Introduction
Stem cell treatments have substantial therapeutic
potential for many pathologies and together with cellular and gene
therapies and biological agents represent an important change in
the therapeutical paradigm, from the ‘silver-bullet’ single
molecules acting at critical crossroads of cellular molecular
pathways (which may be better suited for acute illness) to
combination treatments acting on many more cellular molecules and
pathways at levels closer to physiological functioning and thus
with better overall results especially for chronic conditions.
Furthermore, having the potential to replace lost
tissue and function, stem cells are uniquely positioned to offer
truly regenerative treatments with rapid results and long-term
advantages, and this is especially true when autologous implants
are used. Towards these challenges, considering the scope and the
level of detail of cell-level interactions, a multi-disciplinary
effort including experimental models in various pathologies is
necessary (1-5);
advanced molecular imaging techniques with hybrid PET/CT systems
enables assessment of brain tissue viability contributing to the
in vivo imaging evaluation of response to stem cell-based
therapies. Additionally, recent technological advances in
instrumentation leading to the introduction into clinical practice
of simultaneous PET/MRI systems, together with advances in
radiochemical development of novel PET-tracers, hold promise for
more accurate functional imaging of stroke-related molecular
mechanisms, giving ground for designing more precise and
individualized treatment strategies (6-8). At
the same time much remains to be clarified about their optimal and
individualized production, administration, actions, grafting and
functional integration, and this work aims to bring a contribution
in this regard.
Patients with cerebrovascular accidents (CVA,
stroke) undergo multiple and simultaneous modifications at the
cellular, molecular and genetic level which involve partially
overlapping pathways, through activation or inhibition of specific
molecules. Known so far are: dysfunction of hemostasis, vascular
endothelium and blood-brain barrier; activation and increased
levels of pro-inflammatory and adhesion molecules [cytokines,
nuclear factor-κB (NF-κB), intercellular adhesion molecule (ICAM),
vascular cell adhesion molecule (VCA), leukocyte infiltration,
activation of microglia (1),
functional neuronal impairment, oxidative stress, switching to
anaerobic metabolism by activating hypoxia inducible factor-1α
(HIF-1α)] (9) and apoptosis [B-cell
lymphoma (Bcl), and Bcl-2-associated X protein (Bax)] (10,11).
Post-stroke recovery is further influenced by
excitotoxicity (activation of receptors for N-methyl-D-aspartate
(NMDA), kainate, amino (hydroxy)-methyl-isoxazol-propanoic acid
(AMPA), acidotoxicity and ionic imbalance (Ca, Na, K via
modification of active transport systems or cell (12) lysis), neuroendocrine factors:
insulin-like growth factor-1 (IGF-1) (13-15),
hepatocyte growth factor (HGF) (16), transforming growth factor-β (TGF-β),
nerve growth factor (NGF), brain-derived neurotrophic factor
(BDNF); and other factors (cross-talk between transcription, growth
and neurotrophic factors, neurotransmitters, pathways including
Wnt, notch and hedgehog, bone morphogenetic proteins (BMPs), and
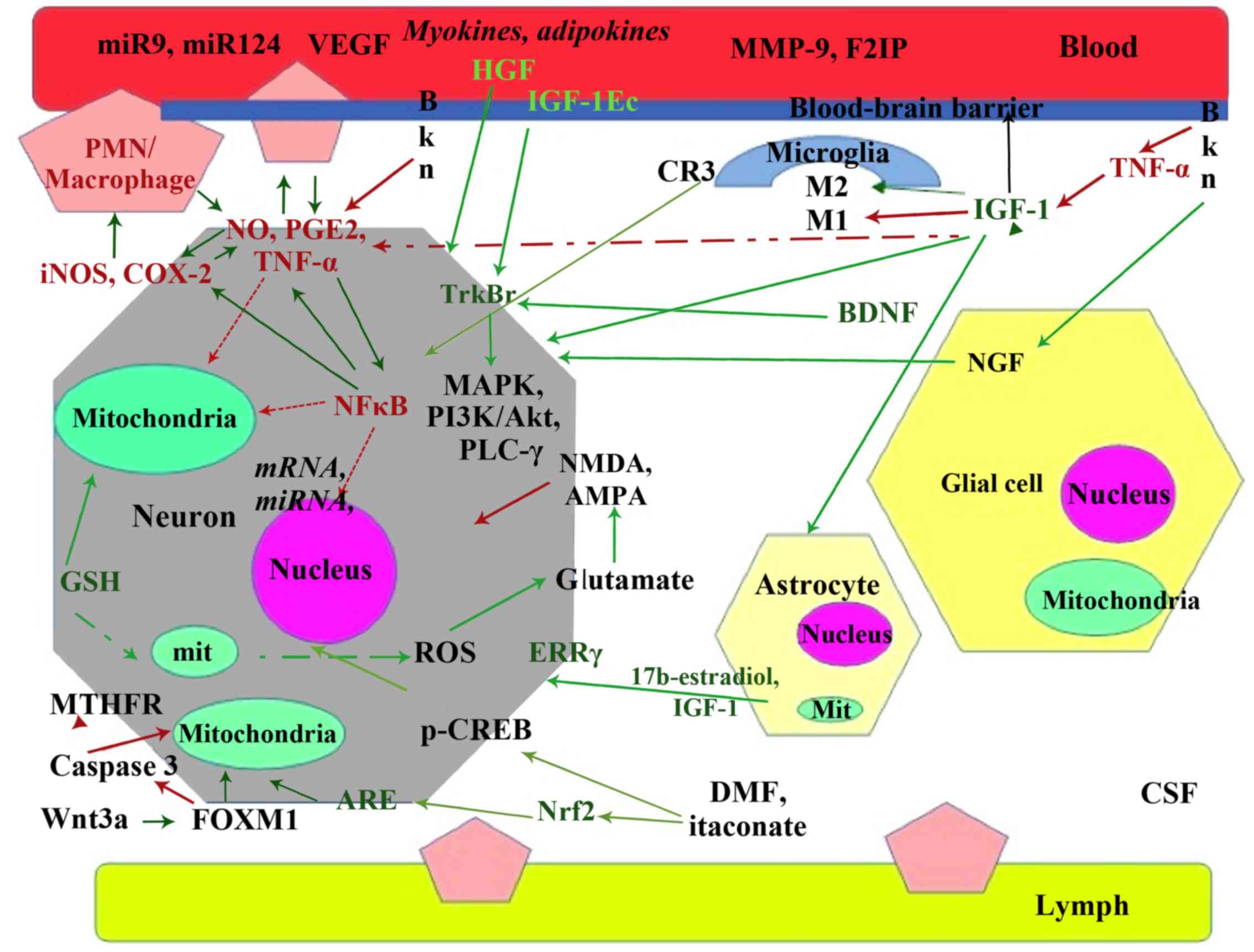
epigenetic modulators (11).
Acting beyond modifications of gene transcription at
epigenetic level, factors influencing stroke recovery at the
genetic level are miRNAs (17) and
genetic variations in molecules such as methyl tetrahidrofolate
reductase (18,19), BDNF (20), apolipoprotein E (APO E), and others
(21) most of these actions are
summarized in Fig. 1.
The complexity of these pathological modifications
which occur simultaneously may explain the fact that as of 2018
over 700 medications for stroke have failed clinical trials
(22).
Current treatments for stroke (thrombolytics and
endovascular thrombectomy) are limited in multiple ways: i)
temporal, to first few hours after stroke; ii) resource-wise, to
tertiary care centers; iii) focus-wise, by addressing mostly the
vascular blockage but not the damaged and regenerating neurons; iv)
scope-wise, by excluding hemorrhagic stroke; and v) patient-wise,
due to co-morbid conditions and the risk for serious, sometimes
fatal complications; these factors make their administration
successful to less than 5% of stroke patients.
A new treatment for stroke which involves a
combination of medications has shown good results in acute and
subacute stroke and can be administered without the above
limitations (23); however in
chronic stroke patients with ample loss of brain tissue (>5
cubic centimeters or >109 neurons), cellular
therapies can provide a better option, with the potential to
improve neurological function years after stroke occurrence.
To achieve functional integration of stem cells in
brain areas affected by stroke many aspects have to be successfully
addressed: migration of administered cells to the injured areas;
differentiation into functional neurons (mostly cholinergic) and
supporting cells including capillaries; adhesion to existing neural
networks and developing new synaptic connections, while at the same
time avoiding new occlusions of capillaries by implanted cells,
excessive multiplication (tumorigenesis), and errant stimulation
(convulsions). On top of these, the procedure should not be overly
complicated, expensive and practically out-of-reach for many
patients.
Our approach relies on selecting and administering a
larger population of stem cells (>100x106) with low
propensity for multiplication (low CD70+) and subsequent
minimal risk for tumorigenesis, and also with more paracrine
actions [cytokines and vascular endothelial growth factor
(VEGF)-secretion] which favor formation of new synapses and
capillaries. Specifically we are employing CD271+ stem
cells, growth factors (GFs) BDNF and IGF-1 added to culture media
and a neurotrophic combination administered iv before and after
stem cell administration; below we summarize the results of
administering this treatment to chronic stroke patients.
Treatment
This is a case series report, resulting from non-standardized
administration of treatments to individual patients on a
case-by-case basis (under the EU rules governing compassionate
care and hospital exemption), not under homogenous inclusion/
exclusion criteria that would be applicable for a clinical study.
The resultant patient data were analysed retrospectively, and the
intention of this article was to provide insights into this type of
treatment, which is currently undergoing further developments
and refinements.
Each patient received a single treatment consisting
of 2-5x106 cells/kg administered via intrathecal
injection (7 patients) or intravenously (1 patient). Cell
suspensions containing adipose-derived stem cells (ASC) were
prepared from patients' own adipose tissue (7 patients) or from a
related donor; this was the case of a 77 year old stroke patient
who received ASCs from a first degree relative.
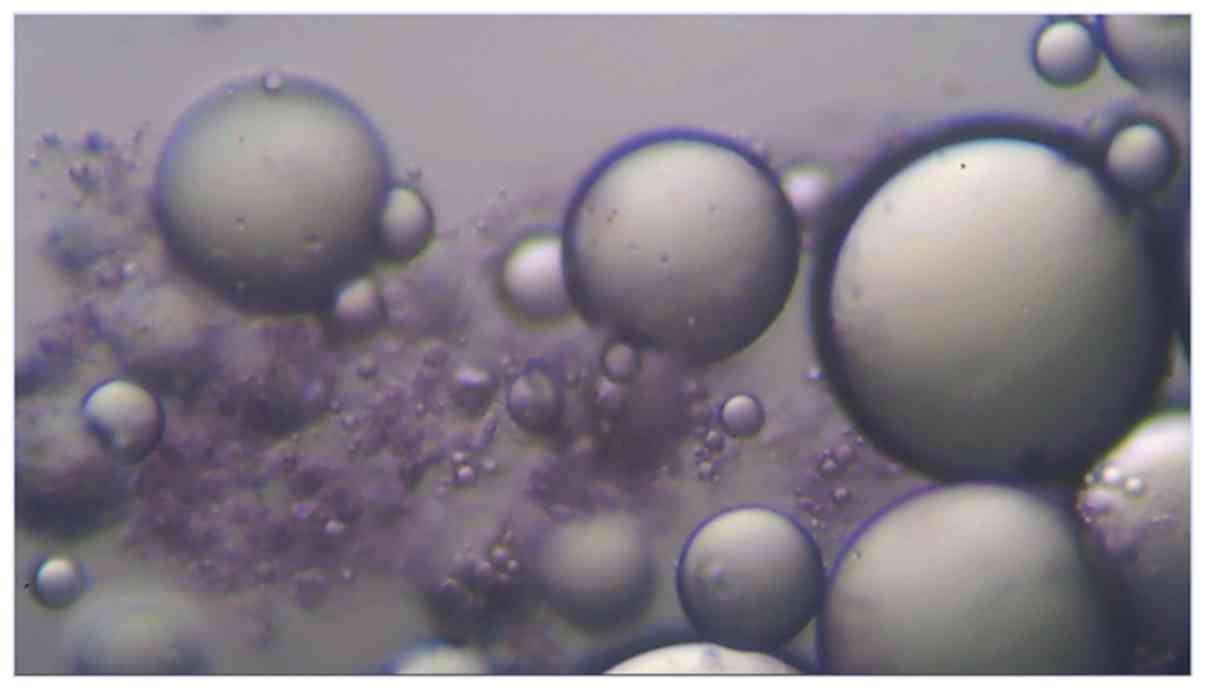
Adipose tissue was harvested via standard
lipoaspiration procedure under local anesthesia with lidocaine,
with the resulting aspirate being a mixture of extracellular matrix
(ECM), GFs and cells, a majority of which are adipocytes, and also
vascular stromal cells and mesenchymal stem cells. A microscopic
image (x200) of the raw lipoaspirate stained with Cresyl Violet
revealing Nissl bodies in mesenchymal stem cells is shown in
Fig. 2.
ECM constituents are type I-VII collagen, elastin,
fibronectin, laminin and glycosaminoglycans, which contain the
following GFs: TGF-β1, platelet-derived growth factor (PDGF), VEGF,
NGF, IGF-1, basic fibroblast growth factor (bFGF), BMP4, epidermal
growth factor (EGF), HGF; interspersed between adipocytes can be
found in mesenchymal stem cells which have the potential to
differentiate in multiple cellular lineage and express self-renewal
genes and specific mesenchymal markers (24).
ASCs cultured with a medium containing 5% autologous
serum express the CD29, CD44, CD73, CD90 and CD105 markers
characteristic of stemness (17) and
their properties are not affected after two freeze cycles; compared
to other sources of stem cells, ASCs have similar phenotype
maintenance as MSCs from bone marrow, also capacity for
differentiation, and secretion of GFs-paracrine actions (25-31).
Furthermore, ASCs had faster proliferation rate and also neural
markers with significantly higher expression than MSCs derived from
bone marrow, skin or umbilical cord (32-35).
Cultured ASCs can start to differentiate into
neuronal precursors as early as 1-3 h (36) after adding insulin, hydrocortisone,
valproic acid and butylated hydroxyanisole, with expression of
nestin, GFAP and NeuN proteins, process augmented by addition of
EGF and bFGF.
Regarding paracrine actions, it is widely agreed
that ASC administration increases levels of neurotrophic factors
such as BDNF irrespective of cellular engraftment (37); similarly a cell-free ASC extract was
shown to modify substantially the expression of many genes involved
in inflammation, immune response and apoptosis (38). Another important action of stem cells
is the increase in VEGF and angiopoetin-1 production in brain
endothelial cells and astrocytes, which increases angiogenesis
(capillary tube formation) and vascular stabilization (39).
Our method for preparing the cellular suspension
focuses on the isolation of ASCs while preserving as much as
possible the GF contained in the lipoaspirate; stem cells are
isolated from the lipoaspirate after treatment with collagenase,
filtration, centrifugation and subsequent incubation with
CD271+ monoclonal antibodies coated microspheres.
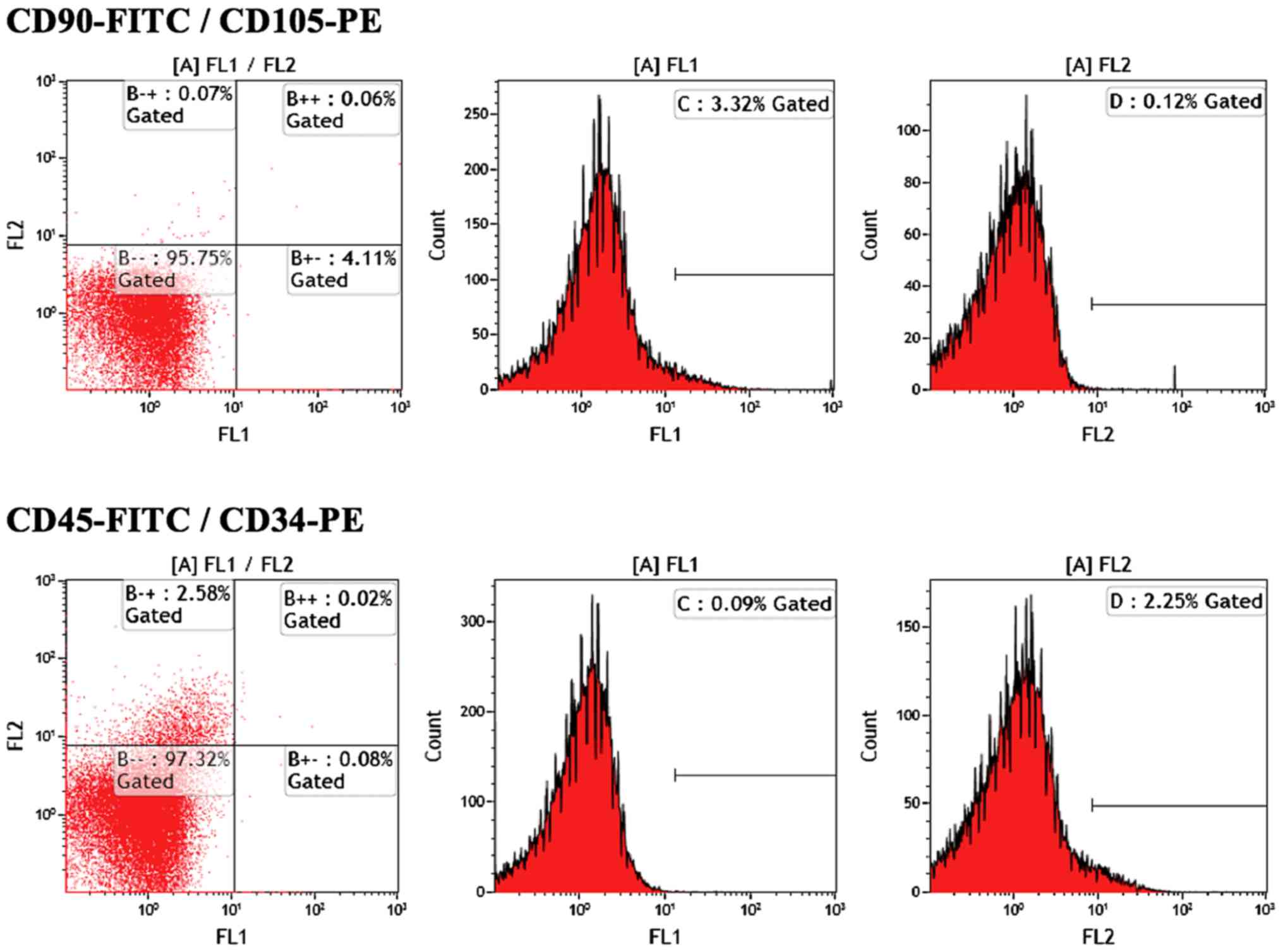
Fig. 3 shows flow
cytometry from a fresly cultured ASC suspension, with cells showing
no CD45 markers, 2% CD34+ and 4% CD90+.
The CD271 surface marker is a cell receptor
belonging to the tumor necrosis factor (TNF) superfamily, also
known as the low-affinity nerve growth factor receptor (LNGFR) or
p75NTR (40) which was shown to be
present on a specific population of stem cells, classically defined
by cell markers CD73, CD90 and CD105 and plastic adherence
(41).
CD271+ stem cells were successfully
obtained from lipoaspirates (42)
and it was shown to be one of the best markers (followed by CD146,
CD106, CD13) for in vitro culturing and expansion of
mesenchymal stem cells; CD271+ stem cells also have the
advantage of producing colony-forming unit-fibroblast (CFU-F), an
important factor influencing grafting and development, activity
which was shown to be absent in the CD271- cells
(43).
CD271+ cells were also shown to have
superior paracrine actions than classic plastic-adherent
mesenchymal stem cells (PA-MSCs) by secreting higher levels of
molecules with chemoattractant actions [monocyte chemoattractant
protein 1 (MCP-1), IL-8, IL-1β], pro-inflammatory [interferon-γ
(IFN-γ), TNF-α], immunosuppressive (IL-10), and differentiation
[granulocyte colony stimulating factor (G-CSF), granulocyte
macrophage colony stimulating factor (GM-CSF)] (40).
To summarize, CD271+ stem cells can be
more easily and efficiently obtained from the respective tissue,
their in vitro enriching potential is among the highest in
the mesenchymal stem cells studied (43) and they have superior potential for
engraftment as a consequence of their immunosuppresive and
lymphohematopoietic properties (40).
For in vitro differentiation of stem cells
into neuronal precursors we used either commercially available
ready-made neuronal medium with no fetal bovine serum, containing
neuronal-specific GFs and penicillin/streptomycin, or have prepared
a culture medium from Dulbecco's Modified Eagle's medium (DMEM) to
which we added neuronal GFs BDNF, IGF-1, ascorbic acid and
aminophylline, which is in line with established neuronal
differentiation protocols (44).
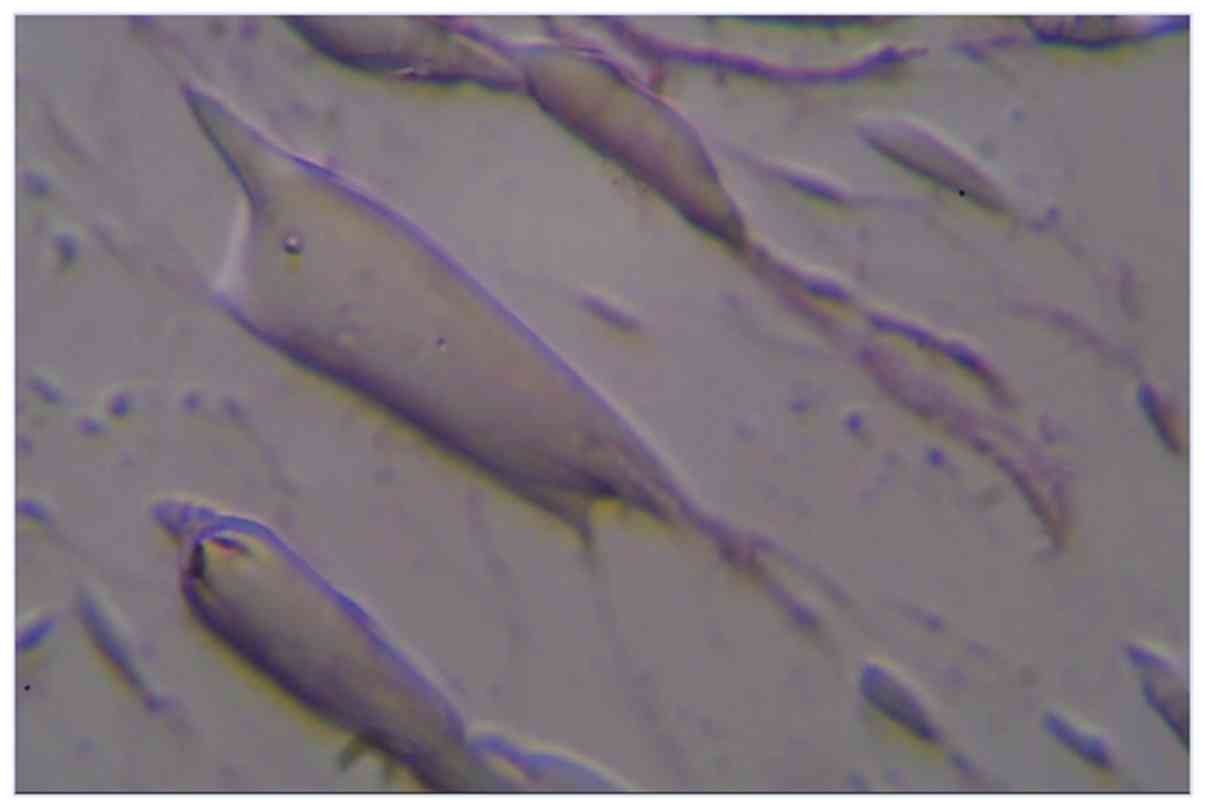
Fig. 4 shows neuronal precursors
with dendritic-like cellular extensions which stain positive with
Cresyl violet, x400.
Patients
ASCs were administered to eight patients who were
treated previously for stroke, which occurred more than 3 months
prior to administration of the stem cell treatment; of these 7
received autologous ASCs and 1 patient of advanced age (77 years
old) was treated with allogeneic cells from a younger first degree
relative. This choice is supported by reports showing that aging
has a deleterious effect on the potential of human MSCs to
differentiate in neurons (45) to
the point that neurogenesis from MSCs is virtually nonexistent in
donors older than 60 years, irrespective of the use of different
protocols which differentiate MSCs to neurons in single or multiple
steps.
Patient demographics, co-morbid conditions and
medication, time of administration of ASCs after stroke, presence
of spasticity, and motor deficit are given in Table I. Of note is that 2 patients were
treated with botulinum toxin for spasticity.
 | Table IInitial patient data. |
Table I
Initial patient data.
| | Age @ CVA, sex | Tx admin after
CVA | Co-morbid
conditions | Spasticity | Motor deficit | Medication |
|---|
| P1 | 54, F | 10 mo | AF, UTI, hepatic
cytolysis, right hemiplegia, reactive depression, anarthria,
expressive aphasia | No | R, aphasia | Concor, tianeptine,
valproic acid |
| P2 | 65, F | 3 mo | Anemia, pressure
ulcers, right femoral fracture, hepatic cytolysis, HBP,
malnutrition | No | L | Indapamide,
amlodipine, piracetam, clopidogrel |
| P3 | 40, F | 31 mo | AF, HBP, septal
hypertrophic cardiomyopathy, severe mitral valve regurgitation;
MTHFR C677T+, factor XIII, PAI 4G/5G | Yes | L | Acenocumarol,
metoprolol, eutirox, baclofen |
| P4 | 35, M | 26 mo | NIDDM, HBP,
nistagmus, dyslipidemia, Thrombophilia MTHFR C677T+,
A1298C+; prothrombin | Yes | L | Acenocumarol,
metoprolol, metformin, atorvastatin |
| P5 | 51, F | 36 mo | Subarachnoid
hemorrhage, left arachnoid cyst 4x9 cm; urinary frequency, vertigo,
spasticity | Yes | L | Baclofen,
betahistine, Aspirin, Feminost |
| P6 | 75, M | 23 mo | Atrial
fibrillation, UTIs, neurological bladder, reactive depression | No | R, aphasia | Dabigatran,
carvedilol, atorvastatin, tianeptin omeprazol |
| P7 | 36, F | 20 mo | Multiple aneurisms
on right middle cerebral, ophtalmic, left cerebellar, supraclinoid,
with surgery and stenting; subarachnoid hemorrhage | Yes | L | HBOT, baclofen,
botulinum toxin |
| P8 | 50, F | 21 mo | Thrombophilia with
homozygous mutations for MTHFR, PAI1, EPCR; dyslipidemia | Yes | R, aphasia | Acenocumarol,
baclofen, Levetiracetam, Fluoxetine botulinum toxin |
Regarding the route of administration, 7 patients
received the cellular suspension via intrathecal injection (lumbar
puncture) and 1 patient via intravenous administration; results are
summarized in Table II.
 | Table IITreatment and results. |
Table II
Treatment and results.
| | No. of
patients | Associated
condition | Route of admin | Cell no. | Allogen | Spasticity
improved | Motor improved | Other |
|---|
| Right
hemiplegia | (3) | Atrial fibrillation
(2); thrombophilia (1) | IT (2) IV (1) |
2.2x106/kg | (1) | Sustained (2) | Minor (1) | Aphasia improved
(2/3) |
| Left
hemiplegia | (5) | Thrombophilia (2),
aneurismal disease (1), brain hemorrhage (1), right femoral
fracture (1) | IT (5) |
3.1x106/kg | | Transient (2)
Sustained (1) | Transient (2) Minor
(1) | Myoclonus (1) |
No correlation was seen (P>0.05; Pearson
correlation r) between the neurologic improvements and number of
ASCs administered, size of ischemic/lacunar brain area, age of
patients, co-morbid conditions, however, due to the small number of
patients we cannot make inferrences on such correlations.
The differences noted were due to the timing of ASC
administration relative to the stroke onset, so that earlier
administration (3 months post-stroke) resulted in the best motor
effects, manifested through sustained but involuntary isometric
contraction of both upper and lower limbs. The other patients had
either no motor improvement or transient improvement in spasticity
(1 point on the modified Ashworth scale) and motor function (1
patient with trombophilia and 1 patient with aneurysmal disease,
both with left hemiparesis) or minor improvements; one patient with
hemorrhagic stroke was able to initiate dorsiflexion of paretic
foot with improved ambulation and stair climbing/descending, which
was helped by a decrease in spasticity, and another patient with
trombophilia and left hemiparesis was able to ambulate better (2
points on Fugl-Meyer on upper extremity).
Aphasia improved in the 2 patients with right
hemiparesis who received ASCs via intrathecal injection, with the
most important improvement observed in a patient progression from
monosyllabic answers to full sentences (6 points increase on Quick
Aphasia Battery); this patient had also improved motor ability of
affected leg and better ambulation (increased balance and
unassisted walking distance).
There was also a difference between the intrathecal
and intravenous administration, with the treatment effects
installed later (approximately 24 h compared to 3-4 h after
intrathecal) and manifested as vertigo, alongside a decrease in
spasticity.
It is worth mentioning that from the two patients
with spasticity who were previously treated with botulinum toxin
and hyperbaric oxygen therapy (HBOT), one had sustained improvement
in spasticity (with iv administration) and another had transient,
episodic amelioration, during which motor ability was significantly
improved (1 point Ashworth Scale and 1 point NIHSS).
Also 2 patients with spasticity had been treated
with HBOT prior to stem cell treatment with minor and transient
improvement in spasticity, but no motor improvement; these two
patients had sustained improvement in spasticity after ASC
treatment.
Side effects were pain after administration starting
3-4 h post intrathecal injection, initially in the lumbar area,
then ascending to cervical area. This was experienced with
different intensity by all patients, and it responded well to
administration of paracetamol, ketoprofen, and/or metamizole.
This pain diminished quickly so that after 24-36 h
there was no need for analgesics.
Three patients experienced transient rise of body
temperature (max 38.5˚C) which also responded well to
paracetamol.
One patient (with allogeneic implant) had a period
of confusion after stem cell administration, which gradually
improved with complete remission after 2 weeks; subsequently this
patient had significant improvement in speech (from monosyllabic
answers to full sentences) and ambulation.
There were no signs of infection either local or
systemic (erythema, edema, fever, leukocytosis, or neurological
symptoms).
Discussion
Administration of MSCs in stroke patients via
intravenous, intra-arterial, intracerebral and intrathecal routes
was performed during the past two decades by many teams in many
countries, mostly with cells from bone marrow, more recently from
adipose tissue and umbilical cord blood (46,47).
Our search in the clinicaltrials.gov database for ‘stem cells’ and
‘stroke’ in January 2020 listed 86 ongoing or completed clinical
studies, but a majority of them although completed had not posted
results; fewer clinical trials to date were performed with ASCs,
and one such example is AMASCIS-01 trial (48) which involved iv administration of
ASCs in up to 19 patients with subacute stroke, and for which also
no results were posted a few years after completion.
Despite the high interest in the field, published
results of MSC treatment in stroke patients are not as numerous and
most have a low number of patients: 5 MSC-treated patients and 25
controls (49) had a significant
difference in Barthel index at 3 and 6 months, but no significant
difference at 12 months or modified Rankin; 16 MSC-treated iv and
36 controls (50) showed that
clinical improvement in the MSC group was associated with serum
levels of stromal cell-derived factor-1 (Sdf-1) and the degree of
involvement of the subventricular region of the lateral ventricle;
in another study 12 stroke patients safely received 4 intrathecal
injections each with modest improvement (51).
A meta-analysis of 7 clinical trials performed until
August 2018 on MSC treatments for stroke patients (52) showed that most studies had less than
32 participants; result-wise there was long-term (at least 6
months) improvement in motor function assessed with the National
Institute of Health Stroke Scale, but not Barthel Index nor
modified Rankin.
While the iv/intra-arterial/intra-thecal
administration of MSCs was mostly done in acute/subacute stroke
patients with modest improvements, small studies showed better
results via neurosurgery (53) so
that 16 of 18 patients had motor improvement at 12 months after
intracerebral implant of MSCs in chronic stroke patients. To our
knowledge there is no known report of administration of
CD271+ cells to stroke patients to which we could
compare our results.
A crucial aspect of stem cell treatment is the
homing of stem cells in the brain after their administration; it
was shown that Sdf-1 levels (46)
elevated in subacute phase of stroke favors homing of stem cells in
ischemic areas and a similar finding was published by a different
team (54), however, we do not know
if measuring the level of Sdf-1 has predictive value for successful
administration of stem cells in specific patients and ASC homing
remains an aspect in need of clarification (55).
A multitude of intertwined factors are acting in
concert and influencing stem cell multiplication and
differentiation; they can be grouped in signaling pathways based on
respective sequences and roles: pro-multiplication-Jak/Stat,
modulated by cytokines such as G-CSF interferons, granulocyte
colony-stimulating factor (G-CSF), and interleukins (IL-6 and
IL-10); Sonic Hedgehog (SHh) and Notch Signaling Pathways,
important in proliferation and differentiation of neural stem
cells; Wnt/β-catenin, important in embryonic development and
tumorigenesis in adult tissues, and PI3K/Akt which is intertwined
with the MAPK/ERK path via protein kinase B (Akt-1) which blocks
apoptosis; and pro-differentiation: MAPK/ERK; activated through
tyrosine kinase and G-protein receptors, and extracellular
effectors such as EGF and FGF; and the TGF-β signaling pathway
which activates apoptosis; finally at epigenetic levels important
roles are held by demethylases (ex ascorbic acid) and histone
deacetylases (ex valproic acid) (56).
Testing limitations make objective verification of
homing and grafting of stem cells in the human brain difficult;
using Technetium-99m-labeled autologous bone-marrow mononuclear
cells (BMMNCs) in 6 patients (55)
with chronic ischemic stroke (days 59-82 post-stroke) of the middle
cerebral artery (MCA); around 2x107 cells were labeled
with 99mTc from a total of 1.25-5x108 and
intraarterial administration into MCA. There were no complications
at the 120-day follow-up; whole body scintigraphy with
Single-Photon Emission Computed Tomography (SPECT) showed at 2 h
post-administration the presence of labeled stem cells in the brain
of all patients, with preference for the infarcted hemisphere.
Interestingly, studies performed in rodents
following iv administration of human ASCs marked with green
fluorescent protein (40,42) showed that at 75 days post infusion
with cultured ASCs after 4 passages, most implanted ASCs were found
in lungs and spleen (approximately 10-30 ASCs per 10.000 resident
cells) followed by brain with 2-10 ASCs/10,000 resident cells, and
there were significant differences in ASC tissue distribution
between animals as well as between the left and right brain
hemispheres in some animals (42).
There was also a different cellular marker expression between cells
at passage 0 (freshly harvested) and passage 4, so that initially
there was a higher proportion of CD34+ and
CD45+ cells (50 and 38%, respectively) vs. passage 4 (2
and 7%, respectively), while the opposite trend was observed for
CD90+ and CD271+ markers, which initially
were present in 52 and 5% of cells, and at passage 4 were
identified in 98 and 62% of cultured ASCs (37). The proportion of hematopoietic vs
neuronal precursor cell markers is important because it seem to
greatly influence the site of ASC grafting, as it was shown that
when CD271+ and CD34+ are co-transplanted in
a 1:1 ratio, they migrate preferentially to the brain, while when
administered in a ratio of 8:1 the stem cells migrated
preferentially to the lungs and to a much lesser extent to the
liver, brain and heart (40). The
difference in stem cell homing may be due to differences in tissue
oxygenation levels in combination with local chemokines; proportion
of CD34+ in the transplanted stem cells can be a crucial
factor when administering stem cell treatments for neurological vs
pulmonary pathologies, especially emphysema, for which as yet,
there is no regenerative therapy available.
In conclusion, we have safely administered
CD271+ mesenchymal stem cells to eight patients with
stroke beginning April 2018; those patients had improvements
especially in areas of spasticity, aphasia, and to a lesser degree
in motor strength and coordination. Improvements varied from
patient to patient; some of these were transient (24-36 h) and some
were long-lasting (one year and continuing). Earliest
administration (3 months post stroke) was followed by best motor
improvement, but other factors were not correlated with patient
improvement.
Side effects were mild to moderate (pain responding
well to non-opiate analgesics) and transient; pain was mostly
absent after 48 h; two patients experienced confusion which lasted
approximately 2 weeks but was followed by significant improvements
in aphasia and spasticity.
Based on above data and the cited literature on mesenchymal
stem cells - of which CD271+ represent a subpopulation - it can
be said that treating chronic stroke patients with
CD271+ stem cells administered intrathecally and/or
intravenously is a safe therapeutic option with good results
especially for spasticity and aphasia; at the same time it needs to
be further studied and improved (e.g., repeated or combined
intrathecal/iv administration, control of stem cell homing by
administering different proportions of
CD271+/CD34+) with the goal of obtaining
ample motor improvements and subsequent functional independence of
the patient.
Note added in proof (added October 6, 2023)
Subsequently to the publication of this article, the following changes have been made, which were not included or reflected in the article as it was originally published. The title of the article has been changed to ‘CD271+ stem cell treatment of patients with chronic stroke: A retrospective case series report’, to reflect that this study should be regarded as a case series report. Secondly, the following text (first paragraph) has been added at the start of the ‘Treatment’ subsection: ‘This is a case series report, resulting from non‑standardized administration of treatments to individual patients on a case‑by‑case basis (under the EU rules governing compassionate care and hospital exemption), not under homogenous inclusion/exclusion criteria that would be applicable for a clinical study. The resultant patient data were analysed retrospectively, and the intention of this article was to provide insights into this type of treatment, which is currently undergoing further developments and refinements.’ Thirdly, on p. 2061, the left‑hand column, the first sentence in the final paragraph of the Discussion has been changed to read as follows: ‘Based on above data and the cited literature on mesenchymal stem cells - of which CD271+ represent a subpopulation - it can be said that treating chronic stroke patients with CD271+ stem cells administered intrathecally and/or intravenously is a safe therapeutic option with good results especially for spasticity and aphasia; at the same time it needs to be further studied and improved (e.g., repeated or combined intrathecal/iv administration, control of stem cell homing by administering different proportions of CD271+/CD34+) with the goal of obtaining ample motor improvements and subsequent functional independence of the patient.’
Acknowledgements
Dr Mihaela Economescu-Chivu (Victor Babes Institute)
helped with the flow cytometry.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
FS: manuscript writing, data collection and
analysis; GP: manuscript planning and data evaluation; GL:
manuscript planning and data evaluation; DAS: manuscript planning
and data evaluation; AT: manuscript planning and data evaluation;
MF: data collection; CB: manuscript planning and evaluation; CB:
manuscript review and data evaluation.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Informed consent was obtained from each patient
prior to procedure.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Claude N, Christakis M and Tsatsakis AM:
Stem cell technologies in toxicology assessments. Toxicology.
270:1–2. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tsatsakis A, Docea AO, Calina D, Tsarouhas
K, Zamfira LM, Mitrut R, Sharifi-Rad J, Kovatsi L, Siokas V,
Dardiotis E, et al: A mechanistic and pathophysiological approach
for stroke associated with drugs of abuse. J Clin Med.
8(1295)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dardiotis E, Aloizou AM, Markoula S,
Siokas V, Tsarouhas K, Tzanakakis G, Libra M, Kyritsis AP, Brotis
AG, Aschner M, et al: Cancer-associated stroke: Pathophysiology,
detection and management (Review). Int J Oncol. 54:779–796.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Devetzi M, Goulielmaki M, Khoury N,
Spandidos DA, Sotiropoulou G, Christodoulou I and Zoumpourlis V:
Genetically modified stem cells in treatment of human diseases:
Tissue kallikrein (KLK1) based targeted therapy (Review). Int J Mol
Med. 41:1177–1186. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yu X, Wang X, Zeng S and Tuo X: Protective
effects of primary neural stem cell treatment in ischemic stroke
models. Exp Ther Med. 16:2219–2228. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bunevicius A, Yuan H and Lin W: The
potential roles of 18F-FDG-PET in management of acute stroke
patients. Biomed Res Int. 2013(634598)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dundar A, Bold MS, Agac B, Kendi AT and
Friedman SN: Stroke detection with 3 different PET tracers. Radiol
Case Rep. 14:1447–1451. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aiello M, Cavaliere C, Marchitelli R,
d'Albore A, De Vita E and Salvatore M: Hybrid PET/MRI methodology.
Int Rev Neurobiol. 141:97–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Grønberg NV, Johansen FF, Kristiansen U
and Hasseldam H: Leukocyte infiltration in experimental stroke. J
Neuroinflammation. 10(115)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kernagis DN and Laskowitz DT: Evolving
role of biomarkers in acute cerebrovascular disease. Ann Neurol.
71:289–303. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Quillinan N, Herson PS and Traystman RJ:
Neuropathophysiology of Brain Injury. Anesthesiol Clin. 34:453–464.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pradeep H, Diya JB, Shashikumar S and
Rajanikant GK: Oxidative stress - assassin behind the ischemic
stroke. Folia Neuropathol. 50:219–230. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dluzniewska J, Sarnowska A, Beresewicz M,
Johnson I, Srai SK, Ramesh B, Goldspink G, Górecki DC and Zabłocka
B: A strong neuroprotective effect of the autonomous C-terminal
peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J. 19:1896–1898.
2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sohrabji F: Estrogen-IGF-1 interactions in
neuroprotection: Ischemic stroke as a case study. Front
Neuroendocrinol. 36:1–14. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Smith PF: Neuroprotection against
hypoxia-ischemia by insulin-like growth factor-I (IGF-I). IDrugs.
6:1173–1177. 2003.PubMed/NCBI
|
|
16
|
Zeng W, Ju R and Mao M: Therapeutic
potential of hepatocyte growth factor against cerebral ischemia
(Review). Exp Ther Med. 9:283–288. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ji Q, Ji Y, Peng J, Zhou X, Chen X, Zhao
H, Xu T, Chen L and Xu Y: Increased brain-specific miR-9 and
miR-124 in the serum exosomes of acute ischemic stroke patients.
PLoS One. 11(e0163645)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jadavji NM, Emmerson JT, MacFarlane AJ,
Willmore WG and Smith PD: B-vitamin and choline supplementation
increases neuroplasticity and recovery after stroke. Neurobiol Dis.
103:89–100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jadavji NM, Emmerson JT, Shanmugalingam U,
MacFarlane AJ, Willmore WG and Smith PD: A genetic deficiency in
folic acid metabolism impairs recovery after ischemic stroke. Exp
Neurol. 309:14–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim JM, Stewart R, Park MS, Kang HJ, Kim
SW, Shin IS, Kim HR, Shin MG, Cho KH and Yoon JS: Associations of
BDNF genotype and promoter methylation with acute and long-term
stroke outcomes in an East Asian cohort. PLoS One.
7(e51280)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wei LK, Au A, Menon S, Gan SH and
Griffiths LR: Clinical relevance of MTHFR, eNOS, ACE, and ApoE gene
polymorphisms and serum vitamin profile among Malay patients with
ischemic stroke. J Stroke Cerebrovasc Dis. 24:2017–2025.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Webb RL, Kaiser EE, Scoville SL, Thompson
TA, Fatima S, Pandya C, Sriram K, Swetenburg RL, Vaibhav K, Arbab
AS, et al: Human neural stem cell extracellular vesicles improve
tissue and functional recovery in the murine thromboembolic stroke
model. Transl Stroke Res. 9:530–539. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Stancioiu F and Makk R: Post-stroke
recovery of motor function with a new combination of medicines - A
pilot study. EJMO. 3:167–181. 2019.
|
|
24
|
Chun SY, Lim JO, Lee EH, Han MH, Ha YS,
Lee JN, Kim BS, Park MJ, Yeo M, Jung B and Kwon TG: Preparation and
characterization of human adipose tissue-derived extracellular
matrix, growth factors, and stem cells: a concise review. Tissue
Eng Regen Med. 16:385–393. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bogdanova A, Berzins U, Nikulshin S,
Skrastina D, Ezerta A, Legzdina D and Kozlovska T: Characterization
of human adipose-derived stem cells cultured in autologous serum
after subsequent passaging and long term cryopreservation. J Stem
Cells. 9:135–148. 2014.PubMed/NCBI
|
|
26
|
Kozlowska U, Krawczenko A, Futoma K, Jurek
T, Rorat M, Patrzalek D and Klimczak A: Similarities and
differences between mesenchymal stem/progenitor cells derived from
various human tissues. World J Stem Cells. 11:347–374.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mazini L, Rochette L, Amine M and Malka G:
Regenerative capacity of adipose derived stem cells (ADSCs),
comparison with mesenchymal stem cells (MSCs). Int J Mol Sci.
20(E2523)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Barilani M, Banfi F, Sironi S, Ragni E,
Guillaumin S, Polveraccio F, Rosso L, Moro M, Astori G, Pozzobon M
and Lazzari L: Low-affinity nerve growth factor receptor (CD271)
heterogeneous expression in adult and fetal mesenchymal stromal
cells. Sci Rep. 8(9321)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Heo JS, Choi Y, Kim HS and Kim HO:
Comparison of molecular profiles of human mesenchymal stem cells
derived from bone marrow, umbilical cord blood, placenta and
adipose tissue. Int J Mol Med. 37:115–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li CY, Wu XY, Tong JB, Yang XX, Zhao JL,
Zheng QF, Zhao GB and Ma ZJ: Comparative analysis of human
mesenchymal stem cells from bone marrow and adipose tissue under
xeno-free conditions for cell therapy. Stem Cell Res Ther.
6(55)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nakao N, Nakayama T, Yahata T, Muguruma Y,
Saito S, Miyata Y, Yamamoto K and Naoe T: Adipose tissue-derived
mesenchymal stem cells facilitate hematopoiesis in vitro and in
vivo: Advantages over bone marrow-derived mesenchymal stem cells.
Am J Pathol. 177:547–554. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Urrutia DN, Caviedes P, Mardones R,
Minguell JJ, Vega-Letter AM and Jofre CM: Comparative study of the
neural differentiation capacity of mesenchymal stromal cells from
different tissue sources: An approach for their use in neural
regeneration therapies. PLoS One. 14(e0213032)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
O'Connor KC: Molecular profiles of
cell-to-cell variation in the regenerative potential of mesenchymal
stromal cells. Stem Cells Int. 2019(5924878)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ikegame Y, Yamashita K, Hayashi S, Mizuno
H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, et
al: Comparison of mesenchymal stem cells from adipose tissue and
bone marrow for ischemic stroke therapy. Cytotherapy. 13:675–685.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Quirici N, Scavullo C, de Girolamo L, Lopa
S, Arrigoni E, Deliliers GL and Brini AT: Anti-L-NGFR and -CD34
monoclonal antibodies identify multipotent mesenchymal stem cells
in human adipose tissue. Stem Cells Dev. 19:915–925.
2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Safford KM, Hicok KC, Safford SD,
Halvorsen YD, Wilkison WO, Gimble JM and Rice HE: Neurogenic
differentiation of murine and human adipose-derived stromal cells.
Biochem Biophys Res Commun. 294:371–379. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chung JY, Kim W, Im W, Yoo DY, Choi JH,
Hwang IK, Won MH, Chang IB, Cho BM, Hwang HS, et al:
Neuroprotective effects of adipose-derived stem cells against
ischemic neuronal damage in the rabbit spinal cord. J Neurol Sci.
317:40–46. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jeon D, Chu K, Lee ST, Jung KH, Ban JJ,
Park DK, Yoon HJ, Jung S, Yang H, Kim BS, et al: Neuroprotective
effect of a cell-free extract derived from human adipose stem cells
in experimental stroke models. Neurobiol Dis. 54:414–420.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Doeppner TR and Hermann DM: Mesenchymal
stem cells in the treatment of ischemic stroke: progress and
possibilities. Stem Cells Cloning. 3:157–163. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kuçi S, Kuçi Z, Kreyenberg H, Deak E,
Pütsch K, Huenecke S, Amara C, Koller S, Rettinger E, Grez M, et
al: CD271 antigen defines a subset of multipotent stromal cells
with immunosuppressive and lymphohematopoietic
engraftment-promoting properties. Haematologica. 95:651–659.
2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Álvarez-Viejo M, Menéndez-Menéndez Y and
Otero-Hernández J: CD271 as a marker to identify mesenchymal stem
cells from diverse sources before culture. World J Stem Cells.
7:470–476. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Meyerrose TE, De Ugarte DA, Hofling AA,
Herrbrich PE, Cordonnier TD, Shultz LD, Eagon JC, Wirthlin L, Sands
MS, Hedrick MA, et al: In vivo distribution of human
adipose-derived mesenchymal stem cells in novel xenotransplantation
models. Stem Cells. 25:220–227. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jones E and McGonagle D: Human bone marrow
mesenchymal stem cells in vivo. Rheumatology (Oxford). 47:126–131.
2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
George S, Hamblin MR and Abrahamse H:
Differentiation of mesenchymal stem cells to neuroglia: in the
context of cell signalling. Stem Cell Rev Rep. 15:814–826.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hermann A, List C, Habisch HJ, Vukicevic
V, Ehrhart-Bornstein M, Brenner R, Bernstein P, Fickert S and
Storch A: Age-dependent neuroectodermal differentiation capacity of
human mesenchymal stromal cells: Limitations for autologous cell
replacement strategies. Cytotherapy. 12:17–30. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Surugiu R, Olaru A, Hermann DM, Glavan D,
Catalin B and Popa-Wagner A: Recent advances in mono- and combined
stem cell therapies of stroke in animal models and humans. Int J
Mol Sci. 20(6029)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gutiérrez-Fernández M, Rodríguez-Frutos B,
Otero-Ortega L, Ramos-Cejudo J, Fuentes B and Díez-Tejedor E:
Adipose tissue derived stem cells in stroke treatment, from bench
to bedside. Discov Med. 16:37–43. 2013.PubMed/NCBI
|
|
48
|
U.S. National Library of Medicine:
Reparative therapy in acute ischemic stroke with allogenic
mesenchymal stem cells from adipose tissue, safety assessment, a
randomised, double blind placebo controlled single center pilot
clinical trial (AMASCIS-01). http://ClinicalTrials.govsimpleClinicalTrials.gov
Identifier: NCT01678534. https://clinicaltrials.gov/ct2/show/NCT01678534?term=stem+cells&cond=stroke&draw=3&rank=16.
Accessed January 10, 2020.
|
|
49
|
Bang OY, Lee JS, Lee PH and Lee G:
Autologous mesenchymal stem cell transplantation in stroke
patients. Ann Neurol. 57:874–882. 2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH
and Bang OY: STARTING collaborators. A long-term follow-up study of
intravenous autologous mesenchymal stem cell transplantation in
patients with ischemic stroke. Stem Cells. 28:1099–1106.
2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Pan K, Deng L, Chen P, Peng Q, Pan J, Wu Y
and Yang Y: Safety and feasibility of repeated intrathecal
allogeneic bone marrow-derived mesenchymal stromal cells in
patients with neurological diseases. Stem Cells Int.
2019(8421281)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Boncoraglio GB, Ranieri M, Bersano A,
Parati EA and Del Giovane C: Stem cell transplantation for ischemic
stroke. Cochrane Database Syst Rev. 5(CD007231)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Steinberg GK, Kondziolka D, Wechsler LR,
Lunsford LD, Kim AS, Johnson JN, Bates D, Poggio G, Case C,
McGrogan M, et al: Two-year safety and clinical outcomes in chronic
ischemic stroke patients after implantation of modified bone
marrow-derived mesenchymal stem cells (SB623): A phase 1/2a study.
J Neurosurg. 131:1462–1472. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hill WD, Hess DC, Martin-Studdard A,
Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE and
Conway SJ: SDF-1 (CXCL12) is upregulated in the ischemic penumbra
following stroke: Association with bone marrow cell homing to
injury. J Neuropathol Exp Neurol. 63:84–96. 2004.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Barbosa da Fonseca LM, Gutfilen B, Rosado
de Castro PH, Battistella V, Goldenberg RC, Kasai-Brunswick T,
Chagas CL, Wajnberg E, Maiolino A, Salles Xavier S, et al:
Migration and homing of bone-marrow mononuclear cells in chronic
ischemic stroke after intra-arterial injection. Exp Neurol.
221:122–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Samoilova EM, Kalsin VA, Kushnir NM,
Chistyakov DA, Troitskiy AV and Baklaushev VP: Adult neural stem
cells: basic research and production strategies for
neurorestorative therapy. Stem Cells Int.
2018(4835491)2018.PubMed/NCBI View Article : Google Scholar
|