Introduction
Radiofrequency catheter ablation (RFCA) is used as
first-line therapy in selected patients with drug-refractory
symptomatic atrial fibrillation (AF) (1,2).
Ablation strategies that target the pulmonary vein antra are the
cornerstone for the majority of AF ablation procedures (3-5).
Twice transseptal punctures with ablating and monitoring pulmonary
venous potential simultaneously are commonly applied in the vast
majority of cardiac electrophysiology centers (6,7).
However, the traditional mapping and ablation techniques without
real-time contact force sensing show poor efficiency on permanent
transmural lesion formation and may lead to excessive X-ray
exposure and procedure time. Persistent iatrogenic atrial septal
defect after transseptal puncture has been observed and
complications associated with septal puncture may also be
increased, as this technique punctures more than one site in fossa
ovalis, particularly in complicated cases (8).
To improve the effectiveness and safety with
reducing fluoroscopy of the AF ablation procedure, a simplified
ablation strategy was developed that combines the single
transseptal puncture technique, fast anatomical mapping (FAM) of
the left atrium (LA), a contact force (CF) sensing catheter, and
the high output stimulation verification technique (9). The present study aimed to demonstrate
the value of this ablation strategy for patients with paroxysmal AF
(PAF).
Patients and methods
Patient selection
A total of 419 PAF patients with non-valvular,
antiarrhythmic drug refractory PAF who underwent de novo
RFCA at Fuwai Hospital between September 2014 and December 2016
were prospectively enrolled in the present study. These patients
were diagnosed with PAF according to the standard clinical
guidelines (10). The present study
was approved by the Institutional Ethics Committee for Biomedical
Research of Fuwai Hospital and registered at Chinese Clinical Trial
Registry (Unique identifier: ChiCTR2000033663). Written informed
consent was obtained from each patient. Patients with non-valvular,
antiarrhythmic drug refractory PAF diagnosed according to the
standard clinical guidelines were included in the present study.
Patients who exhibited a previous AF ablation history, LA size
>55 mm measured by echocardiogram, documented LA thrombus,
severe pulmonary diseases, or previous cardiac surgical history
were excluded from the present study. The details of their clinical
characteristics are presented in Table
I. There were 275 male patients (65.6%) and the average age was
58.7±10.9 years old.
 | Table IBaseline characteristics of patients
(n=419). |
Table I
Baseline characteristics of patients
(n=419).
| Characteristics | Value (%) |
|---|
| Age (years) | 58.7±10.9 |
| Sex | |
|
Male | 275 (65.6%) |
| Duration of AF
(years) | 4.1±4.3 |
| Patients with >1
year of AF | 267(63.7 %) |
| CHA2DS2-VASc
score | |
|
0 | 90 (21.5%) |
|
1 | 131 (31.3%) |
|
2 | 107 (25.5%) |
|
3 | 54 (12.9%) |
|
4 | 23 (5.5%) |
|
5 | 12(2.9%) |
|
6 | 2 (0.5%) |
| Diabetes | 56 (13.4%) |
| Heart failure | 1 (0.2%) |
| Hypertension | 199 (47.5%) |
| Myocardial
infarction | 10 (2.4%) |
| Peripheral vascular
disease | 9 (2.0%) |
| History of
stroke | 26 (6.2%) |
| LVEF (%) | 63.9±6.6 |
| LA size (mm) | 36.5±4.8 |
Simplified electrophysiological
procedures
All procedures were conducted under conscious
sedation, and catheters were typically inserted via the right
femoral vein. After positioning the coronary sinus catheter as an
anatomical landmark, the transseptal puncture was performed under
fluoroscopy using a single 8.5 Fr sheath only (11). FAM of the LA was guided by the
CARTO® 3 system (Biosense Webster, Inc.) using a
PentaRay catheter (Biosense Webster, Inc.). At this stage, a CF
catheter (THERMOCOOL SMARTTOUCH® Catheter; Biosense
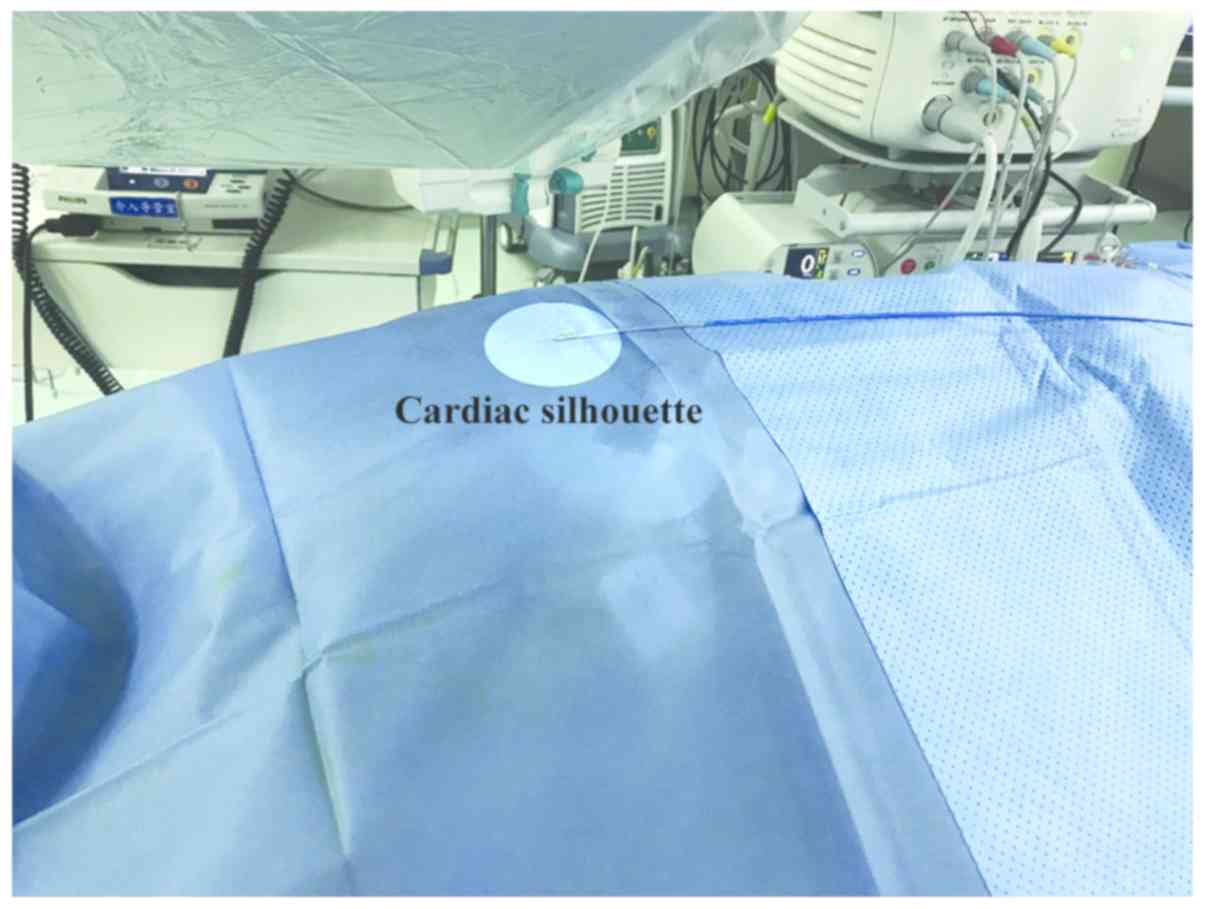
Webster, Inc.) was out of the body but with the tip placed at the
cardiac silhouette of the chest (Fig.
1).
The PentaRay catheter was taken off the sheath when
FAM of LA was accomplished and the CF catheter was inserted into
the LA. Circumferential pulmonary vein isolation (CPVI) was
performed in the present study. The maximal power and temperature
were set as 40 W and 43˚C, respectively. The catheter was
continuously irrigated with saline at a speed of 17 ml/min and the
CF was maintained between 10 and 20 g during the ablation
procedure. Ablation tags were annotated with the CARTO VISITAG™
Module (Biosense Webster, Inc.).
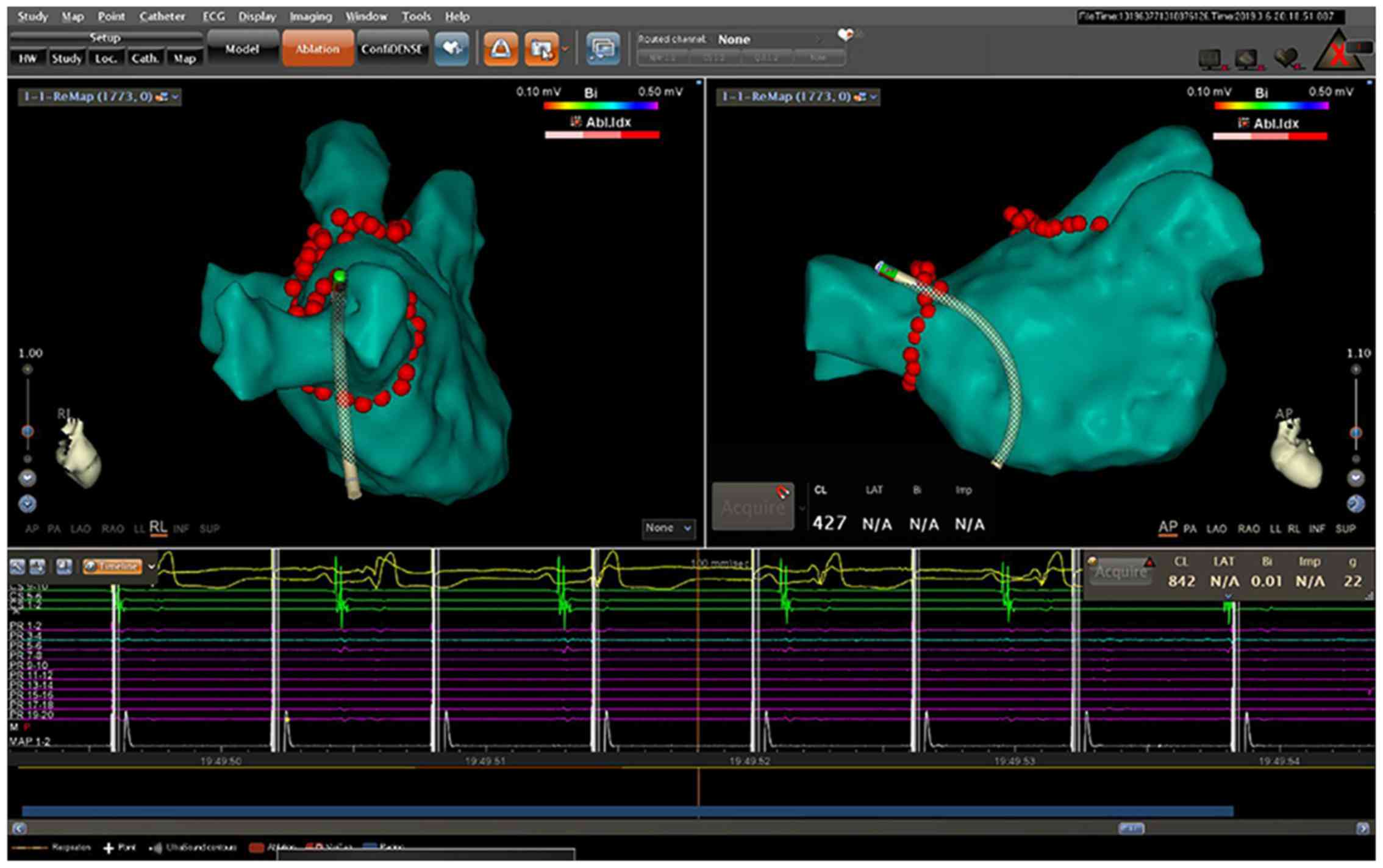
To verify PVI, stimulation with 10 mA outputs along
the ablation lesions was delivered through the distal electrode of
the ablation catheter. Additional ablation was performed if
conduction gaps were identified. A successful procedure was defined
by the absence of LA capture at all pacing sites (Fig. 2).
Post-ablation follow-up
Antiarrhythmic medications, including propafenone
and amiodarone, were administered for 3 months after ablation in
all patients, then terminated if no AF recurred. An
electrocardiogram (ECG) and 24 h Holter were obtained at 1, 3, 6, 9
and 12 months post-ablation during the follow-up. An additional ECG
and Holter were also performed if symptoms suggestive of AF
recurrence occurred. After the 3 month blanking period, arrhythmia
recurrence was defined as any episode (>30 sec duration) of AF
or atrial tachycardia (AT).
Study endpoints
The primary effectiveness endpoint was freedom from
any documented episode of AF/AT, which sustained for >30 sec
during the 12 month follow up and outside a blanking period of 3
months. Secondary endpoints included procedure time and ablation
time, procedure-related complications, and repeated ablation
procedure during follow-up.
Statistical analysis
Continuous data were summarized as mean ± standard
deviation. Categorical data were summarized as counts and
percentages. Comparisons of categorical variables were performed
using χ2 tests. Rates of survival from atrial arrhythmia
recurrence following the 3 month blanking period were estimated
with a Kaplan-Meier model. Cox regression models were used to test
for the significance of patient baseline characteristics and
procedural detail in predicting atrial arrhythmia recurrence rates,
as well as for calculating hazard ratios (HRs) to compare
recurrence risks. All statistical analyses were performed using
SPSS v23.0 software (IBM Corp.).
Results
Procedural parameters
The procedural parameters are summarized in Table II. Entrance/exit block in all PVs
during the procedure were achieved in 415 (99.0%) patients. The
average procedure time was 74.5±9.7 min and the average ablation
time was 27.3±7.8 min. In addition, the average radiation dose was
24.3±25.2 mGy.
 | Table IIProcedural and complication data. |
Table II
Procedural and complication data.
| Factor | Value |
|---|
| Procedure time
(min) | 74.5±9.7 |
| Ablation time
(min) | 27.3±7.8 |
| Fluoroscopy time
(min) | 4.7±3.3 |
| PVI ablation (%) | 419 (100.0) |
| Acute procedural
success (%) | 415 (99.0) |
| AF persisted after
ablation (%) | 0 (0.0) |
| Acute PVI
reconnection (%) | 0 (0.0) |
| Complications | 5 (1.2) |
|
Pericardial
effusion | 1 (0.2) |
|
Arteriovenous
fistulas | 2 (0.5) |
|
Femoral
artery pseudoaneurysm | 2 (0.5) |
Follow-up for effectiveness
At a mean follow-up time of 14.5 ± 4.1 months, 18
(4.3%) patients were unable to be contacted, including one patient
who died due to pulmonary carcinoma without AF recurrence.
Kaplan-Meier analysis estimated that 341 (85.0%) patients were free
from AF/AT during follow-up (Fig.
3). A total of 7 patients underwent repeat ablation procedures
during follow-up. Electric reconduction of PVI was demonstrated
during the repeat procedures, and re-ablation at gaps were
performed.
Multivariable Cox regression modeling demonstrated
that the duration of AF was a significant predictor of recurrence
(Table III). The greatest risk was
an AF duration >1 year, relative to a duration of ≤1 year [HR,
2.0; 95% confidence interval (CI), 1.2-3.2]. A history of
hypertension resulted in a reduced risk, as evidenced by a HR of
<1 (HR, 0.6; 95% CI, 0.4-0.9).
 | Table IIICox regression predictors of
recurrence after a 3 month blanking period. |
Table III
Cox regression predictors of
recurrence after a 3 month blanking period.
| | Hazard Ratio |
|---|
| Parameter
comparison | (95% CI) | χ2
P-value |
|---|
| Duration of AF (>1
year vs. ≤1 year) | 3.0 (1.5, 5.9) | 0.002 |
|
History of
hypertension (Yes vs. no) | 0.7 (0.4, 1.2) | 0.264 |
| History of diabetes
(Yes vs. no) | 1.6 (0.8, 3.1) | 0.145 |
|
Left atrial
size (per mm increase) | 1.0 (1.0, 1.1) | 0.935 |
Complications
The overall procedure-related complication rate was
1.2%, including 1 (0.2%) case of pericardial effusion and 4 (1.0%)
cases of vascular access complications. A total of 2 cases of
arteriovenous fistulas were resolved with only conservative medical
therapy. In addition, 1 case of pericardial effusion required
pericardiocentesis and 2 cases of femoral artery pseudoaneurysm
required puncture, suction and compression. There were no strokes
during the ablation visit or follow-ups (Table II).
Discussion
The present study demonstrated the advantages of a
simplified ablation procedure for PAF of combined single
transseptal puncture, FAM of LA, CF-sensing ablation and the high
output stimulation verification technique among a large number of
patients with PAF. In the current study, the 12 month AF/AT-free
survival rate was improved compared with previous studies (12,13),
while the average procedure time was just 74.5±9.7 min and the
complication rate was controlled at a considerably lower level,
which suggests this simplified and practical strategy is beneficial
in a clinical setting. The multiple-factor analysis demonstrated
that the duration of AF and left atrial size were significant
predictors of recurrence, whereas the history of hypertension
resulted in a reduced risk. Although this finding may initially
appear counter-intuitive, it is supported by a prior study and is
likely due to the protective effect of medications used to treat
hypertension (14).
Radiofrequency ablation for patients with AF
generally requires two transseptal punctures to deliver a
multipolar mapping catheter and an ablation catheter into the left
atrium, respectively. However, this procedure requires a skilled
operator to perform it, and in most cases intracardiac
echocardiography is required (8).
Puncture-related complications and iatrogenic atrial septal defects
are increased followed by an increase in the number of punctures
(8). In a previous study, a modified
transseptal puncture protocol was developed that used only a
coronary sinus catheter as the landmark under fluoroscopy (11). In the present study, all transseptal
procedures were overwhelmingly accomplished by fellows and guided
only by fluoroscopy.
A number of different parameters are known to affect
the transmurality of ablation lesions, including catheter tip
temperature, power output, ablation time and CF. It has previously
been demonstrated that real-time electrogram amplitude and
impedance are poor predictors of the true CF applied (6). It is important to have an accurate
measure of CF because a higher CF may increase the risk of blood
charring (15). CF-guided catheters
can provide stable and moderate CF, allowing for improvements in
ablation safety and effectiveness, while simultaneously reducing
procedure and fluoroscopy times (16). The improved catheter stability leads
to faster transmural lesion formation (17), particularly in the right side PV
(18). Procedures have been
shortened due to faster assessment of appropriate catheter contact,
resulting in the reduction of radiation (14,19-21).
In CF-guided PV isolation, pulmonary vein reconnection remains
primarily attributable to insufficient lesion depth and contiguity
(17). Additionally, since the
achievement of ideal ablation lesions depends on a combination of
CF, power and duration parameters, the integration of these
parameters via an automated algorithm, such as the Visitag with
Ablation Index, may provide a valuable solution to this complex
optimization problem (22-24).
FAM, which is guided by a three-dimensional (3D)
mapping system and a circular or multi-electrode mapping catheter,
also serve a role in CF-guided ablation (25). Traditional point-to-point modeling
cannot rapidly and accurately map the true LA geometry; therefore,
it typically leads to increased fluoroscopy usage in order to
reduce the complication risk (25).
Alternatively, FAM can provide precise LA modeling and electronic
substrate mapping information, leading to fewer manipulation
difficulties and lower radiation (8,26). FAM
guidance has been indicated to allow procedures with nearly zero
fluoroscopy and without compromising the procedure duration,
effectiveness or safety (25,27).
High output stimulation provides a convenient and
reliable approach for the verification of ablation lesions. The
traditional endpoint of PVI is antral disconnection detected by a
circular mapping catheter, which requires complex catheter
manipulation to ensure sufficient contact (22). High output stimulation along the
encircling lesion line without LA capture could also effectively
vivificate the conduction block between all PVs and LA (28). Guided with 3D mapping and CF
monitoring, an operator can ensure ablation line integrity without
concerns regarding poor contact or inaccurate location (9,29).
Supplementary ablation to touch up any residual gaps (LA capture
during high output stimulation along the lesion line) can be
performed immediately, thus decreasing the procedure and
fluoroscopy times.
There are some limitations of the present study.
Firstly, the current study reflects the experience of a single
center in China, and thus may not be representative of results
across sites with differing workflows, levels of operator
experience or patient populations. Secondly, the current study did
not set a control group with the twice transseptal puncture. Giving
the low complication rates and acceptable sinus rhythm maintenance
during follow-up, the choice of this simplified strategy is also a
reasonable option. Additionally, atrial arrhythmia recurrence
estimates could potentially be biased due to patients with an
incomplete follow-up, although the magnitude of this bias could not
be significant due to the low number of these patients with <12
months of follow-up.
In conclusion, the present study demonstrated that
this simplified technique was a simple, safe and effective approach
for PAF ablation therapy. This strategy is a reasonable alternative
for patients experiencing difficulty undergoing twice septal
puncture.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY conceived and designed the study. ZD, LZ, LD, EL
and GC conducted the research and acquired the data. FH and LW
analyzed and interpreted the data. ZD drafted the manuscript. All
authors substantially contributed to the revision of the
manuscript, and approved the final version.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Ethics Committee for Biomedical Research of Fuwai Hospital and
registered at Chinese Clinical Trial Registry (Unique identifier:
ChiCTR2000033663).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kirchhof P, Benussi S, Kotecha D, Ahlsson
A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks
J, et al: ESC Scientific Document Group: 2016 ESC Guidelines for
the management of atrial fibrillation developed in collaboration
with EACTS. Eur Heart J. 37:2893–2962. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
January CT, Wann LS, Alpert JS, Calkins H,
Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD,
Field ME, et al: ACC/AHA Task Force Members: 2014 AHA/ACC/HRS
guideline for the management of patients with atrial fibrillation:
A report of the American College of Cardiology/American Heart
Association Task Force on practice guidelines and the Heart Rhythm
Society. Circulation. 130:e199–e267. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Haïssaguerre M, Shah DC, Jaïs P, Hocini M,
Yamane T, Deisenhofer I, Chauvin M, Garrigue S and Clémenty J:
Electrophysiological breakthroughs from the left atrium to the
pulmonary veins. Circulation. 102:2463–2465. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Verma A, Jiang CY, Betts TR, Chen J,
Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W,
Weerasooriya R, et al: STAR AF II Investigators: Approaches to
catheter ablation for persistent atrial fibrillation. N Engl J Med.
372:1812–1822. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haïssaguerre M, Jaïs P, Shah DC, Takahashi
A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P and
Clémenty J: Spontaneous initiation of atrial fibrillation by
ectopic beats originating in the pulmonary veins. N Engl J Med.
339:659–666. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nakagawa H, Kautzner J, Natale A, Peichl
P, Cihak R, Wichterle D, Ikeda A, Santangeli P, Di Biase L and
Jackman WM: Locations of high contact force during left atrial
mapping in atrial fibrillation patients: Electrogram amplitude and
impedance are poor predictors of electrode-tissue contact force for
ablation of atrial fibrillation. Circ Arrhythm Electrophysiol.
6:746–753. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
De Potter T, Van Herendael H,
Balasubramaniam R, Wright M, Agarwal SC, Sanders P, Khaykin Y,
Latcu CG, Maury P, Pani A, et al: Safety and long-term
effectiveness of paroxysmal atrial fibrillation ablation with a
contact force-sensing catheter: Real-world experience from a
prospective, multicentre observational cohort registry. Europace.
20(FI_3):f410–f418. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fagundes RL, Mantica M, De Luca L, Forleo
G, Pappalardo A, Avella A, Fraticelli A, Dello Russo A, Casella M,
Pelargonio G, et al: Safety of single transseptal puncture for
ablation of atrial fibrillation: Retrospective study from a large
cohort of patients. J Cardiovasc Electrophysiol. 18:1277–1281.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Steven D, Reddy VY, Inada K,
Roberts-Thomson KC, Seiler J, Stevenson WG and Michaud GF: Loss of
pace capture on the ablation line: A new marker for complete
radiofrequency lesions to achieve pulmonary vein isolation. Heart
Rhythm. 7:323–330. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Calkins H, Hindricks G, Cappato R, Kim YH,
Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al:
2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on
catheter and surgical ablation of atrial fibrillation. Heart
Rhythm. 14:e275–e444. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yao Y, Ding L, Chen W, Guo J, Bao J, Shi
R, Huang W, Zhang S and Wong T: The training and learning process
of transseptal puncture using a modified technique. Europace.
15:1784–1790. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Natale A, Reddy VY, Monir G, Wilber DJ,
Lindsay BD, McElderry HT, Kantipudi C, Mansour MC, Melby DP, Packer
DL, et al: Paroxysmal AF catheter ablation with a contact force
sensing catheter: Results of the prospective, multicenter SMART-AF
trial. J Am Coll Cardiol. 64:647–656. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ullah W, McLean A, Tayebjee MH, Gupta D,
Ginks MR, Haywood GA, O'Neill M, Lambiase PD, Earley MJ and
Schilling RJ: UK Multicentre Trials Group**: Randomized
trial comparing pulmonary vein isolation using the SmartTouch
catheter with or without real-time contact force data. Heart
Rhythm. 13:1761–1767. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Widimsky J: Arterial hypertension and
atrial fibrillation: Selecting antihypertensive therapy. Cor Vasa.
54:e248–e252. 2012.
|
|
15
|
Makimoto H, Metzner A, Tilz RR, Lin T,
Heeger CH, Rillig A, Mathew S, Lemeš C, Wissner E, Kuck KH and
Ouyang F: Higher contact force, energy setting, and impedance rise
during radiofrequency ablation predicts charring: New insights from
contact force-guided in vivo ablation. J Cardiovasc Electrophysiol.
29:227–235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lin H, Chen YH, Hou JW, Lu ZY, Xiang Y and
Li YG: Role of contact force-guided radiofrequency catheter
ablation for treatment of atrial fibrillation: A systematic review
and meta-analysis. J Cardiovasc Electrophysiol. 28:994–1005.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bun SS, Ayari A, Latcu DG, Errahmouni A
and Saoudi N: Radiofrequency catheter ablation of atrial
fibrillation: Electrical modification suggesting transmurality is
faster achieved with remote magnetic catheter in comparison with
contact force use. J Cardiovasc Electrophysiol. 28:745–753.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nair GM, Yeo C, MacDonald Z, Ainslie MP,
Alqarawi WA, Nery PB, Redpath CJ, Sadek M, Spence S, Green MS, et
al: Three-year outcomes and reconnection patterns after initial
contact force guided pulmonary vein isolation for paroxysmal atrial
fibrillation. J Cardiovasc Electrophysiol. 28:984–993.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pedrote A, Arana-Rueda E, Arce-León A,
Acosta J, Gómez-Pulido F, Martos-Maine JL, Frutos-López M,
Sánchez-Brotons J and García-Riesco L: Impact of Contact Force
Monitoring in Acute Pulmonary Vein Isolation Using an Anatomic
Approach. A Randomized Study. Pacing Clin Electrophysiol.
39:361–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
El Haddad M, Taghji P, Phlips T, Wolf M,
Demolder A, Choudhury R, Knecht S, Vandekerckhove Y, Tavernier R,
Nakagawa H, et al: Determinants of Acute and Late Pulmonary Vein
Reconnection in Contact Force-Guided Pulmonary Vein Isolation:
Identifying the Weakest Link in the Ablation Chain. Circ Arrhythm
Electrophysiol. 10(e004867)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Marijon E, Fazaa S, Narayanan K, Guy-Moyat
B, Bouzeman A, Providencia R, Treguer F, Combes N, Bortone A,
Boveda S, et al: Real-time contact force sensing for pulmonary vein
isolation in the setting of paroxysmal atrial fibrillation:
Procedural and 1-year results. J Cardiovasc Electrophysiol.
25:130–137. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tanaka N, Inoue K, Tanaka K, Toyoshima Y,
Oka T, Okada M, Inoue H, Nakamaru R, Koyama Y, Okamura A, et al:
Automated Ablation Annotation Algorithm Reduces Re-conduction of
Isolated Pulmonary Vein and Improves Outcome After Catheter
Ablation for Atrial Fibrillation. Circ J. 81:1596–1602.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hussein A, Das M, Chaturvedi V, Asfour IK,
Daryanani N, Morgan M, Ronayne C, Shaw M, Snowdon R and Gupta D:
Prospective use of Ablation Index targets improves clinical
outcomes following ablation for atrial fibrillation. J Cardiovasc
Electrophysiol. 28:1037–1047. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Das M, Loveday JJ, Wynn GJ, Gomes S, Saeed
Y, Bonnett LJ, Waktare JEP, Todd DM, Hall MCS, Snowdon RL, et al:
Ablation index, a novel marker of ablation lesion quality:
Prediction of pulmonary vein reconnection at repeat
electrophysiology study and regional differences in target values.
Europace. 19:775–783. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Reddy VY, Morales G, Ahmed H, Neuzil P,
Dukkipati S, Kim S, Clemens J and D'Avila A: Catheter ablation of
atrial fibrillation without the use of fluoroscopy. Heart Rhythm.
7:1644–1653. 2010.
|
|
26
|
Voskoboinik A, Kalman ES, Savicky Y,
Sparks PB, Morton JB, Lee G, Kistler PM and Kalman JM: Reduction in
radiation dose for atrial fibrillation ablation over time: A
12-year single-center experience of 2344 patients. Heart Rhythm.
14:810–816. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bulava A, Hanis J and Eisenberger M:
Catheter ablation of atrial fibrillation using zero-fluoroscopy
technique: A randomized trial. Pacing Clin Electrophysiol.
38:797–806. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pambrun T, Combes S, Sousa P, Bloa ML, El
Bouazzaoui R, Grand-Larrieu D, Thompson N, Martin R, Combes N,
Boveda S, et al: Contact-force guided single-catheter approach for
pulmonary vein isolation: Feasibility, outcomes, and
cost-effectiveness. Heart Rhythm. 14:331–338. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Eitel C, Hindricks G, Sommer P, Gaspar T,
Kircher S, Wetzel U, Dagres N, Esato M, Bollmann A, Husser D, et
al: Circumferential pulmonary vein isolation and linear left atrial
ablation as a single-catheter technique to achieve bidirectional
conduction block: The pace-and-ablate approach. Heart Rhythm.
7:157–164. 2010.PubMed/NCBI View Article : Google Scholar
|