Introduction
A total of 90% of all cases of oral cancer are
classified as squamous cell carcinoma (SCC), with SCC of the tongue
comprising 50-60% cases worldwide, making it the most common type
of oral cancer (1,2). Tongue cancer can metastasize from the
primary site to cervical regional lymph nodes in the early stage
(3), resulting in a 5-year survival
rate of <50% (4,5). Therefore, the early diagnosis and
treatment of cervical lymph node metastasis in patients with SCC of
the tongue is crucial in order to improve patient survival
rates.
The treatment of oral cancer typically includes
surgery, post-operative adjuvant radiotherapy and chemotherapy
(6). Pre-operative ultrasound is a
non-invasive method used to examine lymph nodes; however,
ultrasound cannot distinguish lymphadenitis from metastatic lymph
nodes (7). Moreover, the current
imaging strategies often result in a missed diagnosis or
misdiagnosis of cancer, especially in patients with early
metastatic lymphoma (8,9). Establishing reliable diagnostic
procedures and standards for the primary foci of tongue cancer and
the detection of metastatic SCC in lymph nodes has important
potential diagnostic and prognostic value for patients with oral
cancer.
Multimodal imaging has become a frequently used
technique in molecular imaging as it can be used to obtain more
accurate images and improve the detection rate of diseases,
including thyroid cancer (10) and
liver fibrosis (11). There has been
an increased interest worldwide in nano-composite materials with
multimodal imaging functions (12-14).
Poly(lactic-co-glycolic acid)(PLGA) is commonly used in the
preparation of ultrasound contrast agents. PLGA is a biodegradable
organic polymer compound with hydrophobic properties, high
spheronization, film formation rates and good biocompatibility
(15,16). As a Food and Drug
Administration-approved material, PLGA has been widely used in
protein research and clinical applications for the delivery of
small molecule drugs and other large molecules (17).
Liquid fluorocarbon perfluorohexane (PFH) is an
aliphatic fluorine-containing compound with a low boiling point
(56˚C) (6). PFH displays good
biocompatibility and is eliminated directly via respiration without
degradation in vivo (18).
Furthermore, the diameter of a nanoparticle contrast agent coated
with liquid fluorocarbon can be increased following liquid-gas
phase change, which results in increased acoustic impedance
followed by increased enhanced ultrasonic signal development
(19).
Superparamagnetic iron oxide (SPIO) nanoparticles
can be coated with a variety of biological macromolecules due to
their small particle size and strong surface modifiability
(20). Additionally, SPIO
nanoparticles display good biological safety and a long circulation
half-life (21). SPIOs integrate
magnetic resonance (MR), ultrasonic and photoacoustic (PA)
multimodal imaging, providing novel ideas and strategies for
disease diagnosis and treatment (22-26).
Norton et al (27)
demonstrated that magnetic SPIO particles vibrate under the action
of acoustic waves, which alters the acoustic impedance of tissues
and enhances backscatter signals and ultrasound imaging. A previous
study reported that PA imaging technology based on the PA effect
has received increasing interest (28). PA imaging technology is a safe and
nondestructive imaging technology that uses a pulsed laser as the
excitation source to irradiate the medium with a light absorber to
generate a PA effect. Moreover, the imaging method reconstructs and
calculates the collected acoustic signals via a signal processing
system to obtain information regarding the internal structure of
tissues (29).
PA imaging technology combines the advantages of
pure optical and acoustic imaging of tissues, thus obtaining tissue
images with high contrast and spatial resolution (30). As a light absorber, SPIO can absorb
laser light at specific wavelengths (31). Laser irradiation of SPIO-coated
particles causes the temperature of the surrounding medium to rise,
causing the medium to expand, generating PA signals (32).
The specific receptor for stromal cell-derived
factor (SDF)1 is CXC chemokine receptor 4 (CXCR4), which is
expressed by fibroblasts, endothelial cells, some inflammatory
cells (natural killer cells, neutrophils, plasma cells and B and T
lymphocytes) and various cancer cells (33). Delilbasi et al (34) demonstrated that all tongue SCC cells
expressed CXCR4 whereas normal squamous epithelium had no or little
expression of CXCR4 and that metastatic SCC cells from the lymph
nodes displayed stronger expression compared with lymphocytes. At
present, CXCR4 is the only known receptor of SDF-1(6).
The CXCR4-C-X-C motif chemokine (CXCL)12 axis serves
an important role in the invasion and metastasis of SCC and has
been indicated to contribute to tumor development. Due to this
(35), it is often used as a
potential target in SCC treatment (36).
The aim of the present study was to prepare
SDF-1-modified nanoparticle contrast agents coated with iron
(II,III) oxide (Fe3O4), the main component of
SPIO (21) and PFH for targeted
multi-modal imaging and to examine their properties in
vitro. The present study provided a potential foundation for
the development of procedures and materials for the diagnosis of
primary tongue cancer and lymph node metastasis.
Materials and methods
Materials
Fe3O4 (cat. no. SOR-10-50)
modified by oleic acid was obtained from Ocean NanoTech, LLC.
Maleimide-polyethylene glycol-poly(lactide
co-glycolide)(MAL-PEG-PLGA; 75:25; 12,000 Da MW) was purchased from
Jinan Daigang Biomaterial Co., Ltd. Polyvinyl alcohol (PVA; 25,000
Da) and PFH were obtained from Sigma-Aldrich, Merck KGaA. Methylene
chloride (CH2Cl2) and isopropyl alcohol were
purchased from Chongqing East Sichuan Chemical Co., Ltd. SDF-1
modified with FITC was obtained from Shanghai Qiangyao
Biotechnology Co., Ltd. The human tongue SCC cell line (SCC-15) was
obtained from the Chongqing Key Laboratory of Oral Diseases and
Biomedical Sciences.
Preparation of
SDF-1/Fe3O4/PLGA/PFH nanoparticles
A double emulsification method was used to prepare
PLGA shells loaded with Fe3O4 and PFH. A
total of 100 mg PLGA and 5 mg Fe3O4 were
added into 2 ml CH2Cl2 and the mixture was
placed in an ice bath with an ultrasound probe at 125 W for 60 sec
until it was fully dissolved. Subsequently, 100 µl PFH and 5 ml of
50 g/l PVA were added to the emulsion, followed by ultrasound
treatment for 5 min (on: 5 sec; off: 5 sec) in an ice bath to
obtain the first emulsion. A total of 10 ml isopropyl alcohol (20
ml/l) was then poured into the first emulsion to solidify the
surface of the nanoparticles. A total of 2 magnetic beads (Beyotime
Institute of Biotechnology,) were placed into the mixture and the
liquid was stirred on a magnetic stirrer for 3 h in an ice bath to
extract CH2Cl2. The sample was then
centrifuged with pure water in a low-temperature (4˚C) high-speed
centrifuge (5 min; 9,391 x g) and the supernatant was discarded.
The precipitate was washed with pure water and the process was
repeated in triplicate. A total of 200 µl SDF-1 (terminal
sulfhydrylation modification and FITC labeling) was added into the
final emulsion and stirred for 4 h at 4˚C. SDF-1 was conjugated to
nanoparticles via thioether bonds. Particles were then centrifuged
at 9,391 x g for 5 min at 4˚C and washed with pure water. The
process was repeated twice to obtain
SDF-1/Fe3O4/PLGA/PFH nanoparticles, which
were stored in the dark at 4˚C until further analysis.
The blank PLGA nanoparticles,
Fe3O4/PLGA/PFH nanoparticles and
SDF-1/PLGA/PFH nanoparticles were all prepared according to the
aforementioned method.
Determination of the basic properties
of the SDF-1/Fe3O4/PLGA/PFH
nanoparticles
Samples were gently stirred with a pipette until
they were fully dispersed and assessed for surface morphology,
distribution and size using a CKX41 light microscope
(magnification, x100); Olympus Corporation) and a JEOL JSM-7800F
scanning electron microscope (magnification, x2,000; Hitachi,
Ltd.). A drop of the nanoparticles (50 ug/ml) was placed on a
silicon panel (5x5 mm) until it dried naturally in the dark
overnight at room temperature. The silicon panel then analyzed via
scanning electron microscopy. A TCS-SP5 laser confocal microscopy
(magnification, x400; Leica Microsystems GmbH) was used to observe
SDF-1 conjugation. A laser particle size meter (Zetasizer 3000HS;
Malvern Instruments, Inc.) was used to detect particle size and
Zeta potential. Determination of the iron content in the samples
(3) was performed via graphite
furnace atomic absorption spectrometry. The wavelength used for
detection was 248.3 nm. The absorption spectrum of the
nanoparticles was detected using an ultraviolet-visible (UV-Vis)
spectrophotometer (CARY 50; Varian Medical Systems) and blank PLGA
nanoparticles were used as the control group.
In vitro imaging experiments
The absorption peaks of nanoparticles were measured
by UV-Vis spectrophotometry, which was performed in previous
experiments (21). The absorption
peaks provided the basis for selecting the excitation laser
wavelength for PA imaging experiments. The
SDF-1/Fe3O4/PLGA/PFH,
Fe3O4/PLGA/PFH, SDF-1/PLGA/PFH and blank PLGA
nanoparticles were divided into 4 groups according to PLGA
concentration (20, 15, 10 and 5 mg/ml). A total of 200 µl of
mixture from each group was placed in 3% agarose gel phantoms and
the PA images and signals values of the groups were collected at
the optimal wavelength (680 nm) using a VEVO LAZR PA imaging system
(Vevo® LAZR; FUJIFILM VisualSonics, Inc.).
Ultrasonic imaging experiment
Ultrasound images of
SDF-1/Fe3O4/PLGA/PFH nanoparticles were
recorded according to 4 different PLGA concentrations (100, 50, 25
and 12.5 mg/ml) at a mechanical index (MI) of 0.6 and under
different MIs (0.2-0.6) at the PLGA concentration of 12.5 mg/ml
using an ultrasound apparatus (Philips iU22; Philips Healthcare). A
total of 1 ml SDF-1/Fe3O4/PLGA/PFH
nanoparticles were injected into a transparent rubber tube and
placed into a water bath with an initial temperature of 37˚C for 6
min to obtain the images. During the process, the temperature was
slowly raised (to 65˚C) and monitored in real time. Using the
B-mode and contrast mode of the ultrasound apparatus, images of
SDF-1/Fe3O4/PLGA/PFH nanoparticles were
obtained at the temperature of 56˚C when the two-dimensional
grayscale ultrasound imaging and contrast-enhanced ultrasound
imaging were strongest. The blank PLGA nanoparticles were treated
using the same method. A DFY ultrasonic image quantitative analysis
diagnostic instrument (Sonomath; Chongqing Ambition Science &
Technologies Co., Ltd.) measured the echo intensity (EI) value of
the samples. Additionally, alterations to
SDF-1/Fe3O4/PLGA/PFH nanoparticle volume in
response to increasing temperature were observed and recorded via
an IX71 light microscope (magnification, x40; Olympus
Corporation).
Cell culture
SCC-15 cells were cultured in DMEM/Nutrient Mixture
F-12 (DMEM-12; Gibco; Thermo Fisher Scientific, Inc.) containing
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin
or streptomycin at 37˚C with 5% CO2. The culture medium
was changed every 2 days. At 80% confluence, cells were
sub-cultured as follows: The culture medium was removed, cells were
washed with PBS 3 times, 1 ml 0.25% trypsin was added and cells
were returned to the incubator for 5 min. A total 3 ml DMEM-12
culture medium was added to the cells for neutralization. The
mixture was pipetted up and down to form a well-mixed suspension
and centrifuged for 3 min at 200 x g and 4˚C. The supernatant was
removed and cells were resuspended in DMEM-12 medium.
Detection of CXCR4 localization and
expression of SCC-15 cells
SCC-15 cells in the logarithmic phase were digested
with 1 ml 0.25% trypsin for 5 min and then centrifuged at 200 x g
for 3 min at 4˚C. Subsequently, cells were inoculated
(5x104 cells/well) into 6-well plates with coverslips.
Cells were cultured in DMEM-12 medium and incubated overnight with
5% CO2 at 37˚C. Cells were washed twice with PBS, fixed
with 4% paraformaldehyde for 15 min at room temperature, washed
with PBS 3 times and blocked with goat serum blocking solution (1%
BSA; Beyotime Institute of Biotechnology) at 37˚C for 30 min to
block non-specific antibody binding. Subsequently, rabbit
anti-human CXCR4 primary antibody (1:200) was added to the cover
glass, which was incubated at 4˚C overnight. After washing 3 times
with PBS, FITC-labelled goat anti-rabbit IgG secondary antibody
(1:100; A0562; Beyotime Institute of Biotechnology) was added to
the cells and incubated at 37˚C for 50 min in the dark. After
rinsing 3 times with PBS, cells were stained with DAPI for 5 min at
room temperature, washed 3 times with PBS and stored at -20˚C until
observation. Cells were observed under a TCS-SP5 laser confocal
microscopy (magnification, x200; Leica Microsystems GmbH).
Targeted experiment
SCC-15 cells in the logarithmic phase were
inoculated (1x105 cells/dish) into a 15 mm laser
confocal dish. Cells were divided into 2 groups: i)
SDF-1/Fe3O4/PLGA/PFH nanoparticle group; and
ii) the blank control group. A total of 2 ml DMEM-12 medium was
added to each dish. Following incubation for 12 h, the medium in
the dish was changed. A total of 2 ml nanoparticles prepared in
DMEM-12 (200 µg/ml) was added to the experimental group and 2 ml
DMEM/F-12 medium was added to the blank control group. Following
incubation for 2 h at 37˚C, cells were washed once with PBS and
nanoparticle targeting was observed using a fully automatic
fluorescence microscope (magnification, x200; Axio Imager.Z2; Carl
Zeiss AG).
Flow cytometry test
SCC-15 cells in the logarithmic phase were
inoculated (1x106 cells/well) into 6-well plates. Cells
were divided into 2 groups: i)
SDF-1/Fe3O4/PLGA/PFH nanoparticle group; and
ii) the blank control group. A total of 2 ml DMEM-12 medium was
added to each dish. Following incubation for 12 h at 37˚C, the
medium in the dish was changed. A total of 2 ml nanoparticles
prepared in DMEM/F-12 medium (200 µg/ml) was added to the
experimental group and 2 ml DMEM/F-12 medium was added to the blank
control group. Following incubation for 2 h at 37˚C, cells were
washed 3 times with PBS, digested with 0.25% pancreatin for 5 min,
centrifuged 3 times at 200 x g for 3 min at 4˚C and the obtained
precipitate was resuspended in PBS for flow cytometry (BD INFLUX;
Becton, Dickinson and Company). Nanoparticles with SDF-1 were
selected as the analyte detector and FITC was used as the analyte
reporter. FlowJo software (version 7.6.5; Becton, Dickinson and
Company) was used for the analysis of flow cytometer data.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 22.0; IBM Corp.). Data are presented as the mean
± standard deviation. Experiments were performed in triplicate.
One-way ANOVA followed by Tukey's post hoc test was used to
determine significant differences between multiple groups. The
Student's t-test was used to analyze differences between two groups
The difference between PA signal values of
SDF-1/Fe3O4/PLGA/PFH and
Fe3O4/PLGA/PFH nanoparticles was analyzed
with unpaired Student's t-test. Difference in EI values of
SDF-1/Fe3O4/PLGA/PFH nanoparticles before and
after the water bath was analyzed using paired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Basic properties of
SDF-1/Fe3O4/PLGA/PFH nanoparticles
The nanoparticle solution was dark brown in
appearance (data not shown). Under a light or scanning electron
microscope, the prepared SDF-1/Fe3O4/PLGA/PFH
nanoparticles were well dispersed when diluted with pure water,
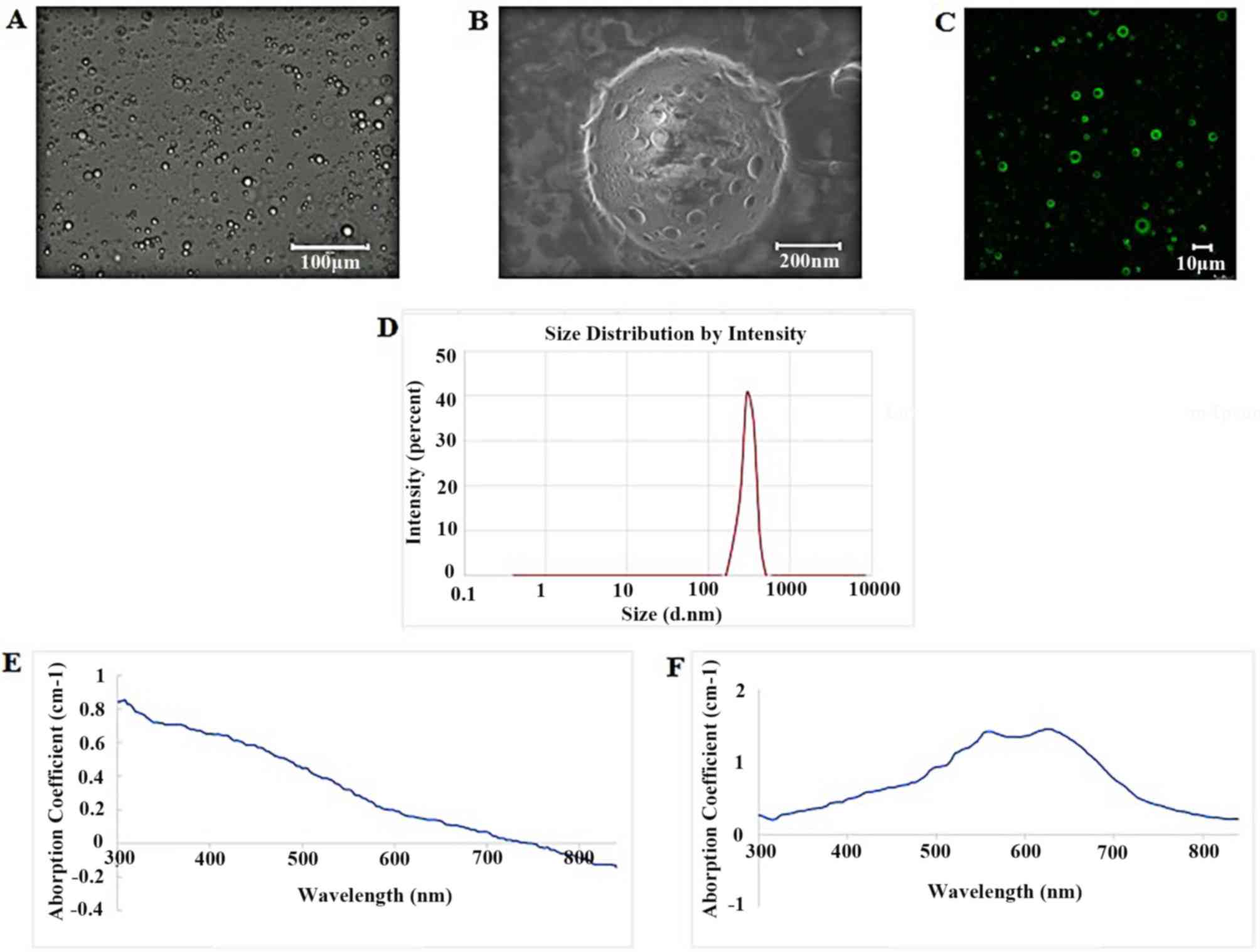
spherical in shape and uniform in size (Fig. 1A and B). Scanning electron microscopy
demonstrated that the surfaces of the nanoparticles were incomplete
and smooth with numerous pores (Fig.
1B). Laser confocal microscopy indicated that SDF-1 was
attached to the PLGA shell of nanoparticles alongside bright green
fluorescence on the surface (Fig.
1C). The average size of the nanoparticles, as measured using a
Malvern particle size meter, was 586.5±124.7 nm (Fig. 1D) and the average zeta potential
was-21.5±2.17 mV. Atomic absorption spectrometry measurements
indicated that there was 35.74 µg/ml of iron in the sample (data
not shown). UV-Vis spectrophotometry detected 2 slight absorption
peaks near 556 and 623 nm for the
SDF-1/Fe3O4/PLGA/PFH nanoparticles (Fig. 1F), whereas the control group
displayed no obvious absorption peaks in detection wavelength bands
(Fig. 1E).
PA imaging experiment
UV-V is spectrophotometry was used to detect
absorption peaks for the SDF-1/Fe3O4/PLGA/PFH
nanoparticles at 556 and 623 nm in the wavelength range of 300-840
nm. However, the excitation laser wavelength range of the PA
instrument used was 680-960 nm and the absorption peak of the
nanoparticles was not within this range. According to its
absorption spectrum, 680 nm was selected as the optimal excitation
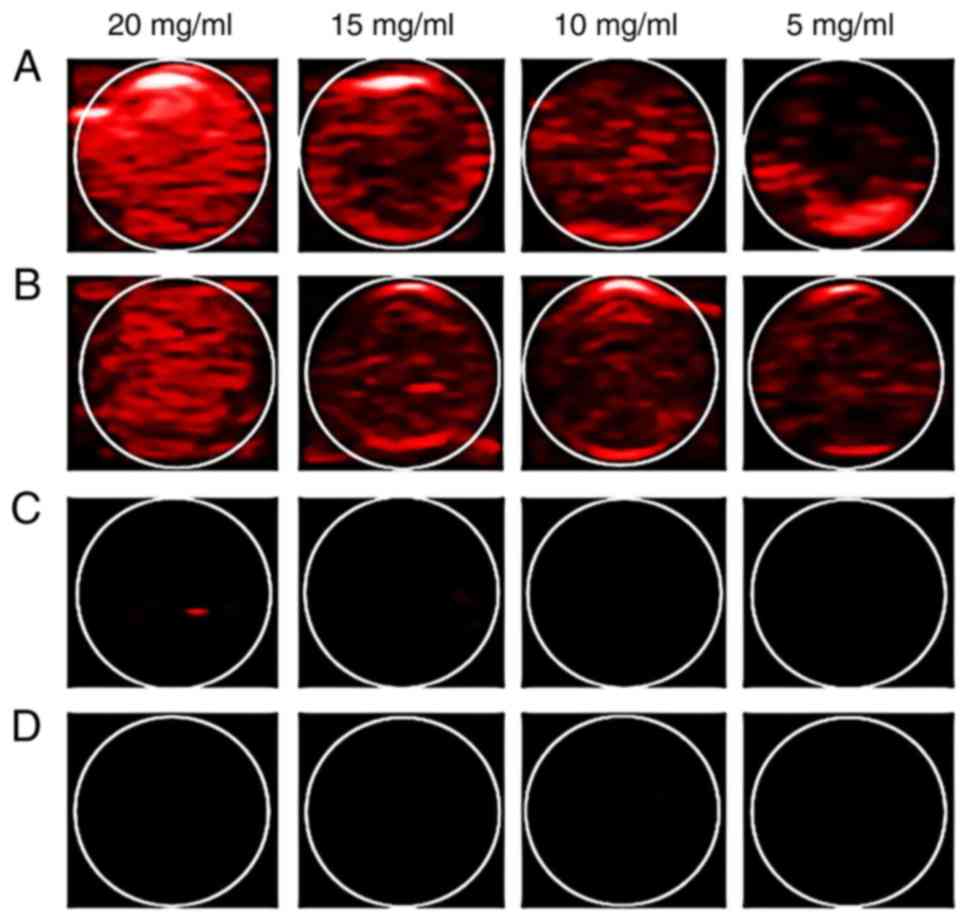
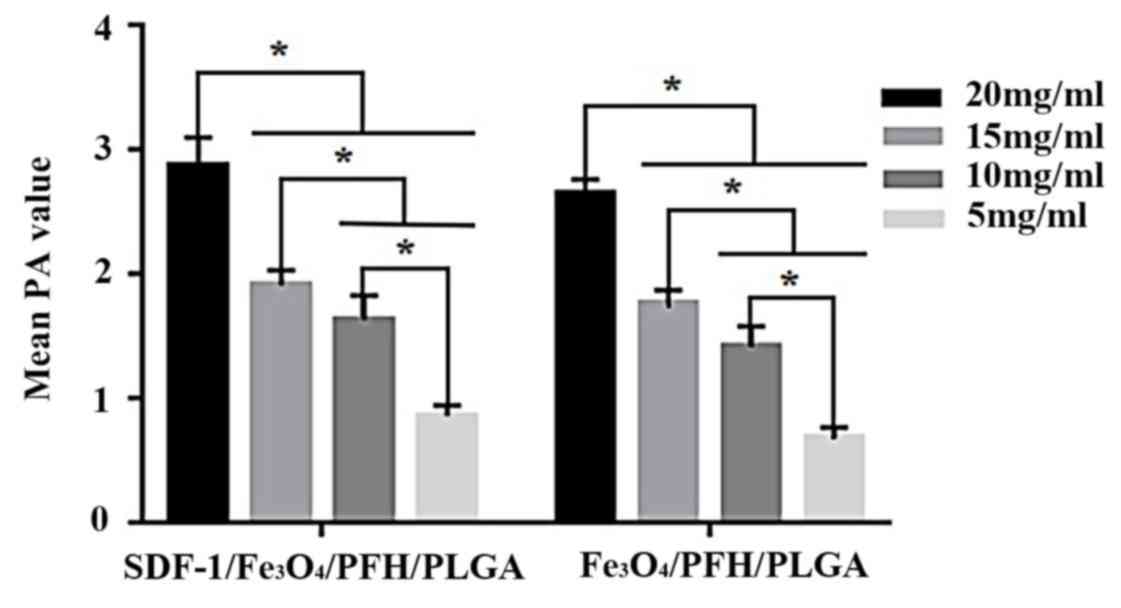
wavelength for the PA imaging experiment. The PA images of 4 groups
and the mean PA value of SDF-1/Fe3O4/PLGA/PFH
and Fe3O4/PLGA/PFH groups are presented in
Figs. 2 and 3 respectively. The
SDF-1/Fe3O4/PLGA/PFH and
Fe3O4/PLGA/PFH nanoparticles displayed
obvious PA signals, whereas there were no obvious PA signals from
SDF-1/PLGA/PFH or blank PLGA nanoparticles. Furthermore, the PA
images of SDF-1/Fe3O4/PLGA/PFH and
Fe3O4/PLGA/PFH nanoparticles were enhanced
compared with SDF-1/PLGA/PFH and blank PLGA nanoparticles (Fig. 2) and the average PA signal values of
each group significantly increased (Fig.
3) with increasing PLGA concentration. Therefore, the results
indicated that Fe3O4 displayed excellent PA
imaging capability and 20 mg/ml nanoparticles emitted the strongest
PA signals in the set of nanoparticles.
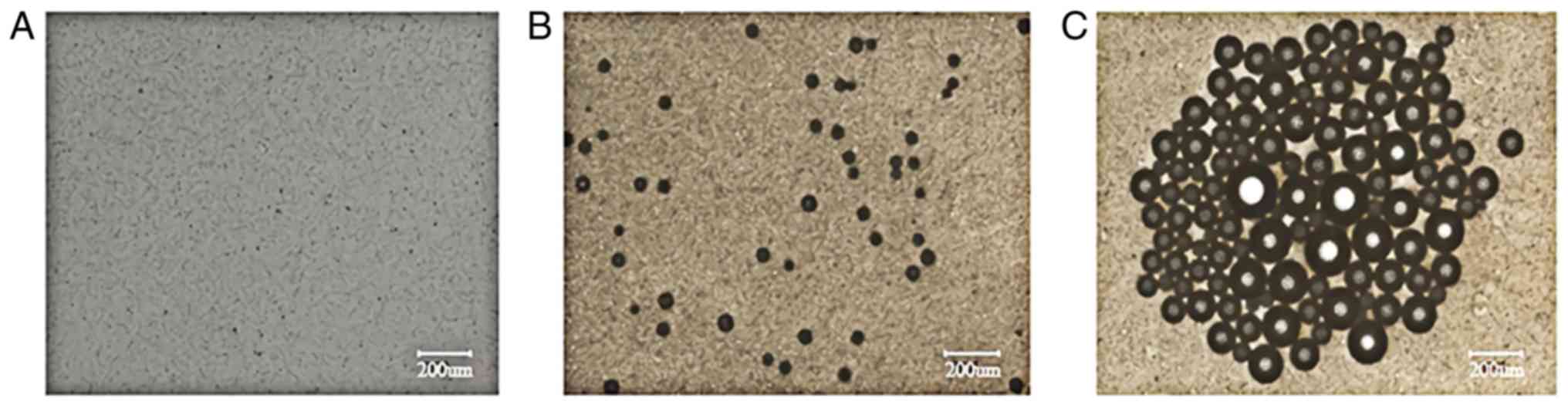
Ultrasonic imaging experiment
The SDF-1/Fe3O4/PLGA/PFH
nanoparticles were divided into 4 groups according to PLGA
concentration (100, 50, 25 and 12.5 mg/ml) and PBS solution was
used as a control group. Samples of the different groups were
embedded into an agarose gel model. The ultrasonic mode of the
ultrasonic diagnostic instrument and an MI value of 0.6 were used
to investigate the imaging conditions of PBS and the different
concentrations of nanoparticles (Fig.
4A-E). Furthermore, ultrasound images for 12.5 mg/ml PLGA in
nanoparticles with a decreased MI value (0.6 to 0.2) were obtained
(Fig. 4E-I). and the corresponding
EI values were measured by DFY quantifier. The results demonstrated
that when the MI value was constant, the ultrasonic signal
displayed a decreasing trend in response to a decrease in
nanoparticle concentration (Table I;
P<0.05). The PBS solution emitted no obvious ultrasonic signal.
Furthermore, when the concentration of nanoparticles was constant,
the ultrasonic signal value decreased in response to a decrease in
MI (EI values were measured in triplicate for each MI value;
Table II; P<0.05). In summary,
the ultrasound imaging performance for
SDF-1/Fe3O4/PLGA/PFH nanoparticles was
satisfactory and the EI value was highest when the MI value was 0.6
and the concentration of PLGA was 100 mg/ml.
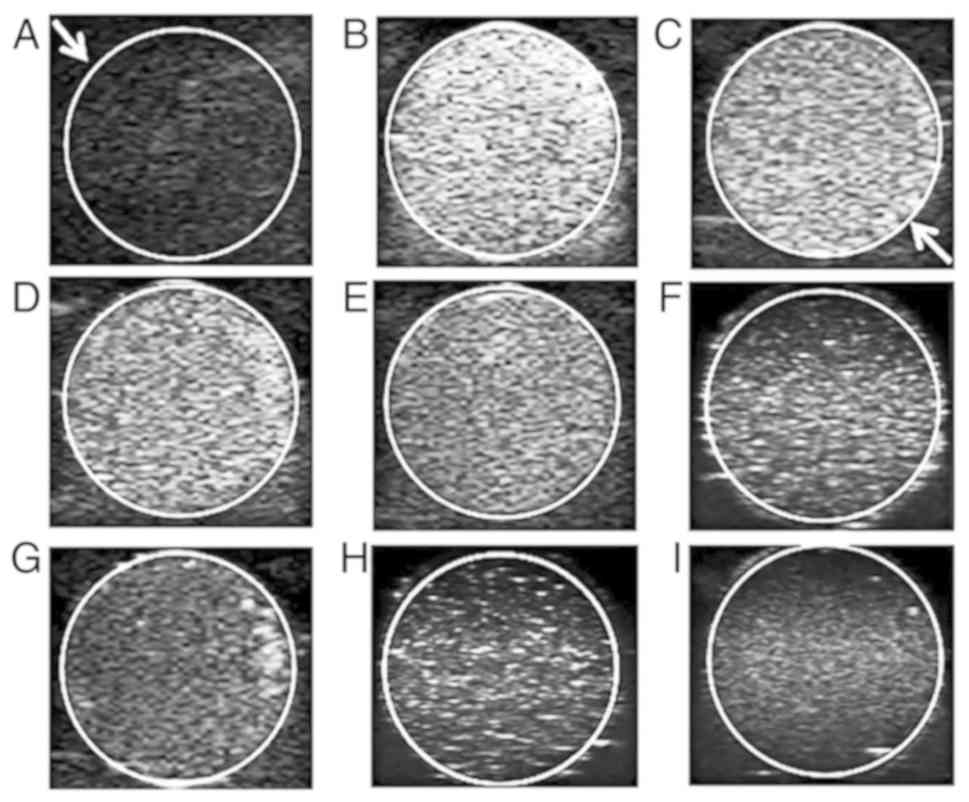 | Figure 4Ultrasonic imaging of
SDF-1/Fe3O4/PLGA/PFH nanoparticles at
different PLGA concentrations and MI values. Images of (A) PBS and
SDF-1/Fe3O4/PLGA/PFH nanoparticles at PLGA
concentrations of (B) 100, (C) 50, (D) 25 and (E) 12.5 mg/ml with a
MI of 0.6. Images of SDF-1/Fe3O4/PLGA/PFH
nanoparticles at MI value of (E) 0.6, (F) 0.5, (G) 0.4, (H) 0.3 and
(I) 0.2 at a PLGA concentration of 12.5 mg/ml. Arrows indicate that
the ultrasound images are shown within the circles. SDF-1, stromal
cell-derived factor 1; Fe3O4, iron (II,III)
oxide; PLGA, poly(lactic-co-glycolic acid); PFH, fluorocarbon
perfluorohexane; MI, mechanical index. |
 | Table IEI values of different PLGA
concentrations at a mechanical index of 0.6. |
Table I
EI values of different PLGA
concentrations at a mechanical index of 0.6.
| PLGA concentration
(mg/ml) | EI |
|---|
| 100a-c | 163.8±3.42 |
| 50a,b | 137.77±1.25 |
| 25a | 116.2±2.98 |
| 12.5 | 99.17±2.76 |
 | Table IIEI values of different MIs at a
concentration of 12.5 mg/ml PLGA. |
Table II
EI values of different MIs at a
concentration of 12.5 mg/ml PLGA.
| MI | EI |
|---|
| 0.6a-d | 99.17±2.76 |
| 0.5a-c | 78.96±3.15 |
| 0.4a,b | 66.25±2.36 |
| 0.3a | 54.59±2.42 |
| 0.2 | 43.73±2.59 |
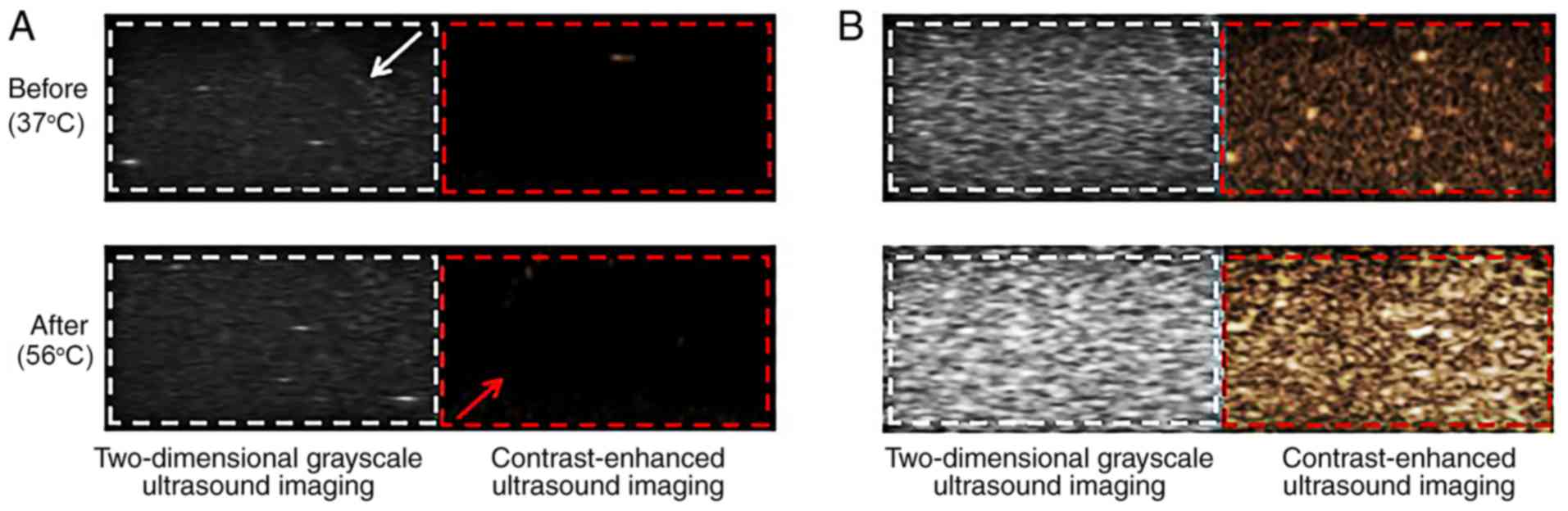
In vitro phase change experiment
The temperature of the water bath used during the
ultrasonic imaging experiment was slowly increased from 37˚C.
Images of SDF-1/Fe3O4/PLGA/PFH nanoparticles
and blank controls were taken before the water bath, when the
contrast-enhanced ultrasound imaging was strongest and collected
using the contrast mode of the ultrasound diagnostic instrument
(Fig. 5). The controls did not
display enhanced images; however, the experimental group displayed
clear two-dimensional grayscale ultrasound images and
contrast-enhanced ultrasound image signals during the heating
process. The degree of two-dimensional grayscale ultrasound imaging
and contrast-enhanced ultrasound imaging in the experimental group
before and after heating, as detected by a DFY quantitative
instrument, was significantly increased (grayscale values were
measured in triplicate for each image; Table III; P<0.05).
 | Table IIIGrayscale values before and after the
water bath in the experimental group. |
Table III
Grayscale values before and after the
water bath in the experimental group.
| Temperature | Two-dimensional
grayscale ultrasound imaging | Contrast-enhanced
ultrasound imaging |
|---|
| Before (37˚C) | 68±1.73 | 32±1.67 |
| After (56˚C) |
152±2.75a |
101±3.09a |
Using an optical microscope, it was observed that
nanoparticles were spherical and well dispersed prior to heating
(Fig. 6A). Following 1 min in the
hot water bath, certain nanoparticles underwent phase
transformation and generated microbubbles (Fig. 6B). When the temperature was increased
to 56˚C, large amounts of microbubbles with increased volume were
observed and the bubbles gradually converged (Fig. 6C). As the temperature continued to
increase to 65˚C, the microbubbles gradually burst and disappeared
(data not shown).
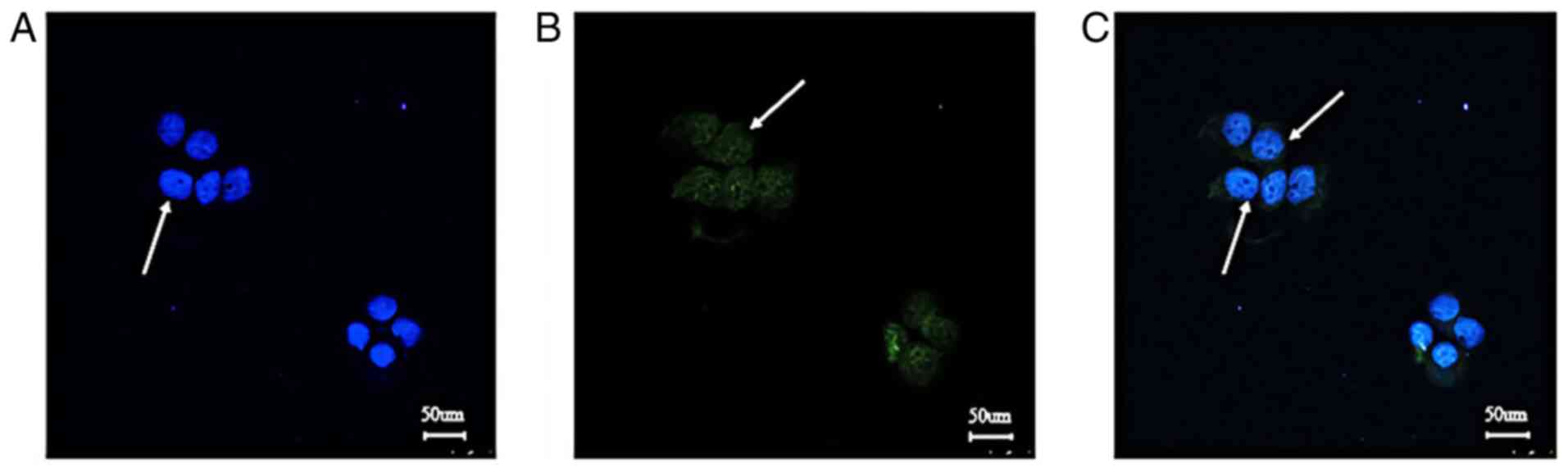
Detection of CXCR4 localization and
expression in SCC-15 cells
In Fig. 7, blue
indicated the nuclei of SCC-15 cells re-stained with DAPI and green
indicated CXCR4 labeled with FITC (as indicated by arrows). The
target protein was expressed in the cell membrane and cytoplasm,
which indicated that CXCR4 was an ideal membrane target
protein.
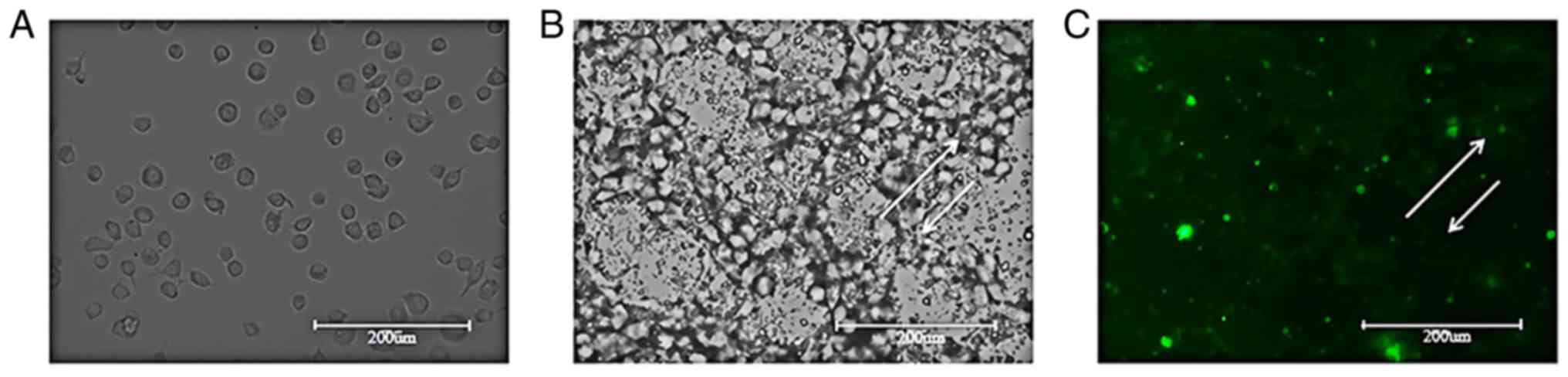
Targeted experiment with SCC-15
cells
SCC-15 cells were observed under a light microscope
and were found to exhibit a tadpole-shaped morphology and were
evenly distributed (Fig. 8A).
Following SDF-1/Fe3O4/PLGA/PFH nanoparticle
addition and incubation for 2 h, SCC-15 cells were observed under a
light microscope. A large number of nanoparticles adhered around
SCC-15 cell membranes and to the cytoplasm (Fig. 8B). A fully-automatic fluorescence
pattern of Fig. 8B from the same
view was generated and bright green fluorescence was observed in
and around the SCC-15 cells (as indicated by the arrows; Fig. 8C). The results demonstrated that
SDF-1/Fe3O4/PLGA/PFH nanoparticles
specifically bound to CXCR4 in SCC-15 cells.
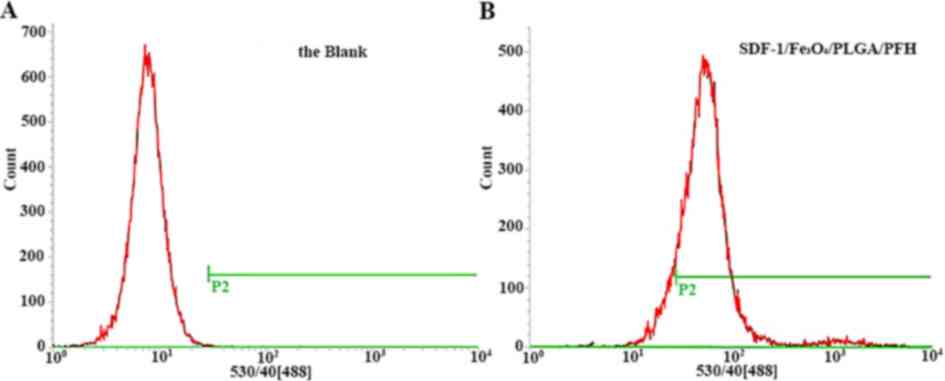
Flow cytometry test
The rate of binding of the targeted nanoparticle
group to SCC-15 cells was 84.44%, while that of the controls was
only 1.1%. The results indicated that the nanoparticles possessed
strong targeted binding ability to SCC-15 cells (Fig. 9).
Discussion
In the present study, a double emulsification method
was used to prepare multifunctional, targeted polymeric
nanoparticles using PLGA as the carrier. PLGA contains rich
functional groups that can promote functionalization of PLGA-based
nanoparticles and biomolecules, and can improve their stability and
functionality (17). PFH and
Fe3O4 were used concurrently as a coating and
the surface of the PGLA shell was linked to FITC-labeled SDF-1 via
thioether bonds, thus forming a nanoparticle with multiple
functions.
The physical characteristics of the particles were
tested using various methods. The solution of the nanoparticles was
observed to be dark brown to the naked eye and the nanoparticles
appeared round and spherical with numerous surface pores when
observed via scanning electron microscopy. The particle size was
measured to be 586.48±124.70 nm. It has been reported that the
microvascular endothelial gap in SCC is 300-780 nm (30); therefore, the nanoparticles could
potentially pass through the microvascular endothelial gap and be
deposited into the target tissue via the CXCR4-CXCL12 axis. The
therapeutic application of the nanoparticles may aid in locating
tumors and metastatic lymphoma more accurately and blocking further
metastasis via these channels. Contrary to the structure of tumor
tissue, the vascular endothelium of normal tissue is tightly
connected and extravasation of nanoparticles is not possible;
however, tumors display enhanced vascular permeability and poor
lymph drainage, which facilitates the retention of nanoparticles
that extravasate into tumor tissue for a prolonged time (37).
Multi-modal imaging has served an increasingly
important role in medical diagnosis and increased focused research
on numerous multi-modality contrast agents with multiple imaging
functions has been conducted (38).
With the rapid development of medical imaging technology,
ultrasound, computed tomography (CT), X-rays and MR imaging have
been widely used. Imaging technology can be used for molecular and
functional imaging of target organs and tissues to obtain
pathological information, thus aiding in the early diagnosis of
diseases (39). MR is frequently
used to diagnose breast cancer (40)
and CT is often used for localized brain tumors, head and neck
cancer, and multiple myeloma (41).
However, the imaging techniques currently used in clinical practice
have limitations, including missed diagnosis, misdiagnosis and they
are not specific to or target lesions (8).
PA imaging is a novel biomedical imaging mode that
combines the advantages of optics and acoustics to improve the
spatial resolution of traditional ultrasound (30). Combining data from two or more
imaging techniques can improve diagnosis accuracy (42).
Fe3O4, a photosensitive
material, absorbs laser light of a specific wavelength and causes
the ambient temperature to rise (31). The thermal expansion of the
surrounding objects produces a PA effect and the signal device
generates PA signals after receiving sound waves (32). Simultaneously,
Fe3O4 magnetic particles can vibrate in
response to acoustic waves, altering the acoustic impedance of
surrounding tissues and enhancing ultrasonic development (43). Additionally, numerous experimental
studies have demonstrated that Fe3O4 improves
the transverse relaxation rate of MR and improves MR imaging
capability (44-46).
Fe3O4 can integrate enhanced PA ultrasound
and MR imaging, is an ideal molecular imaging material and is
biodegradable and safe (21). After
entering the body, Fe3O4 is metabolized by
red blood cells and enters the normal plasma iron pool to
participate in the synthesis of hemoglobin, myoglobin and
cytochrome oxidase, or participate in energy metabolism and
hematopoietic functions (47). In
the in vitro imaging experiments performed in the present
study, the Fe3O4-coated nanoparticles
displayed PA and ultrasound imaging capabilities, which indicated
that they may serve as a multi-modal contrast agent with potential
clinical use for lesion visualization.
PFH is liquid at normal temperature, but when the
temperature rises to its boiling point (56˚C) or above, or the
external pressure decreases to its gasification pressure threshold,
a liquid-gas phase change occurs (6). When PFH enters the gas phase, the
increase of microsphere volume results in the increase of acoustic
impedance, then ultrasonic development is enhanced (48). Commonly used methods to promote
liquid-gas phase transition of liquid fluorocarbon include
temperature-induced phase change (49), acoustic droplet vaporization
(50,51) and optical droplet vaporization
(32). In the present study, a water
bath heating method was used to induce PFH liquid-gas phase change.
When the temperature rose to ~56˚C, numerous phase change
microbubbles were observed using a microscope. Additionally, the EI
values of two-dimensional grayscale and contrast-enhanced
ultrasound images were significantly increased compared with before
heating in the water bat (37˚C). When the temperature rose to 65˚C,
the microbubbles burst and disappeared, weakening the image. As the
phase change temperature of PFH is higher than the body temperature
of the human body, an external water bath heating was used to
promote the phase change; however, this would be difficult to
implement in the human body. Fe3O4, a
photosensitive material, can undergo photo-thermal conversion under
laser radiation to increase the temperature of the surrounding
medium, which may serve as a potential mechanism to promote PFH
phase change in the body (52).
Moreover, a laser has stability and directionality, and can treat
tumors without damaging the surrounding normal tissues via the
principle of thermal ablation, thus providing a direction for
future in vivo experiments (6).
SCC of the tongue accounts for the majority of oral
SCC cases and its high lymph node metastasis rate results in the
low 5-year survival rate (<30%) of patients (53). Several studies have reported that
CXCR4 is widely and highly expressed in oral SCC cells, including
SCC cells of the tongue (30,54,55).
Additionally, it has been demonstrated that the CXCR4-CXCL12 axis
serves an important role in the proliferation, invasion, immune
evasion and metastasis of several types of cancer, including oral
SCC (56,57). The interaction between CXCR12 and its
receptor stimulates downstream signaling pathways to affect tumor
angiogenesis, tumor cell proliferation and chemical resistance,
which suggest that CXCR12 may serve as a potential target for
cancer therapy (58). More
importantly, when SDF-1 on targeted nanoparticles combines with
CXCR4, it can antagonize the tumor-promoting effect caused by
CXCR4-SDF-1(36). The active
targeting effect of SDF-1 allows nanoparticles to remain in tumor
tissues for a prolonged time and allows the rapid release of drugs
using laser local fixed-point radiation, which would increase local
drug concentrations and achieve a chemotherapy effect while
reducing systemic adverse reactions; therefore, the aforementioned
strategy may be used for targeted treatment with long-term
chemotherapy drugs (6). Cellular
immunofluorescence experiments demonstrated that CXCR4 is expressed
in the cell membrane and cytoplasm of SCC-15 cells and is an ideal
membrane protein target. In the present study, SDF-1 was attached
to a PLGA shell by a thioether bond and
SDF-1/Fe3O4/PLGA/PFH nanoparticles were
successfully generated. SCC-15 targeting experiments indicated that
SDF-1/Fe3O4/PLGA/PFH nanoparticles
specifically combined with CXCR4 in SCC-15 cells and bright green
fluorescence was microscopically observed in and around the cells.
Flow cytometry assays indicated that the generated nanoparticles
displayed a high targeting ability. However, whether the expected
target effect can be achieved in vivo requires further
investigation.
In the present study, a targeted nanoparticle
contrast agent with a PA/ultrasonic bimodal imaging function was
successfully generated. Basic physical characteristics of the
nanoparticles were studied in vitro and it displayed PA and
ultrasonic imaging capabilities. The nanoparticles underwent in
vitro phase change under certain conditions to enhance
ultrasonic imaging and specific adherence to tongue SCCs in
vitro. The present work provided support for establishing
procedures and standards to be used for the diagnosis of primary
foci of tongue carcinoma and metastatic SCC of the lymph nodes and
established a foundation for subsequent in vivo
experiments.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Chongqing Social
Livelihood Science and Technology Innovation Project (grant no.
cstc2016shmszx00010), the Science and Technology Research Project
of Chongqing Education Commission (grant no. KJ1600231) and the
Program for Innovation Team Building at Institutions of Higher
Education in Chongqing (grant no. CXTDG201602006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW performed the majority of the experiments and
wrote the manuscript. ZW, LP and SW analyzed and interpreted data.
LQ designed and supervised the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen X, Xu WH, Zhou J and Wang YL: Current
situation of oral squamous cell carcinoma. Stomatology. 37:462–465.
2017.
|
|
2
|
Chen F, Yan L, Lin L, Liu F, Qiu Y, Wang
J, Wu J, Liu F, Huang J, Cai L, et al: Dietary score and the risk
of oral cancer: A case-control study in southeast China.
Oncotarget. 8:34610–34616. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Deshpande N, Needles A and Willmann JK:
Molecular ultrasound imaging: Current status and future directions.
Clin Radiol. 65:567–581. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Riemann M, Knipfer C, Rohde M, Alder W,
Schuster M, Noeth E, Oetter N, Shams N, Neukam FW and Stelzlle F:
Oral squamous cell carcinoma of the tongue: Prospective and
objective speech evaluation of patients undergoing surgical
therapy. Head Neck. 38:993–1001. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chang CC, Yang YJ, Li YJ, Chen ST, Lin BR,
Wu TS, Lin SK, Kuo MY and Tan CT: Corrigendum to ‘MicroRNA-17/20a
functions to inhibit cell migration and can be used a prognostic
marker in oral squamous cell carcinoma’ [Oral Oncol. 49(9) (2013)
923-931]. Oral Oncol. 72:202–203. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xiong J, Feng J, Qiu L, Gao Z, Li P, Pang
L and Zhang Z: SDF-1-loaded PLGA nanoparticles for the targeted
photoacoustic imaging and photothermal therapy of metastatic lymph
nodes in tongue squamous cell carcinoma. Int J Pharm. 554:93–104.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hwang-Bo J, Bae MG, Park JH and Chung IS:
3-O-Acetyloleanolic acid inhibits VEGF-A-induced lymphangiogenesis
and lymph node metastasis in an oral cancer sentinel lymph node
animal model. BMC Cancer. 18(714)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang X, Zhang L, Tan X, Lin Y, Han X,
Wang H, Ming H, Li Q, Liu K and Feng G: Systematic analysis of
genes involved in oral cancer metastasis to lymph nodes. Cell Mol
Biol Lett. 23(53)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Szaniszlo P, Fennewald SM, Qiu S, Kantara
C, Shilagard T, Vargas G and Resto VA: Temporal characterization of
lymphatic metastasis in an orthotopic mouse model of oral cancer.
Head Neck. 36:1638–1647. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wei W, Jiang D, Rosenkrans ZT, Barnhart
TE, Engle JW, Luo Q and Cai W: HER2-targeted multimodal imaging of
anaplastic thyroid cancer. Am J Cancer Res. 9:2413–2427.
2019.PubMed/NCBI
|
|
11
|
Xue LY, Jiang ZY, Fu TT, Wang QM, Zhu YL,
Dai M, Wang WP, Yu JH and Ding H: Transfer learning radiomics based
on multimodal ultrasound imaging for staging liver fibrosis. Eur
Radiol. 30:2973–2983. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rapoport N, Christensen DA, Kennedy AM and
Nam KH: Cavitation properties of block copolymer stabilized
phase-shift nanoemulsions used as drug carriers. Ultrasound Med
Biol. 36:419–429. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Niu C, Wang Z, Lu G, Krupka TM, Sun Y, You
Y, Song W, Ran H, Li P and Zheng Y: Doxorubicin loaded
superparamagnetic PLGA-iron oxide multifunctional microbubbles for
dual-mode US/MR imaging and therapy of metastasis in lymph nodes.
Biomaterials. 34:2307–2317. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li A, Zheng Y, Yu J, Wang Z, Yang Y, Wu W,
Guo D and Ran H: Superparamagnetic perfluorooctylbromide
nanoparticles as a multimodal contrast agent for US, MR, and CT
imaging. Acta Radiol. 54:278–283. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jain RA: The manufacturing techniques of
various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA)
devices. Biomaterials. 21:2475–2490. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li P, Zheng Y, Ran H, Tan J, Lin Y, Zhang
Q, Ren J and Wang Z: Ultrasound triggered drug release from
10-hydroxycamptothecin-loaded phospholipid microbubbles for
targeted tumor therapy in mice. J Control Release. 162:349–354.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ao M, Wang Z, Ran H, Guo D, Yu J, Li A,
Chen W, Wu W and Zheng Y: Gd-DTPA-loaded PLGA microbubbles as both
ultrasound contrast agent and MRI contrast agent-a feasibility
research. J Biomed Mater Res B Appl Biomater. 93:551–556.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lowe KC: Engineering blood: Synthetic
substitutes from fluorinated compounds. Tissue Eng. 9:389–399.
2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cyrus T, Winter PM, Caruthers SD, Wickline
SA and Lanza GM: Magnetic resonance nanoparticles for
cardiovascular molecular imaging and therapy. Expert Rev Cardiovasc
Ther. 3:705–715. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tural B, Ozenbaş M, Atalay S and Volkan M:
Rapid synthesis and characterization of maghemite nanoparticles. J
Nanosci Nanotechnol. 8:861–866. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun Y, Zheng Y, Ran H, Zhou Y, Shen H,
Chen Y, Chen H, Krupka TM, Li A, Li P, et al: Superparamagnetic
PLGA-iron oxide microcapsules for dual-modality US/MR imaging and
high intensity focused US breast cancer ablation. Biomaterials.
33:5854–5864. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Qiao RR, Yang CH and Gao MY:
Superparamagnetic iron oxide nanoparticles: From preparations to in
vivo MRI applications. J Mater Chem. 19:6274–6293. 2009.
|
|
23
|
Mu X, Zhang F, Kong C, Zhang H, Zhang W,
Ge R, Liu Y and Jiang J: EGFR-targeted delivery of DOX-loaded
Fe3O4@ polydopamine multifunctional
nanocomposites for MRI and antitumor chemo-photothermal therapy.
Int J Nanomedicine. 12:2899–2911. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu ZY, Wang Y, Liang CH, Li XH, Wang GY,
Liu HJ and Li Y: In vitro labeling of mesenchymal stem cells with
superparamagnetic iron oxide by means of microbubble-enhanced US
exposure: Initial experience. Radiology. 253:153–159.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Degen CL, Poggio M, Mamin HJ, Rettner CT
and Rugar D: Nanoscale magnetic resonance imaging. Proc Natl Acad
Sci USA. 106:1313–1317. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jun YW, Lee JH and Cheon J: Chemical
design of nanoparticle probes for high-performance magnetic
resonance imaging. Angew Chem Int Ed Engl. 47:5122–5135.
2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Norton SJ and Vo-Dinh T: Imaging the
distribution of magnetic nanoparticles with ultrasound. IEEE Trans
Med Imaging. 26:660–665. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen ZJ, Yang SH and Xing D: In vivo
detection of hemoglobin oxygen saturation and carboxyhemoglobin
saturation with multiwavelength photoacoustic microscopy. Opt Lett.
37:3414–3416. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ermilov SA, Khamapirad T, Conjusteau A,
Leonard MH, Lacewell R, Mehta K, Miller T and Oraevsky AA: Laser
optoacoustic imaging system for detection of breast cancer. J
Biomed Opt. 14(024007)2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li C and Wang LV: Photoacoustic tomography
and sensing in biomedicine. Phys Med Biol. 54:R59–R97.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Grootendorst DJ, Jose J, Fratila RM,
Visscher M, Velders AH, Ten Haken B, Van Leeuwen TG, Steenbergen W,
Manohar S and Ruers TJ: Evaluation of superparamagnetic iron oxide
nanoparticles (Endorem®) as a photoacoustic contrast
agent for intra-operative nodal staging. Contrast Media Mol
Imaging. 8:83–91. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Strohm E, Rui M, Gorelikov I, Matsuura N
and Kolios M: Vaporization of perfluorocarbon droplets using
optical irradiation. Biomed Opt Express. 2:1432–1442.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Delilbasi CB, Okura M, Iida S and Kogo M:
Investigation of CXCR4 in squamous cell carcinoma of the tongue.
Oral Oncol. 40:154–157. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee DJ, Lyshchik A, Huamani J, Hallahan DE
and Fleischer AC: Relationship between retention of a vascular
endothelial growth factor receptor 2 (VEGFR2)-targeted
ultrasonographic contrast agent and the level of VEGFR2 expression
in an in vivo breast cancer model. J Ultrasound Med. 27:855–866.
2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mei L, Liu Y, Zhang Q, Gao HL, Zhang Z and
He Q: Enhanced antitumor and anti-metastasis efficiency via
combined treatment with CXCR4 antagonist and liposomal doxorubicin.
J Control Release. 196:324–331. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Iyer AK, Khaled G, Fang J and Maeda H:
Exploiting the enhanced permeability and retention effect for tumor
targeting. Drug Discov Today. 11:812–818. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ke H, Yue X, Wang J, Xing S, Zhang Q, Dai
Z, Tian J, Wang S and Jin Y: Gold nanoshelled liquid
perfluorocarbon nanocapsules for combined dual modal ultrasound/CT
imaging and photothermal therapy of cancer. Small. 10:1220–1227.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhigang W, Zhiyu L, Haitao R, Hong R,
Qunxia Z, Ailong H, Qi L, Chunjing Z, Hailin T, Lin G, et al:
Ultrasound-mediated microbubble destruction enhances VEGF gene
delivery to the infarcted myocardium in rats. Clin Imaging.
28:395–398. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kuhl CK, Strobel K, Bieling H, Leutner C,
Schild HH and Schrading S: Supplemental breast MR imaging screening
of women with average risk of breast cancer. Radiology.
283:361–370. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ell PJ: PET/CT in oncology: A major
technology for cancer care. Chang Gung Med J. 28:274–283.
2005.PubMed/NCBI
|
|
42
|
Razansky D, Buehler A and Ntziachristos V:
Volumetric real-time multispectral optoacoustic tomography of
biomarkers. Nat Protoc. 6:1121–1129. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu Z, Lammers T, Ehling J, Fokong S,
Bornemann J, Kiessling F and Gätjens J: Iron oxide
nanoparticle-containing microbubble composites as contrast agents
for MR and ultrasound dual-modality imaging. Biomaterials.
32:6155–6163. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim J, Park S, Lee JE, Jin SM, Lee JH, Lee
IS, Yang I, Kim JS, Kim SK, Cho MH and Hyeon T: Designed
fabrication of multifunctional magnetic gold nanoshells and their
application to magnetic resonance imaging and photothermal therapy.
Angew Chem Int Ed Engl. 45:7754–7758. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tian Q, Hu J, Zhu Y, Zou R, Chen Z, Yang
S, Li R, Su Q, Han Y and Liu X: Sub-10 nm Fe3O4@Cu(2-x)S core-shell
nanoparticles for dual-modal imaging and photothermal therapy. J Am
Chem Soc. 135:8571–8577. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ling Y, Wei K, Luo Y, Gao X and Zhong S:
Dual docetaxel/superparamagnetic iron oxide loaded nanoparticles
for both targeting magnetic resonance imaging and cancer therapy.
Biomaterials. 32:7139–7150. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Duguet E, Vasseur S, Mornet S and
Devoisselle JM: Magnetic nanoparticles and their applications in
medicine. Nanomedicine (Lond). 1:157–168. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen YL, Liu FQ, Guo Y, Cheng J, Yang L,
Lu M, Li P, Xu J, Yu T, Wang ZG, et al: PA/US dual-modality imaging
to guide VEGFR-2 targeted photothermal therapy using
ZnPc-/PFH-loaded polymeric nanoparticles. Biomater Sci.
6:2130–2143. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Huang JW, Xu JS, Schmidt C and Xu RX: Heat
sensitive microbubbles for intraoperative assessment of cancer
ablation margin. Multimodal biomedical imaging VII. Int Soc Opt
Photonics. 8216(821604)2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhou Y, Wang Z, Chen Y, Shen H, Luo Z, Li
A, Wang Q, Ran H, Li P, Song W, et al: Microbubbles from
gas-generating perfluorohexane nanoemulsions for targeted
temperature-sensitive ultrasonography and synergistic HIFU ablation
of tumors. Adv Mater. 25:4123–4130. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xu S, Zong Y, Li W, Zhang S and Wan M:
Bubble size distribution in acoustic droplet vaporization via
dissolution using an ultrasound wide-beam method. Ultrason
Sonochem. 21:975–983. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chu M, Shao Y, Peng J, Dai X, Li H, Wu Q
and Shi D: Near-infrared laser light mediated cancer therapy by
photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials.
34:4078–4088. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev.
26:645–662. 2007.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Oliveira-Neto HH, Silva ET, Leles CR,
Mendonca EF, Alencar Rde C, Silva TA and Batista AC: Involvement of
CXCL12 and CXCR4 in lymph node metastases and development of oral
squamous cell carcinomas. Tumour Biol. 29:262–271. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Almofti A, Uchida D, Begum NM, Tomizuka Y,
Iga H, Yoshida H and Sato M: The clinicopathological significance
of the expression of CXCR4 protein in oral squamous cell carcinoma.
Int J Oncol. 25:65–71. 2004.PubMed/NCBI
|
|
56
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rave-Fränk M, Tehrany N, Kitz J, Leu M,
Weber HE, Burfeind P, Schliephake H, Canis M, Beissbarth T,
Reichardt HM and Wolff HA: Prognostic value of CXCL12 and CXCR4 in
inoperable head and neck squamous cell carcinoma. Strahlenther
Onkol. 192:47–54. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Meng W, Xue S and Chen Y: The role of
CXCL12 in tumor microenvironment. Gene. 641:105–110.
2018.PubMed/NCBI View Article : Google Scholar
|