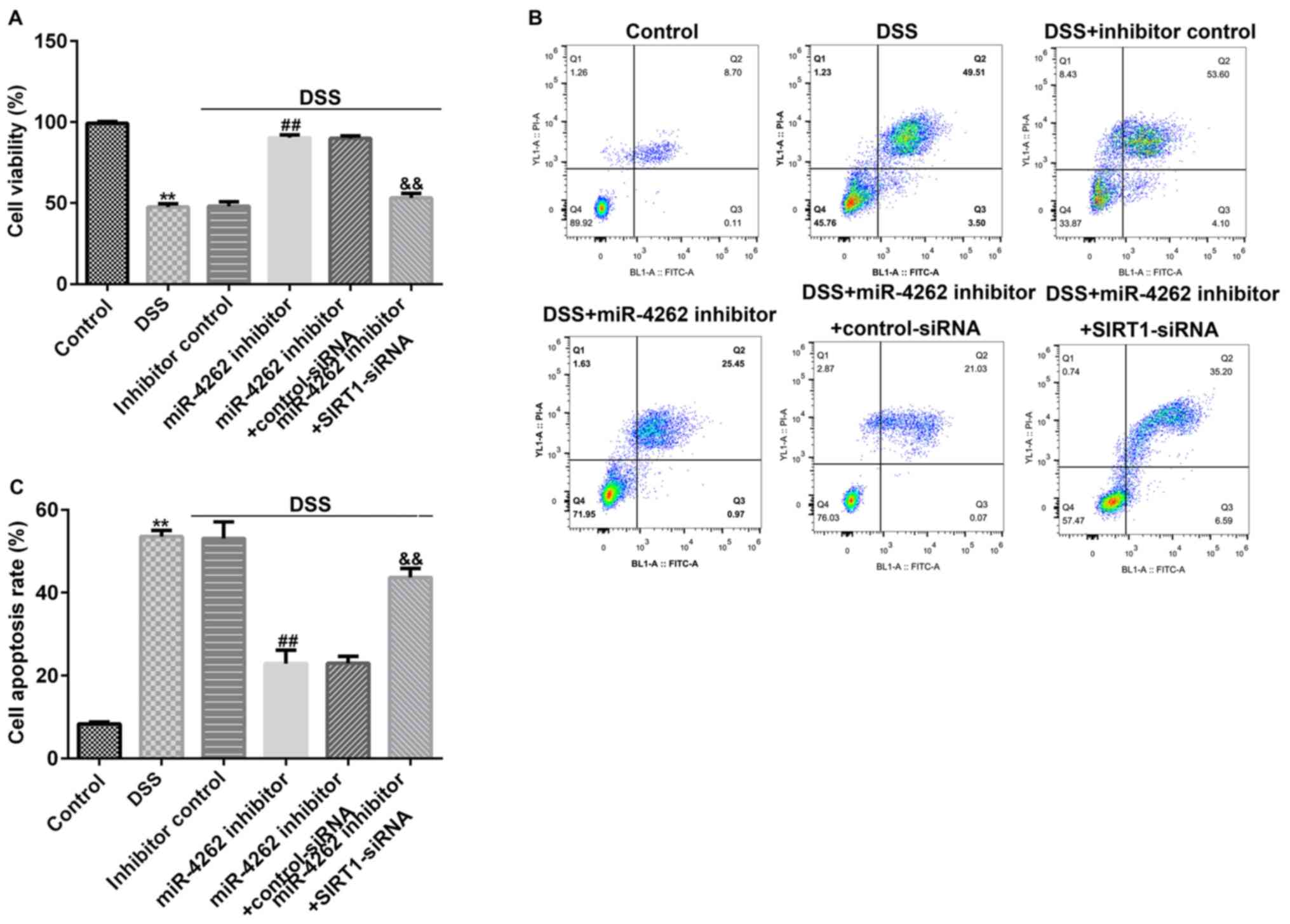 | Figure 6SIRT1-siRNA eliminates the effects of
miR-4262 inhibitor on Caco-2 cell viability and apoptosis. Caco-2
cells were transfected with inhibitor control, miR-4262 inhibitor,
control-siRNA and SIRT1-siRNA for 48 h, and the cells were then
treated with 2% DSS for 4 days. Cells were divided into 6 groups:
Control, DSS, DSS + inhibitor control, DSS + miR-4246 inhibitor,
DSS + miR-4246 inhibitor + control-siRNA, DSS + miR-4246 inhibitor
+ SIRT1-siRNA. (A) MTT was performed to detect Caco-2 cell
viability in the 6 groups. (B) FCM was conducted to measure Caco-2
cell apoptosis in the 6 groups. (C) Results of FCM are displayed as
a bar graph. Three independent experiment were conducted, and the
data are presented as the mean ± SD. **P<0.01 vs.
Control; ##P<0.01 vs. DSS + inhibitor control;
&&P<0.01 vs. DSS + miR-4246 inhibitor +
control-siRNA. miR, microRNA; siRNA, small interfering RNA; DSS,
dextran sulfate sodium; SIRT1, sirtuin 1; PI, propidium iodide. |
miR-4262 inhibitor suppresses NF-κB
pathway activation in DSS-stimulated Caco-2 cells cia regulating
SIRT1
The NF-κB pathway has been confirmed to participate
in the progression of IBD (22).
Finally, western blot analysis was performed to detect the
associated protein expression levels of the NF-κB pathway in
DSS-induced cells. The results revealed that p-p65 expression was
increased (Fig. 7A) and the ratio of
p-p65/p65 was enhanced (Fig. 7B) in
the DSS-treated cells. However, it was then observed that p-p65 was
downregulated and the ratio of p-p65/p65 was decreased in the
DSS-treated Caco-2 cells following transfection with a miR-4262
inhibitor. However, in the DSS + miR-4246 inhibitor + SIRT1-siRNA
group, the expression of p-p65 was enhanced and the ratio of
p-p65/p65 was increased. Collectively, the current results
demonstrate that transfection with a miR-4262 inhibitor exerts
protective effects on IBD cells by inhibiting both the inflammatory
response and Caco-2 cell apoptosis via the regulation of SIRT1,
indicating that miR-4262 may represent a potential target for IBD
treatment.
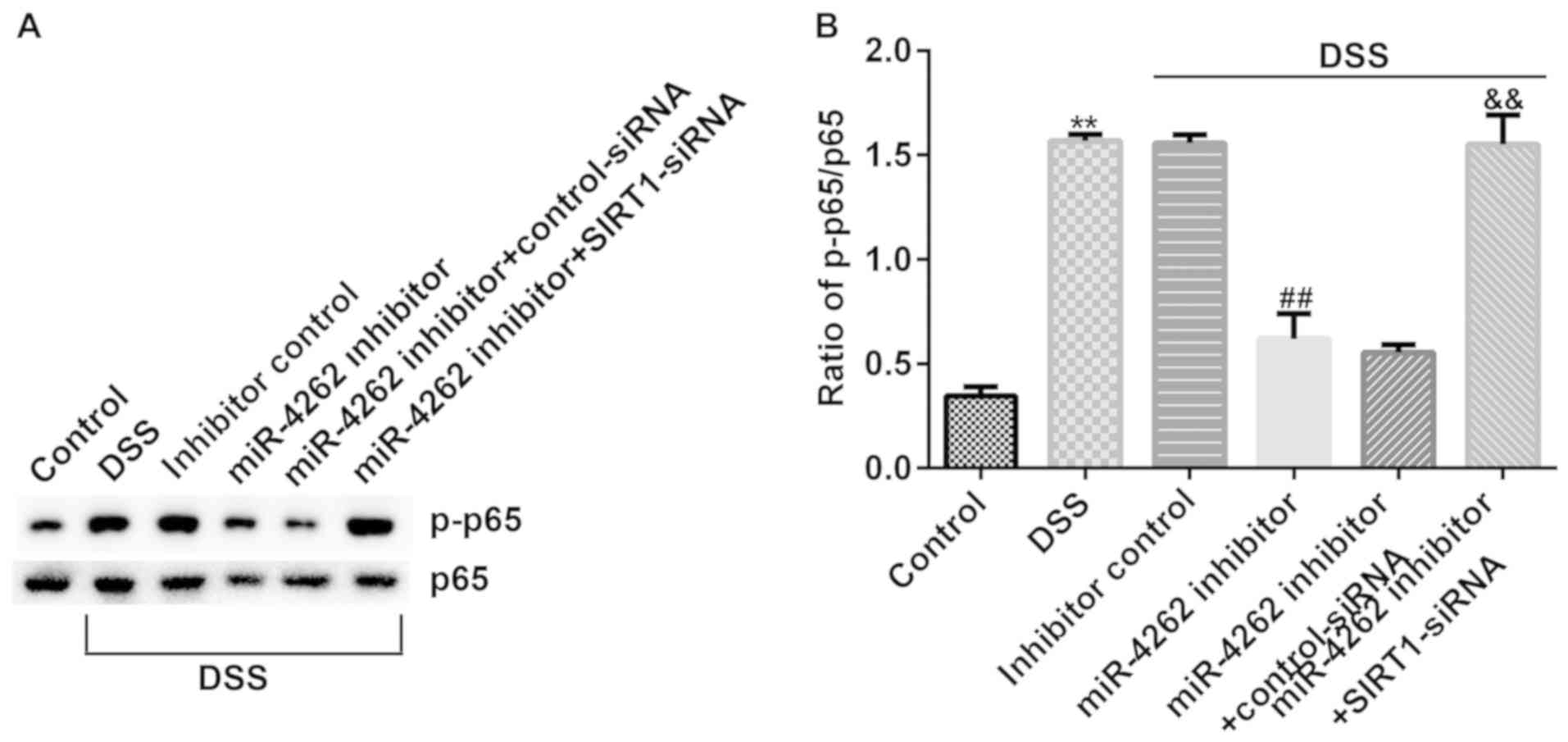 | Figure 7miR-4262 inhibitor alleviates the
activation of the NF-κB pathway in Caco-2 cells and SIRT1-siRNA
reverses the effects. Caco-2 cells were transfected with inhibitor
control, miR-4262 inhibitor, control-siRNA and SIRT1-siRNA for 48
h, and DSS was used to induce the cells for 4 days. Cells were
divided into 6 groups: Control, DSS, DSS + inhibitor control, DSS +
miR-4246 inhibitor, DSS + miR-4246 inhibitor + control-siRNA, DSS +
miR-4246 inhibitor + SIRT1-siRNA. (A) Western blot analysis of p65
and p-p65 expression in different groups. (B) Relative protein
expression of p-p65/p65 was measured. Three independent experiment
were conducted, and the data are presented as the mean ± SD.
**P<0.01 vs. control; ##P<0.01 vs. DSS
+ inhibitor control; &&P<0.01 vs. DSS +
miR-4246 inhibitor + control-siRNA. miR, microRNA; siRNA, small
interfering RNA; DSS, dextran sulfate sodium; SIRT1, sirtuin 1; p-,
phosphorylated. |
Discussion
IBD, a chronic and immune disease, is associated
with hindering intestinal peristalsis, a shortening colon and
inflammation (23). A previous
associated study indicated that chronic inflammation is a main risk
factor in the progression of colorectal cancer and is involved in
the pathogenesis of IBD, while therapies for patients with IBD are
limited, where many patients do not respond to or cannot sustain
treatment with these drugs, which have various side effects
(24). Therefore, the present study
aimed to identify a novel target and to explore the underlying
mechanisms for IBD treatment. DSS is widely adopted to stimulate
colonic inflammation for in vivo and in vitro IBD
models (25,26), and in a DSS mouse IBD model, DSS
appears to be directly toxic to colonic epithelial cells in the
basal crypt (26). Thus, in the
present study, DSS was used to treat Caco-2 cells and the effects
of DSS on Caco-2 cells and IBD tissues were examined.
It has been previously characterized that miRNAs
serve various roles in disease development (27). Moreover, several miRNAs, such as
miR-135a have been confirmed to participate in the progression of
IBD (28). miR-4262, a recently
discovered miRNA, functions as either an oncogene or
tumor-suppressor gene in cancers (29). Numerous studies have demonstrated
that miR-4262 exhibits an abnormal expression in multiple
pathological processes, such as non-small cell lung cancer. For
example, Sun et al (30)
reported that miR-4262 was involved in paclitaxel resistance via
the regulation of PTEN in non-small cell lung cancer. However, the
findings by Song et al (31)
indicated that the levels of miR-4262 were significantly decreased
in osteosarcoma tissues. All these findings suggest that miR-4262
serves a vital role in the development of cancers. However, as far
as is known, the specific mechanisms of action of miR-4262 in IBD
remain to be clarified. In the current study, the effects of
miR-4262 on the progression of IBD were investigated.
In the present study, the level of miR-4262 in IBD
colonic mucosa tissues and normal tissues, as well as in 2%
DSS-stimulated Caco-2 cells and untreated Caco-2 cells was first
determined. It was revealed that the expression of miR-4262 was
significantly higher in IBD colonic mucosa tissues compared with
normal tissues. Moreover, a significant downregulation of miR-4262
in 2% DSS-stimulated Caco-2 cells was observed, compared with
normal cells. These results indicated that miR-4262 may represent a
regulator of IBD. Bioinformatics tools and a dual-luciferase
reporter assay were then adopted to predict the miR-4262 target
gene. The results indicated that miR-4262 directly targeted the
SIRT1 3'-UTR and negatively regulated SIRT1 expression levels. It
was demonstrated that the level of SIRT1 in IBD colonic mucosa
tissues was higher compared with in normal colonic mucosa tissues.
The results suggested that SIRT1 mRNA expression was significantly
reduced in IBD tissues compared with normal tissues. In addition,
the results from RT-qPCR and western blot analysis indicated that
SIRT1 was downregulated in the 2% DSS-treated Caco-2 cells compared
with the control cells, suggesting that SIRT1 participated in the
development of IBD. Based on these data, it was assumed that the
altered expression of miR-4262 may affect the development of IBD.
Furthermore, whether miR-4262 influences SIRT1 expression in IBD
was determined. Inhibitor control, miR-4262 inhibitor,
control-siRNA or SIRT1-siRNA were transfected into Caco-2 cells for
48 h. The data revealed that miR-4262 was downregulated in Caco-2
cells following transfection with miR-4262 inhibitor, and
SIRT1-siRNA significantly decreased the SIRT1 levels. Moreover,
higher mRNA and protein expression level of SIRT1 were obtained in
the miR-4262 inhibitor group; however, these effects were abolished
in the miR-4262 inhibitor + SIRT1-siRNA group. These results
further confirmed that miR-4262 negatively regulates SIRT1
expression via targeting the 3'UTR of SIRT1 in IBD.
Various reports have confirmed that the inflammatory
response is a main characteristic of IBD and is highly associated
with the progression of IBD (20,21,32).
Pro-inflammatory factors, including IL-1β, TNF-α and IL-6 have been
confirmed as important regulators in the occurrence of IBD
(25). DSS-induced murine and cell
models of IBD are often adopted for the investigation of intestinal
inflammatory diseases (18,33). To further investigate the underlying
mechanisms of miR-4262 in IBD, in the present study, Caco-2 cells
were transfected with inhibitor control, miR-4262 inhibitor,
control-siRNA or SIRT1-siRNA for 48 h, followed by 2% DSS treatment
for 4 days. It was demonstrated that DSS induced a greater
production of pro-inflammatory factors in Caco-2 cells, while
miR-4262 inhibitor alleviated the release of DSS-stimulated
inflammatory factors and that these results were reversed by
transfection with SIRT1-siRNA. DSS inhibited Caco-2 cell growth and
induced cell apoptosis compared with the control. Meanwhile,
miR-4262 inhibitor promoted cell growth and decreased cell
apoptosis in DSS-induced Caco-2 cells, and this effect was reversed
in the DSS + miR-4246 inhibitor + SIRT1-siRNA group compared with
the DSS + miR-4246 inhibitor + control-siRNA group. The data
revealed that miR-4246 functions as a marker in IBD progression.
These results are consistent with those of previous studies. Sun
et al (34) reported that
miR-4262 regulated chondrocyte viability, apoptosis and autophagy
by targeting SIRT1 and activating the PI3K/AKT/mTOR signaling
pathway in rats with osteoarthritis. Accumulating studies have
confirmed the role of NF-κB in inflammatory diseases (35,36).
Several reports have demonstrated that miRNAs activate the NF-κB
pathway, which may be associated with the development of
inflammatory diseases (37,38). Therefore, the present study further
validated the effects of NF-κB activity in IBD. Notably, the
present findings revealed that p-p65 expression was increased and
the ratio of p-p65/p65 was enhanced in DSS-treated Caco-2 cells.
Transfection with the miR-4262 inhibitor knocked down p-p65
expression and decreased the ratio of p-p65/p65 in DSS-treated
Caco-2 cells following transfection. However, opposite effects were
observed in the DSS + miR-4246 inhibitor + SIRT1-siRNA group; the
expression of p65 were enhanced and the ratio of p-p65/p65 was
increased. These data demonstrated that miR-4246 inhibitor serves a
protective role in IBD by alleviating apoptosis and activating
NF-κB pathways.
Taken together, the present study, to the best of
our knowledge, was the first to demonstrate the role of miR-4262 in
the development of IBD. The results of the present study
demonstrated the protective effects of transfection with miR-4262
inhibitor on DSS-induced Caco-2 cells, evidenced by inhibiting the
inflammatory response, increasing cell growth and decreasing cells
apoptosis, via regulation of the miR-4262/SIRT1 axis. This
indicates that miR-4262 may represent a potential target for IBD
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD contributed to study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. LS, MD, LY and LX contributed to data collection and
statistical analysis. XX contributed to data collection,
statistical analysis, and manuscript preparation. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All specimens were obtained with written informed
consent and the present study was approved by the Ethics Committee
of Chengdu Women's and Children's Central Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mogilevski T, Burgell R, Aziz Q and Gibson
PR: Review article: The role of the autonomic nervous system in the
pathogenesis and therapy of IBD. Aliment Pharmacol Ther.
50:720–737. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hammer T, Lophaven SN, Nielsen KR, von
Euler-Chelpin M, Weihe P, Munkholm P, Burisch J and Lynge E:
Inflammatory bowel diseases in Faroese-born Danish residents and
their offspring: Further evidence of the dominant role of
environmental factors in IBD development. Aliment Pharmacol Ther.
45:1107–1114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wolf DC, Abraham BP, Afzali A, Allegretti
PD and Arai R: Community perspectives: Combining serology,
genetics, and inflammation markers for the diagnosis of IBD and
differentiation between CD and UC. Gastroenterol Hepatol (N Y).
8:S1–S16. 2012.PubMed/NCBI
|
|
4
|
Liu R, Tang A, Wang X, Chen X, Zhao L,
Xiao Z and Shen S: Inhibition of lncRNA NEAT1 suppresses the
inflammatory response in IBD by modulating the intestinal
epithelial barrier and by exosome-mediated polarization of
macrophages. Int J Mol Med. 42:2903–2913. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pereira TDSF, Brito JAR, Guimarães ALS,
Gomes CC, de Lacerda JCT, de Castro WH, Coimbra RS, Diniz MG and
Gomez RS: MicroRNA profiling reveals dysregulated microRNAs and
their target gene regulatory networks in cemento-ossifying fibroma.
J Oral Pathol Med. 47:78–85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu ZW, Liu YF, Wang S and Li B: miRNA-146a
induces vascular smooth muscle cell apoptosis in a rat model of
coronary heart disease via NF-κB pathway. Genet Mol Res.
14:18703–18712. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang J, Yin K, Lv X, Yang Q, Shao M, Liu X
and Sun H: MicroRNA-24-3p regulates Hodgkin's lymphoma cell
proliferation, migration and invasion by targeting DEDD. Oncol
Lett. 17:365–371. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen Y, Du J, Zhang Z, Liu T, Shi Y, Ge X
and Li YC: MicroRNA-346 mediates tumor necrosis factor α-induced
downregulation of gut epithelial vitamin D receptor in inflammatory
bowel diseases. Inflamm Bowel Dis. 20:1910–1918. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang L, Ma Q, Li Y, Li B and Zhang L:
Inhibition of microRNA-210 suppresses pro-inflammatory response and
reduces acute brain injury of ischemic stroke in mice. Exp Neurol.
300:41–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kumar A, Bhatia HS, de Oliveira AC and
Fiebich BL: microRNA-26a modulates inflammatory response induced by
toll-like receptor 4 stimulation in microglia. J Neurochem.
135:1189–1202. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang K, Ren Y, Liu Y, Zhang J and He JJ:
miR-4262 promotes proliferation and invasion of human breast cancer
cells through directly targeting KLF6 and KLF15. Oncol Res.
25:277–283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Weng L, Ma J, Jia YP, Wu SQ, Liu BY, Cao
Y, Yin X, Shang MY and Mao AW: miR-4262 promotes cell apoptosis and
inhibits proliferation of colon cancer cells: Involvement of
GALNT4. Am J Transl Res. 10:3969–3977. 2018.PubMed/NCBI
|
|
13
|
Zhang D, Li Z, Zhang Y, Tu C, Huo J and
Liu Y: miR-4262 promotes the proliferation of human cutaneous
malignant melanoma cells through KLF6-mediated EGFR inactivation
and p21 upregulation. Oncol Rep. 36:3657–3663. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang L, Duan Z, Liu X and Yuan Y:
N-acetyl-l-cysteine ameliorates the PM2.5-induced oxidative stress
by regulating SIRT-1 in rats. Environ Toxicol Pharmacol. 57:70–75.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sim WC, Kim DG, Lee W, Sim H, Choi YJ and
Lee BH: Activation of SIRT1 by L-serine increases fatty acid
oxidation and reverses insulin resistance in C2C12 myotubes. Cell
Biol Toxicol. 35:457–470. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pan Q, Lou X, Zhang J, Zhu Y, Li F, Shan
Q, Chen X, Xie Y, Su S, Wei H, et al: Genomic variants in mouse
model induced by azoxymethane and dextran sodium sulfate improperly
mimic human colorectal cancer. Sci Rep. 7(25)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Menconi A, Hernandez-Velasco X, Vicuña EA,
Kuttappan VA, Faulkner OB, Tellez G, Hargis BM and Bielke LR:
Histopathological and morphometric changes induced by a dextran
sodium sulfate (DSS) model in broilers. Poult Sci. 94:906–911.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nighot P, Young K, Nighot M, Rawat M, Sung
EJ, Maharshak N, Plevy SE, Ma T and Blikslager A: Chloride channel
ClC-2 is a key factor in the development of DSS-induced murine
colitis. Inflamm Bowel Dis. 19:2867–2877. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Friedrich M, Pohin M and Powrie F:
Cytokine networks in the pathophysiology of inflammatory bowel
disease. Immunity. 50:992–1006. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rogler G: Resolution of inflammation in
inflammatory bowel disease. Lancet Gastroenterol Hepatol.
2:521–530. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
McDaniel DK, Eden K, Ringel VM and Allen
IC: Emerging roles for noncanonical NF-κB signaling in the
modulation of inflammatory bowel disease pathobiology. Inflamm
Bowel Dis. 22:2265–2279. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Soufflet F, Biraud M, Rolli-Derkinderen M,
Lardeux B, Trang C, Coron E, Bruley des Varannes S, Bourreille A
and Neunlist M: Modulation of VIPergic phenotype of enteric neurons
by colonic biopsy supernatants from patients with inflammatory
bowel diseases: Involvement of IL-6 in Crohn's disease.
Neurogastroenterol Motil. 30:2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schenk M, Bouchon A, Seibold F and Mueller
C: TREM-1-expressing intestinal macrophages crucially amplify
chronic inflammation in experimental colitis and inflammatory bowel
diseases. J Clin Invest. 117:3097–3106. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Zhao Q, Liu Y, Tan L, Yan L and Zuo X:
Adiponectin administration alleviates DSS-induced colonic
inflammation in Caco-2 cells and mice. Inflamm Res. 67:663–670.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu X, Chen J, Guan T, Yao H, Zhang W,
Guan Z and Wang Y: Correction to: miRNAs and target genes in the
blood as biomarkers for the early diagnosis of Parkinson's disease.
BMC Syst Biol. 13(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang J, Lian B, Shang Y, Li C and Meng Q:
miR-135a protects dextran sodium sulfate-induced inflammation in
human colorectal cell lines by activating STAT3 signal. Cell
Physiol Biochem. 51:1001–1012. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang H, Jiang H, Zhang H, Liu J, Hu X and
Chen L: miR-4262, low level of which predicts poor prognosis,
targets proto-oncogene CD163 to suppress cell proliferation and
invasion in gastric cancer. Onco Targets Ther. 12:599–607.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun H, Zhou X, Bao Y, Xiong G, Cui Y and
Zhou H: Involvement of miR-4262 in paclitaxel resistance through
the regulation of PTEN in non-small cell lung cancer. Open Biol.
9(180227)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Song K, Liu N, Yang Y and Qiu X:
Regulation of osteosarcoma cell invasion through osteopontin
modification by miR-4262. Tumour Biol. 37:6493–6499.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gerasimidis K, Edwards C, Stefanowicz F,
Galloway P, McGrogan P, Duncan A and Talwar D: Micronutrient status
in children with IBD: True deficiencies or epiphenomenon of the
systemic inflammatory response. J Pediatr Gastroenterol Nutr.
56:e50–e51. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Eichele DD and Kharbanda KK: Dextran
sodium sulfate colitis murine model: An indispensable tool for
advancing our understanding of inflammatory bowel diseases
pathogenesis. World J Gastroenterol. 23:6016–6029. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun W, Li Y and Wei S: miR-4262 regulates
chondrocyte viability, apoptosis, autophagy by targeting SIRT1 and
activating PI3K/AKT/mTOR signaling pathway in rats with
osteoarthritis. Exp Ther Med. 15:1119–1128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schuliga M: NF-kappaB signaling in chronic
inflammatory airway disease. Biomolecules. 5:1266–1283.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lin TH, Pajarinen J, Lu L, Nabeshima A,
Cordova LA, Yao Z and Goodman SB: NF-κB as a therapeutic target in
inflammatory-associated bone diseases. Adv Protein Chem Struct
Biol. 107:117–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hill JM, Clement C, Zhao Y and Lukiw WJ:
Induction of the pro-inflammatory NF-kB-sensitive miRNA-146a by
human neurotrophic viruses. Front Microbiol. 6(43)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pogue AI, Percy ME, Cui JG, Li YY,
Bhattacharjee S, Hill JM, Kruck TP, Zhao Y and Lukiw WJ:
Up-regulation of NF-kB-sensitive miRNA-125b and miRNA-146a in metal
sulfate-stressed human astroglial (HAG) primary cell cultures. J
Inorg Biochem. 105:1434–1437. 2011.PubMed/NCBI View Article : Google Scholar
|