Introduction
Atherosclerosis is a chronic inflammatory disease of
the vascular wall associated with lipid deposition and plaque
fibrosis (1-3). As
a chronic inflammatory disease, the mortality rate caused by
atherosclerosis is rising worldwide (4). Pathologically, dysfunction of
endothelial cells is a critical event in the pathological process
of atherosclerosis, which leads to apoptosis of vascular arterial
walls, atherogenesis, aortic plaque and macrophage infiltration
(5-7). In
addition, atherosclerosis is generally regarded as a lipid-induced
chronic inflammation in the vessel wall, which leads to
inflammatory lesions and apoptosis of endothelial cells (8-10).
Thus, elucidating the possible mechanisms underlying the
inflammatory process and apoptotic signal molecular pathways
involved in the pathology of atherosclerosis are crucial to
understand the occurrence and development of atherosclerosis.
Pterostilbene
(trans-3,5-dimethoxy-4-hydroxystilbene) is a dimethylated
analog of resveratrol that exhibits protective ability against
atherosclerosis (11). Research
indicates that pterostilbene is an anti-inflammatory compound that
causes improvement of atherosclerotic plaque macrophages in
patients with atherosclerosis (12).
Recently, Gao et al (13)
found that pterostilbene can protect rats against acute renal
ischemia reperfusion injury and inhibit oxidative stress and
inflammation via the TLR4/NF-κB signaling pathway. In addition,
atherosclerosis is partly mediated by the dysfunction and apoptosis
of endothelial cells (14).
Meanwhile, the apoptosis of endothelial cells in the artery wall
increases atherosclerotic inflammation and plaques in the formation
of foam cells and necrotic core along with lipid uptake of
macrophages (15-17).
However, understanding the multifactorial process of pterostilbene
in mediating the pathological process of atherosclerotic plaques to
protect endothelial cells against atherosclerosis have not been
well investigated.
The TLR-4/MyD88/NF-κB signaling pathway has been
widely investigated in previous studies (18-20).
Qi et al suggest that suppression of the TLR4/MyD88
signaling pathway confers a protective effect against renal
ischemia/reperfusion injury (21).
Inactivation of the TLR4/MyD88/NF-κB signaling pathway was found to
exhibit potent effects against alcoholic liver fibrosis (22). In addition, Yao et al
demonstrated that downregulating the TLR4/MyD88 signaling pathway
reduced lipopolysaccharide-induced inflammatory liver injury
(23). Furthermore, research also
indicates that suppression of the TLR4/MyD88 signaling pathway
exerts a nephroprotective effect against LPS-induced inflammatory
renal injury, which provides novel insights into the mechanisms of
this therapeutic candidate for the treatment of inflammatory injury
(24). Thus, the TLR-4/MyD88/NF-κB
signaling pathway may be considered as a new potential therapeutic
option for the treatment of inflammatory disease.
In the present study, the potential therapeutic
effects of pterostilbene were investigated where the possible
underlying mechanism in endothelial cells in a rat model of
atherosclerosis was explored. The anti-inflammatory, antioxidant
and anti-apoptotic abilities of pterostilbene in endothelial cells
in the vascular arterial walls were also analyzed in the rat model
of atherosclerosis. These results revealed that pterostilbene
treatment decreased the inflammation, oxidative stress and
apoptosis of arterial endothelial cells and thus prevented rats
against the formation atherosclerotic plaque.
Materials and methods
Establishment of the rat model of
atherosclerosis
The use of experimental animals in the present study
was carried out in accordance with the recommendations in the Guide
for the Care and Use of Laboratory Animals of the National
Institutes of Health (USA) (25).
The animal use protocol was approved by the Committee on the Ethics
of Animal Experiments of the Second Affiliated Hospital of Nanchang
University (Nanchang, Jiangxi, China). Male, 8-week-old
Sprague-Dawley rats with initial body weight of 300-320 g (n=26)
were purchased from the Second Affiliated Hospital of Nanchang
University. All animals were housed with a 12-h light-dark cycle,
at 23±1˚C and 50±5% humidity. All rats had free access to food and
water. All surgeries were performed under anesthesia with
pentobarbital (40 mg/kg), and efforts were made to minimize
suffering of the rats. The rats were fed a 2.5% cholesterol diet
for 8 weeks as described previously (26). The rats were randomly divided into
three experimental groups: healthy group (n=6); group that received
phosphate-buffer saline (PBS) treatment (n=10); group that orally
received pterostilbene
(trans-3,5-dimethoxy-4'-hydroxystilbene,
C16H16O3, ≥99%, purity; 10
mg/kg/day; Great Forest Biomedical, Hangzhou, China; n=10)
treatment once a day. All treatments were continued with a regular
diet for 4 weeks.
Biochemical assays
After 4 weeks of treatment, rats in each group were
injected with pentobarbital for anesthesia, following which 2 ml
blood was taken from the retroorbital venous plexus and were then
sacrificed. Serum samples were obtained using centrifugation at
8,000 x g for 10 min at 4˚C. The concentrations of IL-6 (cat. no.
R6000B), IL-1β (cat. no. RLB00) and TNF-α (cat. no. RTA00) were
determined using enzyme linked immunosorbent assay (ELISA) kits
according to the manufacturer's instructions (Bio-Rad Laboratories,
Inc.). Superoxide dismutase (SOD), catalase (CAT), heme oxygenase-1
(HO-1), malondialdehyde (MDA), myeloperoxidase (MPO), cholesterol
(CHO), high-density lipoprotein cholesterol (HDL-C), total
cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C)
levels were evaluated using a serum biochemical autoanalyzer
(Hitachi 7600 Modular Chemistry Analyzer; Hitachi Ltd.) according
to the manufacturer's instructions.
Histological examination
After sacrifice, half of the aortic arch samples
were obtained, fixed in 10% paraformaldehyde, dehydrated by grading
ethanol, paraffin embedded and cut into 4-µm sections. The sections
then underwent antigen retrieval using eBioscience™ IHC Antigen
Retrieval Solution (cat. no. 00-4955-58, Invitrogen; Thermo Fisher
Scientific, Inc.). Tissue sections were stained with hematoxylin
and eosin (H&E) and then stained with hematoxylin and 0.5% Oil
Red O (Sigma-Aldrich; Merck KGaA) for 1 h at 37˚C. The
histopathological and histomorphometric images were evaluated by
three pathologists and captured using Image-Pro Plus software v2.0
(Media Cybernetics).
TUNEL assay
The apoptosis of cells in tissues was analyzed using
terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine
triphosphate (dUTP)-biotin nick-end labeling (TUNEL) staining kit
(Roche). Tissue sections were stained with TUNEL for 2 h at room
temperature and analyzed using a commercial TUNEL staining kit
(Roche) according to the manufacturer's protocol, following which
they were placed in hematoxylin for 2 min at 37˚C. The sections
were then washed for 2 min with PBS, dipped in 95% ethanol,
absolute ethanol Ⅰ-Ⅱ for 3-5 min, xylene Ⅰ-Ⅱ for 3-5 min and sealed
with neutral resins. Tissues sections were captured using a light
microscope (Olympus Corporation) at magnification, x100.
Cell culture
Endothelial cells were purchased from BeNa
Collection Culture (Beijing, China) and cultured in OptiMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS, Sigma-Aldrich; Merck KGaA), 100 U/ml
penicillin (Sigma-Aldrich; Merck KGaA) and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA) at 37˚C in 5% CO2. Cells
were treated with pterostilbene (1.0 mmol/l for 12 h at 37˚C. All
experiments were performed in triplet.
Cell viability assay
Viability of endothelial cells was analyzed using
the Cell Counting Kit-8 (CCK-8; Sigma-Aldrich; Merck KGaA).
Briefly, endothelial cells at 1x105 cells/ml density
were seeded into six-well plates, along with the addition of 0.2%
H2O2 for 4 h and then incubated with
pterostilbene (1.0 mmol/l) for 12 h at 37˚C. A total of 10 µl CCK-8
solution was added into the cells which were then cultured for 30
min at 37˚C. Cell viability was determined at 450 nm absorbance
using a microplate reader (Bio-Rad Laboratories, Inc.).
Gene knockdown
Endothelial cells (1x105/well) were
seeded into a 6-well plate. After 24 h, the cells were transfected
with 40 pmol siRNA-Nrf2 (5'-GAGUAUGAGCUGGAAAAACUU-3'; Shanghai
GenePharma Co., Ltd.) or siRNA-NC (5'-GACGAGCGGCACGUGCACAUU-3',
Shanghai GenePharma Co., Ltd.) using Lipofectamine®
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Cells were used for further
analyses after a 72-h transfection.
Reactive oxygen species (ROS)
activity
Endothelial cells were cultured in a 6-well plate at
a density of 1.0x105/ml. Cells were treated with
pterostilbene (1.0 mmol/l) for 12 h at 37˚C. Then the cells were
rinsed with PBS, and incubated with 10 µM
2',7'-dichlorodihydrofluorescein diacetate (DCFHDA, Molecular
Probes; Thermo Fisher Scientific, Inc.) for 30 min at 37˚C. Images
of the cells were captured using a Zeiss Inverted Microscope
(magnification, x100; Carl Zeiss). Fluorescence activity was
measured using a fluorometric plate reader (BMG Labtech GmbH) at
excitation 530 nm/emission 485 nm.
Western blot analysis
The treated endothelial cells (1x108)
were lysed in RIPA buffer (Bio-Rad Laboratories, Inc.) and the
lysates were centrifuged at 10,000 x g for 10 min at 4˚C. Protein
concentrations were measured using a BCA Protein Assay kit (Pierce;
Thermo Fisher Scientific, Inc.) and a total of 20 µg protein was
electrophoresed using 10% SDS-PAGE followed by transferred to
polyvinylidene fluoride (PVDF) membranes (EMD Millipore). The
membranes were blocked with 5% bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA), and incubated with the following
primary antibodies: SOD (dilution 1:1,000, cat. no. ab13534,
Abcam), CAT (dilution 1:1,000, cat. no. ab16731, Abcam), HO-1
(dilution 1:1,000, cat. no. ab13243, Abcam), Nrf2 (dilution
1:1,000, cat. no. ab62352, Abcam), TLR-4 (dilution 1:1,000, cat.
no. ab13556, Abcam), MyD88 (dilution 1:1,000, cat. no. ab2064,
Abcam), NF-κB (dilution 1:1,000, cat. no. ab131546, Abcam),
phosphorylated NF-κB (pNF-κB, dilution 1:1,000, cat. no. ab220803,
Abcam), IL-1 (dilution 1:2,000, cat. no. ab200478, Abcam), TNFα
(dilution 1:2,000, cat. no. ab6671, Abcam), IL-17 (dilution
1:2,000, cat. no. ab79056, Abcam) and β-actin (dilution 1:2,000,
cat. no. ab8226, Abcam) for 12 h at 4˚C. Protein was then incubated
with horseradish peroxidase-conjugated secondary antibody (dilution
1:5,000, cat. no. ab205718, Abcam) for 2 h at 37˚C. The bands of
proteins were observed with an enhanced chemiluminescence (ECL)
substrate kit (Beyotime Institute of Biotechnology). Quantitative
expression of proteins was quantified using ImageJ software
(v4.6.2; National Institutes of Health, Bethesda).
Flow cytometry
Apoptosis of endothelial cells was analyzed using
flow cytometry. The treated endothelial cells (1x105)
were stained with 5 µl Annexin V-FITC and 10 µl propidium iodide
(PI) solution for 30 min at 4˚C in the dark. The number of
apoptotic cells was examined by FACS (BD Biosciences). Data
acquisition and analysis were evaluated using a BD Flow Cytometer
v1.0 (BD Biosciences) with NovoExpress® software v1.2
(ACEA Biosciences, Inc.).
Statistical analysis
All data are reported as means ± SD. Statistical
analysis was analyzed by the Student t test or one-way ANOVA
followed by Tukey's test using SPSS software, v19.0 (SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of pterostilbene on body
weight, blood pressure and lipid metabolism in the experimental
rats
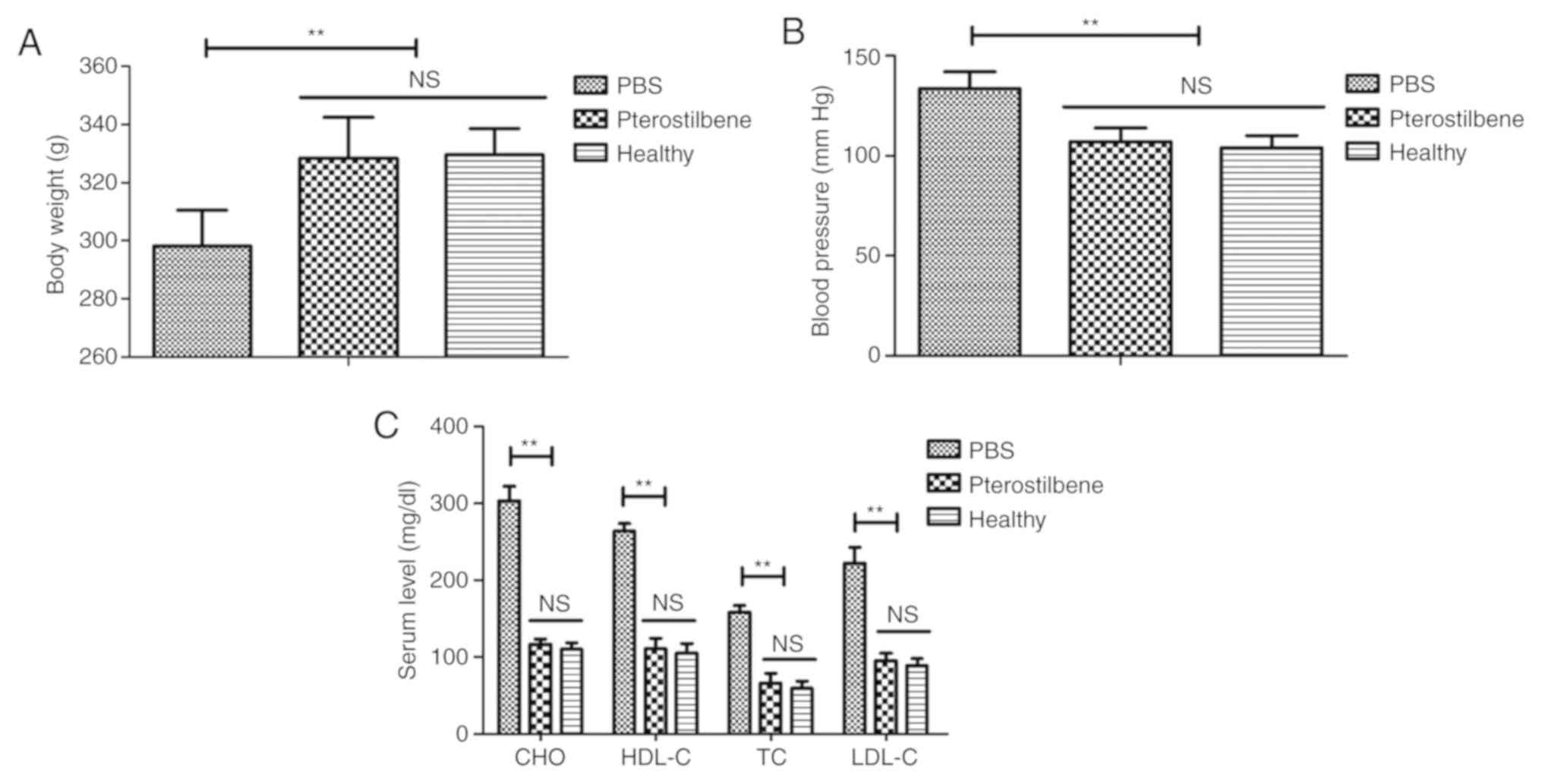
The effects of pterostilbene on body weight, blood
pressure and lipid metabolism were analyzed in the rat model of
atherosclerosis. The results demonstrated that pterostilbene
increased the body weight and reduced blood pressure in the rats
with atherosclerosis when compared to the PBS group (Fig. 1A and B). Pterostilbene treatment decreased CHO,
HDL-C, TC, and LDL-C levels in the plasma of the rats with
atherosclerosis rat compared to these levels in the PBS group
(Fig. 1C).
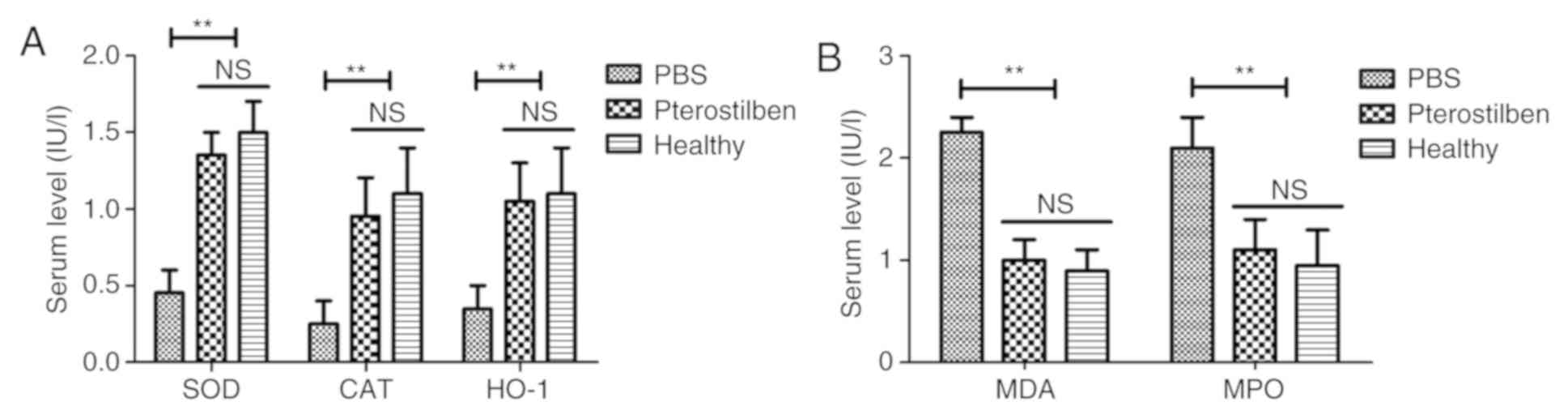
Effect of pterostilbene on oxidative
stress injury in the experimental rats
The antioxidant activity of pterostilbene was
investigated in the rats with atherosclerosis. Serum levels of SOD,
CAT and HO-1 were determined using corresponding kits. The results
revealed that the levels of SOD, CAT and HO-1 were markedly
upregulated in the pterostilbene-treated rats when compared with
the PBS group (Fig. 2A). Conversely,
the MDA and MPO levels in serum were significantly decreased by
pterostilbene treatment when compared to these levels in the PBS
group (Fig. 2B). These results
indicated that pterostilbene can decrease vascular injury-induced
oxidative stress.
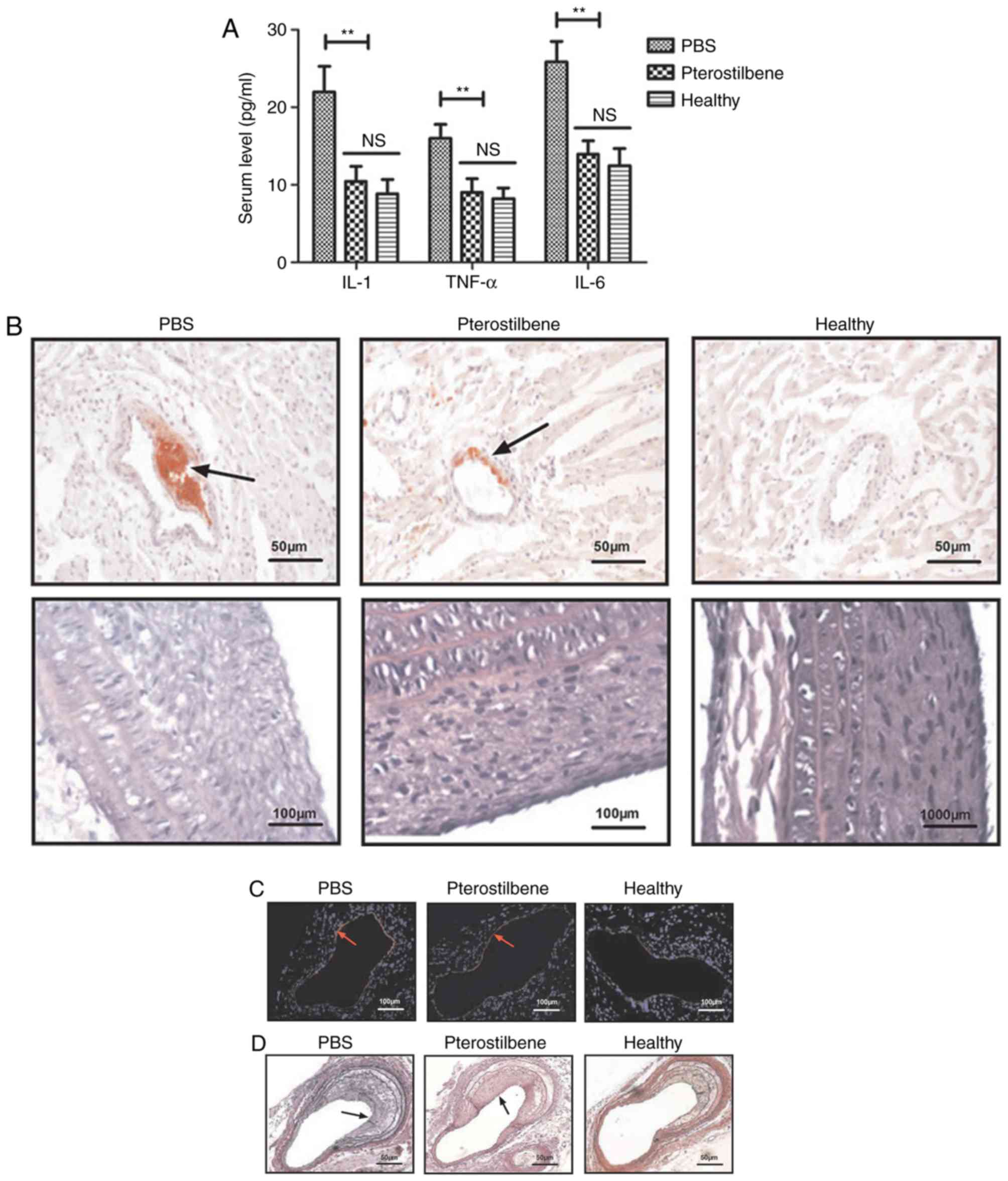
Effect of pterostilbene on
inflammation and pathological features in the experimental
rats
The anti-inflammatory efficacy of pterostilbene was
investigated in the rat model of atherosclerosis. As shown in
Fig. 3A, administration of
pterostilbene decreased serum IL-1, TNF-α and IL-6 levels in the
experimental animals compared to these levels in the PBS group.
Pterostilbene treatment reduced atherogenesis and aortic plaques
(Fig. 3B). Histological analysis
demonstrated that pterostilbene treatment markedly decreased
macrophage infiltration and apoptosis of vascular arterial walls in
the atherosclerosis rat model (Fig.
3C and D).
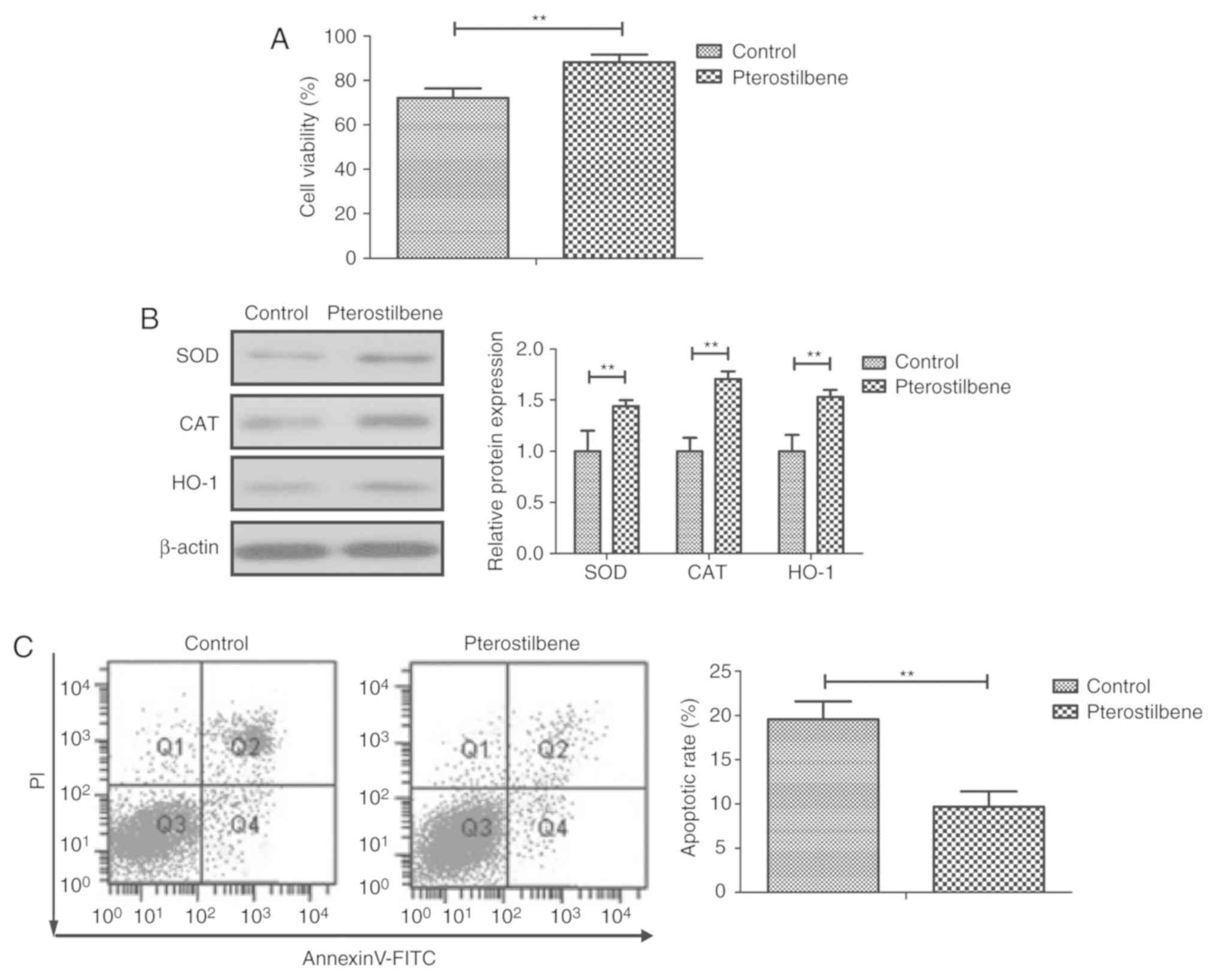
Effects of pterostilbene on cell
viability and apoptosis in endothelial cells
We next investigated the benefits of pterostilbene
in endothelial cells in vitro. The results demonstrated that
pterostilbene increased the viability of endothelial cells when
compared to the control (Fig. 4A).
Administration of pterostilbene significantly attenuated oxidative
stress injury as determined by levels of SOD, CAT and HO-1 and
significantly reduced apoptosis of the endothelial cells as
compared to the control (Fig. 4B and
C). Pterostilbene treatment also
significantly deceased IL-1, TNF-α, and IL-6 expression in the
endothelial cells when compared to the control (Fig. 4D). As illustrated in Fig. 4E, pterostilbene reduced ROS activity
in the endothelial cells compared to the control group.
Pterostilbene inhibits endothelial
cell apoptosis by the Nrf2-mediated TLR-4/MyD88/NF-κB pathway
We finally investigated the possible mechanism
underlying the pterostilbene-mediated inhibition of endothelial
cell apoptosis. Western blot analysis showed that pterostilbene
increased Nrf2 and decreased TLR-4, MyD88, NF-κB expression and
NF-κB phosphorylation in the endothelial cells (Fig. 5A). Nrf2 knockdown (siR-Nrf2)
increased and canceled pterostilbene-mediated regulation of TLR-4,
MyD88, NF-κB expression and NF-κB phosphorylation in the
endothelial cells (Fig. 5B). The
results demonstrated that pterostilbene-mediated inhibition of
endothelial cell apoptosis was abolished via siR-Nrf2 (Fig. 5C).
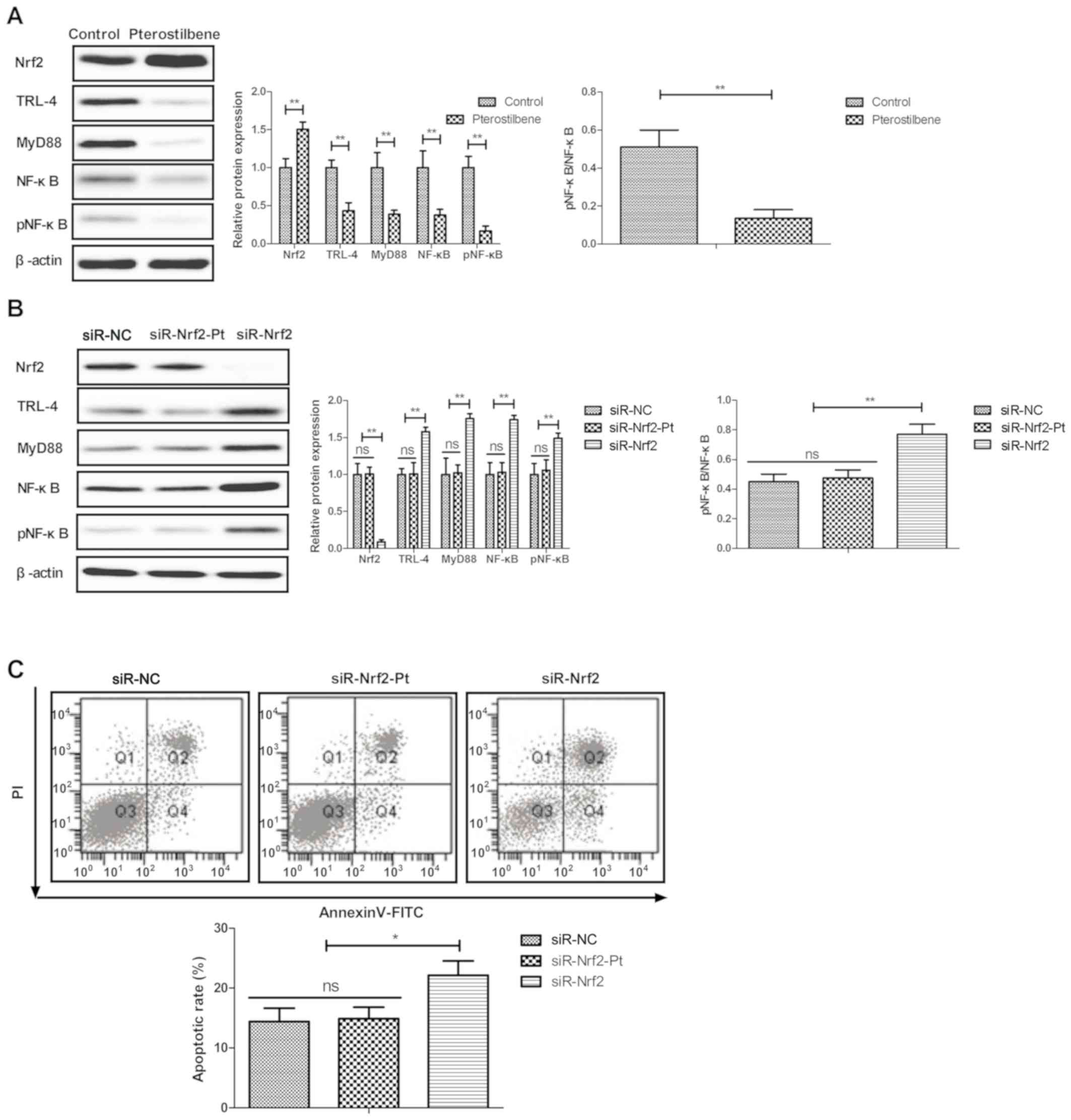 | Figure 5Pterostilbene regulates endothelial
cell apoptosis by the Nrf2-mediated TLR-4/MyD88/NF-κB pathway. (A)
Effect of pterostilbene treatment on Nrf2, TLR-4, MyD88, NF-κB and
pNF-κB expression in endothelial cells. (B) Efficacy of Nrf2
knockdown (siR-Nrf2) on pterostilbene-regulated TLR-4, MyD88, NF-κB
and pNF-κB expression in endothelial cells. (C) Effect of Nrf2
knockdown (siR-Nrf2) on pterostilbene-mediated inhibition of
apoptosis of endothelial cells. *P<0.05,
**P<0.01 vs. the control. NS, not significant. Nrf2,
nuclear factor, erythroid 2 like 2; TLR-4, Toll-like receptor 4;
MyD88, MYD88 innate immune signal transduction adaptor; NF-κB,
nuclear factor κB; pNF-κB, phosphorylated nuclear factor κB. |
Discussion
It is widely recognized that apoptosis of
endothelial cells and inflammatory lesions in artery blood vessels
promote plaque necrosis, which further lead to plaque instability,
thrombosis and atherosclerosis (27). In the present study, we reported the
therapeutic effects of pterostilbene in a rat model of
atherosclerosis. Lipid metabolism, inflammatory cytokines, body
weight, blood pressure and improvement of pathological features
were investigated in atherosclerotic rats after a 4-week
pterostilbene treatment. Treatment with pterostilbene decreased
serum lipid metabolism and attenuated hyperlipidemia and aortic
inflammation in the rats with atherosclerosis, as well as
inhibition of the apoptosis of the endothelial cells, ultimately
improving atherosclerosis. The present study elucidated a novel
mechanism explaining the protective effects of pterostilbene on the
apoptosis of endothelial cells in vascular arterial walls, and
provides insight into the potential mechanisms and strategies for
anti-atherosclerosis treatment. Notably, the findings of the
present study demonstrated that pterostilbene reduced endothelial
cell apoptosis by the Nrf2-mediated TLR-4/MyD88/NF-κB pathway.
Clinically, atherosclerosis is characterized by
dysfunction in lipid homeostasis and endothelial cells in vascular
arterial walls, slow metabolism, impairment of signaling pathways
and increased levels of inflammatory cytokines (28-30).
The anti-adipogenesis mechanism of pterostilbene has been
identified to activate heme oxygenase-1 in 3T3-L1 cells (31). Here, we used a rat model of
atherosclerosis to specify the regulatory effects of pterostilbene
on lipid homeostasis and found that pterostilbene decreased
high-density lipoprotein cholesterol (HDL-C), total cholesterol
(TC) and low-density lipoprotein cholesterol (LDL-C) levels in the
plasma of the atherosclerotic rats, which further led to
improvement in body weight and blood pressure. A previous study
found that pterostilbene decreased cardiac oxidative stress and
inhibited high fat-induced atherosclerosis inflammation (32). We found that pterostilbene attenuated
inflammatory cytokines as evidenced by decreased levels of
interleukin (IL)-1, tumor necrosis factor (TNF)-α and IL-6, which
further decreased endothelial cell apoptosis and oxidative stress
injury. However, the potential molecular mechanisms underlying the
anti-atherogenic effects of pterostilbene require further
elucidation in subsequent research.
Pterostilbene was found to increase protein
expression of Nrf2 in cardiac tissues, which may account for the
prevention of cardiac oxidative stress and inflammation in
fructose-fed rats (32). Chen et
al (33) showed that
pterostilbene protects against uremia serum-induced endothelial
cell damage via activation of the Keap1/Nrf2/HO-1 signaling
pathway. The present study found that treatment with pterostilbene
increased Nrf2, while Nrf2 knockdown canceled the
pterostilbene-mediated inhibition of endothelial cell apoptosis.
The TNF-α mediated-NF-κB signaling pathway is involved in
endothelial cell apoptosis and the progression of atherosclerosis
(34). Hosseini et al
demonstrated that suppression of the TLR-4-MyD88 signaling pathway
is essential for atheroprotection by reducing lesion inflammatory
cytokines TNF-α, IL-1β, and IL-18(35). Our data showed that therapeutic
strategy of pterostilbene protected animals against atherosclerosis
via the Nrf2-mediated TLR-4/MyD88/NF-κB pathway. Moreover,
pterostilbene treatment significantly increased the antioxidant
molecules including catalase (CAT), heme oxygenase1 (HMOX1),
glutathione peroxidase (GPX), and superoxide dismutase (SOD), which
might be a possible anti-apoptotic mechanism of pterostilbene in
endothelial cells. A previous study demonstrated that activating
Nrf2-mediated antioxidant exhibited protective effect against
diabetic live injury via downregulation of the TLR-4/MyD88/NF-κB
pathway (36). Enhancing the Nrf2
pathway was found to protect against LPS-induced sepsis via the
TLR4/MYD88/IκB pathway (37). These
findings are consistent with the fact that TLR-4/MyD88/NF-κB
expression is downregulated by Nrf2 in pterostilbene-treated
endothelial cells that respond to anti-inflammatory and
anti-apoptotic efficacy. However, this study analyzed the total
Nrf2 expression, but did not determine the nuclear and cytoplasmic
fractions in the endothelial cells. Thus, further investigation
needs to differentiate the nuclear and cytoplasmic expression of
Nrf2 in endothelial cells after treatment with pterostilbene in
future research.
In conclusion, data in the present study
demonstrated that pterostilbene exhibited multifaceted effects on
lipid metabolism, reduced inflammation, and decreased endothelial
cell apoptosis in a rat model of atherosclerosis. Administration of
pterostilbene activated the Nrf2 signaling pathway in endothelial
cells by altering downstream expression of the TLR-4/MyD88/NF-κB
pathway, which is involved in antioxidant mechanisms and apoptosis.
In addition, association between pterostilbene and the pathological
features of atherosclerosis was discovered in the present study,
which broadens the clinical implications of pterostilbene for the
treatment of cardiovascular disease. However, additional molecular
mechanisms mediated by pterostilbene warrant further investigate
for targeting atheromatous plaque.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XWX and WHL performed the experiments. KHZ analyzed
data. WMZ designed the study and wrote this manuscript. All authors
read and approved the final version of this manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Third Affiliated Hospital of Nanchang University (approval no.
ZKY2019004; Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yahagi K, Kolodgie FD, Lutter C, Mori H,
Romero ME, Finn AV and Virmani R: Pathology of human coronary and
carotid artery atherosclerosis and vascular calcification in
diabetes mellitus. Arterioscler Thromb Vasc Biol. 37:191–204.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Otsuka F, Yasuda S, Noguchi T and
Ishibashi-Ueda H: Pathology of coronary atherosclerosis and
thrombosis. Cardiovasc Diagn Ther. 6:396–408. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Watson SR and Lessner SM: (Second)
harmonic disharmony: Nonlinear microscopy shines new light on the
pathology of atherosclerosis. Microsc Microanal. 22:589–598.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yamada S, Senokuchi T, Matsumura T, Morita
Y, Ishii N, Fukuda K, Murakami-Nishida S, Nishida S, Kawasaki S,
Motoshima H, et al: Inhibition of local macrophage growth
ameliorates focal inflammation and suppresses atherosclerosis.
Arterioscler Thromb Vasc Biol. 38:994–1006. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martin-Lorenzo M, Balluff B, Maroto AS,
Carreira RJ, van Zeijl RJ, Gonzalez-Calero L, de la Cuesta F,
Barderas MG, Lopez-Almodovar LF, Padial LR, et al: Molecular
anatomy of ascending aorta in atherosclerosis by MS Imaging:
Specific lipid and protein patterns reflect pathology. J
Proteomics. 126:245–251. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Otsuka F, Kramer MC, Woudstra P, Yahagi K,
Ladich E, Finn AV, de Winter RJ, Kolodgie FD, Wight TN, Davis HR,
et al: Natural progression of atherosclerosis from pathologic
intimal thickening to late fibroatheroma in human coronary
arteries: A pathology study. Atherosclerosis. 241:772–782.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Turan TN, Rumboldt Z, Granholm AC, Columbo
L, Welsh CT, Lopes-Virella MF, Spampinato MV and Brown TR:
Intracranial atherosclerosis: Correlation between in-vivo 3T high
resolution MRI and pathology. Atherosclerosis. 237:460–463.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ding L, Hong Y and Peng B: Association
between large artery atherosclerosis and cerebral microbleeds: A
systematic review and meta-analysis. Stroke Vasc Neurol. 2:7–14.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Henrot P, Foret J, Barnetche T, Lazaro E,
Duffau P, Seneschal J, Schaeverbeke T, Truchetet ME and Richez C:
Assessment of subclinical atherosclerosis in systemic lupus
erythematosus: A systematic review and meta-analysis. Joint Bone
Spine. 85:155–163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hsu E and Parthasarathy S:
Anti-inflammatory and antioxidant effects of sesame oil on
atherosclerosis: A descriptive literature review. Cureus.
9(e1438)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nirwane A and Majumdar A: Resveratrol and
pterostilbene attenuated smokeless tobacco induced cardiovascular
aberrations in estrogen deficient female rats. Toxicol Res (Camb).
5:1604–1618. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Y: Pterostilbene, a novel natural
plant conduct, inhibits high fat-induced atherosclerosis
inflammation via NF-κB signaling pathway in Toll-like receptor 5
(TLR5) deficient mice. Biomed Pharmacother. 81:345–355.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gao D, Jing S, Zhang Q and Wu G:
Pterostilbene protects against acute renal ischemia reperfusion
injury and inhibits oxidative stress, inducible nitric oxide
synthase expression and inflammation in rats via the Toll-like
receptor 4/nuclear factor-κB signaling pathway. Exp Ther Med.
15:1029–1035. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li M, Qian M, Kyler K and Xu J:
Endothelial-vascular smooth muscle cells interactions in
atherosclerosis. Front Cardiovasc Med. 5(151)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Verma I, Syngle A, Krishan P and Garg N:
Endothelial progenitor cells as a marker of endothelial dysfunction
and atherosclerosis in ankylosing spondylitis: A cross-sectional
study. Int J Angiol. 26:36–42. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Profumo E, Buttari B, D'Arcangelo D,
Tinaburri L, Dettori MA, Fabbri D, Delogu G and Riganò R: The
nutraceutical dehydrozingerone and its dimer counteract
inflammation- and oxidative stress-induced dysfunction of in vitro
cultured human endothelial cells: A novel perspective for the
prevention and therapy of atherosclerosis. Oxid Med Cell Longev.
2016(1246485)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang T, Hu Q, Shi L, Qin L, Zhang Q and
Mi M: Equol attenuates atherosclerosis in apolipoprotein
E-deficient mice by inhibiting endoplasmic reticulum stress via
activation of Nrf2 in endothelial cells. PLoS One.
11(e0167020)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu H, Zhang G, Huang J, Ma S, Mi K, Cheng
J, Zhu Y, Zha X and Huang W: Atractylenolide I modulates ovarian
cancer cell-mediated immunosuppression by blocking MD-2/TLR4
complex-mediated MyD88/NF-κB signaling in vitro. J Transl Med.
14(104)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vijayan V, Khandelwal M, Manglani K, Gupta
S and Surolia A: Methionine down-regulates TLR4/MyD88/NF-κB
signalling in osteoclast precursors to reduce bone loss during
osteoporosis. Br J Pharmacol. 171:107–121. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ding F, Li F, Li Y, Hou X, Ma Y, Zhang N,
Ma J, Zhang R, Lang B, Wang H and Wang Y: HSP60 mediates the
neuroprotective effects of curcumin by suppressing microglial
activation. Exp Ther Med. 12:823–828. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qi M, Zheng L, Qi Y, Han X, Xu Y, Xu L,
Yin L, Wang C, Zhao Y, Sun H, et al: Dioscin attenuates renal
ischemia/reperfusion injury by inhibiting the TLR4/MyD88 signaling
pathway via up-regulation of HSP70. Pharmacol Res. 100:341–352.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu M, Xu Y, Han X, Yin L, Xu L, Qi Y,
Zhao Y, Liu K and Peng J: Dioscin alleviates alcoholic liver
fibrosis by attenuating hepatic stellate cell activation via the
TLR4/MyD88/NF-κB signaling pathway. Sci Rep.
5(18038)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yao H, Hu C, Yin L, Tao X, Xu L, Qi Y, Han
X, Xu Y, Zhao Y, Wang C and Peng J: Dioscin reduces
lipopolysaccharide-induced inflammatory liver injury via regulating
TLR4/MyD88 signal pathway. Int Immunopharmacol. 36:132–141.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qi M, Yin L, Xu L, Tao X, Qi Y, Han X,
Wang C, Xu Y, Sun H, Liu K and Peng J: Dioscin alleviates
lipopolysaccharide-induced inflammatory kidney injury via the
microRNA let-7i/TLR4/MyD88 signaling pathway. Pharmacol Res.
111:509–522. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sandberg K and Umans JG: Recommendations
concerning the new U.S. National Institutes of Health initiative to
balance the sex of cells and animals in preclinical research. FASEB
J. 29:1646–1652. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gao M, Xin G, Qiu X, Wang Y and Liu G:
Establishment of a rat model with diet-induced coronary
atherosclerosis. J Biomed Res. 31:47–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wei DH, Jia XY, Liu YH, Guo FX, Tang ZH,
Li XH, Wang Z, Liu LS, Wang GX, Jian ZS and Ruan CG: Cathepsin L
stimulates autophagy and inhibits apoptosis of ox-LDL-induced
endothelial cells: Potential role in atherosclerosis. Int J Mol
Med. 31:400–406. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Parolin M, Dassie F, Martini C, Mioni R,
Russo L, Fallo F, Rossato M, Vettor R, Maffei P and Pagano C:
Preclinical markers of atherosclerosis in acromegaly: A systematic
review and meta-analysis. Pituitary. 21:653–662. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Song P, Xia W, Zhu Y, Wang M, Chang X, Jin
S, Wang J and An L: Prevalence of carotid atherosclerosis and
carotid plaque in Chinese adults: A systematic review and
meta-regression analysis. Atherosclerosis. 276:67–73.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Stachyra K, Kiepura A and Olszanecki R:
Air pollution and atherosclerosis-a brief review of mechanistic
links between atherogenesis and biological actions of inorganic
part of particulate matter. Folia Med Cracov. 57:37–46.
2017.PubMed/NCBI
|
|
31
|
Seo YJ, Kim KJ, Koh EJ, Choi J and Lee BY:
Anti-adipogenesis mechanism of pterostilbene through the activation
of heme oxygenase-1 in 3T3-L1 cells. Phytomedicine. 33:7–13.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kosuru R, Kandula V, Rai U, Prakash S, Xia
Z and Singh S: Pterostilbene decreases cardiac oxidative stress and
inflammation via activation of AMPK/Nrf2/HO-1 pathway in
fructose-fed diabetic rats. Cardiovasc Drugs Ther. 32:147–163.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen ZW, Miu HF, Wang HP, Wu ZN, Wang WJ,
Ling YJ, Xu XH, Sun HJ and Jiang X: Pterostilbene protects against
uraemia serum-induced endothelial cell damage via activation of
Keap1/Nrf2/HO-1 signaling. Int Urol Nephrol. 50:559–570.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y,
Zhu J, Ma L, Guo J, Shi H, et al: Exosomes derived from mature
dendritic cells increase endothelial inflammation and
atherosclerosis via membrane TNF-α mediated NF-κB pathway. J Cell
Mol Med. 20:2318–2327. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hosseini H, Li Y, Kanellakis P, Tay C, Cao
A, Liu E, Peter K, Tipping P, Toh BH, Bobik A and Kyaw T: Toll-like
receptor (TLR)4 and MyD88 are essential for atheroprotection by
peritoneal B1a B cells. J Am Heart Assoc. 5(e002947)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yin J, Wang H and Lu G: Umbelliferone
alleviates hepatic injury in diabetic Db/Db mice via inhibiting
inflammatory response and activating Nrf2-mediated antioxidant.
Biosci Rep. 38(BSR20180444)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kong X, Thimmulappa R, Craciun F, Harvey
C, Singh A, Kombairaju P, Reddy SP, Remick D and Biswal S:
Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes
protects against sepsis. Am J Respir Crit Care Med. 184:928–938.
2011.PubMed/NCBI View Article : Google Scholar
|