Introduction
Blood circulation plays a fundamental role in the
body homeostasis by supporting the critical functions including the
transport of water, oxygen, nutrients, and hormones as well as
carbon dioxide and metabolic waste from cells for removal.
With the change of modern lifestyle, people suffer
from chilliness due to mental stress (1) and excessive use of air-conditioning
systems (2). Raynaud's phenomenon
(RP) is a disease caused by vasoconstriction of cutaneous
arterioles induced by cold exposure and mental stress and is
accompanied by such characteristic symptoms as cold sensation in
the limbs, pain, numbness, swelling, and digital ulcers (3,4). Current
pharmacological treatments including calcium channel blockers,
phosphodiesterase type 5 inhibitors, intravenous prostanoids, and
topical nitrates fail to completely control RP and are not well
tolerated by some patients (4).
Aged garlic extract (AGE) is a unique garlic product
prepared through long extraction processes with water-ethanol for
more than 10 months (5). During this
period, many harsh and strong odor substances (i.e., allicin) in
raw garlic are converted into non-irritating water-soluble
sulfur-containing amino acids such as S-allylcysteine (SAC),
S-1-propenylcysteine (S1PC), and
S-allylmercaptocysteine (SAMC) (6,7).
Previous studies have demonstrated the beneficial
effect of AGE on hypertension (8-12),
atherosclerosis (13-15),
metabolic syndrome (16), and
gingivitis (17). Furthermore,
recent studies have revealed that AGE and its constituents, SAC and
S1PC, exert such desirable effects as anti-hypertension (18-22),
cardioprotection (23),
antioxidation (24-27),
antiaging (27,28), immunomodulation (29-31),
anti-fatigue (32,33), anti-stress (34,35),
anti-inflammation (36-39),
and liver protection (40).
Several previous studies show that AGE improves the
peripheral circulatory disturbances. One clinical study using
thermography demonstrated that AGE supplementation increases
microcirculation and improves a variety of symptoms in patients
(41). In another clinical study,
AGE has been shown to enhance cutaneous microcirculation in
patients with the increased risk of cardiovascular events (42). In addition, an animal study
demonstrated that AGE can reverse the decrease in the rectal
temperature of mice induced by cooling (32,43).
Furthermore, administration of S1PC, a characteristic constituent
of AGE increased the blood flow of tail skin in spontaneously
hypertensive rats (SHR) (21).
The above reports indicate that AGE is effective in
improving the peripheral circulation in both animal and human. At
present, however, the mechanism whereby AGE improves the
circulation remains unclear. Hence, this study was conducted with
the aim to identify the active constituent of AGE and to clarify
its mechanism of action using the model of cold-induced reduction
in the tail blood flow of rat.
Materials and methods
Chemicals
AGE was prepared from cloves of garlic (Allium
sativum L.) through the process of rinsing with purified water,
slicing, soaking in ethanol 20-50% (v/v), and extraction/aging for
more than 10 months (5). S1PC was
isolated and purified from AGE using preparative high-performance
liquid chromatography (HPLC), as previously reported (7). SAC and SAMC were synthesized according
to previous methods (7). Chemicals
for synthesis of compounds and post-column HPLC detection reagents
were obtained from Tokyo Chemical Industry. Solvents for HPLC were
purchased from Wako Pure Chemicals Industry. Authentic sulfur
compounds for identification were synthesized and purified
according to the previous report (7).
HPLC analysis
HPLC analysis was performed by post-column HPLC
using a NEXERA X2 system (Shimadzu, Kyoto, Japan) according to the
previous report (7). The column
utilized for separation was a Cadenza CD-C18 column (2.0 x 150 mm,
3 µm; Imtakt Corporation). The sulfur compounds were derivatized
with the hexaiodoplatinate reagents (Tokyo Chemical Industry) and
detected at 500 nm absorbance using diode array detector.
Animals and test substances
Specific pathogen-free male Wistar rats were
obtained at the age of 7 weeks from Japan SLC, Inc (Shizuoka,
Japan). Rats were kept at 23±3˚C and 50±10% humidity under a 12-h
light-dark cycle (light 7:00 a.m.-7:00 p.m.), with free access to
commercially available hard feed (CE-2) and water, until the
experimental use at 10 to 14-weeks of age. AGE was diluted at 0.2
g/ml with distilled water (DW). SAC, S1PC, and SAMC were dissolved
in DW. All test substances (AGE, SAC, S1PC, and SAMC) were
administered to rats in a volume of 10 ml/kg BW using Teflon
feeding needles. The control group was orally administered DW in a
volume of 10 ml/kg BW. Animal experiments were approved by the
Animal Care and Use Committee of Wakunaga Pharmaceutical Co., Ltd.
(approval no. 258). This investigation conformed with the Guide for
the Care and Use of Laboratory Animals published by the US National
Institute of Health (NIH Publication, 8th edition, 2011).
Cold-induced model of reduced tail
blood flow
The cold-induced model of peripheral circulation
disorder was designed based on the rat cooling RP (44) and cold blood stasis syndrome models
(45,46). After being acclimatized to the
experiment room at 23˚C for at least 30 min, rats were examined for
skin blood flow before treatment and then orally administered test
substances. Two hours later, the rats were placed in restrictive
cages (KN-468-B; Natsume) for cooling and submersed in tank filled
with 15˚C water up to the xiphoid processes for 10 min. In the case
of non-cooling experiments, rats were placed in restrictive cages
for 10 min without cooling. After being returned to rearing cages,
the rats were examined for the tail blood flows 1 h after
cooling.
Measurement of tail blood flow
The tail blood flow of rats was measured by using a
contact laser Doppler blood flow meter (FLO-C1; Omegawave). Each
rat was placed in a holder under anesthesia with isoflurane (1
l/min) and its tail blood flow was examined for 3 min. The probe
for the measurement was attached 5 cm apart from the base of the
tail. Rats were cooled for 10 min at 2 h after administering AGE (2
g/kg BW; n=10) or S1PC (6.5 mg/kg BW; n=9), and then measured for
their tail blood flows at 1, 2 and 3 h after cooling. For the
comparison of the effect of three sulfur constituents, rats were
orally administered SAC (7.9 mg/kg BW), SAMC (1.3 mg/kg BW), or
S1PC (0.26, 1.3 and 6.5 mg/kg BW), followed by cooling for 10 min
at 2 h later. The tail blood flow was measured at 1 h after cooling
(n=10). The dose of SAC (7.9 mg/kg BW), SAMC (1.3 mg/kg BW), and
S1PC (6.5 mg/kg BW) was equivalent to the amount contained in AGE
at 2 g/kg BW. In another study, rats were also measured for their
tail blood flow without cooling after oral administration of AGE (2
g/kg BW) or S1PC (6.5 mg/kg BW; n=6).
Measurements of plasma NOx level and
vascular NO-related phosphorylation
Rats were cooled for 10 min at 2 h after
administration of S1PC (6.5 mg/kg BW) or DW (control) and then
anesthetized with isoflurane (1 l/min) to collect the blood from
the orbital vein at 1 h after cooling. Another group of rats was
administered DW and did not receive the cooling treatment
(non-cooling control). After centrifugation of blood samples at
1,500 x g for 15 min at 4˚C, plasma samples were obtained and
stored at -80˚C until analysis. Thoracic aortas of rats were also
isolated, frozen by liquid nitrogen, and stored at -80˚C until
analysis. The plasma content of NOx was determined by using a NOx
colorimetric assay kit (Cayman Chemical Company) according to the
manufacturer's instructions. Rat thoracic aortas were homogenized
using the Multi-Beads shocker (Yasui Kikai) and lysed by a RIPA
lysis buffer (Merck KGaA) containing Halt protease and phosphatase
inhibitor (Thermo Fisher Scientific, Inc.). Western blot analysis
of the lysates was performed as previously described (40). In brief, individual proteins were
separated by SDS-PAGE and immunoblotted with an anti-phospho-eNOS
(Ser1177; Cell Signaling Technology, Inc.), anti-eNOS (Santa Cruz
Biotechnology, Inc.), anti-phospho-AMPKα (Thr172; Cell Signaling
Technology, Inc.), anti-AMPKα (ProteinTech Group, Inc.), and
anti-GAPDH antibody (Wako). Immunoreactive bands were visualized
and analyzed on a V3 Western Workflow (Bio-Rad Laboratories, Inc.)
using Image Lab™ software.
Statistical analysis
The data were expressed as mean ± SEM. Statistically
significant differences between test substance-treated and control
DW-treated groups were determined using Student's t-test or one-way
analysis of variance (ANOVA) followed by Bonferroni's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using WinSTAT (R. Fitch Software).
Results
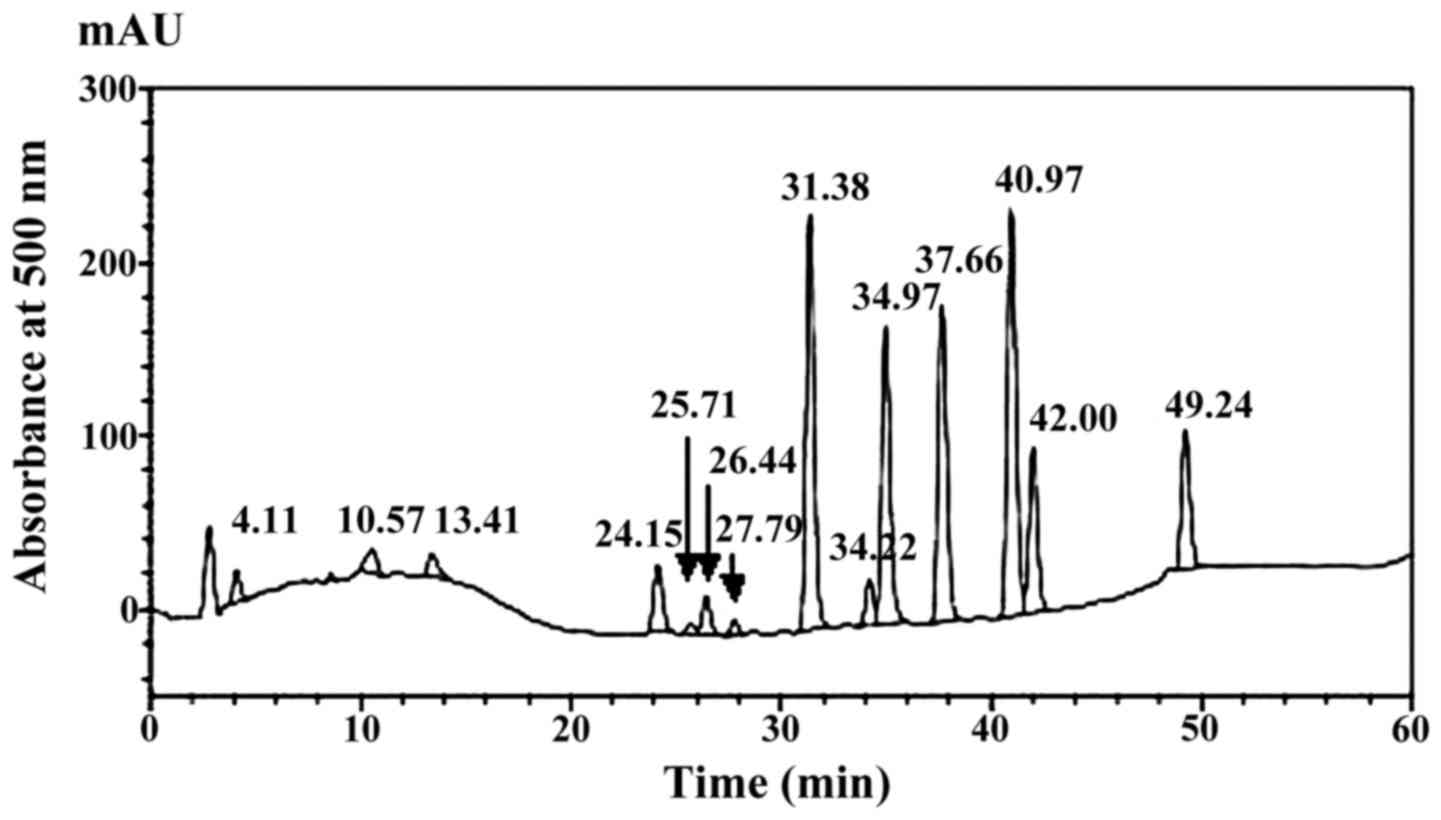
Characterization of hydrophilic sulfur
compounds in AGE
AGE used in this study was analyzed by post-column
HPLC using hexaiodoplatinate reagent for sulfur-specific detection
(7). This analysis detected and
identified 11 hydrophilic sulfur constituents such as
S-methylcysteine, SAC, S1PC, and SAMC which are formed in AGE
through the aging process (7)
(Fig. 1 and Table I).
 | Table ICharacterization of hydrophilic
sulfur compounds in aged garlic extract by post-column HPLC
chromatogram. |
Table I
Characterization of hydrophilic
sulfur compounds in aged garlic extract by post-column HPLC
chromatogram.
| Rt | Mw | Area (%) | Compound |
|---|
| 4.11 | Not determined | 1.29 | Unknown |
| 8.59 | Not determined | 0.12 | Unknown |
| 10.57 | Not determined | 1.52 | Unknown |
| 13.41 | Not determined | 1.36 | Unknown |
| 24.15 | 149 | 3.52 | Methionine |
| 25.71 | Not determined | 0.47 | Unknown |
| 26.44 | 167 | 1.72 |
S-Methylmercaptocysteine |
| 27.79 | Not determined | 0.67 | Unknown |
| 31.38 | 161 | 19.74 |
S-Allylcysteine |
| 34.12 | 161 | 2.12 |
Cis-S-1-propenycysteine |
| 34.97 | 161 | 14.81 |
Trans-S-1-propenycysteine |
| 37.66 | 290 | 16.37 |
γ-Glutamyl-S-allylcysteine |
| 40.97 | 175 | 21.09 |
S-n-Butenylcysteine (internal
standard) |
| 42.00 | 193 | 8.24 |
S-Allylmercaptocysteine |
| 49.24 | 322 | 6.98 |
γ-Glutamyl-S-allylmercaptocysteine |
Effects of AGE and its constituents on
tail blood flow
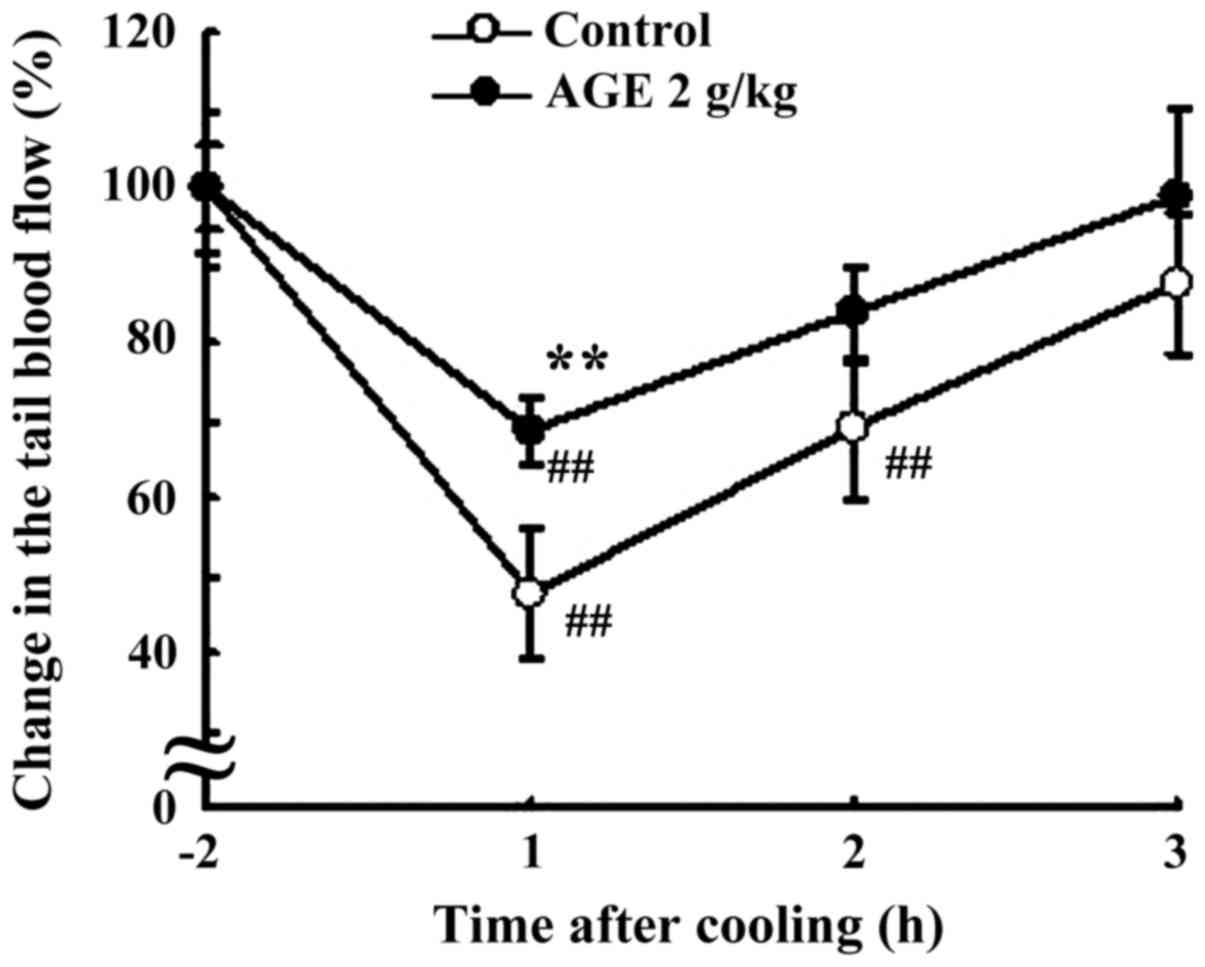
After cooling (15˚C, 10 min), the tail blood flow of
DW-pretreated control rats decreased 53% at 1 h, as compared to the
non-cooling level (P<0.01), and then returned almost to the
initial level at 3 h (Fig. 2). On
the other hand, the tail blood flow of AGE (2 g/kg BW)-pretreated
group was significantly (P<0.01) higher than that of
DW-pretreated control group after cooling and returned to almost
initial non-cooling level (Fig.
2).
The effect of AGE constituents, SAC, S1PC, and SAMC,
on the blood flow was also examined. As shown in Fig. 3, pretreatment with S1PC (6.5 mg/kg
BW) significantly (P<0.05) improved the reduction in the tail
blood flow compared to the pretreatment with DW (control) at 1 h
after cooling. However, pretreatment with SAC or SAMC showed no
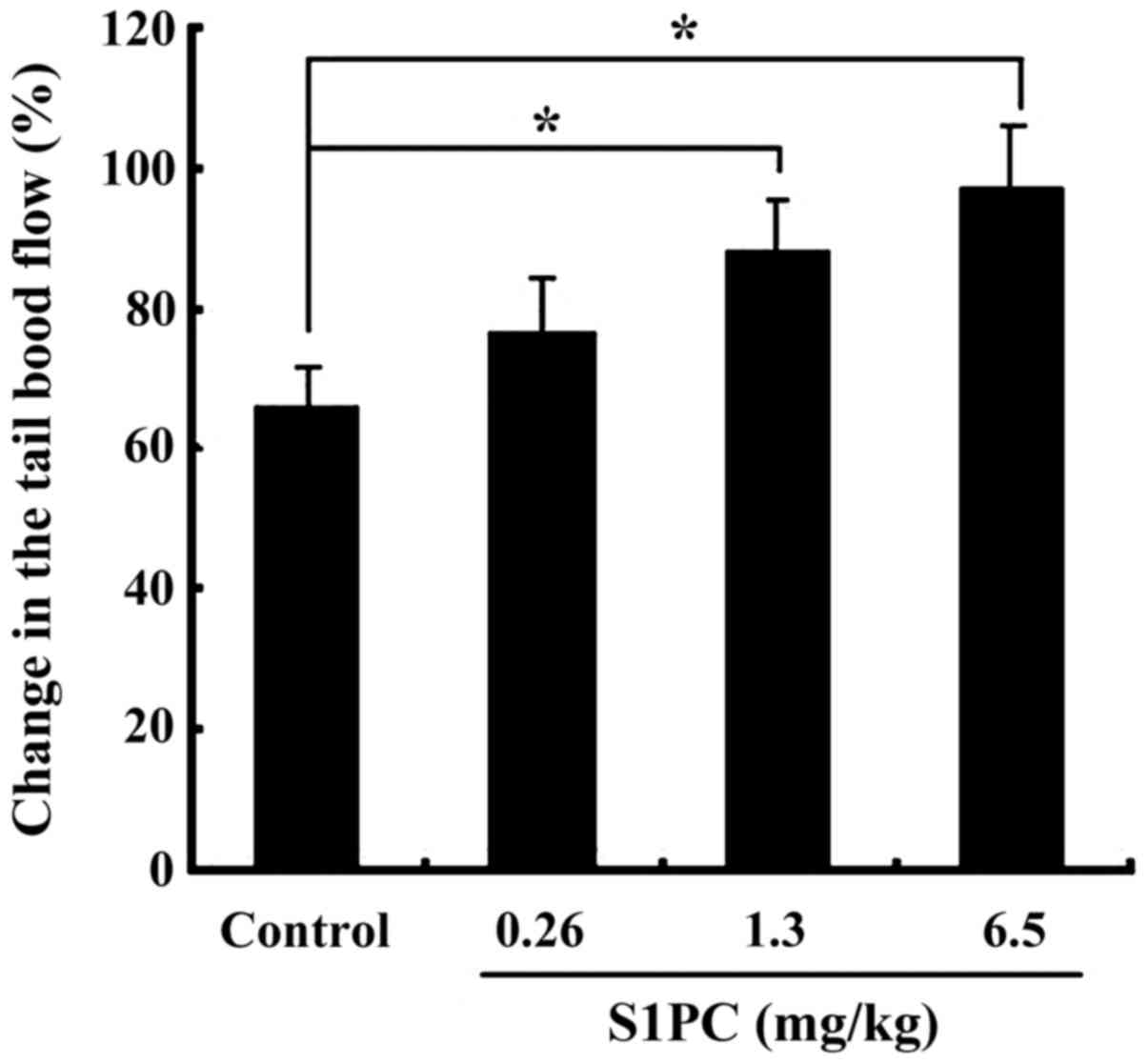
significant improvement. As shown in Fig. 4, the effect of S1PC is
dose-dependent: and the dose of S1PC 6.5 mg/kg BW produced a larger
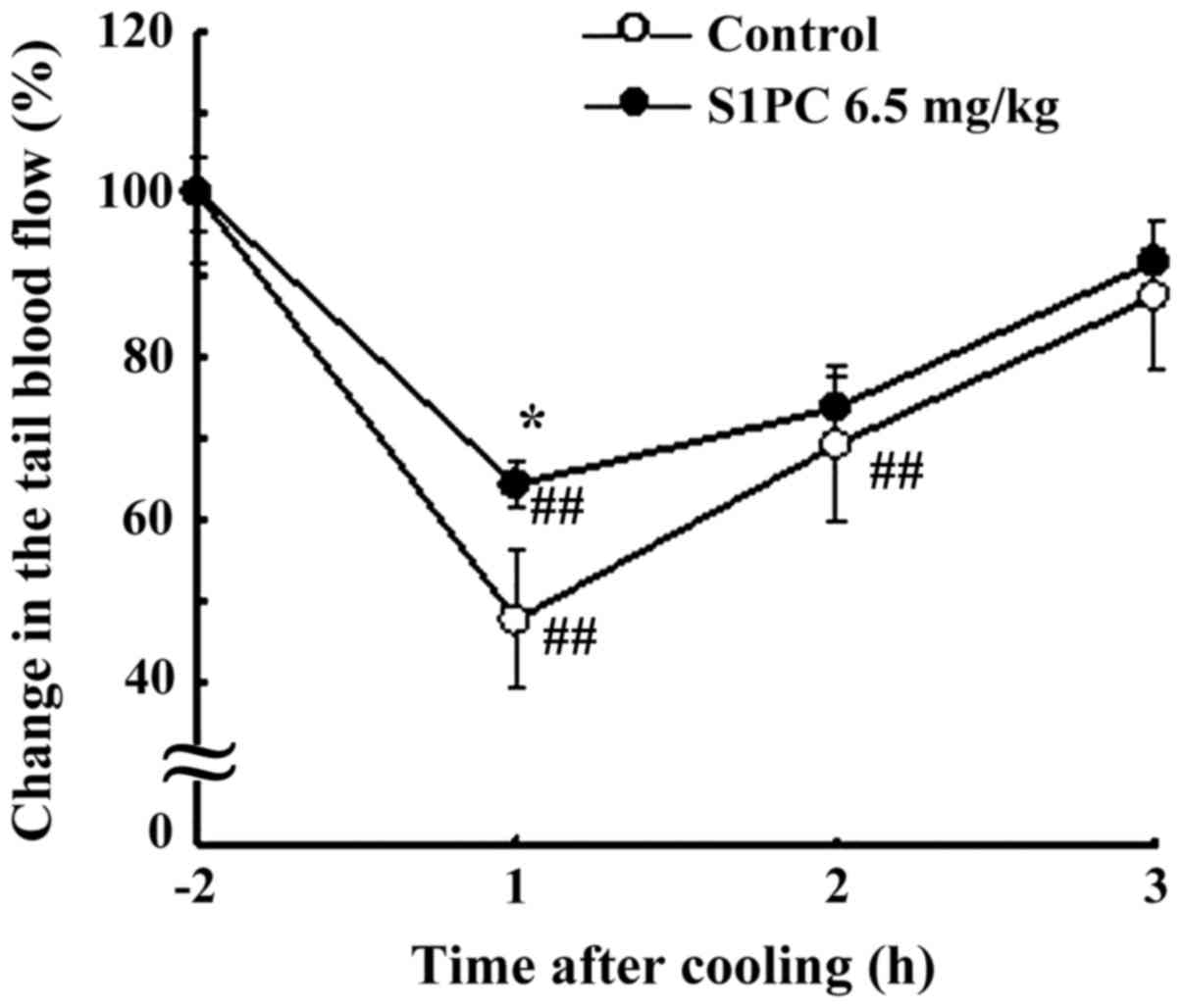
increase of tail blood flow. Fig. 5
showed the time course pattern of tail blood flow in the S1PC (6.5
mg/kg BW)-pretreated group after cooling. At 1 h after cooling,
tail blood flow was significantly higher (P<0.05) in the
S1PC-pretreated group than in the DW-pretreated control group.
Thereafter, no significant difference was observed in the tail
blood flow between the two groups. Both S1PC and AGE pretreatment
had no significant effect on the tail blood flow of non-cooled rats
(data not shown).
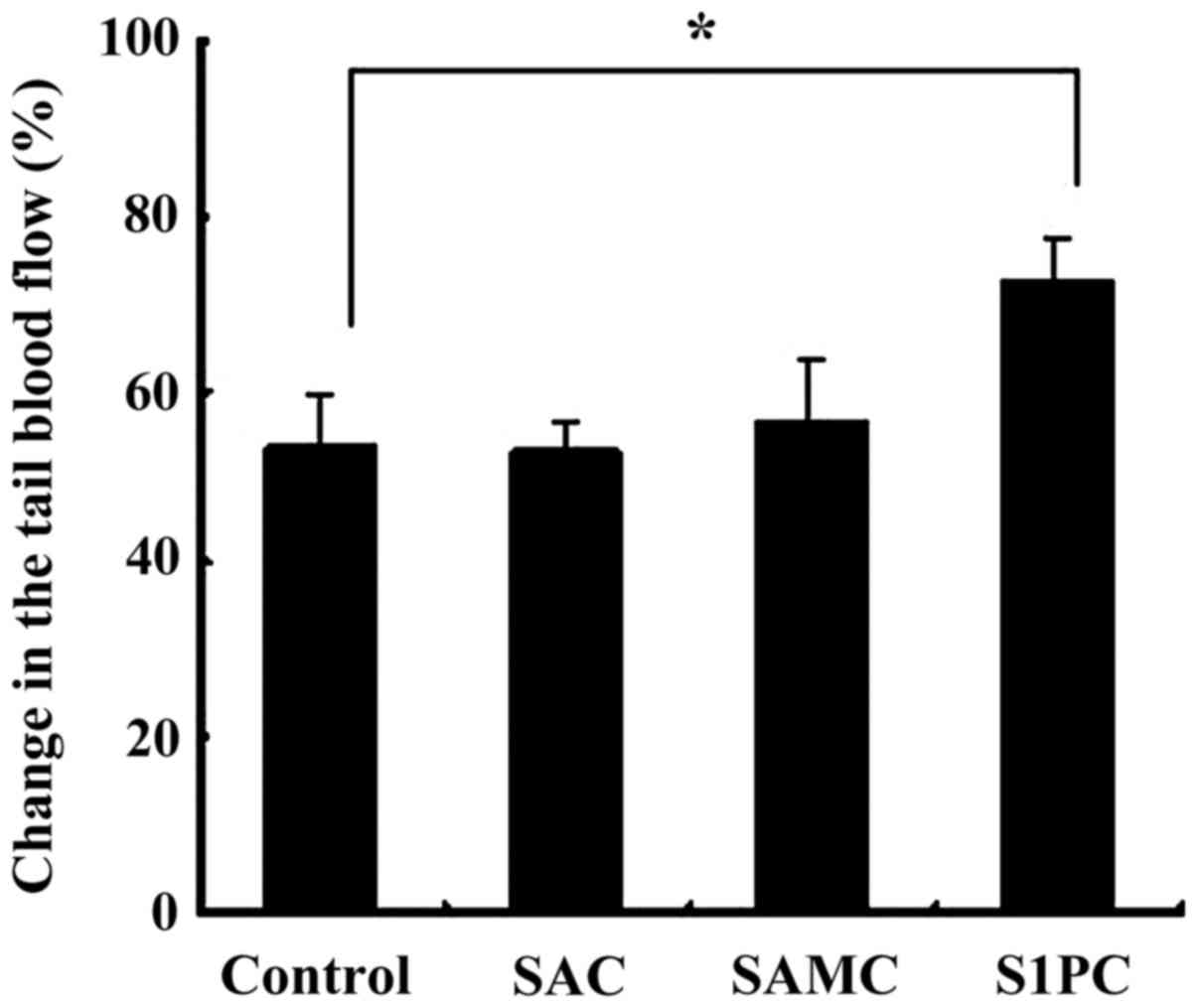 | Figure 3The effect of pretreatment with of
SAC, SAMC, and S1PC on the tail blood flow in cold-treated rats.
SAC, SAMC, and S1PC were orally administered at the dose of 7.9,
1.3 and 6.5 mg/kg BW, respectively, to male Wistar rats 2 h before
cold-treatment (15˚C, 10 min). Distilled water (10 ml/kg BW) was
orally given to control group rats. The tail blood flow was
measured at 2 h before and 1 h after cooling. The level of tail
blood flow at 2 h before cooling is set to 100%. Data are shown as
the means ± SEM (n=10). *Denotes the significant
difference compared with the control group (P<0.05). SAC,
S-allylcysteine; SAMC, S-allylmercaptocysteine; S1PC,
S-1-propenylcysteine. |
Effect of S1PC on NOx concentration in
plasma
NO is known to trigger the vasodilation by
initiating the signaling cascade (47). NO is a gas with a very short plasma
half life of only a few seconds and thus its level is difficult to
measure. It is a common practice to estimate the NO production by
measuring its stable metabolites (NOx). As shown in Table Ⅱ, the
cooling of rat significantly (P<0.01) increased the plasma NOx
concentration as indicated by the comparison between the two groups
of rats with and without cold treatment. Moreover, pretreatment of
rats with S1PC (6.5 mg/kg BW) further increased the plasma NOx
level (P<0.05), compared to non-pretreated rats.
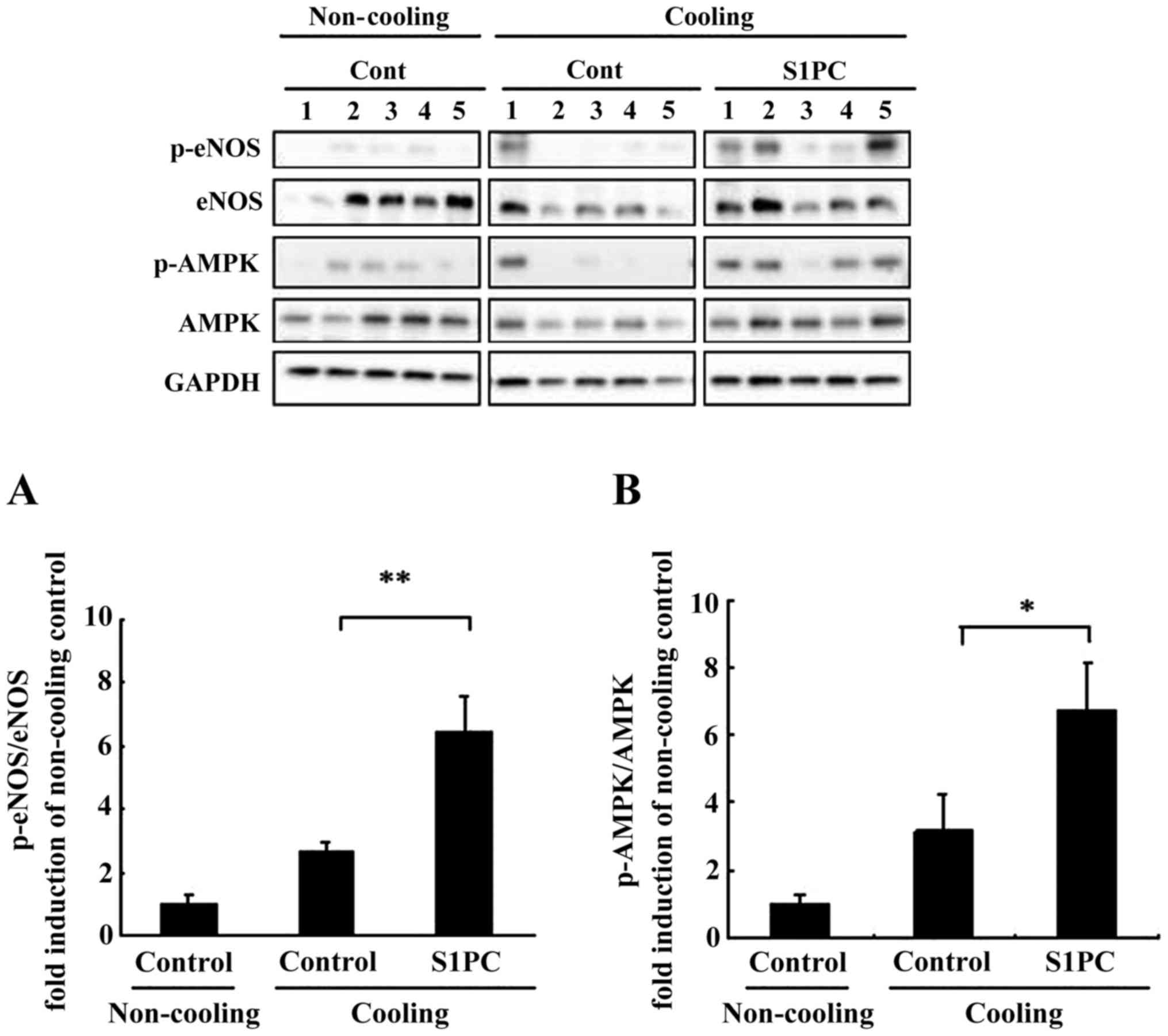
Effect of S1PC on eNOS and AMPK
phosphorylation in aorta
Fig. 6 shows the
effect of S1PC on the level of phosphorylated eNOS and AMPK in
aorta that are key regulatory molecules of NO production in the
vascular endothelium. By western blot analysis, the phosphorylation
level of both eNOS and AMPK was found to be up-regulated by
cooling. Additionally, pretreatment of rat with S1PC (6.5 mg/kg BW)
significantly increased the phosphorylation of eNOS and AMPK
compared to pretreatment with DW.
Discussion
AGE reportedly promotes the recovery of rectal
temperature decline in mice induced by cooling, and its effect is
more potent than that of raw or other garlic preparations (32). Recently it was reported that AGE and
S1PC, a constituent of AGE, significantly increase the tail blood
flow of SHR (21). At present,
however, the underlying mechanism of AGE action has not been fully
elucidated.
In this study, we examined the effects of AGE and
its three characteristic sulfur compounds SAC, S1PC, and SAMC on
the peripheral blood circulation by using a cold-treated rat model;
the tail blood flow of rat was reduced by cooling, and the effect
of pretreatment with AGE and these three constituents was examined
in terms of the recovery of the decreased blood flow. We found that
pretreatment with AGE or S1PC significantly counteracted the
reduction of tail blood flow of the rat caused by cooling. The
effect of S1PC is dose-dependent with the maximal dose of 6.5 mg/kg
BW and specific in the sense that other constituents of AGE, SAC
and SAMC had no effect on the reduction of tail blood flow. These
results suggested that S1PC is a major active constituent
responsible for the effect of AGE to improve the peripheral blood
circulation.
The skin blood flow is regulated by both the
sympathetic vasoconstrictor system and nonadrenergic sympathetic
active vasodilator system to maintain body temperature (48,49).
Under the cold conditions, the vasoconstriction of peripheral
artery is induced by both activation of sympathetic reflex and
reduction of locally produced NO (50), leading to poor circulation and low
body temperature. In addition, Koganezawa et al (51) found that cooling of the rat skin
stimulates presynaptic P2 purinoceptors on sympathetic nerve
terminals and facilitates the release of noradrenaline, thereby
inducing contraction of skin blood vessels via the activation of
α1- and α2-adrenoceptors. Thus it was conceivable that S1PC
improves the blood flow of cooled rat by inhibiting noradrenaline
signaling and/or the enhancement of NO production. However,
Takashima et al (52)
reported that S1PC had no effect on noradrenaline-induced
contraction in isolated rat blood vessels. Thus it is more likely
that S1PC improves the blood flow by acting on NO production system
but not on the pathway of noradrenalin-triggered
vasoconstriction.
The NO production system has a critical role for
vasodilation; NO is produced by eNOS on vascular endothelium, that
increases cGMP levels via activating soluble guanylyl cyclase
present in smooth muscle cells and thereby causes vascular smooth
muscle relaxation (53,54). In addition, it was shown that NO was
involved in the recovery of the reduced human skin blood flow after
cooling (50). Furthermore, a
previous study reported that the NO synthesis inhibitor, L-NAME,
delayed the recovery of tail skin temperature of mice decreased by
cooling (55). Previously, AGE was
shown to increase NO production in endothelial cells (52,56). In
this study, the cooling of rats significantly increased the plasma
NOx concentration (Table II). We
also showed that additional pretreatment with S1PC further
increased the concentration of NO metabolites in the plasma of
cooled rats when compared to DW-pretreated control (Table II). These results suggested that
S1PC improved the reduction of tail blood flow of cooled rats via
enhancing NO production. It was reported that both low temperature
and shear stress induce the activation of eNOS by activating AMPK
(57,58). AMPK is known to phosphorylate the
specific serine residue (Ser1177) of eNOS that is essential for its
activation (58,59). It was also shown that AGE activated
AMPK in both diabetic model TSOD mice (39), and arteriosclerotic model ApoE-KO
mice (36). In addition, S1PC
enhanced the AMPK activity in mouse spleen cells in vitro
(37). In this study, the
phosphorylation level of both eNOS and AMPK was up-regulated by
cooling of rats (Fig. 6). The
additional pretreatment with S1PC further increased the
phosphorylation of eNOS and AMPK in the aorta of cooled rats,
compared to DW-pretreated control (Fig.
6). On the other hand, S1PC did not affect the normal tail
blood flow of non-cooled rats. These results suggest that S1PC
enhances NO production induced by the low temperature and shear
stress through the activation of AMPK, leading to improvement of
peripheral circulatory disturbance.
 | Table IIThe effect of S1PC on the plasma
concentration of NOx in cold-treated rats. |
Table II
The effect of S1PC on the plasma
concentration of NOx in cold-treated rats.
| Group | Plasma NOx
concentration (µM) |
|---|
| No cold
treatment |
2.95±0.57b |
| Cold treatment
only | 6.57±0.60 |
| S1PC pretreatment
and cold treatment |
8.84±0.66a |
In summary, this study showed that pretreatment with
S1PC alleviated the cold-induced reduction of tail blood flow in
rat by increasing the NOx concentration in plasma, and the
phosphorylation of eNOS and AMPK in aorta. The findings suggest
that S1PC may be a useful agent for the treatment of peripheral
circulatory disorders including RP and poor circulation caused by
smoking, aging, and stress.
Acknowledgements
The authors thank Dr Takami Oka of Wakunaga
Pharmaceutical Co., Ltd. for his helpful advice, encouragement, and
critical reading of the manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MU, KK, and JIS designed the experiments, and MU
performed blood flow measurement, and was a major contributor in
writing the manuscript. KK performed measurements of plasma NOx
level. JIS performed measurements of vascular NO-related
phosphorylation. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Wakunaga
Pharmaceutical Co., Ltd. Institutional Animal Care and Use
Committee (approval no. 258). This investigation conformed to the
Guide for the Care and Use of Laboratory Animals published by the
US National Institute of Health (NIH Publication, 8th edition,
2011).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elam M and Wallin BG: Skin blood flow
responses to mental stress in man depend on body temperature. Acta
Physiol Scand. 129:429–431. 1987.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu C, Yavar Z and Sun Q: Cardiovascular
response to thermoregulatory challenges. Am J Physiol Heart Circ
Physiol. 309:H1793–H1812. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
LeRoy EC and Medsger TA Jr: Raynaud's
phenomenon: A proposal for classification. Clin Exp Rheumatol.
10:485–488. 1992.PubMed/NCBI
|
|
4
|
Lis-Święty A: Recent advances in the
workup and management of Raynaud phenomenon. Pol Arch Intern Med.
129:798–808. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
United States Pharmacopeial Convention I.
United States Pharmacopoeia 38 Garlic Fluid Extract USP 28-NF 33.
United States Pharmacopeial Convention, Rockville MD, pp6052-6055,
2015.
|
|
6
|
Colín-González AL, Santana RA, Silva-Islas
CA, Chánez-Cárdenas ME, Santamaría A and Maldonado PD: The
antioxidant mechanisms underlying the aged garlic extract- and
S-allylcysteine-induced protection. Oxid Med Cell Longev.
2012(907162)2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matsutomo T and Kodera Y: Development of
an analytic method for sulfur compounds in aged garlic extract with
the use of a postcolumn high performance liquid chromatography
method with sulfur-specific detection. J Nutr. 146:450S–455S.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ried K, Frank OR and Stocks NP: Aged
garlic extract lowers blood pressure in patients with treated but
uncontrolled hypertension: A randomised controlled trial.
Maturitas. 67:144–150. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ried K, Frank OR and Stocks NP: Aged
garlic extract reduces blood pressure in hypertensives: A
dose-response trial. Eur J Clin Nutr. 67:64–70. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ried K, Travica N and Sali A: The effect
of aged garlic extract on blood pressure and other cardiovascular
risk factors in uncontrolled hypertensives: The AGE at Heart trial.
Integr Blood Press Control. 9:9–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ried K, Travica N and Sali A: The effect
of kyolic aged garlic extract on gut microbiota, inflammation, and
cardiovascular markers in hypertensives: The GarGIC Trial. Front
Nutr. 5(122)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ried K: Garlic lowers blood pressure in
hypertensive subjects, improves arterial stiffness and gut
microbiota: A review and meta-analysis. Exp Ther Med. 19:1472–1478.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Budoff MJ, Ahmadi N, Gul KM, Liu ST,
Flores FR, Tiano J, Takasu J, Miller E and Tsimikas S: Aged garlic
extract supplemented with B vitamins, folic acid and L-arginine
retards the progression of subclinical atherosclerosis: A
randomized clinical trial. Prev Med. 49:101–107. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matsumoto S, Nakanishi R, Li D, Alani A,
Rezaeian P, Prabhu S, Abraham J, Fahmy MA, Dailing C, Flores F, et
al: Aged garlic extract reduces low attenuation plaque in coronary
arteries of patients with metabolic syndrome in a prospective
randomized double-blind study. J Nutr. 146:427S–432S.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gruenwald J, Bongartz U, Bothe G and
Uebelhack R: Effects of aged garlic extract on arterial elasticity
in a placebo-controlled clinical trial using EndoPAT™ technology.
Exp Ther Med. 19:1490–1499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gὁmez-Arbelἁez D, Lahera V, Oubina P,
Valero-Munoz M, Heras NL, Rodriguez Y, Garcia RG, Camacho PA and
Lopez-jaramillo P: Aged garlic extract improves adiponectin levels
in subjects with metabolic syndrome: A double-blind, placebo
controlled, randomized, crossover study. Mediators Inflamm.
285795(2013)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zini A, Mann J, Mazor S and Vered Y: The
efficacy of aged garlic extract on gingivitis - a randomized
clinical trial. J Clin Dent. 29:52–56. 2018.PubMed/NCBI
|
|
18
|
Harauma A and Moriguchi T: Aged garlic
extract improves blood pressure in spontaneously hypertensive rats
more safely than raw garlic. J Nutr. 136 (Suppl 3):769S–773S.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Matsutomo T, Ushijima M, Kodera Y,
Nakamoto M, Takashima M, Morihara N and Tamura K: Metabolomic study
on the antihypertensive effect of S-1-propenylcysteine in
spontaneously hypertensive rats using liquid chromatography coupled
with quadrupole-Orbitrap mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 1046:147–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Matsutomo T, Ushijima M, Kunimura K and
Ohtani M: Metabolomic study reveals the acute hypotensive effect of
S-1-propenylcysteine accompanied by alteration of the plasma
histidine level in spontaneously hypertensive rats. J Pharm Biomed
Anal. 168:148–154. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ushijima M, Takashima M, Kunimura K,
Kodera Y, Morihara N and Tamura K: Effects of S-1-propenylcysteine,
a sulfur compound in aged garlic extract, on blood pressure and
peripheral circulation in spontaneously hypertensive rats. J Pharm
Pharmacol. 70:559–565. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Matsutomo T: Potential benefits of garlic
and other dietary supplements for the management of hypertension
(Review). Exp Ther Med. 19:1479–1484. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chuah SC, Moore PK and Zhu YZ:
S-allylcysteine mediates cardioprotection in an acute myocardial
infarction rat model via a hydrogen sulfide-mediated pathway. Am J
Physiol Heart Circ Physiol. 293:H2693–H2701. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Borek C: Antioxidant health effects of
aged garlic extract. J Nutr. 131 (Suppl 3):1010S–1015S.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yamato O, Tsuneyoshi T, Ushijima M,
Jikihara H and Yabuki A: Safety and efficacy of aged garlic extract
in dogs: upregulation of the nuclear factor erythroid 2-related
factor 2 (Nrf2) signaling pathway and Nrf2-regulated phase II
antioxidant enzymes. BMC Vet Res 29:. 14(373)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tsuneyoshi T: BACH1 mediates the
antioxidant properties of aged garlic extract (Review). Exp Ther
Med. 19:1500–1503. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Moriguchi T, Saito H and Nishiyama N: Aged
garlic extract prolongs longevity and improves spatial memory
deficit in senescence-accelerated mouse. Biol Pharm Bull.
19:305–307. 1996.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ogawa T, Kodera Y, Hirata D, Blackwell TK
and Mizunuma M: Natural thioallyl compounds increase oxidative
stress resistance and lifespan in Caenorhabditis elegans by
modulating SKN-1/Nrf. Sci Rep. 6(21611)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kyo E, Uda N, Kasuga S and Itakura Y:
Immunomodulatory effects of aged garlic extract. J Nutr. 131 (Suppl
3):1075S–1079S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Suzuki J, Yamaguchi T, Matsutomo T, Amano
H, Morihara N and Kodera Y: S-1-Propenylcysteine promotes the
differentiation of B cells into IgA-producing cells by the
induction of Erk1/2-dependent Xbp1 expression in Peyer's patches.
Nutrition. 32:884–889. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Suzuki J, Miki S, Ushijima M and Kodera Y:
Regulation of immune response by S-1-propenylcysteine
through autophagy-mediated protein degradation (Review). Exp Ther
Med. 19:1570–1573. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ushijima M, Sumioka I, Kakimoto M,
Yokoyama K, Uda N, Matsuura H, Kyo E, Suzuki A, Kasuga S, Itakura
Y, et al: Effect of garlic and garlic preparations on physiological
and psychological stress in mice. Phytother Res. 11:226–230.
1997.
|
|
33
|
Morihara N, Ushijima M, Kashimoto N,
Sumioka I, Nishihama T, Hayama M and Takeda H: Aged garlic extract
ameliorates physical fatigue. Biol Pharm Bull. 29:962–966.
2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kasuga S, Ushijima M, Morihara N, Itakura
Y and Nakata Y: Effect of aged garlic extract (AGE) on
hyperglycemia induced by immobilization stress in mice]. Nippon
Yakurigaku Zasshi. 114:191–197. 1999.(In Japanese). PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kyo E, Uda N, Ushijima M, Kasuga S and
Itakura Y: Prevention of psychological stress-induced immune
suppression by aged garlic extract. Phytomedicine. 6:325–330.
1999.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Morihara N, Hino A, Miki S, Takashima M
and Suzuki JI: Aged garlic extract suppresses inflammation in
apolipoprotein E-knockout mice. Mol Nutr Food Res.
61(1700308)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Suzuki JI, Kodera Y, Miki S, Ushijima M,
Takashima M, Matsutomo T and Morihara N: Anti-inflammatory action
of cysteine derivative S-1-propenylcysteine by inducing MyD88
degradation. Sci Rep. 8(14148)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ohtani M and Nishimura T:
Sulfur-containing amino acids in aged garlic extract inhibit
inflammation in human gingival epithelial cells by suppressing
intercellular adhesion molecule-1 expression and IL-6 secretion.
Biomed Rep. 12:99–108. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Miki S, Suzuki JI, Kunimura K and Morihara
N: Mechanisms underlying the attenuation of chronic inflammatory
diseases by aged garlic extract: Involvement of the activation of
AMP-activated protein kinase (Review). Exp Ther Med. 19:1462–1467.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nakagawa S, Kasuga S and Matsuura H:
Prevention of liver damage by aged garlic extract and its
components in mice. Phytother Res. 3:50–53. 1989.
|
|
41
|
Okuhara T: Clinical study of GE on
peripheral circulation. Jpn Pharmacol Ther. 22:3695–3701. 1994.
|
|
42
|
Wlosinska M, Nilsson AC, Hlebowicz J,
Malmsjö M, Fakhro M and Lindstedt S: Aged garlic extract preserves
cutaneous microcirculation in patients with increased risk for
cardiovascular diseases: A double-blinded placebo-controlled study.
Int Wound J. 16:1487–1493. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mizuno I, Sumioka I, Ushijima M, Yasuda K,
Mouri Y, Mizuho O, Ogasawara K and Kyo E: Improvement of peripheral
blood circulation in mouse and human with aged garlic extract
preparation combined with ginseng, oriental bezoar, antler velvet,
cuscuta seed and epimedium herb. Pharmacometrics. 67:371–378.
2004.
|
|
44
|
Guan J, Lin H, Xie M, Huang M, Zhang D, Ma
S, Bian W, Zhan Q and Zhao G: Higenamine exerts an antispasmodic
effect on cold-induced vasoconstriction by regulating the PI3K/Akt,
ROS/α2C-AR and PTK9 pathways independently. Asian Pac J Trop Med.
5:935–938. 2012.
|
|
45
|
Ning SY, Jiang BP, Xu L, Fang TH and Wu
MH: Effect of Liangxuehuayu Recipe on hemorheology in rats with
blood stasis syndrome. Asian Pac J Trop Med. 5:935–938.
2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ryan TJ and Copeman PW: Microvascular
pattern and blood stasis in skin disease. Br J Dermatol.
81:563–573. 1969.PubMed/NCBI View Article : Google Scholar
|
|
47
|
López-López JG, Pérez-Vizcaíno F,
Cogolludo AL, Ibarra M, Zaragozá-Arnáez F and Tamargo J: Nitric
oxide- and nitric oxide donors-induced relaxation and its
modulation by oxidative stress in piglet pulmonary arteries. Br J
Pharmacol. 133:615–624. 2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Johnson JM and Proppe DW: Cardiovascular
adjustments to heat stress. In: Handbook of physiology. Section 4:
Environmental physiology. Fregley MJ and Blatteis CM (eds). Oxford
University Press, New York, NY. pp215–243. 1996.
|
|
49
|
Rowell LB: Human circulation: regulation
during physical stress. Oxford University Press, New York, NY,
1986.
|
|
50
|
Thompson-Torgerson CS, Holowatz LA,
Flavahan NA and Kenney WL: Cold-induced cutaneous vasoconstriction
is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart
Circ Physiol. 292:H1700–H1705. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Koganezawa T, Ishikawa T, Fujita Y,
Yamashita T, Tajima T, Honda M and Nakayama K: Local regulation of
skin blood flow during cooling involving presynaptic P2
purinoceptors in rats. Br J Pharmacol. 148:579–586. 2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Takashima M, Kanamori Y, Kodera Y,
Morihara N and Tamura K: Aged garlic extract exerts
endothelium-dependent vasorelaxant effect on rat aorta by
increasing nitric oxide production. Phytomedicine. 24:56–61.
2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lindauer U, Megow D, Matsuda H and Dirnagl
U: Nitric oxide: A modulator, but not a mediator, of neurovascular
coupling in rat somatosensory cortex. Am J Physiol. 277:H799–H811.
1999.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hodges GJ, Traeger JA III, Tang T, Kosiba
WA, Zhao K and Johnson JM: Role of sensory nerves in the cutaneous
vasoconstrictor response to local cooling in humans. Am J Physiol
Heart Circ Physiol. 293:H784–H789. 2007.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhao Y, Vanhoutte PM and Leung SWS:
Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci. 129:83–94.
2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Morihara N, Sumioka I, Moriguchi T, Uda N
and Kyo E: Aged garlic extract enhances production of nitric oxide.
Life Sci. 71:509–517. 2002.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Binti Md Isa K, Kawasaki N, Ueyama K,
Sumii T and Kudo S: Effects of cold exposure and shear stress on
endothelial nitric oxide synthase activation. Biochem Biophys Res
Commun. 412:318–322. 2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhang Y, Lee TS, Kolb EM, Sun K, Lu X,
Sladek FM, Kassab GS, Garland T Jr and Shyy JY: AMP-activated
protein kinase is involved in endothelial NO synthase activation in
response to shear stress. Arterioscler Thromb Vasc Biol.
26:1281–1287. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y,
Wang X, Sun Y, Lopez BL, Christopher TA, Peterson JM, et al:
C1q/TNF-related proteins, a family of novel adipokines, induce
vascular relaxation through the adiponectin
receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler
Thromb Vasc Biol. 31:2616–2623. 2011.PubMed/NCBI View Article : Google Scholar
|