Introduction
As most human autoimmune disorders, autoimmune
thyroiditis (AIT) (chronic lymphocytic thyroiditis/Hashimoto's
thyroiditis) results from a combination of genetic predisposition
and environmental triggers (1).
Clinical and epidemiologic evidence point to excessive iodine
intake as the environmental agent responsible for the thyroid
autoimmunity induction (2,3). The role of iodine in the homeostatic
regulation of thyroid function was first demonstrated >50 years
ago. However, the precise mechanism of regulation remains unclear
(4). High doses of iodide suppress
the functional activity of the thyrocytes (Wolff-Chaikoff effect),
inhibiting the iodination of the thyroid protein fraction and
decreasing the concentration of thyroid hormones in serum (5). It has been demonstrated that a single
injection of a high dose of iodide inhibits the biosynthesis of
thyroid hormones at several levels (6,7). The
sensitivity to the stimulating action of thyroid-stimulating
hormones decreases and the expression and activity of
thyroperoxidase (the enzyme catalyzing iodination of thyroglobulin
in the presence of iodide and hydrogen peroxide) are suppressed
(8). Finally, the NADPH-oxidase
reaction producing H2O2 (the limiting step in
the iodide metabolism) is also suppressed (7,9). The
necrotic effect is increased in case of selenium (Se) deficiency
(10,11). However, thyroid cells have their own
antioxidant system. Thus, in the case of iodine excess, the
expression of antioxidative enzymes increases (12). Therefore, the first iodine-induced
thyroiditis has been transient in most experimental animals, except
for genetically modified animals prone to develop AIT, such as
non-obese diabetic (NOD) mice. They present important areas of
destroyed thyroid tissue which are replaced by inflammatory tissue
(13).
Experimental autoimmune thyroiditis (EAT) has been
used to simulate human autoimmune thyroid disease for decades
(14). EAT can be easily induced in
genetically susceptible strains of mice by excess iodine ingestion
(15) or by immunization with mouse
thyroglobulin (16).
However, iodine excess alone has also been used to
induce EAT in insusceptible murine models, including Wistar rats
(17,18), as well as in other animals (19). Iodine excess is a cheap and efficient
method for EAT induction.
The aim of the present study was to assess the
effects of inorganic Se supplementation on thyroid morphology in
EAT induced by the administration of potassium iodide (KI) in
Wistar rats.
Materials and methods
Animals
A total of 48 Wistar adult rats (24 females weighing
160±20 g and 24 males weighing 180±20 g) were used for the present
study. The animals were obtained from the ‘Victor Babes’ National
Institute of Research Development in the Pathology Domain and
Biomedical Sciences (Bucharest, Romania). Wistar rats were housed
under standard conditions at the Biobase for research animals of
‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania) and were fed with standard food. The rats were housed in
clean and ventilated polyurethane cages; 2 rats were placed in each
cage. All rats were maintained under standard conditions of
temperature (20±40̊C), relative humidity of 55±10%, and light/dark
cycles of 12/12 h consecutively. Access to food and water was ad
libidum. The acclimatization of the rats lasted 7 days prior to
the study initiation. The study was approved by the Ethics
Committee of ‘Grigore T. Popa’ University of Medicine and
Pharmacy.
EAT and Se administration
As AIT is more common in females (3:1) (20-22),
it was investigated whether the same susceptibility of the female
sex also occurs in the animal model of Wistar rats. The animals
were randomized into groups according to four treatment regimens:
C0, two control groups for each sex; C1, two (male and female)
groups that received KI for 56 days (0.2 mg per animal in drinking
water); C2, two (male and female) groups that received concomitant
KI and sodium selenite (0.5 mg/kg body weight of sodium selenite
administered in drinking water) for 56 days; and C3, two (male and
female) groups that received KI for 56 days and afterwards sodium
selenite for another 56 days (Table
I).
 | Table IStudy group allocation and treatment
regimens. |
Table I
Study group allocation and treatment
regimens.
| Factors | C0 | C1 | C2 | C3 |
|---|
| Sex
(males/females) | 6/6 | 6/6 | 6/6 | 6/6 |
| KI
administration | No | 56 days of KI | 56 days of KI | 56 days of KI |
| Na-Se
administration | No | No | 56 days of Na-Se,
concomitant with KI administration | 56 days of Na-Se,
after the KI administration |
| Total days of
treatment/observation | 7 days | 56 days | 56 days | 112 days |
Even though no dose-finding study was performed, a
previous report (23) concerning
sodium selenite administration in Wistar rats has shown consistent
toxic effects of sodium selenite at a dose of >1 mg/kg body
weight (using the same administration method: ad libitum in
drinking water) (23,24).
Tissue collection and analysis
General anesthesia was performed with a combination
of ketamine (60 mg/kg body weight) and xylazine (8 mg/kg body
weight) administered intraperitoneally. Thyroid tissues were
collected for pathology analysis, in accordance with the Council
Directive 63/2010/EU on the protection of animals used for
scientific purposes, after 7 days in control groups, 56 days in C1
and C2 groups, and 112 days in C3 groups. Tissue samples were
harvested from the neck area containing the anterior muscular plan,
the thyroid and parathyroid tissue and the tracheal tissue with the
cartilage ring. In order to keep the thyroid intact, tissue samples
were fixed in 10% formaldehyde at 24̊C for 24 h. The tissue samples
were embedded in paraffin and cut into 4-µm thick sections. Next,
the tissue samples were stained with hematoxylin and eosin
(H&E) for 40 min, or van Gieson's (VG) stain for 15 min at room
temperature 22-24̊C. The morphometric evaluation was performed
using a light microscope, with x10 objective. For positive and
negative control, thyroid and parathyroid tissues were
immunohistochemically stained with synaptophysin, chromogranin,
thyroglobulin and thyroid transcription factor (TTF1) at the ‘Prof.
Dr. Gioconda Dobrescu’ Department of Pathology, ‘Sf. Spiridon’
County Hospital (Iasi, Romania). Parathyroid tissues showed diffuse
negative immunoreactivity for chromogranin, synaptophysin and TTF1;
parafollicular C cells were positive for chromogranin and
synaptophysin, and thyroid tissue presented diffuse nuclear
immunoreactivity for TTF1 and positive cytoplasmic immunoreactivity
for thyroglobulin. Immunohistochemistry was performed at room
temperature between 22-24̊C. The overnight staining technique was
carried out at 4̊C with a maximum staining duration of 24 h.
The sections were examined and photographed using
Nikon Eclipse E600 (Nikon Corporation) and the Lucia Net program,
equipped with the Nikon Digital Net Camera DN100 image capture
system (Nikon Corporation) and morphometric analysis software
(morphometric software LUCIA Net v.16.2®; Laboratory
Imaging s.r.o., with NIS Elements 3.0®) at the ‘Prof.
Dr. Gioconda Dobrescu’ Department of Pathology. The morphometric
analysis of the thyroid tissues was made from 5 successive images
captured from each lobe (digital pictures of 10 non-adjacent 10x
fields). The interpretation of histopathological sections and the
acquisition of images for all studied animals were performed by a
single examiner (DGCA).
Morphopathology parameter
assessment
The mean size of thyroid follicles was assessed by
measuring the maximum diameter of ≥20 thyroid follicles per case in
various areas of the thyroid gland. The mean size of the thyroid
follicular epithelium was assessed by measuring the size of the
follicular epithelium in fixed positions (at 12, 5 and 7 o'clock)
in 20 follicles per case.
The morphological evaluation of thyroid follicles
was performed in 20 thyroid follicles for each case and included a
scoring system from 0 to 3 for each of the following parameters:
Presence of inflammation, vascular congestion, resorption vacuoles,
interfollicular space and interstitial collagen deposits (Table II). A final thyroiditis score was
calculated as the sum of the inflammation score, vascular
congestion, fibrosis and resorption vacuoles scores, and the
results corresponded to either normal thyroid morphology (final
score 0-3), mild thyroiditis (final score 4-6), moderate
thyroiditis (final score 7-9) or severe thyroiditis (final score
10-12) (Table II).
 | Table IIScoring system used for the
morphopathological evaluation of the AIT. |
Table II
Scoring system used for the
morphopathological evaluation of the AIT.
| | Scoring system | |
|---|
| Parameter | 0 | 1 | 2 | 3 | Refs. |
|---|
| Inflammation | Normal
morphology | Mild destruction of
thyroid follicles with few lymphocytes attacking ~ 2 or 3 thyroid
follicles | Focal foci with
moderate destruction of the thyroid follicles in 10-40% of the
total area | Severe destruction
of thyroid follicles with inflammatory infiltration in >40% of
the total area | (21,22,35),
adapted |
| Vascular
congestion | Normal
morphology | Mild vascular
distention in the capsular blood vessels | Moderate congestion
in the capsular and intraglandular blood vessels in 10-40% of the
total area | Severe congestion
in the capsular and intraglandular blood vessels in >40% of the
total area | (20,33),
adapted |
| Resorption
vacuoles | Normal
morphology | Resorption vacuoles
present in <10% of the follicular cavities | Resorption vacuoles
present in 10-40% of the follicular cavities | Resorption vacuoles
present in >40% of the follicular cavities | (20,33),
adapted |
| Interfollicular
space | Normal
morphology | Interfollicular
spaces observed in <10% of the glandular surface | Interfollicular
spaces observed in 10-40% of the glandular surface | Interfollicular
spaces observed in >40% of the glandular surface | (20,33),
adapted |
| Interstitial
collagen deposits | Normal
morphology | Discreet
collagenization of the thyroid capsule | Thickened capsule
with fine pericapsular septae in the interstitial space | Important capsular
fibrosis with thick pericapsular septae and perifollicular
fibrosis | (20,33),
adapted |
| Thyroiditis
score | Normal thyroid
morphology: 0-3 Mild thyroiditis: 4-6 Moderate thyroiditis: 7-9
Severe thyroiditis: 10-12 | (20,33,36-38),
adapted |
Statistical analysis
All statistical analyses were performed using SPSS
v24.0 software (IBM Corp.). Skewness and kurtosis (-2<P<2)
tests, the tests of normality in frequentist statistics, were used
to examine the distribution of continuous variables. For multiple
comparisons of normally distributed data, two-way ANOVA was
performed with Tukey's HSD post hoc test. If the normality
assumption was not satisfied, Kruskal-Wallis test and
Dunn-Bonferroni post hoc test were carried out. Associations
between categorical variables were assessed by Chi-square test.
The results are presented in Tables III and IV and Figs.
1 and 2. Specifically, Figs. 1 and 2
present the data on thyroid morphofunctional parameters, i.e., the
mean size of thyroid follicles and follicular epithelium. Table III presents the comparison of the
thyroid morphology results between male and female rats in each
treatment group. Association matrixes of the morphopathological
parameters assessed within each study group are presented in
Table IV (vascular congestion,
resorption vacuoles, interfollicular space, interstitial collagen
deposits).
 | Table IIIComparison of thyroid morphology
results between male and female rats in each treatment group. |
Table III
Comparison of thyroid morphology
results between male and female rats in each treatment group.
| | C0 | C1 | C2 | C3 |
|---|
| | Males | Females | Males | Females | Males | Females | Males | Females |
|---|
| Score | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
|---|
| Inflammation |
|
0 Normal
morphology | 6 | 100 | 6 | 100 | 6 | 100 | 4 | 66.7 | 6 | 100 | 6 | 100 | 5 | 83.3 | 6 | 100 |
|
1 Mild
follicular destruction | - | - | - | - | - | - | 1 | 16.7 | - | - | - | - | 1 | 16.7 | - | - |
|
2 Moderate
follicular destruction | - | - | - | - | - | - | 1 | 16.7 | - | - | - | - | - | - | - | - |
|
3 Severe
follicular destruction | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
|
P-value | - | 0.333 | - | 0.296 |
| Vascular
congestion |
|
0 Normal
morphology | 6 | 100 | 6 | 100 | - | - | - | - | - | - | - | - | 3 | 50 | 5 | 83.3 |
|
1 Mild
vascular distention | - | - | - | - | 1 | 16.7 | 2 | 33.3 | 3 | 50 | 3 | 50 | - | | 1 | 16.7 |
|
2 Moderate
vascular congestion | - | - | - | - | 1 | 16.7 | 2 | 33.3 | 3 | 50 | 3 | 50 | 1 | 16.7 | - | - |
|
3 Severe
vascular congestion | - | - | - | - | 4 | 66.7 | 2 | 33.3 | - | - | - | - | 2 | 33.3 | - | - |
|
P-value | - | 0.513 | - | 0.212 |
| Resorption
vacuoles |
|
0 Normal
morphology | 6 | 100 | 4 | 66.7 | - | - | - | - | 6 | 100 | 6 | 100 | 1 | 16.7 | 6 | 100 |
|
1 <10% of
follicular cavities | - | - | 2 | 33.3 | 2 | 33.3 | 3 | 50 | - | - | - | - | 3 | 50 | - | - |
|
2 10-40% of
follicular cavities | - | - | - | - | 2 | 33.3 | 2 | 33.3 | - | - | - | - | 1 | 16.7 | - | - |
|
3 >40% of
follicular cavities | - | - | - | - | 2 | 33.3 | 1 | 16.7 | - | - | - | - | 1 | 16.7 | - | - |
|
P-value | 0.439 | 0.766 | - | 0.036 |
| Interfollicular
space |
|
0 Normal
morphology | 6 | 100 | 6 | 100 | - | - | - | - | 6 | 100 | 6 | 100 | 4 | 66.7 | 6 | 100 |
|
1 <10% of
glandular surface | - | - | - | - | 3 | 50 | 4 | 66.7 | - | - | - | - | - | - | - | - |
|
2 10-40% of
glandular surface | - | - | - | - | 1 | 16.7 | 2 | 33.3 | - | - | - | - | 2 | - | - | - |
|
3 >40% of
glandular surface | - | - | - | - | 2 | 33.3 | - | - | - | - | - | - | - | - | - | - |
|
P-value | - | 0.290 | - | 0.439 |
| Interstitial
collagen deposits |
|
0 Normal
morphology | 6 | 100 | 5 | 83.3 | - | - | 1 | 16.7 | - | - | - | - | 1 | 16.7 | 5 | 83.3 |
|
1 Mild
capsular collagenization | - | - | 1 | 16.7 | 2 | 33.3 | 5 | 83.3 | 3 | 50 | - | - | 2 | 33.3 | 1 | 16.7 |
|
2 Thickened
capsule | - | - | - | - | 4 | 66.7 | - | - | 2 | 33.3 | 3 | 50 | 3 | 50 | - | - |
|
3 Important
capsular fibrosis | - | - | - | - | - | - | - | - | 1 | 16.7 | 3 | 50 | - | - | - | - |
|
P-value | 0.296 | 0.043 | 0.122 | 0.049 |
| Final score of
thyroiditis |
|
0-3 Normal
thyroid morphology | 6 | 100 | 6 | 100 | - | - | - | - | 4 | 66.7 | 1 | 16.7 | 2 | 33.3 | 6 | 100 |
|
4-6 Mild
thyroiditis | - | - | - | - | 1 | 16.7 | 3 | 50 | 2 | 33.3 | 5 | 83.3 | 3 | 50 | - | - |
|
6-9 Moderate
thyroiditis | - | - | - | - | 3 | 50 | 3 | 50 | - | - | - | - | 1 | 16.7 | - | - |
|
10-12 Severe
thyroiditis | - | - | - | - | 2 | 33.3 | - | - | - | - | - | - | - | - | - | - |
|
P-value | - | 0.223 | 0.079 | 0.049 |
 | Table IVAssociation matrixes of
morphopathological parameter results assessed within each study
group. |
Table IV
Association matrixes of
morphopathological parameter results assessed within each study
group.
| Males | C1 | C2 | C3 | C0 | Females | C1 | C2 | C3 | C0 | Males vs.
females |
|---|
| Vascular congestion
scoring |
|
C1 | - | | | | C1 | - | | | | 0.513 |
|
C2 | 0.049 | - | | | C2 | 0.301 | - | | | - |
|
C3 | 0.020 | 0.029 | - | | C3 | 0.025 | 0.011 | - | | 0.212 |
|
C0 | 0.001 | 0.002 | 0.050 | - | C0 | 0.007 | 0.002 | 0.500 | - | - |
|
C1+2+3 | | | | 0.001 | C1+2+3 | | | | 0.049 | 0.407 |
| Resorption vacuoles
scoring |
|
C1 | - | | | | C1 | - | | | | 0.766 |
|
C2 | 0.007 | - | | | C2 | 0.001 | - | | | - |
|
C3 | 0.661 | 0.036 | - | | C3 | 0.001 | - | - | | 0.036 |
|
C0 | 0.007 | - | 0.036 | - | C0 | 0.050 | 0.439 | 0.439 | - | 0.439 |
|
C1+2+3 | | | | 0.001 | C1+2+3 | | | | 0.050 | 0.036 |
| Interfollicular
spaces scoring |
|
C1 | - | | | | C1 | - | | | | 0.290 |
|
C2 | 0.007 | - | | | C2 | 0.002 | - | | | - |
|
C3 | 0.025 | 0.439 | - | | C3 | 0.002 | - | - | | 0.439 |
|
C0 | 0.007 | - | 0.439 | - | C0 | 0.002 | - | - | - | - |
|
C1+2+3 | | | | | C1+2+3 | | | | | |
| Interstitial
collagen deposits scoring |
|
C1 | - | | | | C1 | - | | | | 0.043 |
|
C2 | 0.393 | - | | | C2 | 0.004 | - | | | 0.043 |
|
C3 | 0.497 | 0.439 | - | | C3 | 0.003 | 0.040 | - | | 0.122 |
|
C0 | 0.002 | 0.007 | 0.014 | - | C0 | 0.003 | 0.040 | - | - | 0.296 |
|
C1+2+3 | | | | 0.001 | C1+2+3 | | | | 0.050 | 0.050 |
| Final thyroiditis
scoring |
|
C1 | - | | | | C1 | - | | | | 0.223 |
|
C2 | 0.025 | - | | | C2 | 0.105 | - | | | 0.079 |
|
C3 | 0.049 | 0.393 | - | | C3 | 0.002 | 0.019 | - | | 0.049 |
|
C0 | 0.007 | 0.439 | 0.049 | - | C0 | 0.002 | 0.019 | - | - | - |
|
C1+2+3 | | | | 0.046 | C1+2+3 | | | | 0.033 | 0.474 |
Results
Mean size of thyroid follicles
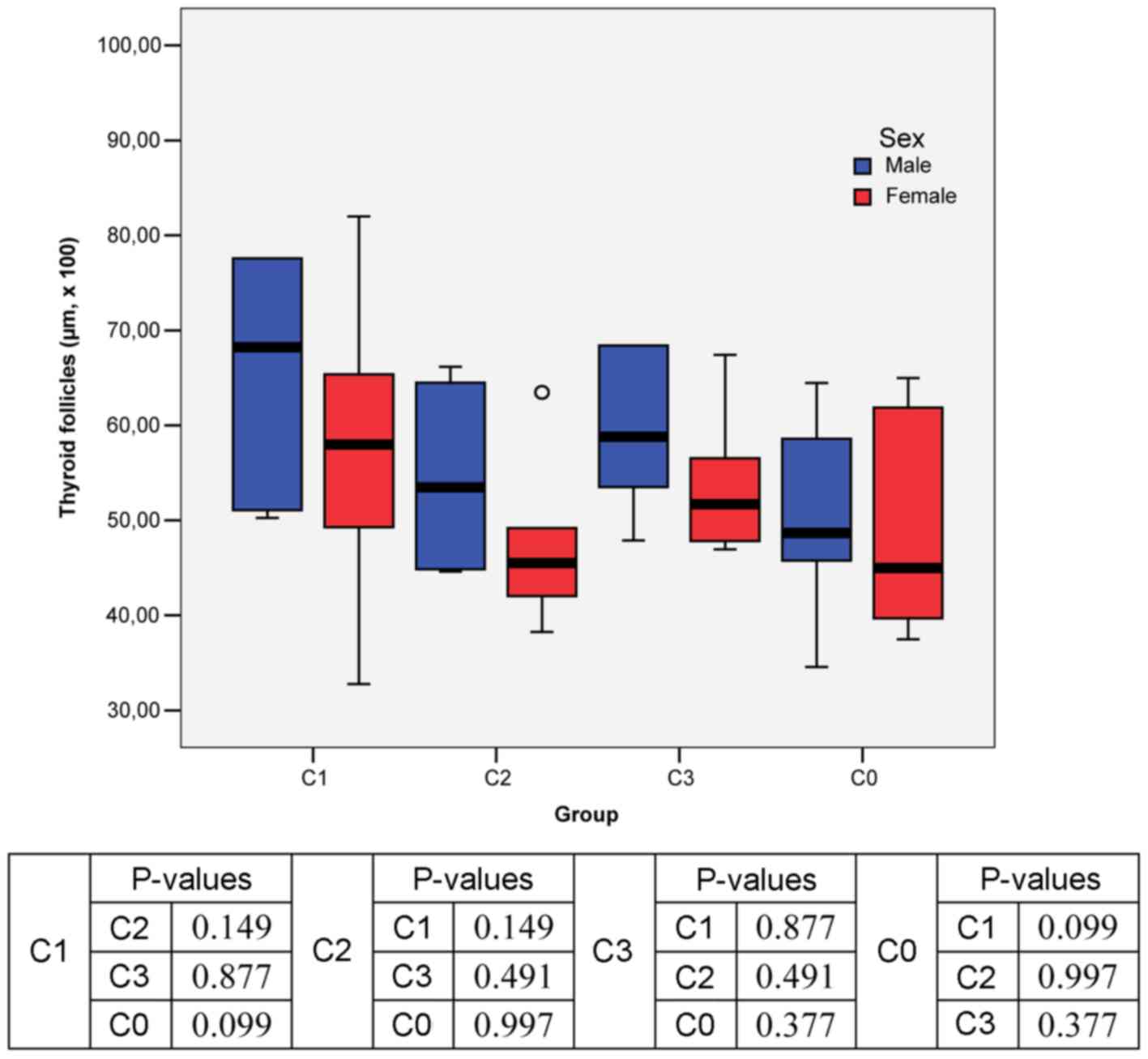
Regarding the size of thyroid follicles, the
normality tests revealed the following aspects: The analysis of the
entire study group showed that the mean value (56.48±17.05 µm x100)
was far different than the median value (52.40 µm x100); Skewness
(skw = 2.105) and Kurtosis (krt = 6.605) test results >2
suggested that the assumption of normality was not satisfied for
the entire range of values; however, in C0, C1, C3, for both male
and female subgroups, continuous values were confirmed.
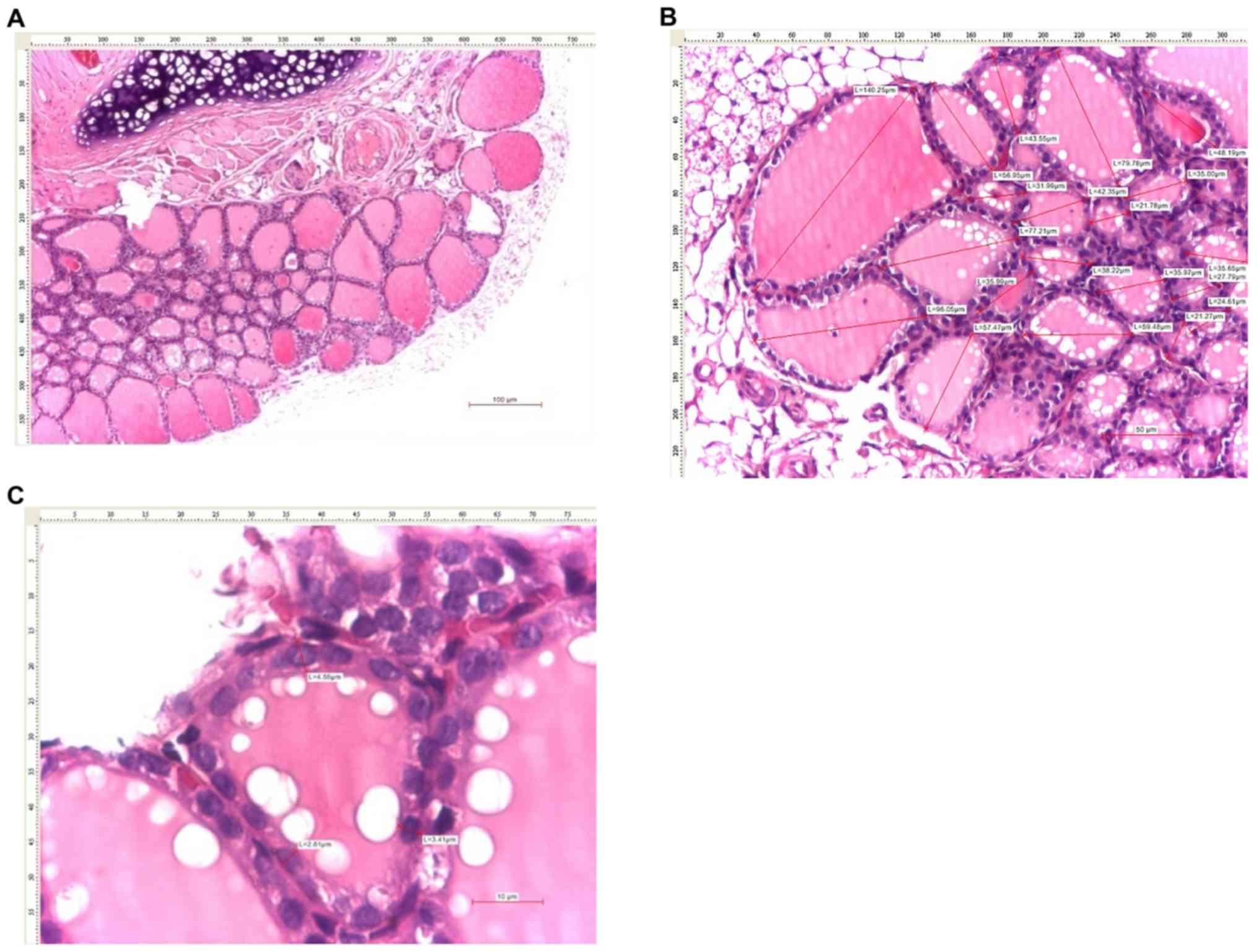
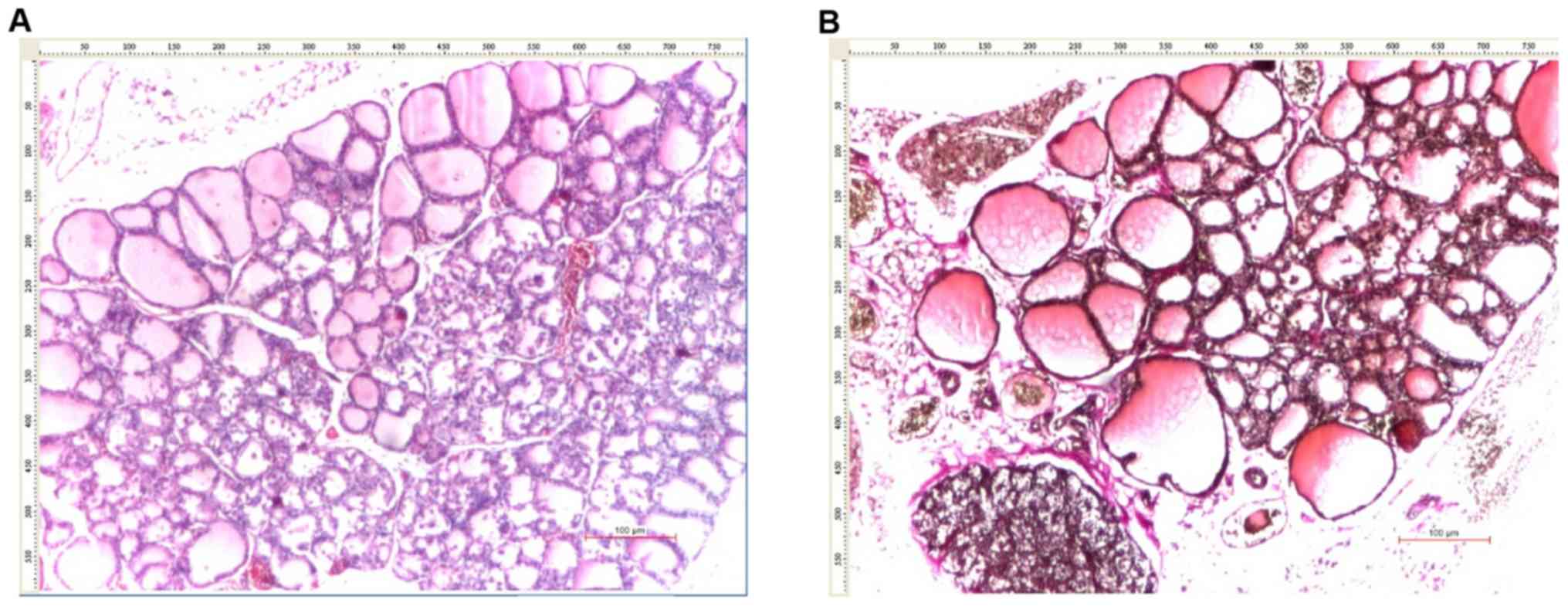
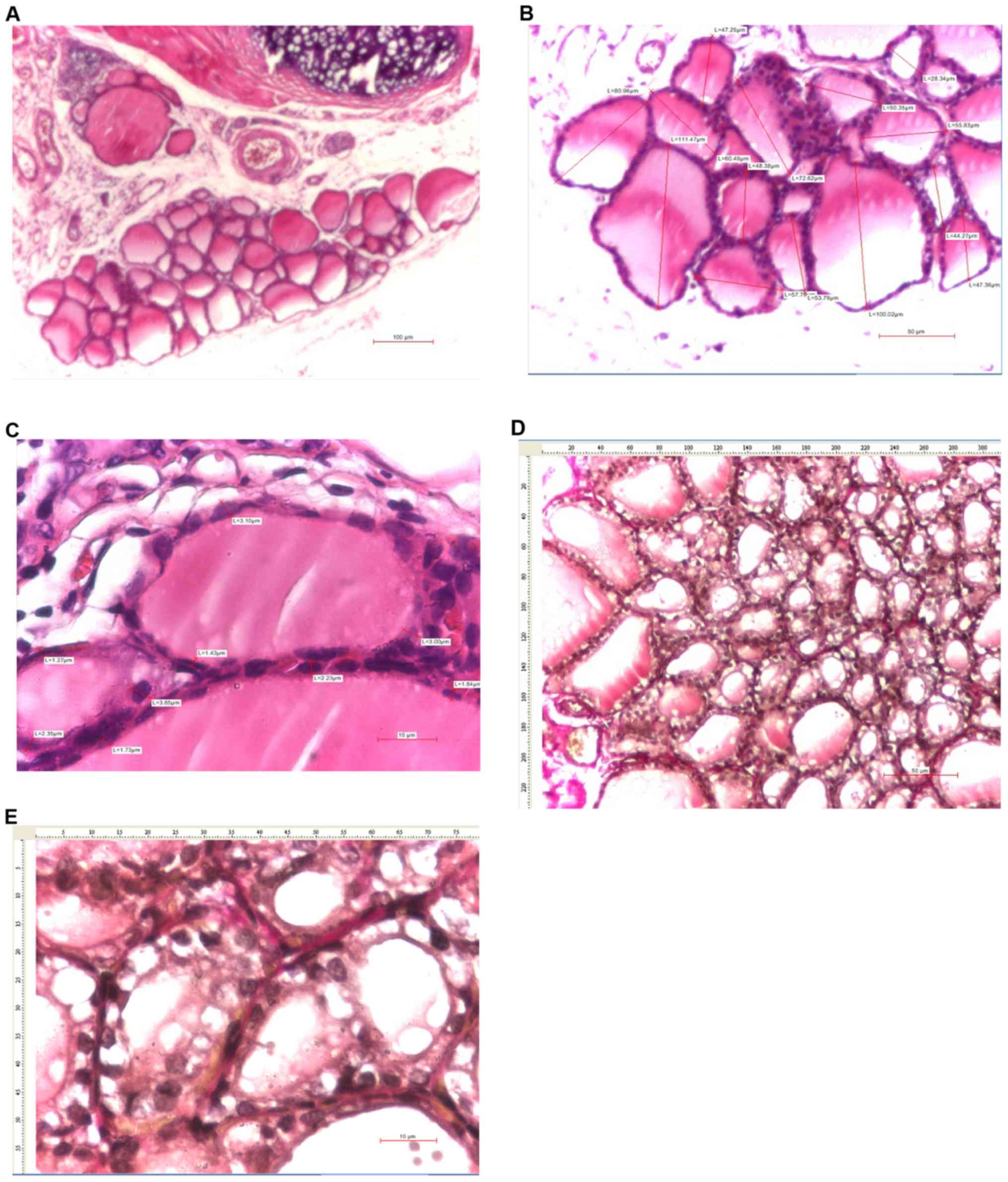
The male rat thyroid morphology (Figs. 1, and 3A and B)
showed that C1 group had higher mean value of thyroid follicles
than C0 group (73.82 vs. 50.13 µm x100) and C2 group (73.82 vs.
53.74 µm x100). In C3 group, the mean value was higher than that
recorded in the control group C0 (65.86 vs. 50.13 µm x100). The
lowest mean value of the thyroid follicles was registered in the
control group C0 (Fig. 1).
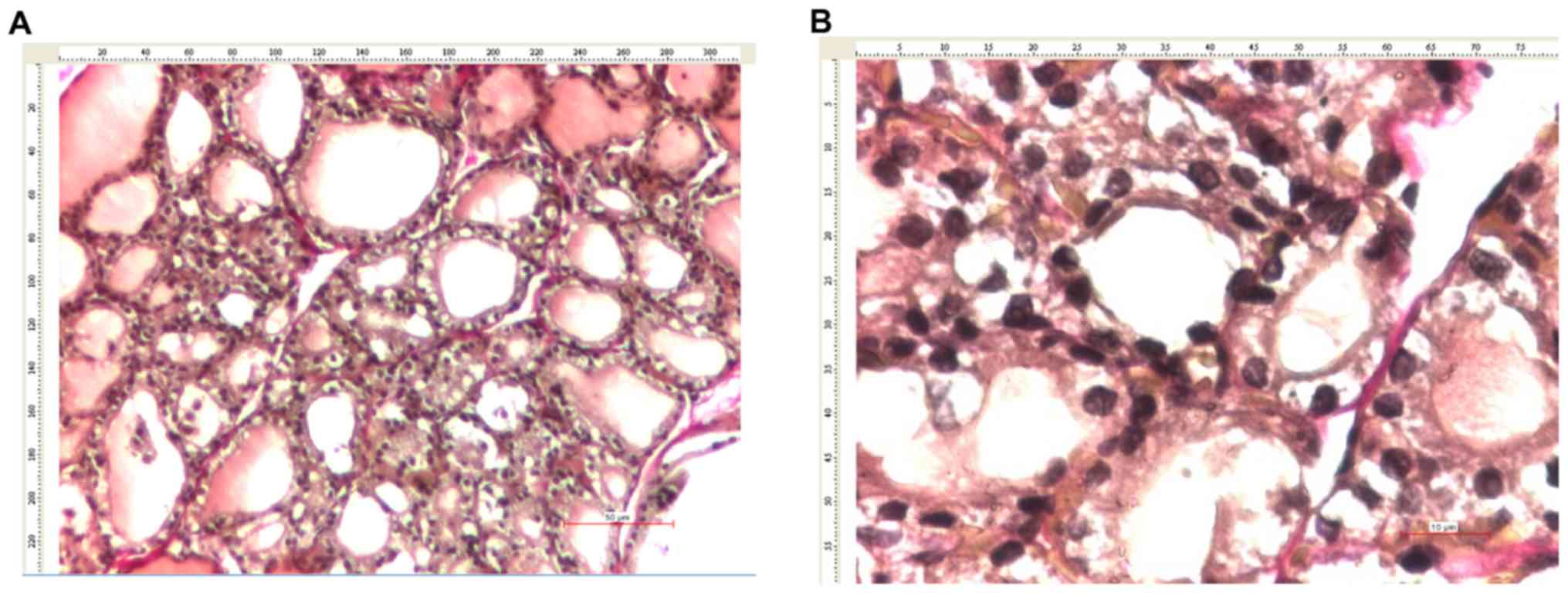
In female rats, the highest mean value of thyroid
follicles was recorded in C1 group (57.56 µm x100) and the lowest
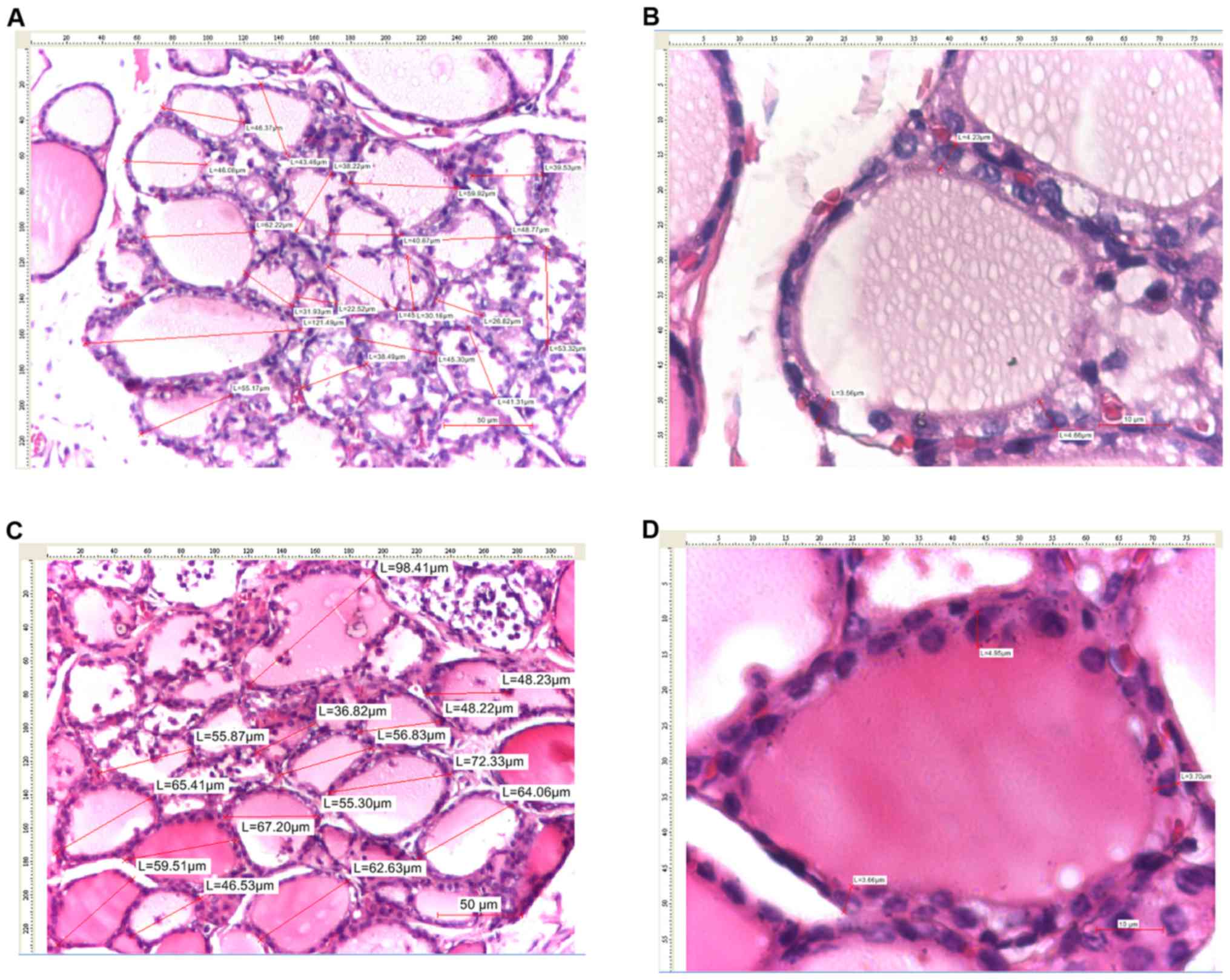
in C2 group (47.32 µm x100; Figs. 1
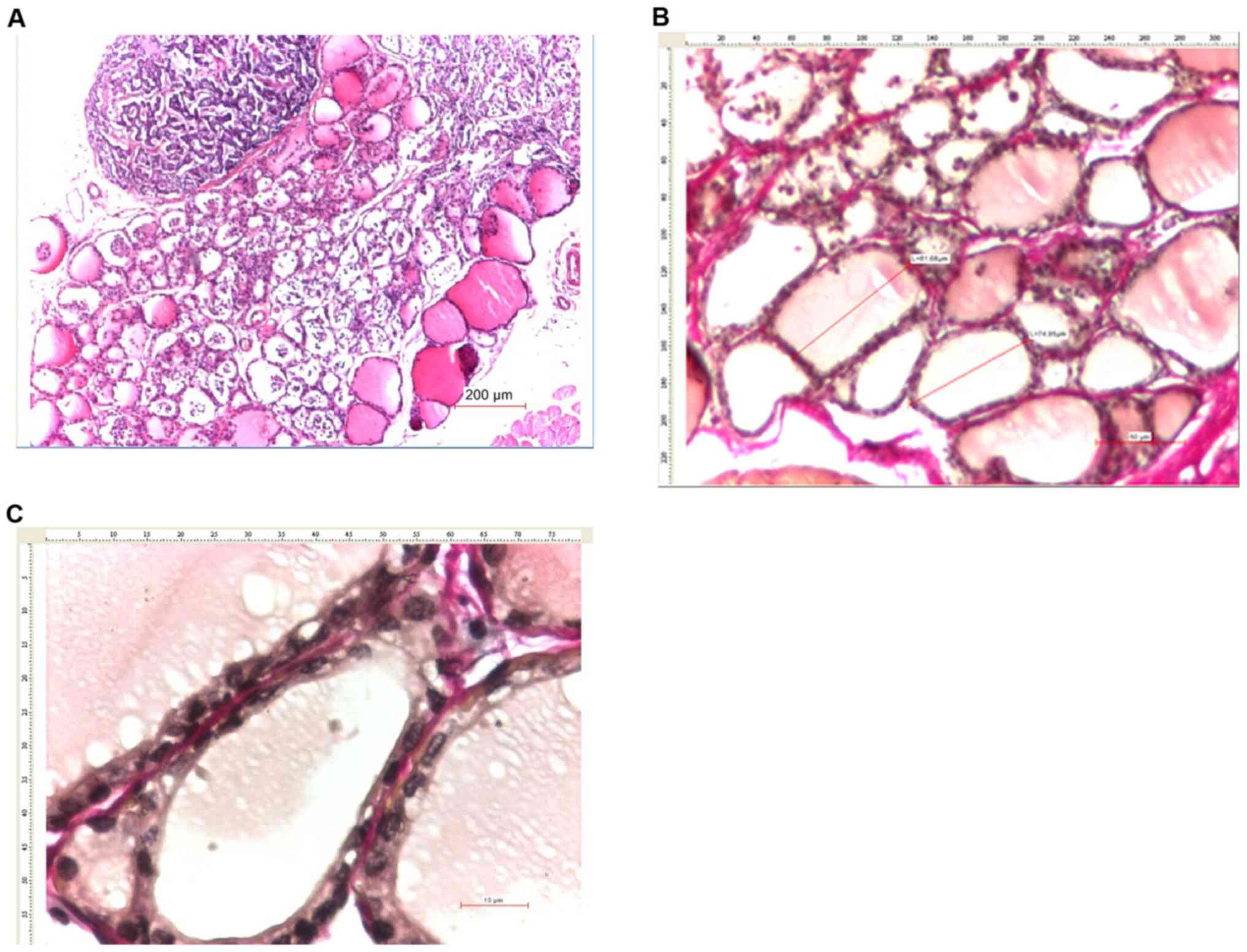
and 4A and C, 7B, and
8B).
 | Figure 7Thyroid images of female rats with
sequential administration of KI and sodium selenite. (A) Thyroid
tissue (H&E, x4), (B) measurements of the maximum diameter of
thyroid follicles (H&E, x10) (20 thyroid follicles were
measured in total), (C) measurements of follicular epithelium
heights (H&E, x40), (D) resorption vacuoles (VG, x20) and (E)
follicular epithelium details (VG, x40). KI, potassium iodine;
H&E, hematoxylin and eosin; VG, van Gieson's stain. |
The results of two-way ANOVA showed no statistically
significant differences in the mean size of thyroid follicles
analyzed by sex and intervention group (Fig. 1).
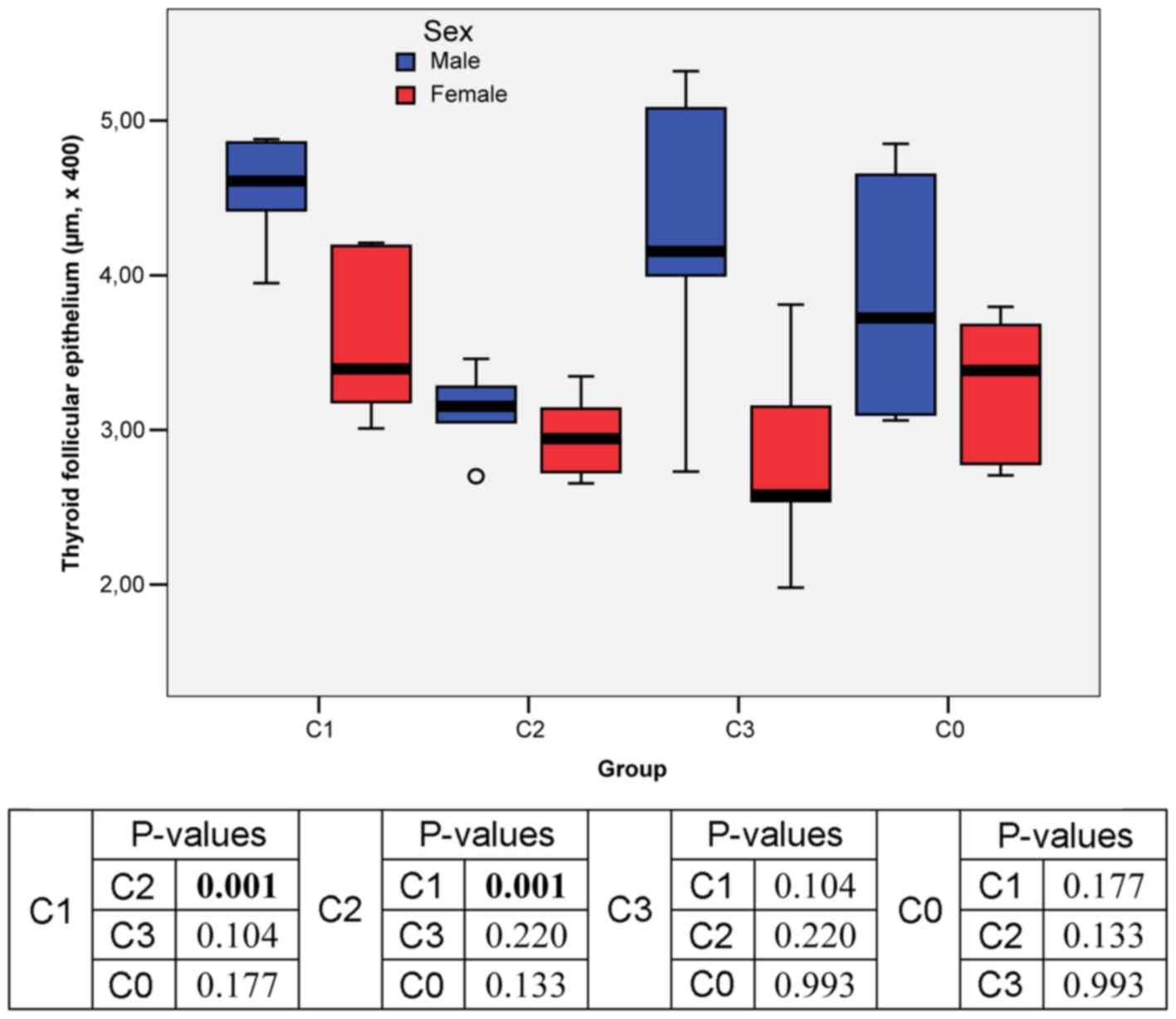
Mean size of the thyroid follicular
epithelium
The values for the size of follicular epithelium in
the entire study group were homogeneous, thus significance tests
could be applied for these continuous variables: The mean value
(3.55±0.80 µm x400) was close to the median value (3.29 µm x400);
Skewness (skw = 0.472) and Kurtosis (krt = -0.649) test results
were comprised in the interval [-2, +2].
In male rats, the highest mean value of the thyroid
follicular epithelium was recorded in C1 group (only KI
administration; 4.56 µm x100) and the lowest mean value in C2 group
(3.13 µm x100) (Figs. 3C and
5B).
In female rats, the highest mean value of the
thyroid follicular epithelium was recorder in C1 group (3.56 µm
x100) and the lowest mean value was recorded in C3 group (2.77 µm
x100) (Figs. 2, 4B and D,
7C and E, and 8C).
Two-way ANOVA results showed statistically
significant differences in the mean size of thyroid follicular
epithelium analyzed by sex and intervention group only between C1
(only KI adminsitration) and C2 (concomitant KI and sodium selenite
administration) groups (Fig. 2).
Inflammation assessment
The results on thyroid inflammation revealed that
there were no significant differences between sex or treatment
regimens in the study groups (C1, P=0.333; C3, P=0.296; Table III).
Vascular congestion
In male rats, significant differences were found
between groups: In C1 group, severe vascular congestion was
observed in 66.7% of male rats; in C2 group, 50% of rats had
moderate vascular congestion; whereas in C3 group, 50% of male rats
had normal vascular morphology (P=0.001; Table III).
In female rats, significant differences were also
identified between groups: In C1 group, the same percentage of
cases (33.3%) presented severe, moderate and mild vascular
modifications; in C2 group, 50% of female rats had moderate
vascular congestion; whereas in C3 group, 83.3% of the cases had
normal vascular morphology (P=0.049). However, within each of the
study groups, no statistically significant differences in terms of
sex were confirmed (C1, P=0.513; C3, P=0.212; Tables III and IV).
Resorption vacuoles
In male rats, significant differences were confirmed
between the groups: In C1 group, resorption vacuoles assessment
revealed equal percentages of cases (33.3%) with score 1 (<10%),
2 (10-40%) and 3 (>40%); in C2 group, all cases had normal
morphology; and in C3 group, 50% of the male rats had resorption
vacuoles <10% (P=0.001; Table
III).
In female rats, significant differences were also
found between groups: In C1 group, 50% of female rats had
resorption vacuoles <10%; and in C2 and C3 groups, all cases had
normal morphology (P=0.05; Tables
IV and 7D). Significant sex differences were observed only in
C3 group (P=0.036; Table III).
Interfollicular space
All rats (regardless of sex) in C1 group (only KI
administration) presented interfollicular spaces. The
interfollicular space score for C1 male rats (treated only with KI)
was significantly different than that in C2 group (concomitant KI
and Se administration, P=0.007), C3 group (subsequent KI and Se
administration, P=0.025) and C0 group (control, P=0.007) (Table IV). Scores of 2 (10-40% of glandular
surface) and 3 (>40% of glandular surface) were particularly
recorded in 50% of male rats (one rat with score 2 and two rats
with score 3), whereas all the female rats presented only scores of
1 (<10% of the glandular surface) and 2 (Table III).
Interstitial collagen deposits
In male rats, 66.7% of the cases in the C1 group had
moderate fibrosis; in C2 group, 50% of male rats had mild collagen
deposits and only 33.3% moderate fibrosis; whereas in C3 group,
only 33.3% of cases had mild collagen deposits and 50% moderate
fibrosis (P=0.001; Table III).
In female rats, significant differences between
groups were also confirmed: In C1 group, 83.3% of the rats had
discrete collagen deposits; in C2 group, 50% had important collagen
deposits; and in C3 group, 83.3% had normal morphology (P=0.05;
Table IV). Concerning the
morphology of interstitial collagen deposits, significant sex
differences were observed only within C1 (P=0.043) and C3 (P=0.049)
groups (Table III).
Thyroiditis final score
In males, significant differences between treatment
regimens were confirmed: In C1 group (only KI administration), 50%
of the rats developed moderate thyroiditis and 33.3% severe
thyroiditis; in C2 group (concomitant KI and Se administration),
33.3% of the male rats developed mild thyroiditis; and in C3 group
(subsequent KI and Se administration), 50% of cases had mild
thyroiditis and 16.7% moderate thyroiditis (P=0.046; Table IV and Fig. 5A).
Female rats demonstrated significant differences in
overall thyroid morphology: In C1 group, 50% of cases developed
moderate thyroiditis; in C2 group, 83.3% of female rats had mild
thyroiditis; whereas all cases in C3 group had normal morphology
(P=0.033; Table IV and Figs. 6A and 7A).
Regarding final thyroiditis score, significant sex
differences were recorded only in C3 group where all females had
normal thyroid morphology, similar to the female control group
(P=0.049; Table III and Fig. 8A).
Discussion
In the present study, the protective role of Se on
thyroid morphology in iodine-induced AIT in Wistar rats was
confirmed. Se effect was more evident in female rats, as the
subsequent administration of Se after iodine exposure determined
minimum modifications on the thyroid morphology, preserving the
normal aspect of the thyroid gland.
Iodine plays an important role in the induction and
modulation of thyroid autoimmunity. Several studies have assessed
the prevalence of thyroid antibodies and autoimmune hypothyroidism
in patients which are located in iodine-replete versus in
iodine-deficient areas (25-27).
After iodine prophylaxis in iodine deficient areas,
a 4-fold increase in the prevalence of anti-thyroid antibodies has
been reported (28). According to a
Danish survey, following the administration of 500 µg/day iodine
dose for 6 months, AIT occurred in 20% of the healthy individuals
that were included in the study (29).
The same results were obtained using
NOD.H2® mice as experimental animal models for
genetically determined AIT. Iodine enrichment in these mice showed
increased incidence and severity of the disease in a dose-dependent
manner (1). Several possible
mechanisms have been described by which iodine could trigger AIT.
Iodine exposure leads to increased iodination of thyroglobulin and,
therefore, to increased antigenicity (immunogenicity) by creating
new iodine-containing epitopes or by discovering cryptic epitopes.
This facilitates antigen exposure (antigen processing or antigen
presentation) and, thus, increases T cell receptor binding and
activation of T cells, respectively (1).
Secondly, increased iodine exposure determines the
increase of reactive oxygen species (ROS) in thyrocytes. ROS may
increase the expression of intracellular adhesion molecule 1 in the
thyroid follicular cell, which subsequently attracts
immunocompetent cells to the thyroid gland. The binding of ROS to
the phospholipidic membrane may induce injury to the thyroid and
the release of auto-antigens (28).
In addition, iodine excess promotes apoptosis of thyroid follicular
cells by inducing the expression of TRAIL (TNF-related
apoptosis-inducing ligand) necrosis factor and its receptor, death
receptor-5, in the thyroid. There is also in vitro evidence
of the iodine's influence on the immune system cells as it may
increase dendritic cell maturation, and increase the number of T
cells, as well as the production of immunoglobulins (28).
In the present study, the EAT in adult male and
female Wistar rats was induced by KI administration in the drinking
water. Most studies in the literature, which sought to induce AIT,
used genetically modified animal models, NOD.H2®
(1,13) and BB/W (Bio Breeding/Worcester) rats
(30,31). NOD mice and BB/W rats are animal
models generally used for the study of type 1 diabetes.
NOD.H2® is a genetically animal model predisposed to
develop AIT over time. Iodine administration increases the
prevalence of AIT, earlier occurrence, and a more severe form of
disease (32). A similar study was
performed on BB/W rats by looking at the effect of excess iodine on
thyroid function and on immunological phenomena that trigger AIT.
The results of the study showed an increase in the number of
dendritic cells and lymphocyte infiltrate in animals receiving
additional iodine in drinking water (31). Both the increase in the number of
dendritic cells and the lymphocyte infiltrate are possible
mechanisms involved in triggering AIT.
In a study on NOD.H2® mice of different
ages, it was observed that the prevalence and severity of AIT
increased with age in genetically predisposed animals (32). In the present study, only young adult
Wistar rats were used, with no genetic predisposition to influence
the onset or the form of the disease. This was also preferred in
order to have a homogenous group and to avoid possible age-induced
changes in the experiment.
Regarding the presence of thyroid inflammation in
both male and female Wistar rats, no significant changes between
the treatment regimens were described, the results being similar to
those in the control group. The absence of inflammation could be
explained by the short period of iodine administration or by
insufficient iodine quantities. Similar studies that obtained
inflammatory changes had administered iodine up to 12 weeks
(32-34).
The evolution of follicular epithelioum size shows
potential benefits of Se treatment as favourable statistically
significant differences were observed between the measured
parameter in the group treated with concomitant sodium selenite and
KI administration, in comparison with the group administered with
only iodide.
Significant changes were observed in the groups
treated with KI and Se compared with the KI treated groups, with
forms of thyroiditis less aggressive in both males and females
treated with Se. In rats with sequentially KI and sodium selenite
administration, the same favourable outcomes were not obtained as
in the case of the concomitantly treated groups; this effect was
only observed in males. The females initially treated with KI and
subsequently with Se had a surprising evolution, the results being
almost identical to those of the control group, as they no longer
had AIT. Experimental studies have shown that the antigen that
initiates AIT in animal models is thyroglobulin, regardless of
species (studies in mice, rats and birds) (35). The thyroglobulin allografts affect
the susceptibility to thyroiditis. Moreover, modulating genes
related to the X-chromosome have been highlighted, which could
explain the different responses in AIT not only in animals, but
also in humans (36). The
effectiveness of Se supplementation proved to be different
depending on the time of treatment initiation and sex.
Autoimmune thyroid disease is highly prevalent, with
the highest female-to-male ratio among all autoimmune diseases
(37). There is a large body of
evidence that moderate amounts of estrogen may enhance immunologic
reactivity to self-antigens (38,39).
However, as AIT is frequently diagnosed after menopause, the
X-chromosome seems to be the source of enhanced susceptibility
rather than sex steroid levels. For example, X-chromosome
inactivation has been associated with autoimmune thyroid disease
(40). However, there have been
reports in men that confirm a connection between estradiol levels
(or estradiol to testosterone ratio) and thyroid autoimmunity
(41,42).
In males, Se supplementation has been shown to be
more effective with concomitant administration of KI. Males have
been presented with less aggressive forms of the disease than those
who had received successive administration (initially with KI and
subsequently with Se). Se administration, in both concomitant and
then successively treated groups, contributed to milder forms of
AIT, compared with the group not supplemented with Se.
Moreover, an extremely important aspect was observed
in the groups of female rats in which, unlike male rats, Se
supplementation proved to be very effective. In the group of
females treated successively, the thyroid morphological aspect was
identical to the morphological appearance of the control group,
which did not show AIT, thus advocating the remission of the
KI-induced disease. Concomitant administration resulted in a
significant improvement in thyroid morphology.
Overall, the results of the present study revealed
the effectiveness of Se supplementation in both co-administration
with KI and sequential administration in female and male sex alike.
Significant sex differences were recorded in the groups initially
treated with KI and subsequently with Se (P=0.049): While in
females histological appearance of the thyroid was normal in the
whole group, in males only 33% had normal thyroid, the rest having
mild (50%) or medium (17%) thyroiditis. In the rest of the study
groups, the differences were not statistically significant
(Table III).
This was especially observed in females due to the
hormonal features involved in the AIT pathogenesis. It is known
that estrogen increases (while androgen decreases) the response of
the hypothalamic-pituitary-adrenocortical axis to stress, and
activation of this axis is more pronounced in women than in men,
which explains the higher incidence of autoimmune thyroid disease
in women (43).
There is a number of limitations in the present
study. A low number of Wistar rats was used in each study group,
although valid for statistical analysis following previous
scientific research protocols. In addition, a Se dose-finding study
was not performed; however, no clinical signs of Se toxicity were
observed during the study at the administered dosages. An
additional limitation is represented by the fact that all
histopathological sections and the acquisition of images of the
studied animals were performed by a single examiner and no Cohen's
kappa could be established to confirm the rater's reliability.
The present study showed the effective results of Se
supplementation on restoring the normal thyroid morphology in
iodine induced AIT in Wistar rats.
In conclusion, the impact of induction of AIT on
Wistar rats (induced by KI administration) is higher in males than
in females, although the latter are more prone to the disease.
Males develop more severe forms; the difference is primarily due to
the modulating role of estrogens.
Se supplementation has been shown to be effective,
resulting in improved forms of AIT. The timing of Se administration
has also been proven to be important and concomitant administration
of KI and sodium selenite is associated with the return of thyroid
morphology to normal in most cases.
Acknowledgements
Professional editing, linguistic and technical
assistance was provided by Irina Radu, Individual Service Provider,
certified translator in Medicine and Pharmacy (certificate
credentials: Series E, no. 0048).
Funding
The study was supported by an internal grant from
‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania) (no. 29.243/20.12.2013). The funders had no role in the
study design, data collection and analysis, decision to publish, or
preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during the study are
included in this published article.
Authors' contributions
CP, DGCA, IV, OB and ILS were involved in the
conception and design of the study. CP, DGCA, IV and OB acquired
the data. DGCA was involved in the analysis and interpretation of
the histological data. The statistical analysis and overall
interpretation of data was performed by CP, DGCA, IV, IA and ILS.
IV, IA, DGCA and CP drafted the manuscript. CP, DGCA, IV and IA
revised critically the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rose NR, Bonita R and Burek CL: Iodine: An
environmental trigger of thyroiditis. Autoimmun Rev. 1:97–103.
2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Markou K, Georgopoulos N, Kyriazopoulou V
and Vagenakis AG: Iodine-induced hypothyroidism. Thyroid.
11:501–510. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Foley TP Jr: The relationship between
autoimmune thyroid disease and iodine intake: A review. Endokrynol
Pol. 43):53–69. 1992.PubMed/NCBI
|
|
4
|
Wolff J and Chaikoff IL: Plasma inorganic
iodide as a homeostatic regulator of thyroid function. J Biol Chem.
174:555–564. 1948.PubMed/NCBI
|
|
5
|
Paul T, Meyers B, Witorsch RJ, Pino S,
Chipkin S, Ingbar SH and Braverman LE: The effect of small
increases in dietary iodine on thyroid function in euthyroid
subjects. Metabolism. 37:121–124. 1988.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Denef JF, Many MC and van den Hove MF:
Iodine-induced thyroid inhibition and cell necrosis: Two
consequences of the same free-radical mediated mechanism? Mol Cell
Endocrinol. 121:101–103. 1996.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vitale M, Di Matola T, D'Ascoli F, Salzano
S, Bogazzi F, Fenzi G, Martino E and Rossi G: Iodide excess induces
apoptosis in thyroid cells through a p53-independent mechanism
involving oxidative stress. Endocrinology. 141:598–605.
2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cardoso LC, Martins DCL, Figueiredo MDL,
Rosenthal D, Vaisman M, Violante AHD and Carvalho DP:
Ca(2+)/nicotinamide adenine dinucleotide phosphate-dependent
H(2)O(2) generation is inhibited by iodide in human thyroids. J
Clin Endocrinol Metab. 86:4339–4343. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mahmoud I, Colin I, Many MC and Denef JF:
Direct toxic effect of iodide in excess on iodine-deficient thyroid
glands: Epithelial necrosis and inflammation associated with
lipofuscin accumulation. Exp Mol Pathol. 44:259–271.
1986.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Contempre B, Denef JF, Dumont JEMM and
Many MC: Selenium deficiency aggravates the necrotizing effects of
a high iodide dose in iodine deficient rats. Endocrinology.
132:1866–1868. 1993.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Contempre B, Le Moine O, Dumont JE, Denef
JF and Many MC: Selenium deficiency and thyroid fibrosis. A key
role for macrophages and transforming growth factor beta
(TGF-beta). Mol Cell Endocrinol. 124:7–15. 1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chiu-Ugalde J, Wirth EK, Klein MO, Sapin
R, Fradejas-Villar N, Renko K, Schomburg L, Köhrle J and Schweizer
U: Thyroid function is maintained despite increased oxidative
stress in mice lacking selenoprotein biosynthesis in thyroid
epithelial cells. Antioxid Redox Signal. 17:902–913.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kolypetri P, Noel NA, Carayanniotis KA and
Carayanniotis G: Iodine content of thyroglobulin in
Nod.H2® mice developing iodine-accelerated autoimmune
thyroiditis. Hormones (Athens). 9:151–160. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arata N, Ando T, Unger P and Davies TF:
By-stander activation in autoimmune thyroiditis: Studies on
experimental autoimmune thyroiditis in the GFP®
fluorescent mouse. Clin Immunol. 121:108–117. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rasooly L, Burek CL and Rose NR:
Iodine-induced autoimmune thyroiditis in NOD-H-2® mice.
Clin Immunol Immunopathol. 81:287–292. 1996.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Čiháková D, Sharma RB, Fairweather D,
Afanasyeva M and Rose NR: Animal models for autoimmune myocarditis
and autoimmune thyroiditis. In: Autoimmunity: Methods and
Protocols. Perl A (ed). Humana Press, Totowa, NJ, pp175-193,
2004.
|
|
17
|
Pitsiavas V, Smerdely P, Li M and Boyages
SC: Amiodarone induces a different pattern of ultrastructural
change in the thyroid to iodine excess alone in both the BB/W rat
and the Wistar rat. Eur J Endocrinol. 137:89–98. 1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao J, Lin X, Liu X, Yang Q, Zhang Z,
Jiang Q and Bian J: Effect of combined excess iodine and
low-protein diet on thyroid hormones and ultrastructure in Wistar
rats. Biol Trace Elem Res. 155:416–422. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bagchi N, Brown TR, Urdanivia E and
Sundick RS: Induction of autoimmune thyroiditis in chickens by
dietary iodine. Science. 230:325–327. 1985.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hassanin KM, Abd El-Kawi SH and Hashem KS:
The prospective protective effect of selenium nanoparticles against
chromium-induced oxidative and cellular damage in rat thyroid. Int
J Nanomedicine. 8:1713–1720. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Roubaty C, Bedin C and Charreire J:
Prevention of experimental autoimmune thyroiditis through the
anti-idiotypic network. J Immunol. 144:2167–2172. 1990.PubMed/NCBI
|
|
22
|
Cui SL, Yu J and Shoujun L: Iodine Intake
Increases IP-10 Expression in the serum and thyroids of rats with
experimental autoimmune thyroiditis. Int J Endocrinol.
2014(581069)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Risher J (ed): Toxicological Profile for
Selenium (Update). DIANE Publishing, Darby, PA, 2011. https://books.google.com/books?id=AEVdvRbs6xEC&pgis=1.
|
|
24
|
Solcan C, Ciobanu C and Cuciureanu R:
Dose-dependent subacute toxicity of sodium selenite in male Wistar
rats. Jökull J. 63:57–69. 2013.
|
|
25
|
Effraimidis G and Wiersinga WM: Mechanisms
in endocrinology: autoimmune thyroid disease: old and new players.
Eur J Endocrinol. 170:R241–R252. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vanderpump MP, Tunbridge WM, French JM,
Appleton D, Bates D, Clark F, Evans JG, Hasan DM, Rodgers H,
Tunbridge F, et al: The incidence of thyroid disorders in the
community: A twenty-year follow-up of the Whickham Survey. Clin
Endocrinol (Oxf). 43:55–68. 1995.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Laurberg P, Pedersen KM, Hreidarsson A,
Sigfusson N, Iversen E and Knudsen PR: Iodine intake and the
pattern of thyroid disorders: A comparative epidemiological study
of thyroid abnormalities in the elderly in Iceland and in Jutland,
Denmark. J Clin Endocrinol Metab. 83:765–769. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fountoulakis S, Philippou G and Tsatsoulis
A: The role of iodine in the evolution of thyroid disease in
Greece: From endemic goiter to thyroid autoimmunity. Hormones
(Athens). 6:25–35. 2007.PubMed/NCBI
|
|
29
|
Nielsen E, Greve K, Larsen JC, Meyer O,
Krogholm K and Hansen M: Iodine, Inorganic and Soluble Salts. The
Danish Environmental Protection Agency, Copenhagen K, Denmark,
2014. https://www2.mst.dk/Udgiv/publications/2014/01/978-87-93026-87-2.pdf.
|
|
30
|
Yanagisawa M, Hara Y, Satoh K, Tanikawa T,
Sakatsume Y, Katayama S, Kawazu S, Ishii J and Komeda K:
Spontaneous Autoimmune Bio Breeding/Worcester Thyroiditis in
(BB/W). Rat. 33:851–861. 1986.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li M, Eastman CJ and Boyages SC: Iodine
induced lymphocytic thyroiditis in the BB/W rat: Early and late
immune phenomena. Autoimmunity. 14:181–187. 1993.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Barin JG, Talor MV, Sharma RB, Rose NR and
Burek CL: Iodination of murine thyroglobulin enhances autoimmune
reactivity in the NOD.H2 mouse. Clin Exp Immunol. 142:251–259.
2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhu YP, Bilous M and Boyages SC: Excess
iodine induces the expression of thyroid solid cell nests in
lymphocytic thyroiditis-prone BB/W rats. Autoimmunity. 20:201–206.
1995.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lupachik SV, Nadol'nik LI, Netsetskaya ZV
and Vinogradov VV: Effect of long-term injection of high doses of
potassium iodide on iodine metabolism in rat thyroid gland. Biochem
Suppl Ser B: Biomed Chem. 1:53–57. 2007.
|
|
35
|
Ruwhof C and Drexhage HA: Iodine and
thyroid autoimmune disease in animal models. Thyroid. 11:427–436.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Amara IB, Bouaziz H, Guermazi F and Zeghal
N: Effect of selenium on hypothyroidism induced by methimazole
(MMI) in lactating rats and their pups. Acta Biol Hung. 61:145–157.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jacobson DL, Gange SJ, Rose NR and Graham
NMH: Epidemiology and estimated population burden of selected
autoimmune diseases in the United States. Clin Immunol
Immunopathol. 84:223–243. 1997.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xiang Y, Jin Q, Li L, Yang Y, Zhang H, Liu
M, Fan C, Li J, Shan Z and Teng W: Physiological low-dose oestrogen
promotes the development of experimental autoimmune thyroiditis
through the up-regulation of Th1/Th17 responses. J Reprod Immunol.
126:23–31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kincade PW, Medina KL, Smithson G and
Scott DC: Pregnancy: A clue to normal regulation of B
lymphopoiesis. Immunol Today. 15:539–544. 1994.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yin X, Latif R, Tomer Y and Davies TF:
Thyroid epigenetics: X chromosome inactivation in patients with
autoimmune thyroid disease. Ann N Y Acad Sci. 1110:193–200.
2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chailurkit LO, Aekplakorn W and
Ongphiphadhanakul B: The relationship between circulating estradiol
and thyroid autoimmunity in males. Eur J Endocrinol. 170:63–67.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen Y, Chen Y, Xia F, Wang N, Chen C, Nie
X, Li Q, Han B, Zhai H, Jiang B, et al: A higher ratio of estradiol
to testosterone is associated with autoimmune thyroid disease in
males. Thyroid. 27:960–966. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Falgarone G, Heshmati HM, Cohen R and
Reach G: Mechanisms in endocrinology. Role of emotional stress in
the pathophysiology of Graves' disease. Eur J Endocrinol.
168:R13–R18. 2012.PubMed/NCBI View Article : Google Scholar
|