Introduction
Osteoarthritis (OA) is a common chronic and
irreversible joint disease (1,2) that
causes alterations to the morphology, structure and function of
chondrocytes (3). Overexpression of
inflammatory mediators, including cytokines [interleukin (IL)-1β],
reactive oxygen species and matrix-degrading enzymes, leads to
progressive deterioration of cartilage, synovium and subchondral
bone (4). At present, no effective
treatments to prevent or reverse progressive joint injury are
available (5).
AG is an inducible nitric oxide synthase (iNOS)
inhibitor, which can regulate the activity and expression levels of
iNOS (6). iNOS inhibitors have been
used to alleviate articular cartilage injury, pain and inflammation
in a surgical model of OA (7). In
addition, AG has various pharmacological effects, including
anti-inflammatory effects that can modulate OA and articular
inflammation (8). Additionally, it
has been reported that AG protects against colonic inflammation by
inhibiting the expression of NF-κB/p65(9). In a previous study, AG treatment
reduced the protein expression levels of iNOS and p65 in the liver
of an animal model of diabetes (10). Furthermore, AG can reduce the level
of osteocyte apoptosis during non-traumatic osteonecrosis (11). Although the pharmacological effects
of AG have been studied in numerous cell types (12-17),
it remains unclear whether AG can influence iNOS and
cyclooxygenase-2 (COX-2) expression, and the NF-κB signaling
pathway in rat chondrocytes.
The pathogenesis of OA is related to the activation
of a number of different molecular pathways, such as the NF-κB and
MAPK pathways (18). Activation of
the NF-κB signaling pathway directly affects the pathogenesis and
development of OA (19). Activated
NF-κB molecules trigger increases in the levels of other
proinflammatory cytokines, for example IL-1β, tumor necrosis factor
α and IL-6, leading to increased extracellular degradation and
reduced synthesis of collagen and proteoglycans, which further
contribute to the onset of OA (20).
The activation of NF-κB has been hypothesized to be closely
associated with IL-1β-stimulated OA (20). Furthermore, it has been hypothesized
that the proinflammatory responses stimulated by IL-1β stimulate
chondrocytes during OA, leading to increases in COX-2 and iNOS
expression by activating NF-κB (21-23).
In addition, NF-κB promotes articular cartilage breakdown by
inducing the expression of matrix metalloproteinases and ADAMTs in
OA chondrocytes (20,24). Elevated levels of NF-κB in OA
chondrocytes lead to the overexpression of COX2 and iNOS, which
further contribute to cartilage inflammation (25,26) and
degeneration of articular cartilage (24).
Targeted therapies that interfere with NF-κB
signaling may serve as a novel therapeutic strategy for the
treatment of OA. In the present study, IL-1β-treated articular
chondrocytes were used to investigate whether AG inhibited iNOS and
COX-2 expression, and the NF-κB signaling pathway.
Materials and methods
Chondrocyte isolation and culture
Sprague-Dawley rats (age, 2-3 weeks; weight, 41±3.5
g; sex, male:female, 1:1) obtained from The Second Affiliated
Hospital of Harbin Medical University (Harbin, China) were
anaesthetized with isoflurane and sacrificed by cervical
dislocation. Articular cartilage from the femoral heads and knees
was isolated, and ground into small pieces under sterile
conditions. All experiments were approved by the Departmental
Animal Care and Use Committee at Northeast Agricultural
University.
As previously described (27), cartilage debris was isolated with
trypsin (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C for 30 min,
and subsequently rinsed with PBS containing penicillin and
streptomycin solution. The tissue was digested with collagenase
Type II (Gibco; Thermo Fisher Scientific, Inc.) in PBS at 37˚C for
4 h. Subsequently, the supernatant was collected and centrifuged at
500 x g, 27˚C for 7 min. The harvested articular chondrocytes were
placed in 25 m2 culture flasks containing DMEM
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin, and were
incubated at 37˚C with 5% CO2. When the monolayer on the
bottom of the culture flask reached 80-90% confluence, the cells
were passaged and the second-generation chondrocyte cells were
isolated for subsequent experiments.
Chondrocyte treatment
To assess cell viability, chondrocytes were seeded
in 96-well plates (5x104/ml, 100 µl/well), treated with
0, 0.3, 1, or 3 mM AG (Sigma-Aldrich; Merck KGaA) for 24, 48 and 72
h at 37˚C and evaluated using a Cell Counting Kit-8 (CCK-8) assay
(Dojindo Molecular Technologies, Inc.), as described below. For the
evaluation of iNOS and COX-2 expression, chondrocyte cells were
exposed to the following conditions for 24 h at 37˚C: i) 10 ng/ml
IL-1β (PeproTech, Inc.) alone or ii) co-treatment with different
concentrations of AG (0.3, 1, 3 mM) and 10 ng/ml IL-1β for 24 h,
after treatment with 10 ng/ml IL-1β for 24 h alone. The activity of
the NF-κB signaling pathway was assessed in chondrocytes treated
with either 10 ng/ml IL-1β alone for 0.5 h or co-treated with AG
(0.3, 1 or 3 mM) and 10 ng/ml IL-1β at 37˚C for 2 h. Rat
chondrocytes cultured without AG and IL-1β were used as
controls.
Cell viability assay
The cytotoxicity of AG was determined using a CCK-8
assay. The assay was conducted on chondrocytes treated with varying
concentrations of AG (0.3, 1 or 3 mM) for 24, 48, and 72 h, as
aforementioned. Cells were incubated with 10 µl CCK-8 reagent at
37˚C for 2 h. Subsequently, the absorbance at a wavelength of 450
nm was determined using a microplate reader.
Western blot analysis
AG-treated chondrocyte cells were washed with PBS
and collected with cell scrapers. Total protein was extracted from
AG-treated chondrocyte cells using RIPA lysis buffer (Beyotime
Institute of Biotechnology) by centrifugation at 10,000 x g for 15
min at 4˚C. Total protein was quantified using a bicinchoninic acid
assay. Protein (15 µl/lane) was separated by 10% SDS-PAGE and
transferred to nitrocellulose membranes. Subsequently, the
membranes were blocked with 5% skim-milk at room temperature for 1
h. The membranes were incubated at 4˚C overnight with primary
antibodies targeted against: p65 (cat. no. 8242; 1:1,000; Cell
Signaling Technology, Inc.), NF-κβ inhibitor α (IκBα; cat. no.
9242; 1:1,000; Cell Signaling Technology, Inc.), inhibitor of
NF-κβ-β (IKKβ; cat. no. ab124957; 1:1,000; Abcam), phosphorylated
(p)-p65 (cat. no. 3033; 1:1,000; Cell Signaling Technology, Inc.),
p-IκBα (cat. no. 9246; 1:1,000; Cell Signaling Technology, Inc.),
p-IKKβ (cat. no. ab59195; 1:1,000; Abcam), iNOS (cat. no. WL0992a;
1:500; Wanleibio Co., Ltd.), COX-2 (cat. no. WL01750; 1:500;
Wanleibio Co., Ltd.) and GAPDH (cat. no. TA-08; 1:3,000; OriGene
Technologies, Inc.). Subsequently, the membranes were washed with
TBST. Following primary incubation, the membranes were incubated
for 2 h at 37˚C with horseradish peroxidase-conjugated secondary
antibody goat anti-mouse IgG and horseradish peroxidase-conjugated
secondary antibody goat anti-rabbit IgG (cat. nos. ZB2305 and
ZB-2301, respectively; 1:1,000; ZSGB-BIO). Protein bands were
visualized using an ECL kit (Beyotime Institute of Biotechnology)
and the signals were analyzed using a Tanon detection system (Tanon
Science and Technology Co., Ltd.). GAPDH was used as the loading
control. The densities of bands were analyzed using ImageJ (version
1.51; National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the chondrocyte cells
using the BioSci™ Tissue/Cultured Cell Total RNA Extraction kit
(Dakewe Bioengineering Co., Ltd.), according to the manufacturer's
instructions. Total RNA (1 µg), dNTPs (cat. no. AD101-12; Beijing
TransGen Biotech Co., Ltd.), primer (cat. no. AH101-02; Beijing
TransGen Biotech Co., Ltd.) and TransScript®II reverse
transcriptase (cat. no. AH101-02; Beijing TransGen Biotech Co.,
Ltd.) were used to synthesize cDNA. The temperature protocol was as
follows: 50˚C for 30 min and 85˚C for 5 sec. Quantitative PCR
(qPCR) was performed using SuperReal PreMix Plus (SYBR Green; cat.
no. FP205; Tiangen Biotech Co., Ltd.) according to the
manufacturer's protocols. The following thermocycling conditions
used for qPCR: 15 min at 95˚C for initial denaturation; followed by
40 cycles at 95˚C for 15 sec, 60˚C for 32 sec, and 72˚C for 5 min.
The following primer pairs were used for qPCR: GAPDH forward,
5'-GATGCCCCCATGTTTGTGAT-3' and reverse,
5'-GGCATGGACTGTGGTCATGAG-3'; iNOS forward,
5'-GAGACGCACAGGCAGAGGTTG-3' and reverse,
5'-AGCAGGCACACGCAATGATGG-3'; and COX-2 forward,
5'-AGAAGCGAGGACCTGGGTTCA C-3' and reverse,
5'-ACACCTCTCCACCGATGACCTG-3'. Protein levels were quantified using
the 2-ΔΔCq method (28) and normalized to the internal
reference gene GAPDH.
Immunofluorescence assay
After treating with 10% FBS, Second-generation
chondrocytes cultured with DMEM supplemented with 0.05% FBS were
treated with 10 ng/ml IL-1β, or co-incubated with 10 ng/ml IL-1β
and 1 mM AG for 2 h at 37˚C. Subsequently, the cells were washed
with PBS and fixed with 4% paraformaldehyde for 1 h at 37˚C. The
cells were washed with PBS, blocked with 10% goat serum (cat. no.
AR1009; 0.3% Triton, 1:10; Boster Biological Technology) for 1 h at
room temperature and rinsed with PBS. Subsequently, the cells were
incubated with primary antibodies against p-p65 (cat. no. 3033;
1:100; Cell Signaling Technology, Inc.) overnight at 4˚C. Following
primary incubation, the cells were gently washed and then incubated
with a horseradish peroxidase-conjugated secondary antibody goat
anti-rabbit IgG (cat. no. ZB-2301; 1:250; OriGene Technologies,
Inc.) for 2 h at 37˚C. Subsequently, the cells were stained with
DAPI (Beyotime Institute of Biotechnology) at room temperature for
15 min. Cells were rinsed and observed under a fluorescence
microscope in six randomly-selected fields (magnification,
x400).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 18.0; SPSS, Inc.). Data are presented as the mean
± standard deviation. And all experiments were performed at least
three times. Differences were assessed using one-way ANOVA followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
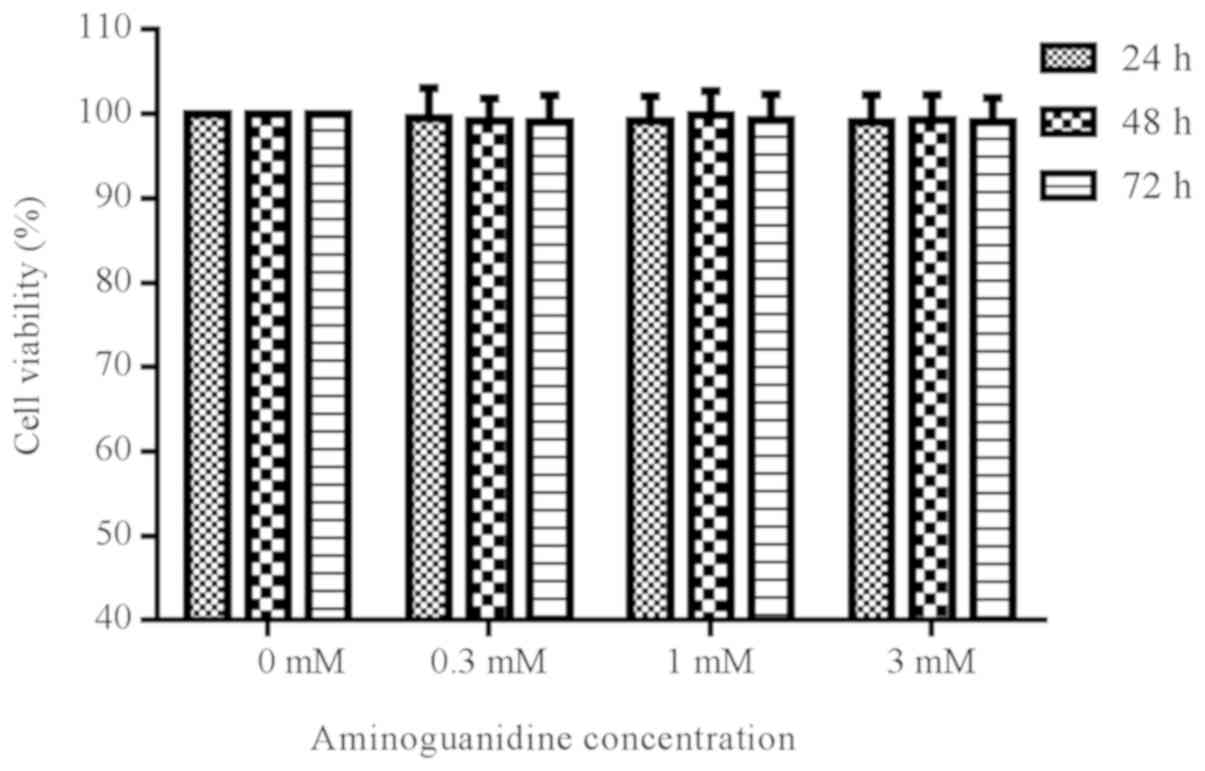
Effect of AG on cell viability
To determine whether different concentrations of AG
were cytotoxic to rat chondrocytes, cells were treated with AG at
varying concentrations (0.3, 1 or 3 mM) for 24, 48, and 72 h. The
results suggested that treatment with AG did not significantly
alter the viability of the cells, as measured by a CCK-8 assay
(P>0.05; Fig. 1).
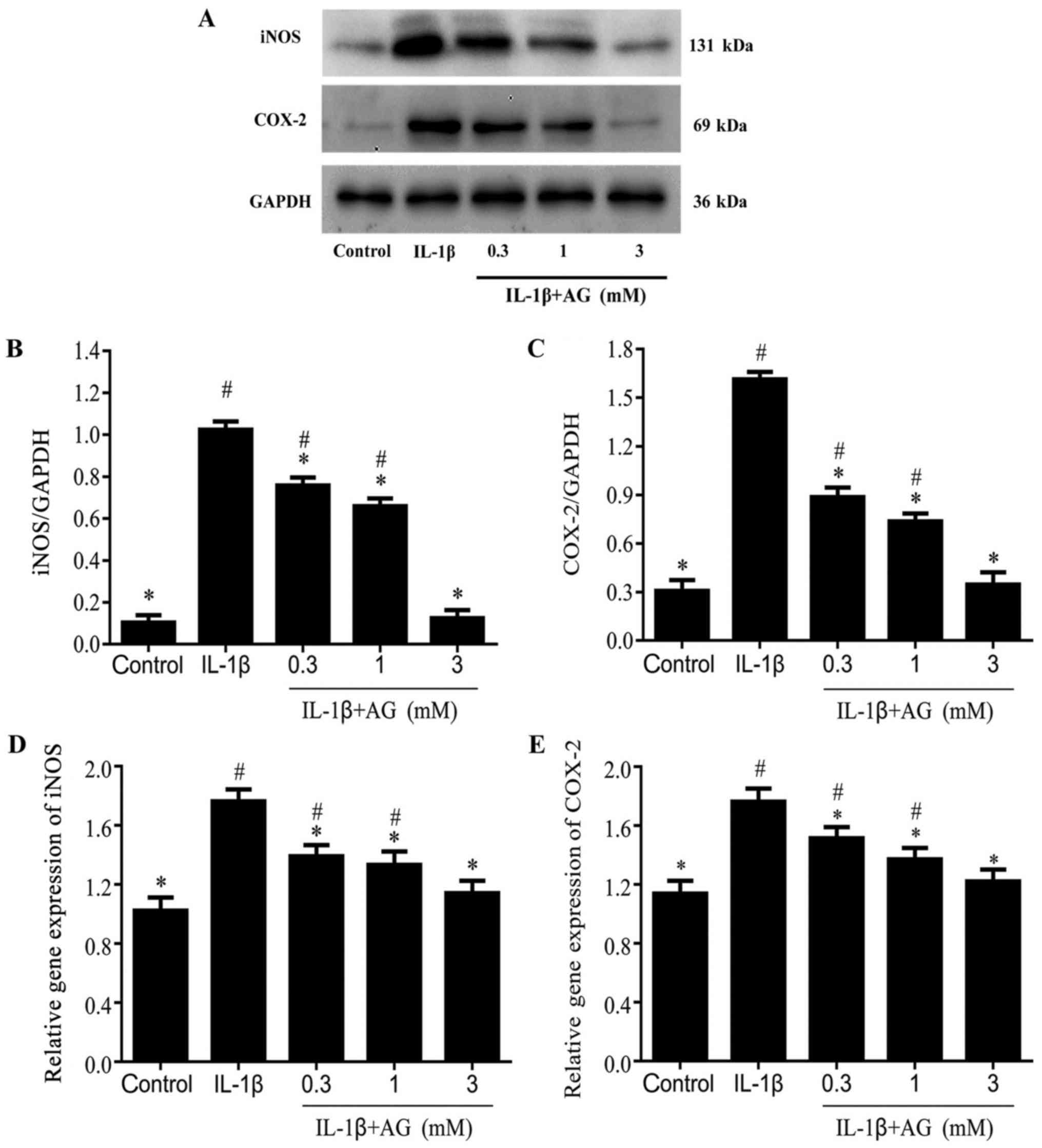
Effect of AG on IL-1β-induced
expression levels of iNOS and COX2 in chondrocytes
Chondrocytes treated with IL-1β displayed
significantly increased COX-2 and iNOS expression levels compared
with the control cells (Fig. 2A-E).
Compared with cells stimulated with IL-1β alone, the protein
expression levels of iNOS and COX-2 were decreased in a
dose-dependent manner by AG (P<0.05; Fig. 2A-C). The gene expression levels of
iNOS and COX-2 were also decreased in a dose-dependent manner by AG
(P<0.05; Fig. 2D and E).
Effect of AG on the activity of NF-κB
in chondrocytes induced by IL-1β
Western blot analysis suggested that the levels of
p-IKKβ and p-IκBα were significantly increased in IL-1β-stimulated
chondrocytes compared with the control cells (P<0.05; Fig. 3A, C
and E). Moreover, the protein levels
of p-IKKβ and p-IκBα were significantly reduced in chondrocytes
co-treated with AG and IL-1β (P<0.05; Fig. 3C and E). Interestingly, the lowest p-IKKβ and
p-IκBα protein expression levels were observed in chondrocytes
treated with 1 mM AG (Fig. 3C and
E). Chondrocyte treatment with IL-1β
significantly reduced the protein expression levels of IκBα
(P<0.05; Fig. 3D) and IKKβ levels
(P<0.05; Fig. 3B) compared with
the control cells. Chondrocytes co-cultured with AG and IL-1β
displayed decreased expression levels of IKKβ and IκBα compared
with the IL-1β group (Fig. 3B and
D). Similar to the results obtained
for p-IKKβ/IKKβ and p-IκBα/IκBα, the highest inhibitory effect of
AG on IL-1β-induced p-IKKβ/IKKβ and p-IκBα/IκBα alterations was
observed in cells treated with 1 mM AG (Fig. 3C and D).
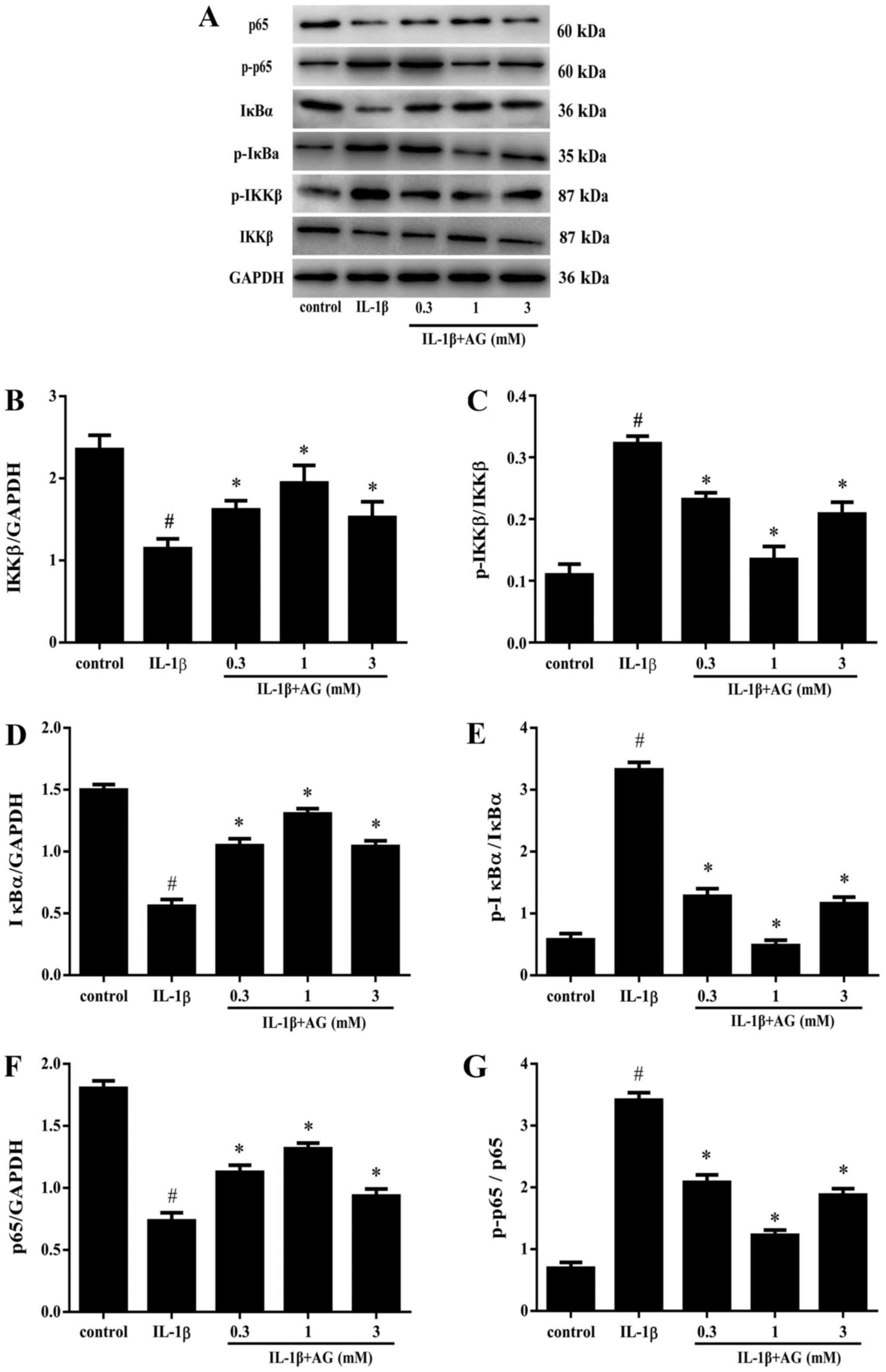 | Figure 3Protein levels of IKKβ, p-IKKβ, IκBα,
p-IκBα, p65 and p-p65 in the different treatment groups. (A)
Western blotting was performed to assess the protein expression
levels of p-IKKβ, p-IκBα, IKKβ, IκBα, p65, and p-p65. Densitometric
analysis of western blotting for (B) IKKβ, (C) p-IKKβ/IKKβ, (D)
IκBα, (E) p-IκBα/IκBα, (F) p65 and (G) p-p65/p65.
#P<0.05 vs. the control group. *P<0.05
vs. the IL-1β group. IKKβ, inhibitor of NF-κβ-β; p, phosphorylated;
IκBα, NF-κβ inhibitor α; IL, interleukin; AG, aminoguanidine. |
Treatment with IL-1β significantly reduced the
protein expression levels of NF-κB p65 in chondrocytes compared
with the control cells (Fig. 3F). By
contrast, co-treatment with AG and IL-1β resulted in significantly
higher expression levels of p65 compared with cells stimulated with
IL-1β alone (P<0.05; Fig. 3F).
Compared with cells stimulated with IL-1β alone, co-treatment with
0.3, 1 or 3 mM AG and IL-1β significantly reduced the p-p65/p65
(P<0.05; Fig. 3G). Importantly,
the greatest decrease in p-p65 levels was observed in the group
treated with 1 mM AG (Fig. 3G).
Taken together, the results suggested that AG inhibited the
activity of NF-κB in chondrocytes activated by IL-1β and that AG
displayed the strongest inhibitory effect at a concentration of 1
mM.
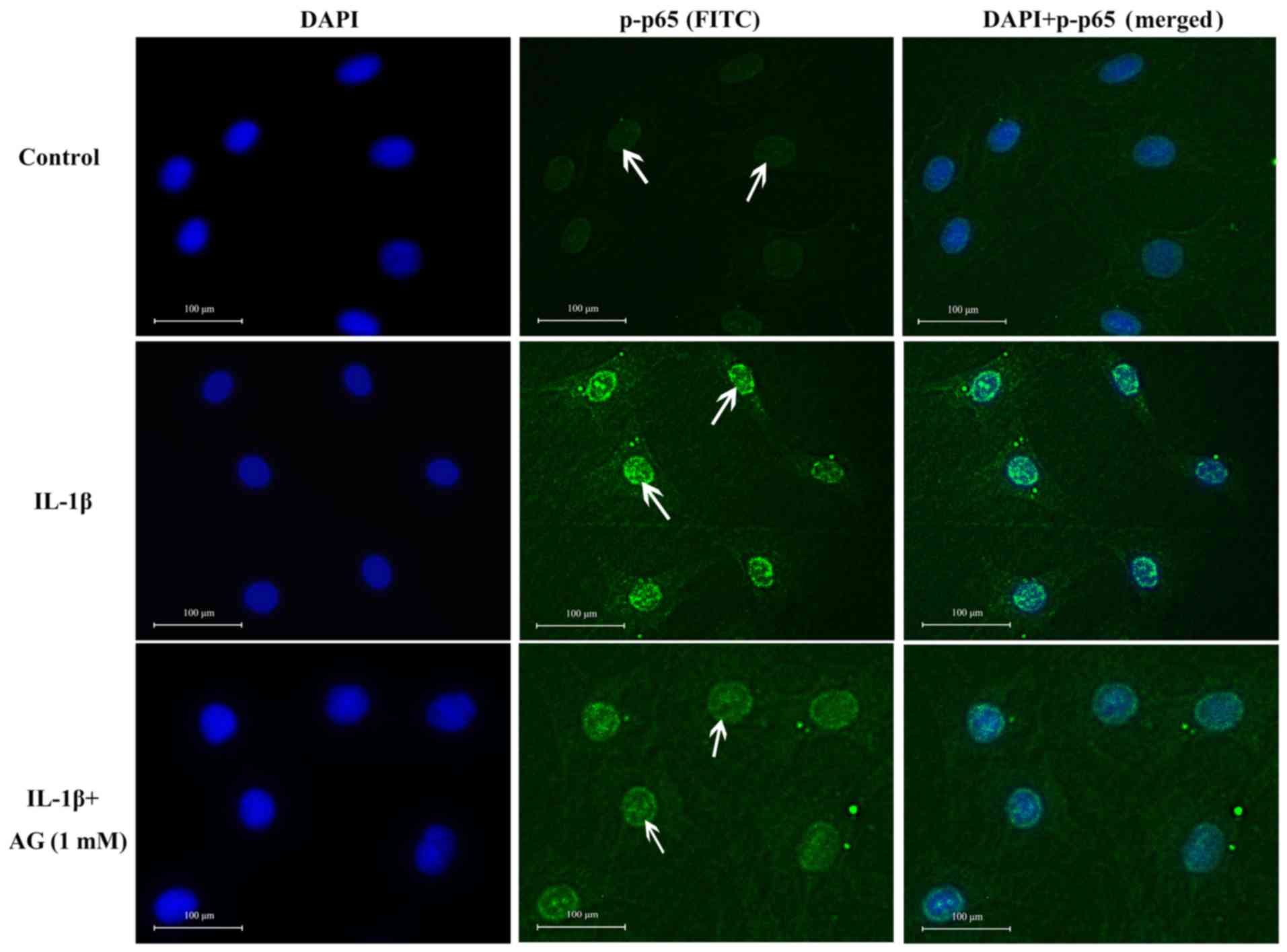
Effect of AG on the nuclear
translocation of NF-κB p65
No p65 staining was observed in the nuclei of
untreated control cells, indicated by no green fluorescence inside
the nucleus (Fig. 4). However,
IL-1β-stimulated chondrocytes displayed p65 staining in the
nucleus, but not in the cytoplasm (Fig.
4). However, IL-1β-stimulated chondrocytes displayed p-p65
staining in the nucleus. Therefore, strong green fluorescence was
observed in the nucleus (Fig. 4). In
cells co-treated with 1 mM AG and 10 ng/ml IL-1β, the intensity of
green fluorescence in the nucleus decreased. The levels of
cytoplasmic staining were also low in this group (Fig. 4). Therefore, these results suggested
that the entry of p-p65 into the nucleus was limited in cells
co-treated with AG and IL-1β.
Discussion
OA is a chronic joint disease (1) and at present, no effective preventive
or therapeutic drugs are available for the treatment of the disease
(2). Therefore, the development of
effective compounds for the treatment of OA is important. In the
present study, the potential of AG, a compound that inhibits iNOS,
to prevent or delay the progression of OA was investigated.
The roles of AG as an inhibitor of oxidation
(29), apoptosis (30,31) and
inflammation (32-34)
have been widely recognized and accepted. In addition to these
well-recognized roles, AG can inhibit the generation of
isoproterenol-induced reactive oxygen species, and restore levels
of antioxidant superoxide dismutase, glutathione and catalase in
the heart (35). AG can also
suppress the production of nitric oxide and the expression of iNOS
in osteocytes, leading to the release of cytochrome C and the
induction of osteocyte apoptosis (11). Moreover, AG can decrease the
accumulation of glycosylation products, which can lead to
endoplasmic reticulum stress-induced cell apoptosis (36). Furthermore, AG mediates articular
inflammatory processes by downregulating IL-1β production in human
osteoarthritic synovial tissue and cartilage (8). Therefore, in the present study, the
possible associations between the inhibitory effect of AG on
inflammation and the NF-κB signaling pathway were investigated.
IL-1β is involved in the pathogenesis of OA and it
may result in marked alterations to the cartilage, including matrix
degradation, inflammation, chondrocyte phenotypic changes and
chondrocyte apoptosis (26,37-39).
Previous studies have reported that 10 ng/ml IL-1β induces an
inflammatory response in chondrocytes (27,40);
therefore, 10 ng/ml was used as the working concentration of IL-1β
in the present study. IL-1β induces iNOS and COX-2 expression,
promotes the synthesis of inflammatory mediators, including
prostaglandin E2 and nitric oxide, and stimulates articular
chondrocytes to produce high levels of NF-κB (26). Therefore, in the present study,
chondrocytes were stimulated with IL-1β to mimic the
pathophysiology of OA. The results of the present study were
consistent with previous studies, suggesting that IL-1β induced
increased iNOS and COX-2 expression (37,41,42).
iNOS is not only an inflammatory mediator, but is
also essential for the initiation and promotion of the inflammatory
response (43). iNOS can
significantly increase the production of nitric oxide, which leads
to destruction of articular cartilage and chondrocyte apoptosis
(44). In addition, iNOS can
regulate other inflammatory processes, for example, cortisol can
interact with or induce iNOS to increase the extent of DNA damage,
and the formation of reactive oxygen/nitrogen species (45). Increased gene and protein expression
levels of iNOS and COX-2 contribute to pain and joint inflammation
in patients with OA (46). Moreover,
current OA treatment strategies focus on the use of
anti-inflammatory drugs that reduce COX-2 levels, which can
alleviate joint pain and swelling (47). Therefore, reducing the expression of
iNOS and COX-2 in chondrocytes could potentially ameliorate joint
inflammation and the degeneration of articular cartilage in
patients with OA. In a recent study, treatment with kaempferol
reduced the expression levels of iNOS and COX-2 in a dose-dependent
manner in IL-1β-stimulated rat chondrocytes (23). Consistently, the results of the
present study indicated that AG decreased the gene and protein
expression levels of iNOS and COX-2 in IL-1β-stimulated rat
articular chondrocytes. Therefore, the present study suggested that
AG might reduce inflammatory responses.
Furthermore, the present study suggested that the
expression of COX-2 and iNOS may be closely associated with the
activation of the NF-κB signaling pathway. In a study conducted by
Lianxu et al (39), an small
interfering RNA targeted against NF-κB p65 reduced iNOS and COX-2
expression levels in rat chondrocytes stimulated by IL-1β.
Additionally, IL-1β-mediated iNOS expression was reduced following
suppression of NF-κB activity in rat chondrocytes (48). The aforementioned studies suggested
that increased iNOS and COX-2 protein expression levels in
IL-1β-stimulated rat chondrocytes are dependent upon the activation
of NF-κB. Furthermore, it has been reported that the protein
expression level of IκBα and the activity of NF-κB are decreased by
IKKβ phosphorylation (49).
Activated NF-κB translocates into the nucleus to induce the
expression of iNOS and COX-2(50)
and other proinflammatory cytokines, such as NO and PGE2(26), which further aggravate OA symptoms.
The results reported in the aforementioned studies were consistent
with the results of the present study, which indicated that IL-1β
successfully induced an inflammatory response in chondrocytes.
Therefore, suppressing NF-κB activity might serve as a novel
therapeutic option for OA (11,25,36,46,47). In
the present study, AG inhibited IKKβ phosphorylation, IκBα
degradation and NF-κB/p65 activation. Similar effects have been
reported with pomegranate fruit extract (51). Pomegranate fruit extract inhibited
IL-6 production, the expression of IKKβ mRNA, the degradation of
IκBα and the nuclear translocation of NF-κB/p65 in OA chondrocytes.
Furthermore, AG inhibited expression of COX-2 and iNOS, and similar
effects have been observed with chrysin (52), suggesting that there is a common
mechanism of action among these drugs. The present study suggested
that AG inhibited NF-κB activation and suppressed the inflammatory
response in IL-1β-stimulated chondrocytes. Therefore, it was
hypothesized that AG inhibited nuclear translocation of NF-κB/p65
by inhibiting the phosphorylation of IκBα and IKKβ, thereby
reducing the expression of iNOS and COX-2, and suppressing the
inflammatory response. Collectively, these results indicated that
AG has the potential to protect articular chondrocytes. Further
investigation into the clinical application of AG is required.
In conclusion, AG downregulated iNOS and COX-2
expression by blocking the NF-κB signaling pathway; therefore, AG
may protect chondrocytes. Additionally, 1 mM AG was the most
effective concentration for the inhibition of inflammation.
Furthermore, the present study indicated that AG may serve as a
potential therapeutic for OA, therefore, providing the theoretical
basis for future clinical studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Key
R&D Program of China (grant no. 2017YFD0502200) and the Major
National Science and Technology Special and Key Research
Projects-Provincial Funding Projects (grant no. GX18B023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM designed the study and prepared the manuscript.
LG designed the study and produced the final draft of manuscript
before submitting. YM, TM and XS analyzed the data. YM, ZZ, HB, YL,
HH, RY and YW performed the experiments and processed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Departmental
Animal Care and Use Committee at Northeast Agricultural
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Madry H and Cucchiarini M: Advances and
challenges in gene-based approaches for osteoarthritis. J Gene Med.
15:343–355. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Litwic AE, Parsons C, Edwards MH,
Jagannath D, Cooper C and Dennison EM: Comment on: Inflammatory
mediators in osteoarthritis: A critical review of the state-of-the
art, prospects, and future challenges. Bone. 106:28–29.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McCulloch K, Litherland GJ and Rai TS:
Cellular senescence in osteoarthritis pathology. Aging Cell.
16:210–218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hafez HM, Ibrahim MA, Ibrahim SA, Amin EF,
Goma W and Abdelrahman AM: Potential protective effect of
etanercept and aminoguanidine in methotrexate-induced
hepatotoxicity and nephrotoxicity in rats. Eur J Pharmacol.
768:1–12. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Balaganur V, Pathak NN, Lingaraju MC, More
AS, Latief N, Kumari RR, Kumar D and Tandan SK: Effect of
S-methylisothiourea, an inducible nitric oxide synthase inhibitor,
in joint pain and pathology in surgically induced model of
osteoarthritis. Connect Tissue Res. 55:367–377. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shirazi I, Yaron I, Wollman Y, Blum M,
Chernihovsky T, Judovich R, Iaina A and Yaron M: Down regulation of
interleukin 1beta production in human osteoarthritic synovial
tissue and cartilage cultures by aminoguanidine. Ann Rheum Dis.
60:391–394. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Farghaly HS and Thabit RH: L-arginine and
aminoguanidine reduce colonic damage of acetic acid-induced colitis
in rats: Potential modulation of nuclear factor-κB/p65. ClinExp
Pharmacol Physiol. 41:769–779. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Di Naso FC, Rodrigues G, Dias AS, Porawski
M, Fillmann H and Marroni NP: Hepatic nitrosative stress in
experimental diabetes. J Diabetes Complications. 26:378–381.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang J, Kalhor A, Lu S, Crawford R, Ni JD
and Xiao Y: iNOS expression and osteocyte apoptosis in idiopathic,
non-traumatic osteonecrosis. Acta Orthop. 86:131–141.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim J, Kim CS, Sohn E, Lee YM, Jo K, Shin
SD and Kim JS: Aminoguanidine protects against apoptosis of retinal
ganglion cells in Zucker diabetic fatty rats. Eur Rev Med Pharmacol
Sci. 18:1573–1578. 2014.PubMed/NCBI
|
|
13
|
Li W, Hu Q, Ren X, He P, Xu H, Dai H and
Chen Z: Aminoguanidine suppresses methylglyoxal-mediated
oxygen-glucose deprivation injury in human brain microvascular
endothelial cells. Zhejiang Da Xue Xue Bao Yi Xue Ban. 42:261–266.
2013.(In Chinese). PubMed/NCBI
|
|
14
|
Tian M, Qing C, Niu Y, Dong J, Cao X, Song
F, Ji X and Lu S: Effect of aminoguanidine intervention on
neutrophils in diabetes inflammatory cells wound healing. Exp Clin
Endocrinol Diabetes. 121:635–642. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sliman SM, Eubank TD, Kotha SR, Kuppusamy
ML, Sherwani SI, Butler ES Kuppusamy P, Roy S, Marsh CB, Stern DM
and Parinandi NL: Hyperglycemic oxoaldehyde, glyoxal, causes
barrier dysfunction, cytoskeletal alterations, and inhibition of
angiogenesis in vascular endothelial cells: Aminoguanidine
protection. Mol Cell Biochem. 333:9–26. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Saiko P, Graser G, Giessrigl B, Lackner A,
Grusch M, Krupitza G, Basu A, Sinha BN, Jayaprakash V, Jaeger W, et
al: A novel N-hydroxy-N'-aminoguanidine derivative inhibits
ribonucleotide reductase activity: Effects in human HL-60
promyelocytic leukemia cells and synergism with
arabinofuranosylcytosine (Ara-C). Biochem Pharmacol. 81:50–59.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zheng B, Zheng T, Wang L, Chen X, Shi C
and Zhao S: Aminoguanidine inhibition of iNOS activity ameliorates
cerebral vasospasm after subarachnoid hemorrhage in rabbits via
restoration of dysfunctional endothelial cells. J Neurol Sci.
295:97–103. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013.
|
|
20
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liao S, Zhou K, Li D, Xie X, Jun F and
Wang J: Schisantherin A suppresses interleukin-1β-induced
inflammation in human chondrocytes via inhibition of NF-κB and
MAPKs activation. Eur J Pharmacol. 780:65–70. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rasheed N, Alghasham A and Rasheed Z:
Lactoferrin from camelus dromedarius inhibits nuclear transcription
factor-κB Activation, Cyclooxygenase-2 expression and prostaglandin
E2 production in stimulated human chondrocytes. Pharmacognosy Res.
8:135–141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhuang Z, Ye G and Huang B: Kaempferol
alleviates the interleukin-1β-induced inflammation in rat
osteoarthritis chondrocytes via suppression of NF-κB. Med Sci
Monit. 23:3925–3931. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen YJ, Tsai KS, Chan DC, Lan KC, Chen
CF, Yang RS and Liu SH: Honokiol, a low molecular weight natural
product, prevents inflammatory response and cartilage matrix
degradation in human osteoarthritis chondrocytes. J Orthop Res.
32:573–580. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou HF, Yan H, Pan H, Hou KK, Akk A,
Springer LE, Hu Y, Allen JS, Wickline SA and Pham C: Peptide-siRNA
nanocomplexes targeting NF-κB subunit p65 suppress nascent
experimental arthritis. J Clin Invest. 124:4363–4374.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Wang D, Qiao J, Zhao X, Chen T and Guan D:
Thymoquinone inhibits IL-1β-induced inflammation in human
osteoarthritis chondrocytes by suppressing NF-κB and MAPKs
signaling pathway. Inflammation. 38:2235–2241. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Y, Wang J, Song X, Bai H, Ma T, Zhang
Z, Li X, Jiang R, Wang G, Fan X, et al: Effects of baicalein on
IL-1β-induced inflammation and apoptosis in rat articular
chondrocytes. Oncotarget. 8:90781–90795. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ahmad R, Ahmad N, Naqvi AA, Exarchou V,
Upadhyay A, Tuenter E, Foubert K, Apers S, Hermans N and Pieters L:
Antioxidant and Antiglycating Constituents from Leaves of Ziziphus
oxyphylla and Cedrela serrata. Antioxidants(Basel).
5(9)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Orman D, Vardi N, Ates B, Taslidere E and
Elbe H: Aminoguanidine mitigates apoptosis, testicular seminiferous
tubules damage, and oxidative stress in streptozotocin-induced
diabetic rats. Tissue Cell. 47:284–290. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wright C, Iyer AKV, Kulkarni Y and Azad N:
S-Nitrosylation of Bcl-2 negatively affects autophagy in lung
epithelial cells. J Cell Biochem. 117:521–532. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Madhu BP, Singh KP, Saminathan M, Singh R,
Tiwari AK, Manjunatha V, Harish C and Manjunathareddy GB:
Correlation of inducible nitric oxide synthase (iNOS) inhibition
with TNF-α, caspase-1, FasL and TLR-3 in pathogenesis of rabies in
mouse model. Virus Genes. 52:61–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shafaroodi H, Hassanipour M, Mousavi Z,
Rahimi N and Dehpour AR: The effects of sub-chronic treatment with
pioglitazone on the septic mice mortality in the model of cecal
ligation and puncture: Involvement of nitric oxide pathway. Acta
Med Iran. 53:608–616. 2015.PubMed/NCBI
|
|
34
|
Tian M, Qing C, Niu Y, Dong J, Cao X, Song
F, Ji X and Lu S: Aminoguanidine cream ameliorates skin tissue
microenvironment in diabetic rats. Arch Med Sci. 12:179–187.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Parthasarathy A, Gopi V, Devi KMS, Balaji
N and Vellaichamy E: Aminoguanidine inhibits ventricular fibrosis
and remodeling process in isoproterenol-induced hypertrophied rat
hearts by suppressing ROS and MMPs. Life Sci. 118:15–26.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lenin R, Mohan V and Balasubramanyam M:
SEAP activity serves for demonstrating ER stress induction by
glucolipotoxicity as well as testing ER stress inhibitory potential
of therapeutic agents. Mol Cell Biochem. 404:271–279.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen WP, Wang YL, Tang JL, Hu PF, Bao JP
and Wu LD: Morin inhibits interleukin-1β-induced nitric oxide and
prostaglandin E2 production in human chondrocytes. Int
Immunopharmacol. 12:447–452. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li R, Cai L, Ding J, Hu CM, Wu TN and Hu
XY: Inhibition of hedgehog signal pathway by cyclopamine attenuates
inflammation and articular cartilage damage in rats with
adjuvant-induced arthritis. J Pharm Pharmacol. 67:963–971.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lianxu C, Hongti J and Changlong Y:
NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2,
NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced
chondrocytes. Osteoarthritis Cartilage. 14:367–376. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Huang X, Pan Q, Mao Z, Zhang R, Ma X, Xi Y
and You H: Sinapic acid inhibits the IL-1 β-induced inflammation
via MAPK downregulation in rat chondrocytes. Inflammation.
41:562–568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ma Z, Wang Y, Piao T and Liu J:
Echinocystic acid inhibits IL-1β-induced COX-2 and iNOS expression
in human osteoarthritis chondrocytes. Inflammation. 39:543–549.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ansari MY and Haqqi TM: Interleukin-1β
induced stress granules sequester COX-2 mRNA and regulates its
stability and translation in human OA chondrocytes. Sci Rep.
6(27611)2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Suschek CV, Schnorr O and Kolb-Bachofen V:
The role of NOS in chronic inflammatory processes in vivo: Is it
damage-promoting, protective, or active at all? Curr Mol Med.
4:763–775. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gómez R, Scotece M, Conde J, Lopez V, Pino
J, Lago F, Gómez-Reino JJ and Gualillo O: Nitric oxide boosts TLR-4
mediated lipocalin 2 expression in chondrocytes. J Orthop Res.
31:1046–1052. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Flaherty RL, Owen M, Fagan-Murphy A,
Intabli H, Healy D, Patel A, Allen MC, Patel BA and Flint MS:
Glucocorticoids induce production of reactive oxygen
species/reactive nitrogen species and DNA damage through an iNOS
mediated pathway in breast cancer. Breast Cancer Res.
19(35)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang SN, Xie GP, Qin CH, Chen YR, Zhang
KR, Li X, Wu Q, Dong WQ, Yang J and Yu B: Aucubin prevents
interleukin-1 beta induced inflammation and cartilage matrix
degradation via inhibition of NF-κB signaling pathway in rat
articular chondrocytes. Int Immunopharmacol. 24:408–415.
2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chin KY: The spice for joint inflammation:
Anti-inflammatory role of curcumin in treating osteoarthritis. Dove
Medical Press. 10:3029–3042. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lei M, Wang JG, Xiao DM, Fan M, Wang DP,
Xiong JY, Chen Y, Ding Y and Liu SL: Resveratrol inhibits
interleukin 1β-mediated inducible nitric oxide synthase expression
in articular chondrocytes by activating SIRT1 and thereby
suppressing nuclear factor-κB activity. Eur J Pharmacol. 674:73–79.
2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ying X, Chen X, Cheng S, Shen Y, Peng L
and Xu HZ: Piperine inhibits IL-β induced expression of
inflammatory mediators in human osteoarthritis chondrocyte. Int
Immunopharmacol. 17:293–299. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Stuhlmeier KM: The anti-rheumatic gold
salt aurothiomalate suppresses interleukin-1beta-induced hyaluronan
accumulation by blocking HAS1 transcription and by acting as a
COX-2 transcriptional repressor. J Biol Chem. 282:2250–2258.
2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Haseeb A, Khan NM, Ashruf OS and Haqqi TM:
A polyphenol-rich pomegranate fruit extract suppresses NF-κB and
IL-6 expression by blocking the activation of IKK and NIK in
primary human chondrocytes. Phytother Res. 31:778–782.
2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zheng W, Tao Z, Cai L, Chen C, Zhang C,
Wang Q, Ying X, Hu W and Chen H: Chrysin attenuates IL-1β-induced
expression of inflammatory mediators by suppressing NF-κB in human
osteoarthritis chondrocytes. Inflammation. 40:1143–1154.
2017.PubMed/NCBI View Article : Google Scholar
|