Introduction
The fracture healing process involves many complex
steps, and ultimately restores the structure and function of the
fracture site (1,2). The healing process begins immediately
after a fracture occurs, and the local inflammatory reaction is a
critical part of early healing (3).
Moreover, the inflammatory response plays a vital role in the
promotion and coordination of local osseous tissue regeneration
(3). With the passive release of
locally damaged tissue and the active release of inflammatory
cells, the expression levels of inflammatory factors including
tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6, IL-11
and IL-18 are significantly elevated (4). Systemic and local release of these
inflammatory factors have an important effect on biological
processes such as vascular regeneration, cell recruitment and
cartilaginous callus production (4).
Among these inflammatory factors, TNF-α and IL-1
have major functions in the regulation of fracture healing, and
their secretion levels vary at different time points (4-6).
These two master regulators of inflammation reach first peak levels
between 24 h and 7 days after the fracture occurs (4-6).
The process of cell recruitment into the hematoma is primarily
activated by inflammatory factors at this stage (5,6). Then,
the expression levels of TNF-α and IL-1 decrease until the
regulation of osseous tissue regeneration is required (5,6). The
second increase in these two regulators often occurs 4 weeks after
fracture, and the specific time point and duration of this increase
are related to the individual healing rate and age of the patient
(5,6).
Although direct fracture healing has been reported,
indirect fracture healing is the main route of fracture healing
(2,7). Both endochondral ossification and
intramembranous ossification play important roles in indirect
fracture healing, but intramembranous ossification is faster during
bone regeneration (8-10).
In order to form new osseous tissue that can resist rotational and
shear forces, stem cells in the osteotylus differentiate into
osteoblasts and bone cells, which then gradually mineralize
(8-10).
Mesenchymal stem cells (MSCs) can serve as both a source of
bone-shaped osteoblasts and osteoprogenitor cells (8,9), as well
as having a paracrine effect on bone tissue regeneration (10). During the process of osseous tissue
regeneration, the influence of inflammatory factors on osteogenic
differentiation of bone marrow MSCs (BMMSCs) has been investigated
(4). In inflammatory
microenvironments, IL-1 and TNF-α have a prominent role in
inflammatory osteolysis (11). While
TNF-α has similar activity to that of IL-1β, the expression of
IL-1β is relatively higher in inflamed osseous tissues (12). As a major regulator of fracture
healing, IL-1β reportedly promotes osteogenic differentiation of
BMMSCs (7,13,14). It
has been shown that the use of non-steroidal anti-inflammatory
drugs during the fracture healing phase leads to prolonged healing,
which is consistent with the reported osteogenic effect of
inflammatory factors (15-18).
However, previous studies have revealed that IL-1β inhibits stem
cell differentiation into osteoblasts, and this inhibitory effect
is enhanced with increasing IL-1β concentration (19). In addition, the mechanism via which
IL-1β affects osteogenic differentiation has been previously
investigated, and the involvement of several signaling pathways has
been identified (20-23).
Bone morphogenetic protein/Smad (BMP/Smad) is a classical signaling
pathway that is often activated during osteogenic differentiation
of stem cells and plays a key role in early osteogenesis and late
mineralization (24). However,
whether the BMP/Smad signaling pathway participates in the effect
of IL-1β on osteogenic differentiation is not fully understood
(19,25).
The aim of the present study was to investigate the
effect on the osteogenic differentiation of mouse bone marrow
mesenchymal stem cells (MBMMSCs) at various concentrations, in
order to assess whether IL-1β has a positive effect on fracture
healing. Using systematic experiments, the present study examined
the maximum concentration of IL-1β that would be required to
produce a positive effect, and identified the role of the BMP/Smad
pathway after IL-1β stimulation.
Materials and methods
Cell culture
All animal experiments in this study were performed
in accordance with the guidelines of Anhui Medical University, and
were approved by the Ethics Committee at Anhui Medical University.
In this experiment, five male C57BL/6 mice provided by the Anhui
Laboratory Animal Center (age, 6 weeks; weight, 18-22 g) were used.
Animals were housed at a temperature of 20-25˚C, 50-60% humidity in
a 12/12 h light/dark cycle and access to food and water. The main
aim was to obtain primary cells (BMMSCs) from the mice. Euthanasia
was performed by the inhalation of CO2 (100%) using a
closed euthanasia device, which was placed in a ventilated
environment. Before mice entered the device, low concentrations of
CO2 were prepassed into the device. After mice entered
the chamber, 100% CO2 was gradually introduced. After
determining the mortality of the mouse, CO2 (100%) was
continually passed for 2 min. If mice did not breathe spontaneously
for 3 min and there was no blink reflex, mortality was confirmed.
Primary MBMMSCs were isolated and expanded as previously described
(26). Euthanized mice were
sterilized in 70% ethanol for 15 min, and cells were isolated from
the femur and tibiae bone marrow, and then cultured in a T75 flask.
To form complete medium, 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin (Hyclone; GE Healthcare Life
Sciences) were added to the α modification of Eagle's minimum
essential medium (Hyclone; GE Healthcare Life Sciences). Primary
cells were cultured in complete medium in an incubator containing
5% CO2 at 37˚C until the cells were fully confluent. The
medium was changed every 3 days, and MBMMSCs at passage 3 were used
in the subsequent experiments. MBMMSCs at passage 2 were identified
by fluorescence activated cell sorting staining. Antibodies against
CD90, CD105, CD34 and CD45 (2 µg/ml; BD Biosciences) were used for
staining (30 min at 25˚C) and a flow cytometer (BD FACSCalibur; BD
Biosciences) was used for the detection (Fig. S1).
Cell proliferation
The effect of IL-1β (R&D Systems, Inc.) on the
proliferation of MBMMSCs was detected using a Cell-Counting Kit-8
(CCK-8) assay (Dojindo Molecular Technologies, Inc.) according to
the manufacturer's instructions. MBMMSCs (5x103
cells/well) were plated into 96-well plates containing IL-1β at
various concentrations (0, 0.01, 0.1, 1 or 10 ng/ml) for 1, 3 or 5
days at 37˚C. Cell culture medium was removed and 100 µl 10% CCK-8
solution was added to each well. After 2 h of incubation at 37˚C,
absorbance of the wells was measured at 450 nm.
Cell apoptosis
The effect of IL-1β on MBMMSC apoptosis was assessed
using flow cytometry via double-staining for Annexin V-FITC and PI
(BD Biosciences). MBMMSCs were seeded into 24-well plates
(8x104 cells/well) and collected 48 h after IL-1β
treatment (0, 0.01, 0.1, 1, or 10 ng/ml) at 37˚C. Cells were washed
twice with PBS and resuspended in 200 µl 1X binding buffer (BD
Biosciences) at a density of 5x105 cells/ml. Then, 5 µl
Annexin V-FITC and 10 µl PI were added to the tube and incubated
for 15 min in the dark at 25˚C. A flow cytometer (BD FACSCalibur;
BD Biosciences) was used for the detection.
Assessment of alkaline phosphatase
(ALP) activity and mineralized matrix formation
To assess the effect of IL-1β on osteogenic
differentiation, MBMMSCs were seeded in 24-well plates
(8x104 cells/well). After the cells began to adhere to
the plates, osteogenic medium (complete medium containing 10 mM
β-glycerophosphate, 50 nM ascorbic acid and 100 nM dexamethasone;
Sigma-Aldrich; Merck KGaA) containing IL-1β at different
concentrations (0, 0.01, 0.1, 1 or 10 ng/ml) was added to induce
differentiation.
For ALP staining, MBMMSCs were washed twice with PBS
after 7 days of treatment. After fixing with 4% paraformaldehyde
for 30 sec at 25˚C, the cells were stained according to the
manufacturer's instructions of the ALP staining kit (Beyotime
Institute of Biotechnology). To quantitatively analyze ALP
activity, MBMMSCs were washed twice with PBS and lysed with 1%
Triton X-100 for 15 min. ALP activity was measured based on the
absorbance at 405 nm, and was normalized to total protein
concentration using a bicinchoninic acid assay (Thermo Fisher
Scientific, Inc.).
In addition, MBMMSCs were stained with Alizarin Red
(Wuhan Servicebio Technology Co., Ltd.) to assess mineralized
matrix deposition for bone nodule formation after 21 days of IL-1β
treatment. MBMMSCs were washed three times with PBS, fixed with 4%
paraformaldehyde for 15 min at 25˚C and stained with 1 Alizarin Red
dye solution according to the manufacturer's instructions. For
further quantitative analysis, a 10% chlorinated 16 alkyl pyridine
solution of sodium phosphate (pH 7.0; Sigma-Aldrich; Merck KGaA)
was added to dissolve the dye for 30 min at 25˚C, and the
absorbance was measured at 620 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
To determine the effect of IL-1β on downstream
osteogenic genes during osteogenic induction, RT-qPCR was conducted
to evaluate the relative expression of genes at different stages.
Total RNA was extracted from MBMMSCs using TRIzol®
reagent (Thermo Fisher Scientific, Inc.). All sample concentrations
were normalized, and cDNA was synthesized using PrimeScript RT
Master mix according to the manufacturer's instructions (Takara
Bio, Inc.). qPCR was performed using SYBR® Premix EX Taq
according to the manufacturer's instructions (Takara Bio, Inc.).
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95˚C for 30 sec; followed by 40 cycles of
95˚C for 5 sec and 60˚C for 30 sec. The primers used are shown in
Table I. GAPDH was used as the
internal reference gene for normalization. The 2-ΔΔCq
method was used for quantification (27).
 | Table IPrimer sequences for reverse
transcription-quantitative PCR. |
Table I
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| RUNX2 |
GACTGTGGTTACCGTCATGGC |
ACTTGGTTTTTCATAACAGCGGA |
| ALP |
AGAAGTTCGCTATCTGCCTTGCCT |
TGGCCAAAGGGCAATAACTAGGGA |
| COL1A1 |
GCTCCTCTTAGGGGCCACT |
CCACGTCTCACCATTGGGG |
| BSP |
TGCTAACTGCGCAAACATACC |
AGGGGGTGTGATAAAGGAACG |
| BMP2 |
ACTACACGAAAGCAGTGGGAA |
GCATCTGTTGCGAAACACT |
| OCN |
TAGCAGACACCATGAGGACCATCT |
CCTGCTTGGACATGAAGGCTTTGT |
| OPN |
AGCAAGAAACTCTTCCAAGCAA |
GTGAGATTCGTCAGATTCATCCG |
| GAPDH |
AGGTCGGTGTGAACGGATTTG |
TGTAGACCATGTAGTTGAGGTCA |
Western blot analysis
MBMMSCs in 6-well plates (4x105
cells/well) were lysed with RIPA buffer containing a protease
inhibitor cocktail (Thermo Fisher Scientific, Inc.) and
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology). The concentration of total protein was determined
using a bicinchoninic acid assay. Equal amounts of protein (20
µg/lane) in each group were separated using 10% SDS-PAGE and
transferred to PVDF membranes (EMD Millipore). After incubation in
5% skimmed milk for 1 h at 25˚C to block non-specific binding, the
membranes were incubated with primary antibodies (1:1,000 dilution)
against Smad1, Smad5, Smad4 and phosphorylated (p)-Smad1/5 (cat.
no. 12656; Cell Signaling Technology, Inc.) and GAPDH (cat. no.
5174; Cell Signaling Technology, Inc.) for 8 h at 4˚C. This was
followed by incubation with horseradish peroxidase (HRP)-conjugated
secondary antibody (1:2,000; cat. no. 7074; Cell Signaling
Technology, Inc.) in Tris-buffered saline/0.1% Tween-20 at room
temperature for 1 h. The immunoreactive bands were visualized using
Immobilon Western HRP (EMD Millipore). The Tanon 5200
chemiluminescent imaging system (Tanon Science and Technology Co.,
Ltd.) was used for densitometric analysis.
Treatment with signaling pathway
inhibitor
To further evaluate the role of BMP/Smad signaling
in osteogenic differentiation under IL-1β treatment, the BMP/Smad
signaling pathway was blocked using LDN193189 (Selleck Chemicals).
MBMMSCs were pre-stimulated with 0.2 µM LDN193189 for 2 h at 37˚C
and then stimulated with 0.1 ng/ml IL-1β at 37˚C until the time of
detection (Western blot analysis at 7 days, ALP analysis at 7 days,
mineralization analysis at 21 days and qPCR analysis at 7
days).
Statistical analysis
Data are presented as the mean ± SD from ≥3
independent experiments. The results were analyzed by one-way ANOVA
using SPSS 23.0 software (IBM Corp.). Dunnett's multiple
comparisons test was used as the post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
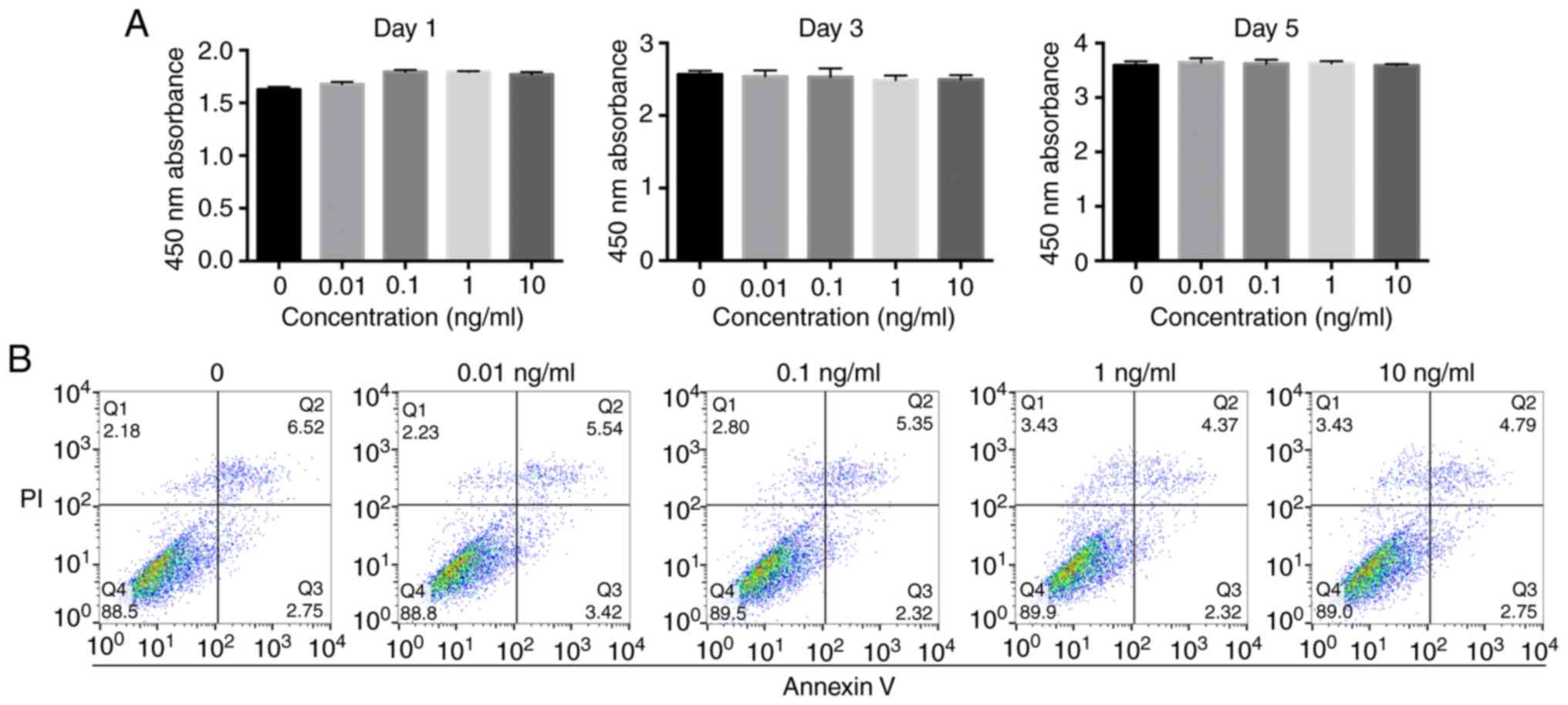
Effect of IL-1β on MBMMSC
proliferation and apoptosis
Prior to investigating the effect of IL-1β on
osteogenic differentiation, it was important to determine whether
it affects the proliferation of MBMMSCs. It was found that MBMMSCs
treated with various concentrations of IL-1β maintained similar
proliferation rates compared with the control group (Fig. 1A). Moreover, it was demonstrated that
IL-1β did not induce cytotoxicity to MBMMSCs at the highest
concentration (20 ng/ml). In addition, the present results
suggested that IL-1β at different concentrations did not cause
significant early apoptosis of MBMMSCs (Fig. 1B).
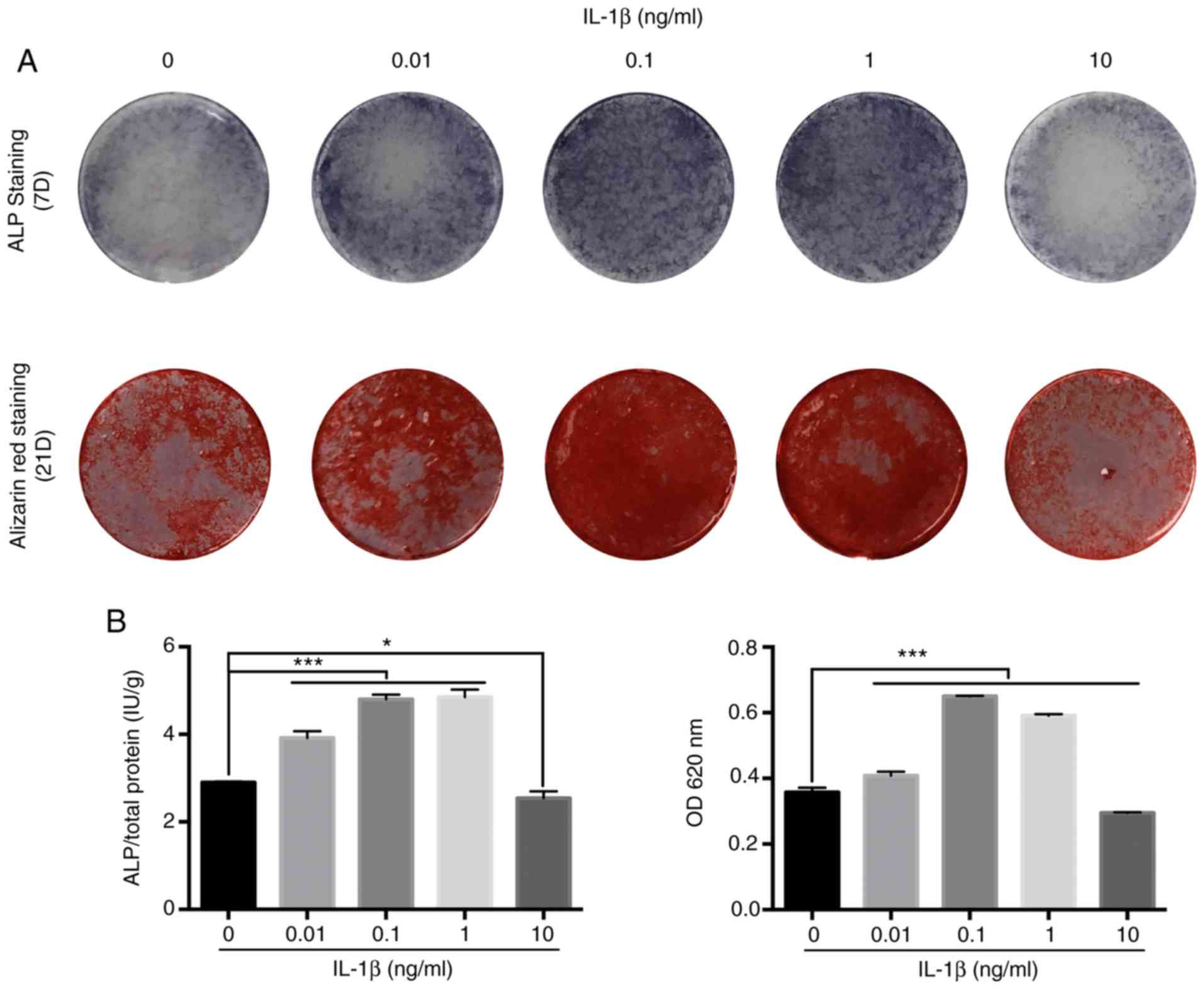
IL-1β enhances osteogenic
differentiation of MBMMSCs within a certain concentration
range
To assess the influence of IL-1β on osteogenic
differentiation of MBMMSCs, ALP assay, Alizarin Red staining and
quantitative tests were performed. It was demonstrated that IL-1β
caused osteogenic differentiation at very low concentrations (0.01
ng/ml; Fig. 2A). Furthermore, with
increasing IL-1β concentration, the osteogenic effect of IL-1β was
increased, and similar results were obtained at 0.1 and 1 ng/ml.
When the IL-1β concentration continued to increase, its osteogenic
effect on MBMMSCs began to decrease, and showed a relative
inhibitory effect at 10 ng/ml. Moreover, quantitative analysis
results were similar (Fig. 2B), thus
indicating that IL-1β promoted the osteogenic differentiation of
MBMMSCs within a certain range, but this effect disappeared after
exceeding this range.
IL-1β upregulates the expression of
osteogenic genes
The transcriptional level of the osteogenic genes is
altered after activation of various signaling pathways (24,28,29).
Therefore, the mRNA expression levels of the downstream osteogenic
differentiation markers Runx2, ALP, collagen 1A1 (COL1A1), bone
sialoprotein (BSP), BMP2, osteocalcin (OCN) and osteopontin (OPN),
were determined using RT-qPCR. It was found that Runx2 mRNA
expression was increased after 3 days of IL-1β treatment. On day 7,
the mRNA expression levels of ALP, COL1A1, BSP and BMP2 in the
IL-1β-treated MBMMSCs were significantly increased compared with
the control group, and the mRNA expression levels of OCN and OPN
began to increase. By day 14, the expression levels of OCN and OPN
were found to further increase. Similar to osteogenic
differentiation, the expression of osteogenic genes in MBMMSCs did
not increase after stimulation with 10 ng/ml IL-1β, and the overall
expression levels were lower compared with the control group
(Fig. 3). Therefore, the present
results suggested that IL-1β may regulate osteogenic
differentiation at the mRNA level.
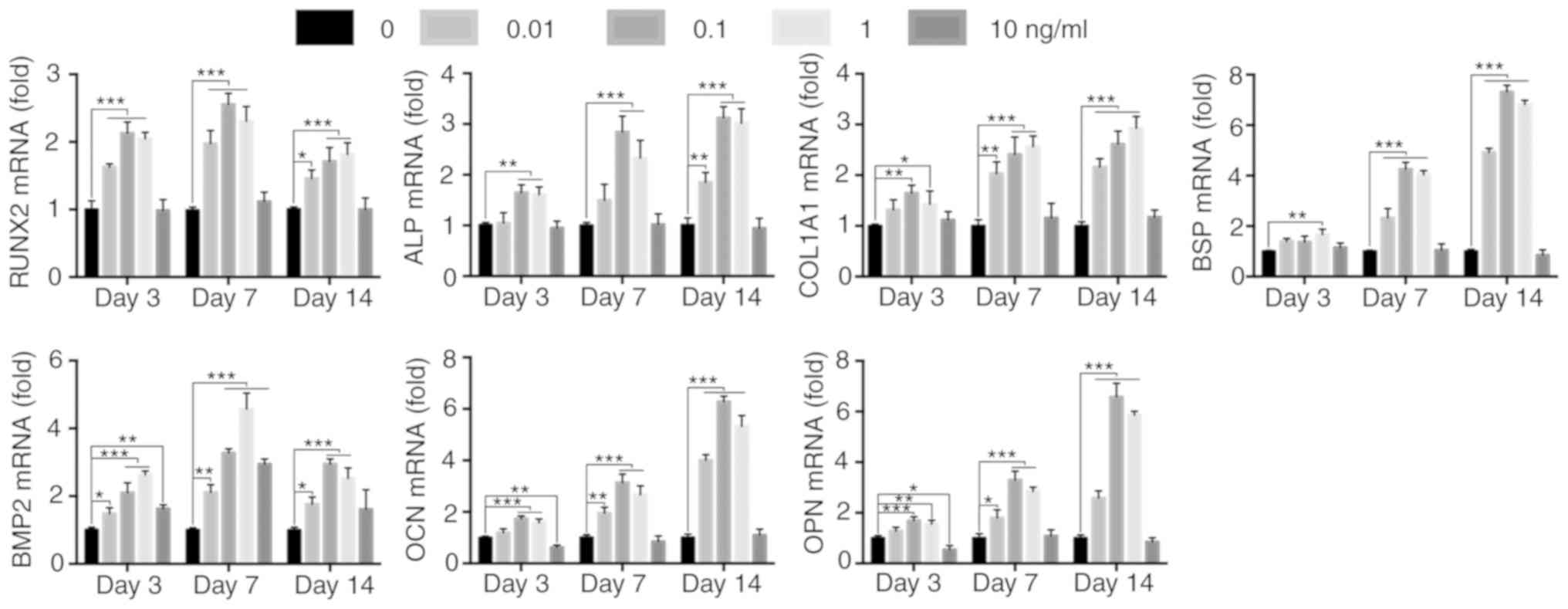 | Figure 3Effect of IL-1β on downstream
osteogenic genes. mRNA expression levels of RUNX2, ALP, COL1A1,
BSP, BMP2, OCN and OPN at 3, 7 and 14 days. Expression levels were
normalized to GAPDH. *P<0.05, **P<0.01,
***P<0.001. IL, interleukin; ALP, alkaline
phosphatase; BMP, bone morphogenetic protein; OCN, osteocalcin;
OPN, osteopontin; BSP, bone sialoprotein; RUNX2, runt-related
transcription factor 2; COL1A1, type I collagen. |
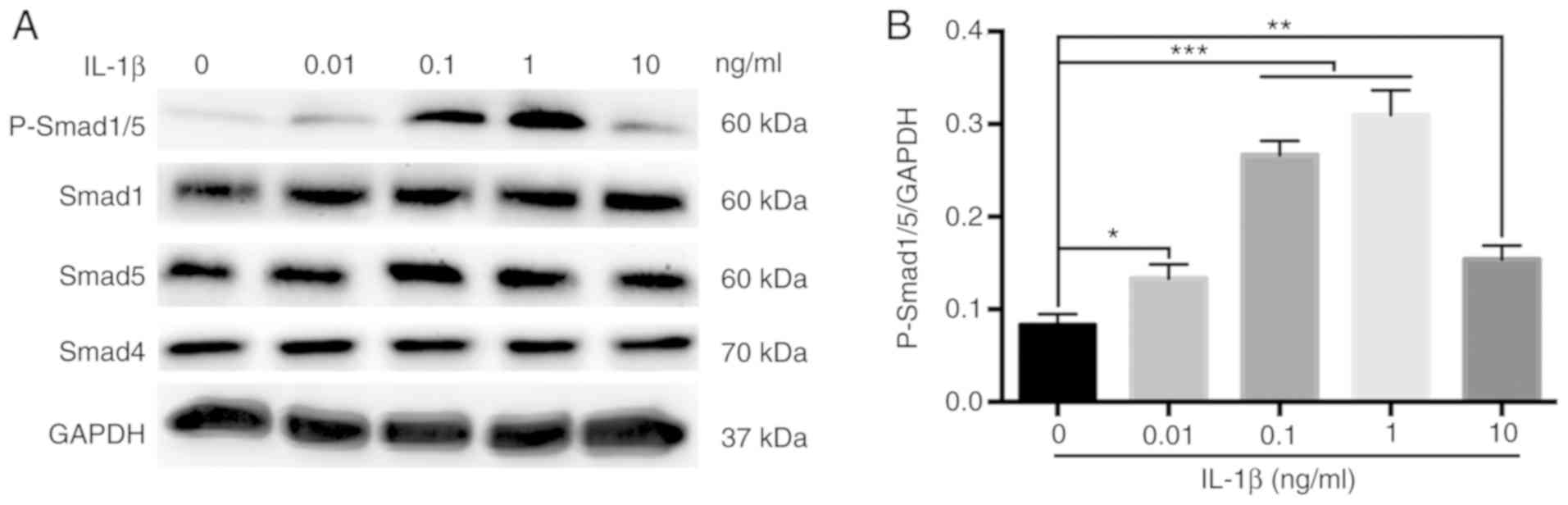
Activation of the BMP/Smad signaling
pathway in MBMMSCs
To assess whether the BMP/Smad classical osteogenic
pathway is involved in the effect of IL-1β on the osteogenic
differentiation of MBMMSCs, the activation of Smad signaling was
examined by Western blot analysis during osteogenesis. It was
demonstrated that the phosphorylation of Smad1/5, which represents
the activation level of the BMP/Smad pathway (30,31), was
significantly increased after 7 days of IL-1β stimulation (Fig. 4A and B). However, the protein expression levels
of Smad1, Smad4 and Smad5 were unchanged after IL-1β treatment
(Fig. 4). Moreover, the
phosphorylation level of Smad1/5 showed a downward trend at 10
ng/ml IL-1β. Thus, the present results indicated that IL-1β
regulated osteogenic differentiation of MBMMSCs via the BMP/Smad
signaling pathway.
Inhibition of the BMP/Smad signaling
pathway suppresses IL-1β-induced osteogenic differentiation
LDN193189 is a transforming growth factor
(TGF)-β/Smad inhibitor that is often used to inhibit BMP2-induced
p-Smad1/5/9 (32-34).
Western blot analysis was performed to evaluate Smad
phosphorylation levels after 7 days. It was identified that
LDN193189 significantly reduced the protein expression level of
p-Smad1/5 stimulated by 0.1 ng/ml IL-1β, returning to levels
similar to the control group (Fig.
5). It was also found that blocking the BMP/Smad signaling
pathway decreased ALP and mineralization staining, and similar
results were obtained from the quantitative assays (Fig. 5C and D). Moreover, these results were further
supported by the results of the RT-qPCR (Fig. 5E). Collectively, the present results
suggested that the BMP/Smad signaling pathway may have an important
role in IL-1β-induced osteogenic differentiation.
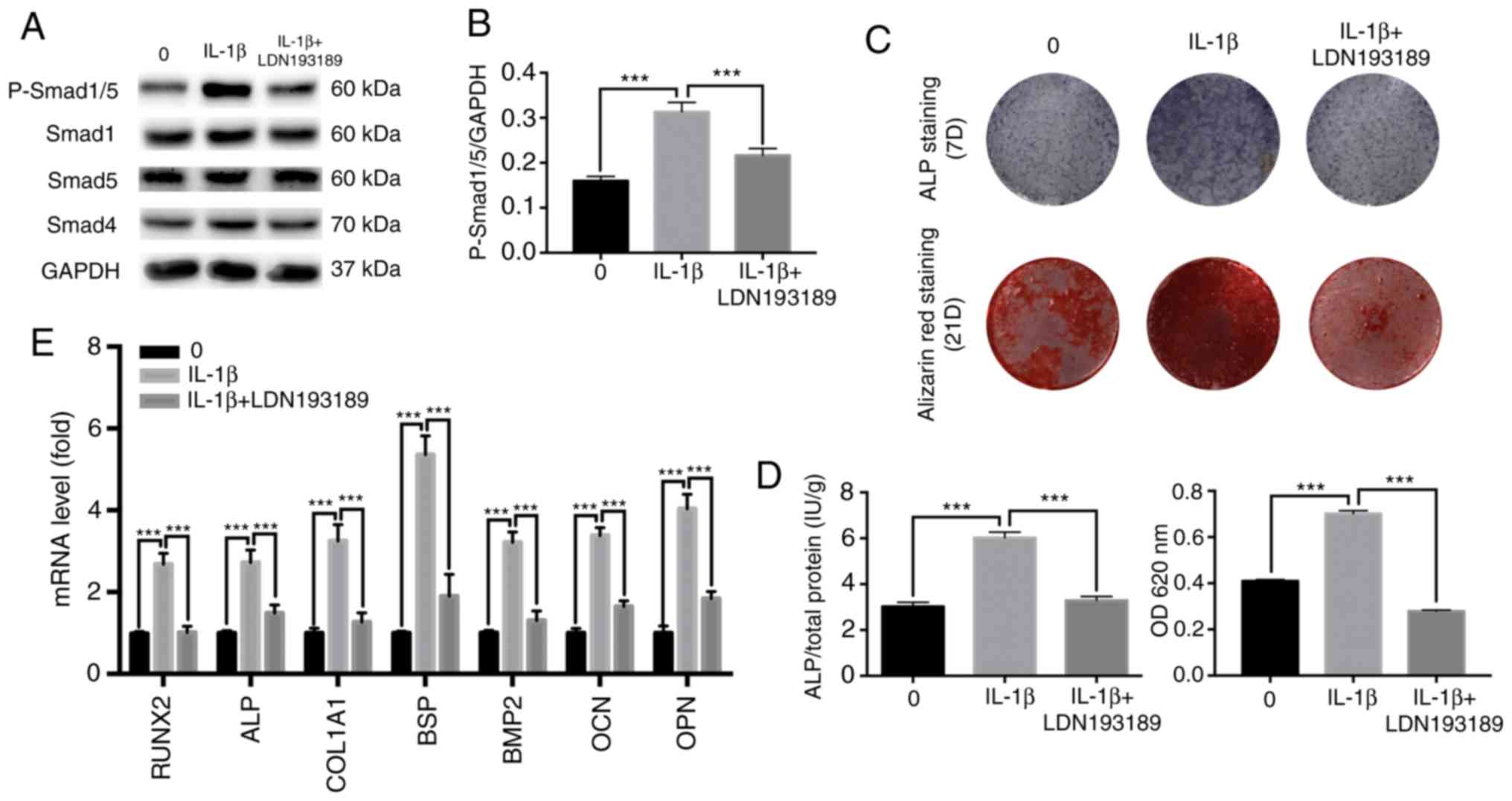 | Figure 5Effect of TGF-β/Smad inhibitor on the
osteogenic differentiation of MBMMSCs. MBMMSCs were treated with
osteogenic differentiation medium in the presence of 0.1 ng/ml
IL-1β along with the inhibitor of TGF-β/Smad LDN193189. (A) Western
blot analysis results for the levels of p-Smad1/5, and total Smad1,
Smad5 and Smad4 at 7 days. (B) Quantitative results of
p-Smad1/5/GAPDH. (C) Entire plate views of ALP staining at 7 days
and alizarin red staining at 21 days. (D) Quantitative evaluation
of ALP activity and alizarin red staining results. (E) mRNAs
expression levels of RUNX2, ALP, COL1A1, BSP, BMP2, OCN and OPN at
7 days. Expression levels were normalized to GAPDH.
***P<0.001. p-, phosphorylated; IL, interleukin; ALP,
alkaline phosphatase; BMP, bone morphogenetic protein; OCN,
osteocalcin; OPN, osteopontin; BSP, bone sialoprotein; RUNX2,
runt-related transcription factor 2; COL1A1, type I collagen;
MBMMSCs, mouse bone marrow mesenchymal stem cells. |
Discussion
The present study investigated the role of IL-1β in
osteogenic differentiation during fracture healing and assessed the
conditions under which IL-1β exerted a positive effect on MBMMSCs.
Several controversial issues, which had been highlighted in
previous studies, were addressed by the present results (14,19,35).
Firstly, it was found that IL-1β did not promote the proliferation
of MBMMSCs, or induce cytotoxicity and apoptosis within a certain
concentration range. Furthermore, IL-1β positively regulated the
osteogenic differentiation of MBMMSCs within a certain
concentration range, and when the concentration was too high,
osteogenic differentiation did not occur and was inhibited.
Moreover, it was demonstrated that the BMP/Smad signaling pathway
was activated in IL-1β-mediated osteogenic regulation.
Prior to investigating the effect of IL-1β on
osteogenic differentiation, the present study first assessed its
effect on MBMMSC proliferation and apoptosis. It was demonstrated
that IL-1β concentrations within a certain range did not
significantly promote or inhibit the proliferation of MBMMSCs,
which eliminated interference in the subsequent osteogenic
differentiation experiments. Moreover, previously reported
concentrations of IL-1β, which are shown to contribute to
osteogenic differentiation of various cells, are within this
selected range (7,14,19,25). In
addition, as early cell apoptosis cannot pass endpoint monitoring
methods such as CCK-8(26), the
present study examined whether IL-1β induced cell membrane damage
in MBMMSCs by evaluating Annexin V on the cell membrane surface.
The present results suggested that IL-1β stimulation did not induce
significant early apoptosis in MBMMSCs, thus establishing the basis
for subsequent experiments.
During the process of fracture healing, the sterile
inflammatory response plays an important role at all stages
(4-6). A
variety of inflammatory factors act as executors of inflammatory
response, exerting a variety of regulatory functions in fracture
healing (4-6).
Among them, the regulation of IL-1β on new bone formation has been
extensively studies; however, there are discrepancies between these
results (6,7,14). The
main reasons for these inconsistencies may include unclear
concentration ranges and differentiation of experimentally selected
cells. The present study selected BMMSCs, which is an important
source of new osseous tissue, to assess the regulatory role of
IL-1β in osteogenic differentiation within a sufficiently large
concentration range. The present results suggested that the
addition of IL-1β at 0.01-1 ng/ml under osteogenic induction
significantly enhanced the osteogenic differentiation of MBMMSCs,
and this was demonstrated by the mRNA expression levels of
downstream osteogenic genes. Unlike IL-1β, other inflammatory
factors such as TNF-α do not cause similar osteogenic promoting
effects (36-39).
Previous findings have shown that TNF-α significantly inhibits
osteogenic differentiation potential of MSCs at different
concentrations (36-39).
In the present study, when IL-1β concentration was increased to 10
ng/ml, osteogenic promotion disappeared and osteogenic genes were
downregulated. Thus, the present results indicated that IL-1β
promoted new bone formation during fracture healing only within a
specific concentration range. Furthermore, excess IL-1β may not be
beneficial to fracture healing and may produce adverse results.
From the overall perspective of the fracture healing process, the
aseptic inflammatory response is essential, but excessive
inflammation hinders fracture healing (4-6).
Therefore, further animal experiments are required to investigate
the effect of IL-1β on fracture healing in vivo. In an
animal model, IL-1β could be loaded onto the surface of implants by
internal fixation and surface modification to reach a stable local
IL-1β concentration at the fracture site (40).
Multiple signaling pathways are involved in
regulating the osteogenic differentiation of BMMSCs, such as the
Wnt/β-catenin (20),
mitogen-activated protein kinase (21), phosphoinositide 3-kinase (22) and estrogen receptor pathways
(23). BMP/Smad, one of the
classical osteogenic signaling pathways, has been reported to
participate in IL-1β-induced differentiation of periodontal cells
(19). However, it has also been
reported that the BMP/Smad signaling pathway is not involved in the
regulation of the differentiation potential of BMMSCs by IL-1β
(25). If IL-1β acts via the
BMP/Smad pathway, signaling will be initiated with ligand-induced
oligomerization of serine/threonine receptor kinases and
phosphorylation of the cytoplasmic signaling molecules Smad1/5/9
(19,30,31).
Furthermore, carboxy-terminal phosphorylation of Smads by activated
receptors results in their binding with the common signaling
transducer Smad4, and causes their translocation to the nucleus
(30,31). In the present study p-Smad1/5, which
shares the same binding site as p-Smad1/5/9 (30,31), was
used as an indication of BMP/Smad activation. To further
demonstrate that IL-1β regulated osteogenic differentiation of
MBMMSCs via the BMP/Smad signaling pathway, a TGF-β/Smad inhibitor
was used to block the activity of this pathway. The impaired
osteogenic effect identified in suggested that the BMP/Smad pathway
may play a vital role in the regulation of MBMMSC osteogenic
differentiation by IL-1β.
However, there are several limitations to the
present study that require discussion. Firstly, MBMMSCs were
selected as the research subject, however to obtain results that
are clinically application the use of human cells may be more
beneficial. Therefore, future experiments will use human BMMSCs.
Furthermore, the intensity of BMP/Smad activation stimulated by
IL-1β at different concentrations may not completely correspond to
the observed osteogenic effects. When the IL-1β concentration >1
ng/ml, its osteogenic effect was reduced, but the expression level
of p-Smad1/5 was still relatively high. Thus, the present results
suggested that other signaling pathways may be activated and
participate in the regulation of osteogenic differentiation by
IL-1β, and this requires further investigation.
In conclusion, the present results indicated that
IL-1β enhanced the osteogenic differentiation potential of MBMMSCs
within a certain concentration range. It was also demonstrated that
IL-1β activated the classical BMP/Smad osteogenic signaling pathway
and its osteogenic induction effect was lost when the concentration
was too high. Therefore, the present results suggested that
regulation of the BMP/Smad signaling pathway by IL-1β may be a
novel therapeutic for the treatment of fracture healing.
Supplementary Material
Fluorescence activated cell sorting
staining of mouse bone marrow mesenchymal stem cells. (A) Positive
markers. (B) Negative markers.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW, ZN, JY, ML, LL, XP, LX and XW carried out the
experiments. HW wrote the manuscript. SF and XW designed the
experiments. SF revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments in the present study were
performed in accordance with the Laboratory Animal Management
Regulations, and were approved by the Ethics Committee at Anhui
Provincial Hospital, Anhui Medical University
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Behonick DJ, Xing Z, Lieu S, Buckley JM,
Lotz JC, Marcucio RS, Werb Z, Miclau T and Colnot C: Role of matrix
metalloproteinase 13 in both endochondral and intramembranous
ossification during skeletal regeneration. PLoS One.
2(e1150)2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marsell R and Einhorn TA: The biology of
fracture healing. Injury. 42:551–555. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Loi F, Córdova LA, Pajarinen J, Lin TH,
Yao Z and Goodman SB: Inflammation, fracture and bone repair. Bone.
86:119–130. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gerstenfeld LC, Cullinane DM, Barnes GL,
Graves DT and Einhorn TA: Fracture healing as a post-natal
developmental process: Molecular, spatial, and temporal aspects of
its regulation. J Cell Biochem. 88:873–884. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cho TJ, Gerstenfeld LC and Einhorn TA:
Differential temporal expression of members of the transforming
growth factor beta superfamily during murine fracture healing. J
Bone Miner Res. 17:513–520. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lange J, Sapozhnikova A, Lu C, Hu D, Li X,
Miclau T III and Marcucio RS: Action of IL-1beta during fracture
healing. J Orthop Res. 28:778–784. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mumme M, Scotti C, Papadimitropoulos A,
Todorov A, Hoffmann W, Bocelli-Tyndall C, Jakob M, Wendt D, Martin
I and Barbero A: Interleukin-1β modulates endochondral ossification
by human adult bone marrow stromal cells. Eur Cell Mater.
24:224–236. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bielby RC, Boccaccini AR, Polak JM and
Buttery LD: In vitro differentiation and in vivo mineralization of
osteogenic cells derived from human embryonic stem cells. Tissue
Eng. 10:1518–1525. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sottile V, Thomson A and McWhir J: In
vitro osteogenic differentiation of human ES cells. Cloning Stem
Cells. 5:149–155. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Furuta T, Miyaki S, Ishitobi H, Ogura T,
Kato Y, Kamei N, Miyado K, Higashi Y and Ochi M: Mesenchymal stem
cell-derived exosomes promote fracture healing in a mouse model.
Stem Cells Transl Med. 5:1620–1630. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Graves DT and Cochran D: The contribution
of interleukin-1 and tumor necrosis factor to periodontal tissue
destruction. J Periodontol. 74:391–401. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stashenko P, Jandinski JJ, Fujiyoshi P,
Rynar J and Socransky SS: Tissue levels of bone resorptive
cytokines in periodontal disease. J Periodontol. 62:504–509.
1991.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Loebel C, Czekanska EM, Staudacher J,
Salzmann G, Richards RG, Alini M and Stoddart MJ: The calcification
potential of human MSCs can be enhanced by interleukin-1β in
osteogenic medium. J Tissue Eng Regen Med. 11:564–571.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sonomoto K, Yamaoka K, Oshita K, Fukuyo S,
Zhang X, Nakano K, Okada Y and Tanaka Y: Interleukin-1β induces
differentiation of human mesenchymal stem cells into osteoblasts
via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2
pathway. Arthritis Rheum. 64:3355–3363. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bhattacharyya T, Levin R, Vrahas MS and
Solomon DH: Nonsteroidal antiinflammatory drugs and nonunion of
humeral shaft fractures. Arthritis Rheum. 53:364–367.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
O'Connor JP, Capo JT, Tan V, Cottrell JA,
Manigrasso MB, Bontempo N and Parsons JR: A comparison of the
effects of ibuprofen and rofecoxib on rabbit fibula osteotomy
healing. Acta Orthop. 80:597–605. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Burd TA, Hughes MS and Anglen JO:
Heterotopic ossification prophylaxis with indomethacin increases
the risk of long-bone nonunion. J Bone Joint Surg Br. 85:700–705.
2003.PubMed/NCBI
|
|
18
|
Krischak GD, Augat P, Sorg T, Blakytny R,
Kinzl L, Claes L and Beck A: Effects of diclofenac on periosteal
callus maturation in osteotomy healing in an animal model. Arch
Orthop Trauma Surg. 127:3–9. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mao CY, Wang YG, Zhang X, Zheng XY, Tang
TT and Lu EY: Double-edged-sword effect of IL-1β on the
osteogenesis of periodontal ligament stem cells via crosstalk
between the NF-κB, MAPK and BMP/Smad signaling pathways. Cell Death
Dis. 7(e2296)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Saidak Z, Le Henaff C, Azzi S, Marty C, Da
Nascimento S, Sonnet P and Marie PJ: Wnt/β-catenin signaling
mediates osteoblast differentiation triggered by peptide-induced
α5β1 integrin priming in mesenchymal skeletal cells. J Biol Chem.
290:6903–6912. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thouverey C and Caverzasio J: Focus on the
p38 MAPK signaling pathway in bone development and maintenance.
Bonekey Rep. 4(711)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guntur AR and Rosen CJ: The skeleton: a
multi-functional complex organ: New insights into osteoblasts and
their role in bone formation: The central role of PI3Kinase. J
Endocrinol. 211:123–130. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vico L and Vanacker JM: Sex hormones and
their receptors in bone homeostasis: Insights from genetically
modified mouse models. Osteoporos Int. 21:365–372. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee KW, Yook JY, Son MY, Kim MJ, Koo DB,
Han YM and Cho YS: Rapamycin promotes the osteoblastic
differentiation of human embryonic stem cells by blocking the mTOR
pathway and stimulating the BMP/Smad pathway. Stem Cells Dev.
19:557–568. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ye W, Fei XM, Tang Y, Xu XX and Zhu Y:
IL-1β-treated bone marrow mesenchymal stem cells enhances
osteogenetic potential via NF-κB pathway. Zhongguo Shi Yan Xue Ye
Xue Za Zhi. 25:890–895. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
26
|
Liu X, Qu X, Wu C, Zhai Z, Tian B, Li H,
Ouyang Z, Xu X, Wang W, Fan Q, et al: The effect of enoxacin on
osteoclastogenesis and reduction of titanium particle-induced
osteolysis via suppression of JNK signaling pathway. Biomaterials.
35:5721–5730. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4(16009)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang YJ, Zhang HQ, Han HL, Zou YY, Gao QL
and Yang GT: Taxifolin enhances osteogenic differentiation of human
bone marrow mesenchymal stem cells partially via NF-κB pathway.
Biochem Biophys Res Commun. 490:36–43. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schmierer B and Hill CS: TGFbeta-SMAD
signal transduction: Molecular specificity and functional
flexibility. Nat Rev Mol Cell Biol. 8:970–982. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xiao YT, Xiang LX and Shao JZ: Bone
morphogenetic protein. Biochem Biophys Res Commun. 362:550–553.
2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ying X, Sun L, Chen X, Xu H, Guo X, Chen
H, Hong J, Cheng S and Peng L: Silibinin promotes osteoblast
differentiation of human bone marrow stromal cells via bone
morphogenetic protein signaling. Eur J Pharmacol. 721:225–230.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Peart TM, Correa RJ, Valdes YR, Dimattia
GE and Shepherd TG: BMP signalling controls the malignant potential
of ascites-derived human epithelial ovarian cancer spheroids via
AKT kinase activation. Clin Exp Metastasis. 29:293–313.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vogt J, Traynor R and Sapkota GP: The
specificities of small molecule inhibitors of the TGFβ and BMP
pathways. Cell Signal. 23:1831–1842. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang RL, Yuan Y, Tu J, Zou GM and Li Q:
Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways
converge on Runx2 to regulate BMP-2-induced osteoblastic
differentiation. Cell Death Dis. 5(e1187)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Qiu X, Wang X, Qiu J, Zhu Y, Liang T, Gao
B, Wu Z, Lian C, Peng Y, Liang A, et al: Melatonin rescued reactive
oxygen species-impaired osteogenesis of human bone marrow
mesenchymal stem cells in the presence of tumor necrosis
factor-alpha. Stem Cells Int. 2019(6403967)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang X, Sun H, Liao H, Wang C, Jiang C,
Zhang Y and Cao Z: MicroRNA-155-3p mediates TNF-α-inhibited
cementoblast differentiation. J Dent Res. 96:1430–1437.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chang J, Liu F, Lee M, Wu B, Ting K, Zara
JN, Soo C, Al Hezaimi K, Zou W, Chen X, et al: NF-κB inhibits
osteogenic differentiation of mesenchymal stem cells by promoting
β-catenin degradation. Proc Natl Acad Sci USA. 110:9469–9474.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang N, Wang G, Hu C, Shi Y, Liao L, Shi
S, Cai Y, Cheng S, Wang X, Liu Y, et al: Tumor necrosis factor α
suppresses the mesenchymal stem cell osteogenesis promoter miR-21
in estrogen deficiency-induced osteoporosis. J Bone Miner Res.
28:559–573. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang BG, Myers DE, Wallace GG, Brandt M
and Choong PF: Bioactive coatings for orthopaedic implants-recent
trends in development of implant coatings. Int J Mol Sci.
15:11878–11921. 2014.PubMed/NCBI View Article : Google Scholar
|