Introduction
Anesthetic-induced cognitive dysfunction is a
frequent complication following major surgery (1), and the typical symptoms include memory
deficits and impaired information processing after surgery. Some
patients with anesthetic-induced cognitive dysfunction can regress,
but an increasing number of cases have been found to involve
long-term cognitive dysfunction that leads to a significantly
decreased quality of life in patients (2). For example, it is reported that ~30-50%
patients suffer from postoperative cognitive dysfunction after
cardiac surgery, and only 45% of them can recover to baseline
cognitive function by one year after surgery (3). Isoflurane is a generally used
anesthetic that has been reported to induce cognitive dysfunction
in patients after surgery (4).
Previous studies have provided evidence for neuroinflammation as a
key event in the pathogenesis of isoflurane-induced cognitive
dysfunction (5). For example,
increased inflammatory cytokines have been observed to be induced
by anesthesia in brain after surgery (6), and isoflurane-induced cognitive
dysfunction can be attenuated by suppressing neuroinflammation
(7).
Tetramethylpyrazine (TMP) is an alkaloid extracted
from the roots of Ligusticum chuanxiong Hort (8), and has important biological actions,
including anti-inflammatory, cardioprotective, antioxidant,
immune-enhanced and neuroprotective effects (9,10).
Moreover, TMP has been reported to exert a neuroprotective role in
Parkinson's disease (11) and
cerebral ischemia/reperfusion (12).
However, whether TMP can also improve the cognitive dysfunction
induced by isoflurane remains unknown. Previous studies have
revealed that TMP can improve certain diseases by inhibiting
inflammatory reactions, especially neuroinflammation. For example,
Fu et al (13) found an
antidepressant effect of TMP in a chronic unpredictable mild stress
mouse model via the suppression of the Toll-like receptor 4
inflammatory signaling pathway. In addition, Wang et al
(14) demonstrated that TMP exerts a
protective role against traumatic brain injury by attenuating
neuroinflammation. Thus, it was hypothesized that TMP may regulate
the inflammatory reaction in isoflurane-induced cognitive
dysfunction.
MicroRNAs (miRNAs/miRs) are a group of small
non-coding RNAs with extensive biological function in various human
diseases, such as metabolic homoeostasis (15). Numerous miRNAs have been identified
as key molecules in neurodevelopment, such as miR-200a and miR-302c
(16). In addition, certain miRNAs
exhibit neuroprotective effects by regulating neuroinflammation
(17). miR-150 has been suggested to
be an inhibitor of neuroinflammation (18), and it can ameliorate neuropathic pain
by targeting AKT3, which is a major regulator of the inflammatory
response (19). However, to the best
of our knowledge, the relationship between miR-150 and
anesthetic-induced cognitive dysfunction has rarely been
reported.
Previous studies have examined the role of miRNAs in
the protective mechanisms of traditional Chinese herbal medicines
in various human diseases, such as miR-294(20). To investigate novel approaches to
attenuate the impairment in cognition induced by isoflurane, the
present study aimed to evaluate the protective role of TMP against
isoflurane-induced cognitive dysfunction in rats, and to further
identify the underlying mechanism by assessing the role of miR-150
and its effects on neuroinflammation.
Materials and methods
Animals and exposure to
isoflurane
In total, 100 male Sprague-Dawley at 7 weeks of age
(220-260 g) rats were purchased from the Animal Center of Chinese
Academy of Sciences, and were housed in animal facilities with free
access to water and food under a standard environment
(25±1°C, 60-65% humidity and 12/12 h light/dark cycle).
The rats were randomly designed into two groups: Control (n=10) and
isoflurane groups (n=90). For isoflurane exposure, the rats were
housed in anesthetic chambers containing 2% isoflurane
(Sigma-Aldrich; Merck KGaA) mixed with 100% oxygen for 4 h. The
rats in the control group were placed in the animal facilities with
room air. All the animal experiments were in accordance with the
guidelines of the National Institutes of Health and Association for
Assessment and Accreditation of Laboratory Animal Care (21), and were approved by the Animal Care
and Use Committee of Dongying Hospital of Traditional Chinese
Medicine Hospital.
Animal treatment
The rats that received isoflurane exposure were
further assigned to eight groups (10 rats/group): Isoflurane + DMSO
vehicle, isoflurane + TMP, isoflurane + miR-negative control
(miR-NC), isoflurane + miR-150 mimic, isoflurane + miR-150
inhibitor, isoflurane + TMP + miR-NC, isoflurane + TMP + miR-150
inhibitor and isoflurane groups. For DMSO or TMP treatment, the
rats were intraperitoneally injected with DMSO (1 ml) or 20 mg/kg
TMP (Nanjing Zelang Medical Technology Co., Ltd.) at 24 h after the
isoflurane treatment (22). The
miR-150 mimic, miR-150 inhibitor and miR-NC (2 nM) were synthesized
by Shanghai GenePharma Co., Ltd. and injected into the rats by
lateral cerebroventricular injection to regulate the expression of
miR-150 in vivo 24 h before isoflurane exposure,
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) was used for
transfection according to the manufacturer's instructions.. The
sequences for the vectors were as follows: miR-150 mimic,
5'-UCUCCCAACCCUUGUACCAGUG-3'; miR-150 inhibitor,
5'-CACUGGUACAAGGGUUGGGAGA-3'; and miR-NC,
5'-UUCUCCGAACGUGUCACGU-3'. The detailed treatment for the groups is
summarized in Table I. After
treatment, five rats from each group were subjected to the Morris
water maze test to evaluate the cognitive function, and hippocampi
were collected from the remaining five rats after sacrifice by
cervical dislocation.
 | Table ITreatment in different groups. |
Table I
Treatment in different groups.
| Groups | Treatment |
|---|
| Control (n=10) | Room air |
| Isoflurane
(n=10) | 2% isoflurane
exposure |
| Isoflurane + DMSO
(n=10) | 2% isoflurane
exposure and DMSO injection |
| Isoflurane + TMP
(n=10) | 2% isoflurane
exposure and injection with 20 mg/kg TMP |
| Isoflurane + miR-NC
(n=10) | 2% isoflurane
exposure and injection with 2 nM miR-NC |
| Isoflurane +
miR-150 mimic (n=10) | 2% isoflurane
exposure and injection with 2 nM miR-150 mimic |
| Isoflurane +
miR-150 inhibitor (n=10) | 2% isoflurane
exposure and injection with 2 nM miR-150 inhibitor |
| Isoflurane + TMP +
miR-NC (n=10) | 2% isoflurane
exposure and injection with 20 mg/kg TMP and 2 nM miR-NC |
| Isoflurane + TMP +
miR-150 mimic (n=10) | 2% isoflurane
exposure and injection with 20 mg/kg TMP and 2 nM miR-150
mimic |
| Isoflurane + TMP +
miR-150 inhibitor (n=10) | 2% isoflurane
exposure and injection with 20 mg/kg TMP and 2 nM miR-150
inhibitor |
Morris water maze performance
This study evaluated the cognitive function of the
rats using a Morris water maze test, which was performed using a
method previously described (23).
Briefly, 48 h post-isoflurane exposure, the rats in each group were
subjected to the hidden-platform training for 5 days with 2 trials
per day, and the platform was removed on the 6th day. A
computerized tracking system (Actimetrics software version 2.6;
Actimetrics) was used to monitor the trials, and the performance,
including latency to find the hidden platform, distance traveled,
time spent in each quadrant and number of platform crossing, of the
rats in different groups was recorded and compared. After the test,
the rats (weight, 250-300 g) were sacrificed by cervical
dislocation under anesthesia with 50 mg/kg pentobarbitone.
ELISA
The expression levels of the pro-inflammatory
cytokines IL-1β (cat. no. RLB00), IL-6 (cat. no. R6000B) and TNF-α
(cat. no. RTA00) in the hippocampus collected from the rats in each
group were detected using the rat ELISA kit (Invitrogen; Thermo
Fisher Scientific, Inc.), following the manufacturer's
instruction.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNAs in the hippocampus were extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), which were then used for RT to synthesize cDNA using the
PrimeScript RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocols, the conditions were 37˚C for 15 min and
98˚C for 5 min. Hippocampal expression levels of miR-150 and the
mRNA expression of AKT3 were measured using RT-qPCR, which was
performed using a SYBR green I Master Mix kit (Invitrogen; Thermo
Fisher Scientific, Inc.) on a 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The PCR conditions
were as follows: 95˚C for 5 min, followed by 40 cycles of 95˚C for
30 sec, 60˚C for 30 sec, and 72˚C for 40 sec and then a final
extension at 72˚C for 5 min. The final expression levels were
calculated using the 2-ΔΔCq method (24) and normalized to U6 or GAPDH. Primers
used in the current study are as follows: miR-150, forward:
5'-TGCGGTCTCCCAACCCTTG-3' and reverse, 5'-CCAGTGCAGGGTCCGAGGT-3';
AKT3, forward, 5'-TGTGGATTTACCTTATCCCCTCA-3' and reverse,
5'-GTTTGGCTTTGGTCGTTCTGT-3'; U6, forward,
5'-CGCTTCGGCAGCACATATACTAAAATTGGAAC-3' and reverse:
5'-GCTTCACGAATTTGCGTGTCATCCTTGC-3'; GAPDH, forward,
5'-AGAAGGCTGGGGCTCATTTG-3' and reverse,
5'-AGGGGCCATCCACAGTCTTC-3'.
Cell culture
Human neuroblastoma SH-SY5Y cells (cat. no.
ATCC®CRL-2266™) were purchased from the
American Type Culture Collection. Cells were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a humidified
incubator with 5% CO2.
Dual-luciferase reporter assay
The potential targets of miR-150 were predicted
using TargetScan 7.2 (http://www.targetscan.org/vert_72/), which are
presented in Table II, and the
3'-untranslated region (UTR) of AKT3 had the complementary sequence
of miR-150. To assess their interaction, a dual-luciferase reporter
assay was conducted. The wild-type (WT) and mutant type (MUT)
3'-UTR of AKT3 were cloned into pGL3 reporter vector (50 ng;
Promega Corporation) (Sangon Biotech Co., Ltd.) and then were
respectively co-transfected into SH-SY5Y cells with miR-150 mimic,
miR-150 inhibitor or miR-NC (200 ng) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), following the manufacturer's instruction. After
cell transfection for 48 h, the relative luciferase activity was
measured using the Promega Dual-luciferase Reporter Assay kit
(Promega Corporation). Renilla luciferase activity was used
as the internal control.
 | Table IIList of the predicted target genes of
miR-150 commonly predicted in TargetScan 7.2 database. |
Table II
List of the predicted target genes of
miR-150 commonly predicted in TargetScan 7.2 database.
| | Predicted target
genes of miR-150 |
|---|
| | Target gene | Gene name |
|---|
| 1 | MYB | v-myb avian
myeloblastosis viral oncogene homolog |
| 2 | ADIPOR2 | Adiponectin
receptor 2 |
| 3 | HILPDA | Hypoxia inducible
lipid droplet-associated |
| 4 | TADA1 | Transcriptional
adaptor 1 |
| 5 | MTCH2 | Mitochondrial
carrier 2 |
| 6 | MBD6 | methyl-CpG binding
domain protein 6 |
| 7 | BASP1 | Brain abundant,
membrane attached signal protein 1 |
| 8 | ZBTB4 | Zinc finger and BTB
domain containing 4 |
| 9 | ZMAT2 | Zinc finger,
matrin-type 2 |
| 10 | PLP2 | Proteolipid protein
2 (colonic epithelium-enriched) |
| 11 | FOXD3 | Forkhead box
D3 |
| 12 | ZFP91 | ZFP91 zinc finger
protein |
| 13 | NKX2-4 | NK2 homeobox 4 |
| 14 | PTP4A1 | Protein tyrosine
phosphatase type IVA, member 1 |
| 15 | KCNIP1 | Kv channel
interacting protein 1 |
| 16 | SGMS1 | Sphingomyelin
synthase 1 |
| 17 | EIF4B | Eukaryotic
translation initiation factor 4B |
| 18 | ETF1 | Eukaryotic
translation termination factor 1 |
| 19 | PRICKLE2 | Prickle homolog 2
(Drosophila) |
| 20 | STX5 | Syntaxin 5 |
| 21 | ZEB1 | Zinc finger E-box
binding homeobox 1 |
| 22 | ACVR1B | Activin A receptor,
type IB |
| 23 | ARIH2 | Ariadne RBR E3
ubiquitin protein ligase 2 |
| 24 | AKT3 | v-akt murine
thymoma viral oncogene homolog 3 |
| 25 | PDIA6 | Protein disulfide
isomerase family A, member 6 |
| 26 | GABRA4 | γ-aminobutyric acid
A receptor, α4 |
| 27 | CDAN1 | Codanin 1 |
| 28 | FOXO4 | Forkhead box
O4 |
| 29 | UBE2R2 |
Ubiquitin-conjugating enzyme E2R 2 |
| 30 | DYRK1A | Dual-specificity
tyrosine-(Y)-phosphorylation regulated kinase 1A |
| 31 | HMGA2 | High mobility group
AT-hook 2 |
| 32 | MBTD1 | mbt domain
containing 1 |
| 33 | EP300 | E1A binding protein
p300 |
| 34 | RORB | RAR-related orphan
receptor B |
| 35 | GOSR1 | Golgi SNAP receptor
complex member 1 |
| 36 | NEFM | Neurofilament,
medium polypeptide |
| 37 | FOXP1 | forkhead box
P1 |
| 38 | SRGAP3 | SLIT-ROBO Rho
GTPase activating protein 3 |
| 39 | HIF-1α | Hypoxia inducible
factor-1 α subunit (basic helix-loop-helix transcription
factor) |
| 40 | LMO4 | LIM domain only
4 |
| 41 | EPHB2 | EPH receptor
B2 |
| 42 | NEGR1 | Neuronal growth
regulator 1 |
| 43 | UST |
Uronyl-2-sulfotransferase |
| 44 | ATP8A2 | ATPase,
aminophospholipid transporter, class I, type 8A, member 2 |
| 45 | TMCC1 | Transmembrane and
coiled-coil domain family 1 |
| 46 | RAD23B | RAD23 homolog B
(S. cerevisiae) |
| 47 | PAPD5 | PAP associated
domain containing 5 |
| 48 | CPD | Carboxypeptidase
D |
| 49 | EBF3 | Early B-cell factor
3 |
| 50 | SMARCD1 | SWI/SNF related,
matrix associated, actin dependent regulator of chromatin,
subfamily d, member 1 |
| 51 | ITSN1 | Intersectin 1 (SH3
domain protein) |
| 52 | GDI1 | GDP dissociation
inhibitor 1 |
| 53 | FAM134C | Family with
sequence similarity 134, member C |
| 54 | TBC1D20 | TBC1 domain family,
member 20 |
| 55 | MMP14 | Matrix
metallopeptidase 14 (membrane-inserted) |
| 56 | GABRG2 | γ-aminobutyric acid
A receptor, γ2 |
| 57 | ADAM19 | ADAM
metallopeptidase domain 19 |
| 58 | EIF4E | Eukaryotic
translation initiation factor 4E |
| 59 | TET3 | tet methylcytosine
dioxygenase 3 |
| 60 | PKHD1 | Polycystic kidney
and hepatic disease 1 (autosomal recessive) |
| 61 | PAPPA |
Pregnancy-associated plasma protein A,
pappalysin 1 |
| 62 | CHD2 | Chromodomain
helicase DNA binding protein 2 |
| 63 | CC2D1B | Coiled-coil and C2
domain containing 1B |
| 64 | PRRT2 | Proline-rich
transmembrane protein 2 |
| 65 | MMP16 | Matrix
metallopeptidase 16 (membrane-inserted) |
Statistical analysis
Data are presented as the mean ± SD, and were
analyzed using SPSS 21.0 (IBM Corp.) and GraphPad Prism 7.0
software (GraphPad Software, Inc.). The differences between groups
were assessed using Student's t-test or one-way ANOVA, followed by
Tukey's or Bonferroni post hoc test. Moreover, behavioral data were
analyzed using a mixed ANOVA and a Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was repeated at least three times.
Results
Neuroprotective effects of TMP on
isoflurane-induced cognitive dysfunction
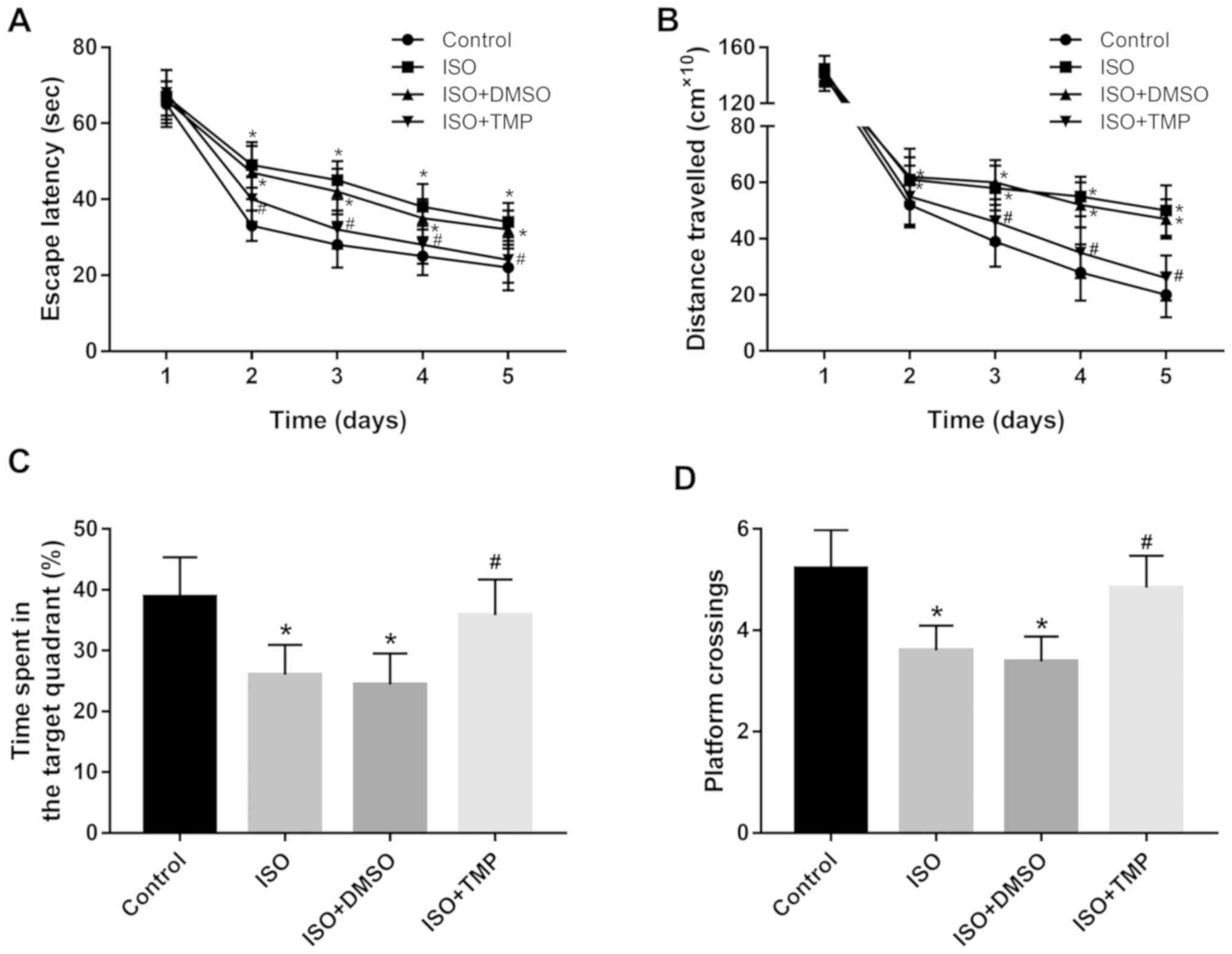
A Morris water maze test was performed to evaluate
the effects of isoflurane and TMP treatment on the cognitive
function in the rats. In rats exposed to isoflurane, the latency to
find the hidden platform and the distance travelled during the test
were significantly increased compared with the control group (all
P<0.05; Fig. 1A and B), indicating an impairment in the spatial
learning ability of the rats. After removing the hidden platform,
it was found that the rats exposed to isoflurane spent less time in
target quadrant and crossed through the original platform location
fewer times compared with the control group (all P<0.05;
Fig. 1C and D), suggesting that the memory ability of
the rats was impaired. Moreover, the injection of TMP significantly
rescued the isoflurane-induced cognitive dysfunction, as
demonstrated by the improved spatial learning and memory indicators
in the Morris water maze test (all P<0.05; Fig. 1).
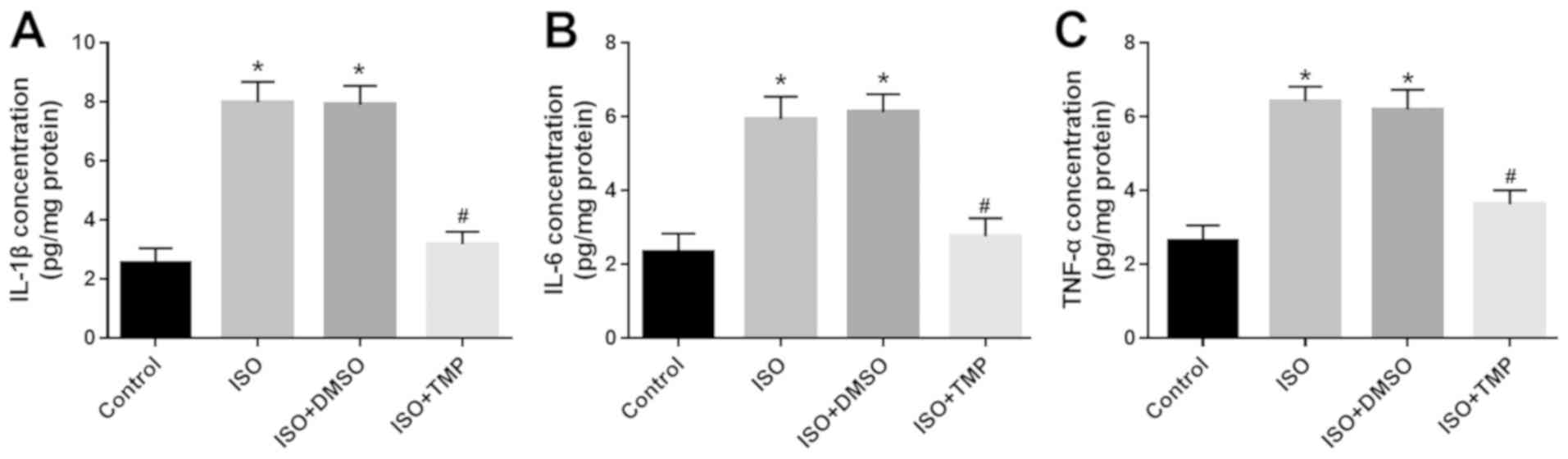
Anti-inflammatory effect of TMP in
rats exposed to isoflurane
The impairment in cognitive function induced by
isoflurane is accompanied by enhanced inflammatory responses
(1). The hippocampal levels of the
pro-inflammatory cytokines IL-1β, IL-6 and TNF-α were all increased
by exposure to isoflurane (all P<0.05; Fig. 2). However, in the rats that received
TMP treatment, the isoflurane-induced increases in IL-1β, IL-6 and
TNF-α levels were significantly reversed (all P<0.05; Fig. 2).
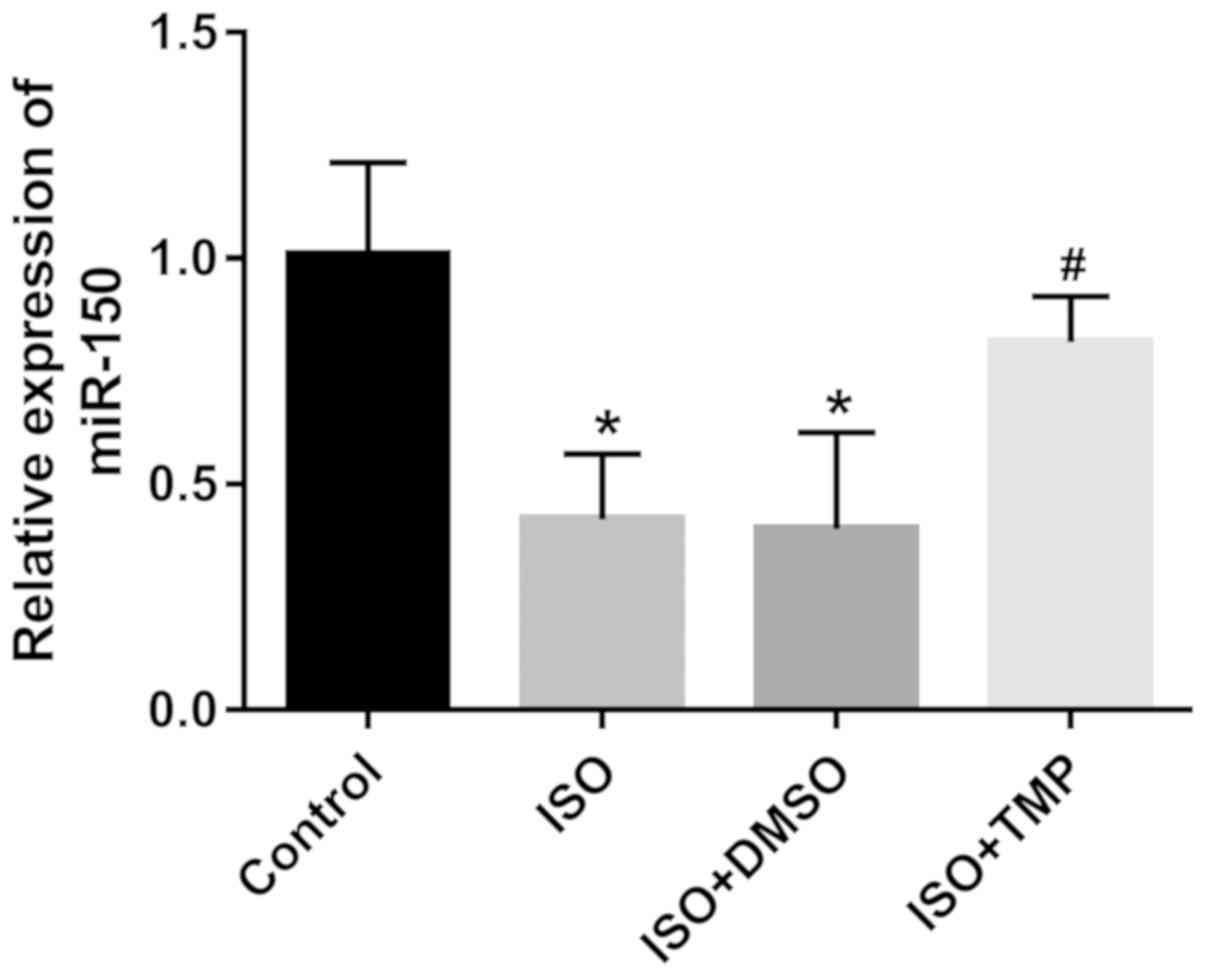
Dysregulation of miR-150 in rats under
isoflurane anesthesia and TMP treatment
The aberrant expression of miR-150 has been
identified in neuropathic pain (19). The results demonstrated that the
expression of miR-150 in the hippocampus was significantly
decreased in the rats exposed to isoflurane compared with control
group (P<0.05; Fig. 3), while
this effect was partially abrogated by TMP treatment (P<0.05;
Fig. 3).
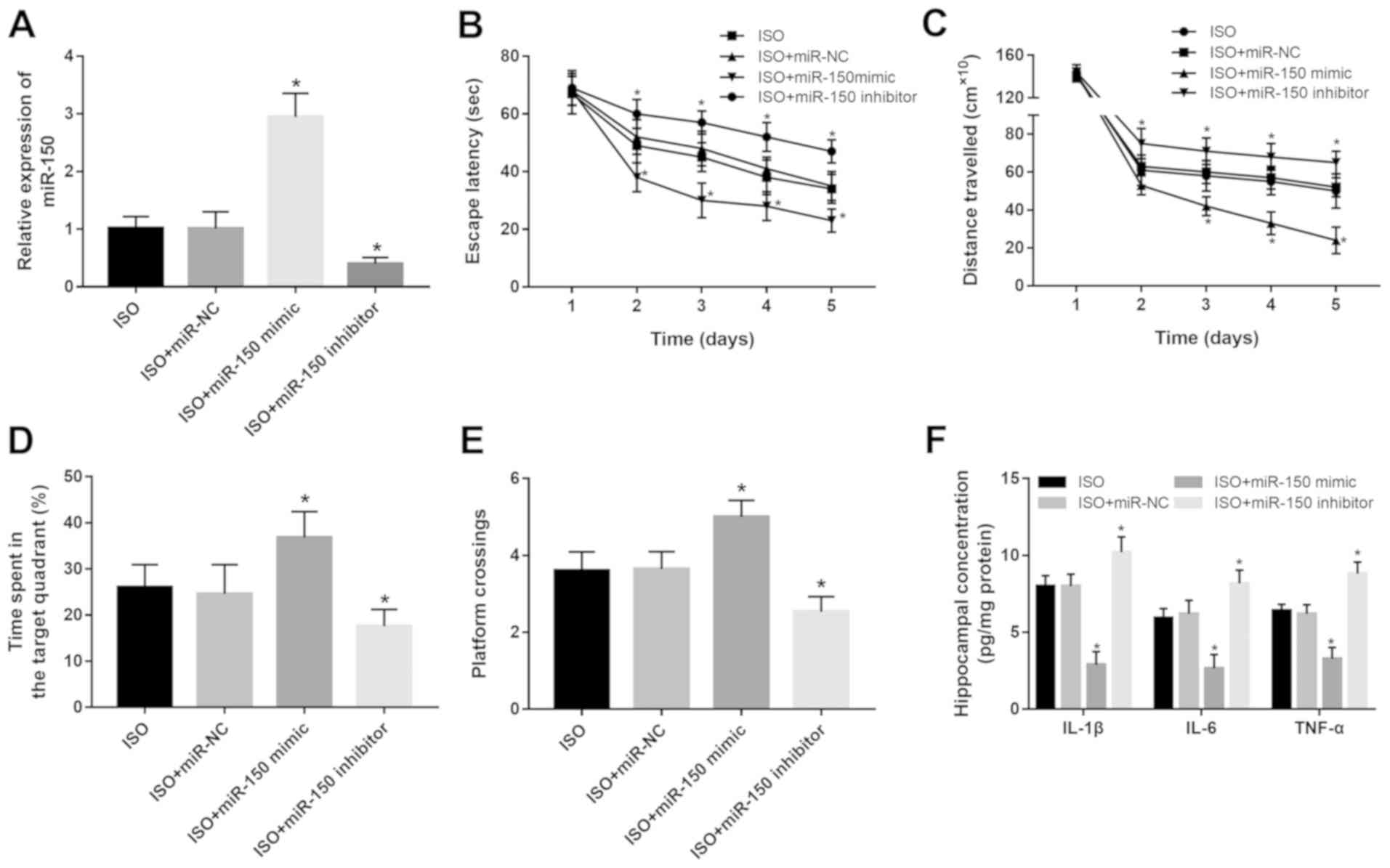
Effects of miR-150 on
isoflurane-induced cognitive dysfunction and neuroinflammation
Based on the decreased expression of miR-150 in rats
exposed to isoflurane, the effects of miR-150 on the impaired
cognitive function and neuroinflammation induced by isoflurane were
further investigated. The expression of miR-150 was upregulated by
the miR-150 mimic but was downregulated by the miR-150 inhibitor in
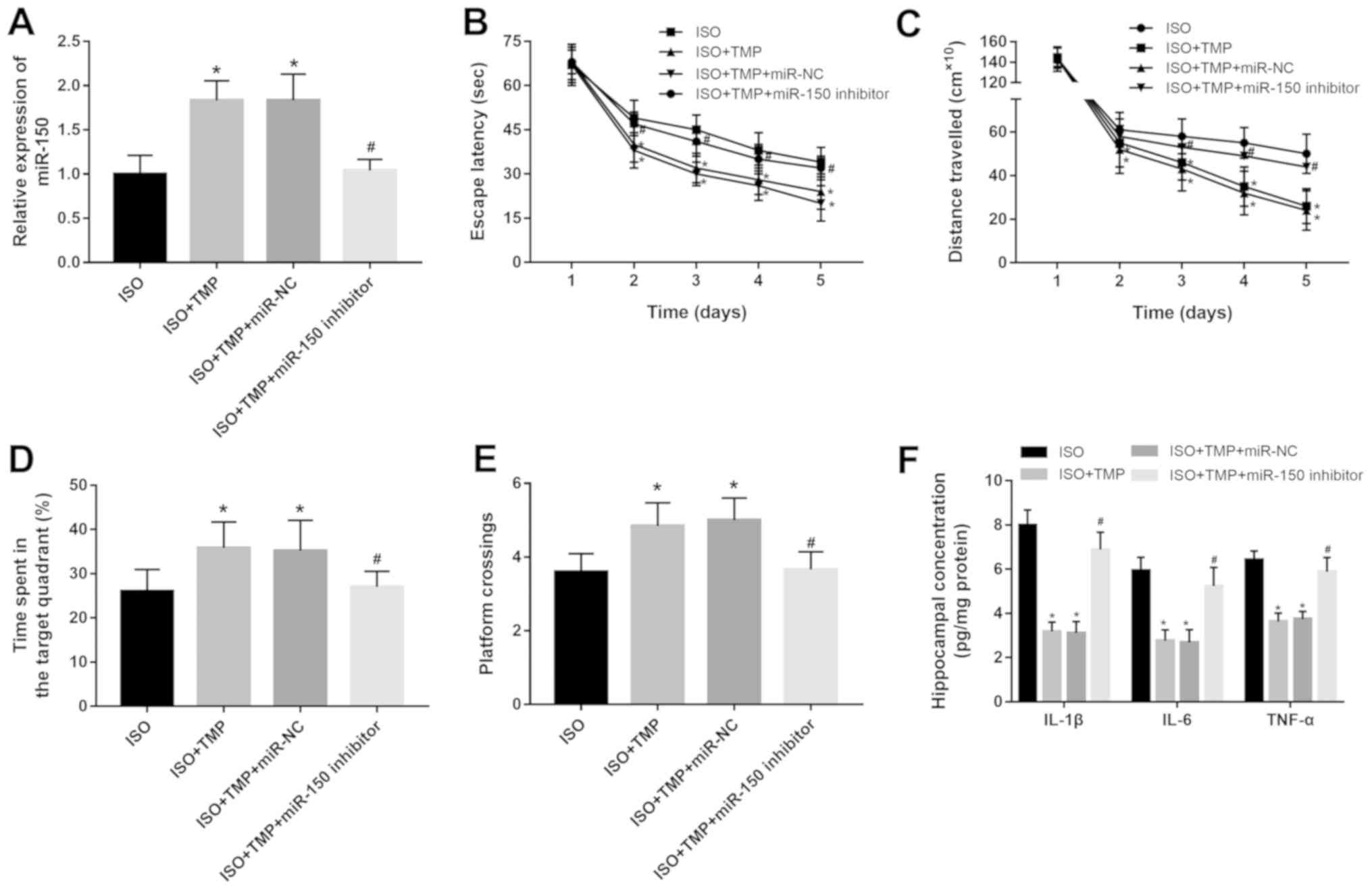
hippocampal tissues in vivo (both P<0.05; Fig. 4A). It was found that
isoflurane-induced spatial learning and memory impairments were all
reversed by the overexpression of miR-150, but were aggravated by
the knockdown of miR-150 (all P<0.05; Fig. 4B-E). Moreover, the hippocampal
concentrations of IL-1β, IL-6 and TNF-α were ameliorated by the
upregulation of miR-150, but the opposite results were obtained in
the rats with miR-150 knockdown (all P<0.05; Fig. 4F).
TMP exerts neuroprotective effects by
regulating miR-150 in rats exposed to isoflurane
Considering the regulatory activity of TMP on the
expression of miR-150 in the isoflurane-treated rats, the role of
miR-150 in the neuroprotective effect of TMP in rats was evaluated.
In the rats exposed to isoflurane, the increased expression of
miR-150 induced by TMP was successfully inhibited by the miR-150
inhibitor (P<0.05; Fig. 5A).
Furthermore, the results obtained from the Morris water maze test
indicated that the neuroprotective effects of TMP on the cognitive
function in the rats exposed to isoflurane were significantly
attenuated by the knockdown of miR-150 (all P<0.05; Fig. 5B-E). With regards to
neuroinflammation, the decreased hippocampal levels of IL-1β, IL-6
and TNF-α induced by TMP were all upregulated by the miR-150
inhibitor (all P<0.05; Fig.
5F).
AKT3 serves as a target gene of
miR-150
miR-150 mimic transfection significantly increased
the expression of miR-150 in SH-SY5Y cells, but miR-150 inhibitor
transfection had an opposite effect (all P<0.05; Fig. 6A). A complementary sequence of
miR-150 was found in the 3'-UTR of AKT3 (Fig. 6B). The luciferase analysis results
demonstrated that the overexpression of miR-150 could inhibit the
relative luciferase activity, but the knockdown of miR-150 could
promote the luciferase activity in the WT group (all P<0.05;
Fig. 6C). However, no significant
changes were identified in the luciferase activity in MUT groups
(all P>0.05).
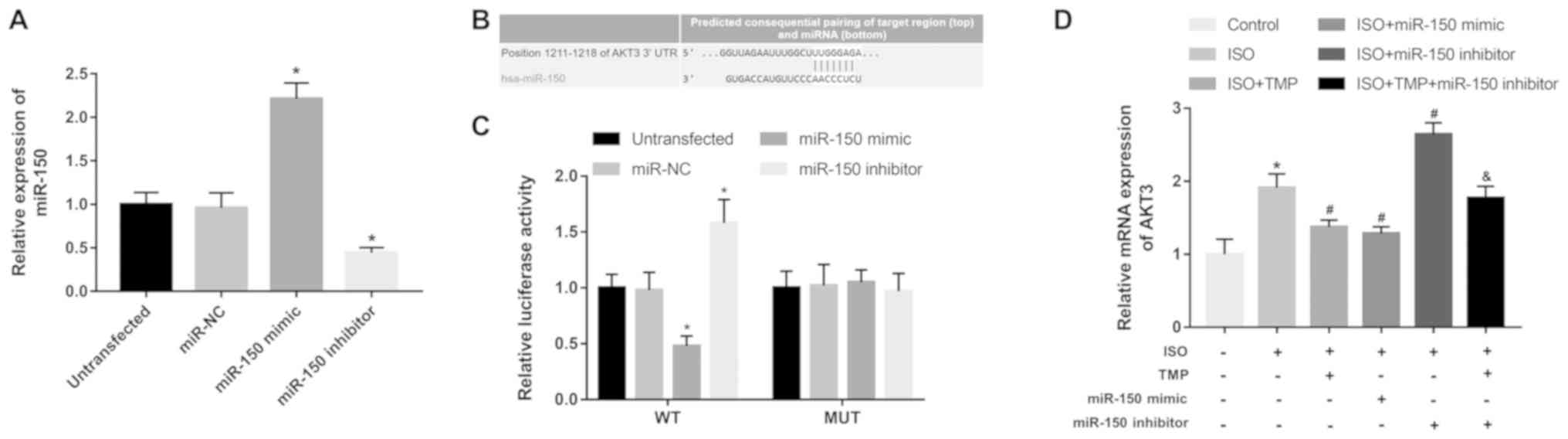 | Figure 6miR-150 directly regulates the
expression of AKT3. (A) miR-150 mimic transfection significantly
increased the expression of miR-150 in SH-SY5Y cells, but miR-150
inhibitor transfection has an opposite effect. (B) Complementary
sequence of miR-150 in the 3'-UTR of AKT3. (C) Luciferase activity
analysis to assess the interaction between miR-150 and AKT3.
*P<0.05 vs. untransfected group. (D) ISO exposure led
to increased AKT3 expression, while this inhibition was rescued by
TMP treatment. The overexpression of miR-150 could inhibited,
whereas the knockdown of miR-150 could enhanced the expression of
AKT3. The TMP-induced inhibition in AKT3 expression was reversed by
the knockdown of miR-150. *P<0.05 vs. Control;
#P<0.05 vs. ISO; &P<0.05 vs. ISO +
TMP. ISO, isoflurane; TMP, tetramethylpyrazine; UTR, untranslated
region; NC, negative control; miR, microRNA; WT, wild-type; MUT,
mutant. |
The relative mRNA expression of AKT3 in the
hippocampus collected from the experimental rats was measured. It
was demonstrated that isoflurane exposure led to increased
expression of AKT3, while TMP treatment could inhibit this increase
in AKT3 expression (all P<0.05; Fig.
6D). Furthermore, via the in vivo regulation of miR-150,
the mRNA expression of AKT3 in the isoflurane-treated rats was
inhibited by the overexpression of miR-150, but was enhanced by the
knockdown of miR-150 compared with the isoflurane group (all
P<0.05; Fig. 6D). In the
isoflurane-treated rats that received both TMP and miR-150
inhibitor administration, the TMP-induced decrease in AKT3
expression was reversed by the knockdown of miR-150 (P<0.05;
Fig. 6D).
Discussion
Anesthetic-induced cognitive dysfunction remains a
challenging health problem for doctors after major surgery
(25). The present study focused on
the protective effects of the traditional Chinese herbal medicine
TMP in rats exposed to isoflurane and evaluated the underlying
mechanisms. The current results suggested that the TMP treatment in
rats ameliorated the impairment in cognitive function induced by
isoflurane exposure, and that anesthetic-induced neuroinflammation
was also attenuated by TMP. In addition, it was found that the
hippocampal expression of miR-150 was suppressed by isoflurane
exposure, but was enhanced after TMP treatment. The overexpression
of miR-150 in rats led to improved cognitive function and
neuroinflammation, while the knockdown of miR-150 abrogated the
protective effects of TMP against isoflurane-induced cognitive
dysfunction and neuroinflammation.
Previous studies have revealed the clinical
potential of traditional Chinese herbal medicines in various human
diseases, such as hepatitis and neurodegenerative disease (26). Some active components of Chinese
medicine can exert significantly protective effects for the
treatment of cognitive function impairment induced by anesthetics.
For example, Chen et al (1)
provided evidence for apigenin as a medicine that attenuates
cognitive dysfunction induced by isoflurane in aged rats. Moreover,
Zhang et al (27)
demonstrated the neuroprotective role of ginsenoside Rg1 in rats
exposed to isoflurane, while Chen et al (28) revealed that vitexin exerts a
protective effect against neurotoxicity induced by isoflurane. The
neuroprotective role of salidroside has also been reported in rats
expose to isoflurane (29). These
aforementioned studies provide evidence for the important roles of
traditional Chinese medicine in neuroprotective effects in rats
exposed to isoflurane.
TMP is a widely used and analyzed traditional
Chinese medicine in human diseases, such as liver fibrosis
(30) and prostate cancer (31). The effects of TMP on the nervous
system have been reported in oxygen-glucose deprivation-induced
mortality (8) and ischemic stroke
(32). However, whether TMP is
involved in the neuroregulation in rats exposed to isoflurane
remains elusive. In the current study, a Morris water maze test was
performed to evaluate the effect of TMP on the spatial learning and
memory abilities of rats. It was demonstrated that the impaired
cognitive function induced by isoflurane in rats was significantly
ameliorated by TMP treatment. Thus, it was concluded that TMP
exerts a protective effect against isoflurane-induced cognitive
dysfunction.
Neuroinflammation is a key event during impairments
of learning and memory in rats exposed to anesthetics (33). The neuroprotective effects of
apigenin (1) and ginsenoside
Rb1(34) in rats exposed to
isoflurane are achieved by attenuating neuroinflammation. TMP has
been shown to be closely associated with inflammation, and its
anti-inflammatory effect has been well described in vivo
(35). The present results also
provide evidence that TMP exerts an anti-neuroinflammatory role in
rats exposed to isoflurane, and suggested that TMP may ameliorate
isoflurane-induced cognitive dysfunction by attenuating
neuroinflammation. However, the changes in systemic inflammatory
responses were not analyzed, which was one of the limitations of
the current study. Despite the presence of a blood-brain barrier,
it has been reported that the circulating immune system is also
closely associated with neuroinflammation (36). Thus, further studies should
investigate the effects of TMP on systemic inflammatory responses
to further identify the regulatory mechanisms.
Previous studies have investigated the role of
miRNAs in the mechanisms involved in the protective effects of
traditional Chinese medicine. For instance, Liu et al
(37) revealed that curcumin can
enhance sensitivity to radiation in prostate cancer cells via the
regulation of miR-143. Moreover, Fan et al (38) demonstrated that emodin protects
against hyperglycemia-induced injury in PC-12 cells via the
upregulation of miR-9. TMP has been reported to alleviate spinal
cord injury via the regulation of miR-214-3p (39) and relieve hypoxia-caused damage via
the downregulation of miR-449a (40). However, the number of functional
miRNAs involved in the development of isoflurane-induced cognitive
dysfunction remains unknown.
miR-150 has been reported to exert protective
effects in neuropathic pain by suppressing neuroinflammation
(19). Moreover, aberrant expression
of miR-150 has been observed in multiple sclerosis after treatment
with curcumin (41). In the present
study, it was found that the hippocampal expression of miR-150 was
significantly decreased in rats exposed to isoflurane, and that
this inhibitory effect was relieved by treatment with TMP,
suggesting the potential role of miR-150 in the development of
isoflurane-induced injury and in the biological function of TMP.
Further experiments were performed to verified this conclusion, the
results of which indicated that the overexpression of miR-150 in
rats improved the impairment in cognitive function and inflammatory
reaction induced by isoflurane exposure. Furthermore, the knockdown
of miR-150 abrogated the protective effects of TMP on spatial
learning and memory abilities, as well as neuroinflammation in
rats. Collectively, these results suggested that miR-150 may be a
mediator of the neuroprotective effects of TMP via
neuroinflammation in rats exposed to isoflurane.
miRNAs are involved in the development of various
human disease by regulating their target genes (42). In the present study, a number of
candidate target genes were predicted using TargetScan for miR-150,
and AKT3 was selected based on previous studies demonstrating its
crucial role in neurological function (43,44).
AKT3 is a major isoform of the AKT family of serine/threonine
protein kinases, which is predominant in the brain (45). The dysregulation of AKT3 has been
widely reported to be involved in the regulation of learning and
memory deficits in several diseases (43,44).
Additionally, AKT3 has been shown to be a target gene of
miR-150(19), which is one of the
major molecules of the PI3K/AKT signaling pathway. In the present
study, it was demonstrated that AKT3 was a direct target of
miR-150, and the results indicated the negatively regulatory effect
of miR-150 on the expression of AKT3 in rats exposed to isoflurane
and administrated with TMP. It was also found that inhibited AKT3
expression induced by TMP could be rescued by the knockdown of
miR-150, which suggested that miR-150 may mediate the
neuroprotective effect of TMP in isoflurane-treated rats by
targeting AKT3. PI3K/AKT signaling is considered an important
pathway in regulating neuroinflammation (46). Yang et al (47) revealed that TMP can ameliorate the
injury induced by acute myocardial ischemia and attenuated the
inflammatory response by regulating the PI3K/AKT signaling pathway.
These previous studies combined with the current findings suggest
that TMP may protect isoflurane-induced cognitive dysfunction via
the miR-150/AKT signaling, and this conclusion warrants further
investigation.
Alongside AKT3, other targets of miR-150 have
previously been reported, such as glioma-associated oncogene
homolog 1 (Gli1) (48) and hypoxia
inducible factor-1α (HIF-1α) (49).
For instance, Peng et al (50) found that Gli1 was involved in the
cerebral ischemia/reperfusion injury after isoflurane
post-conditioning. Moreover, HIF-1α has been reported to serve an
important role in the blood-brain barrier disruption induced by
isoflurane (51), and it could
mediate the biological function of TMP in the improvement of tibial
dyschodroplasia (52) and
endothelial dysfunction (53).
However, whether these aforementioned genes also participate the
neuroprotective effects of TMP mediated by miR-150 remains unknown,
and future studies should focus on the potential targets and
signaling to further confirm the mechanisms underlying the
functional role of TMP and miR-150 in isoflurane-induced cognitive
dysfunction.
In conclusion, the present study provided evidence
that TMP and miR-150 exert protective roles against the cognitive
dysfunction induced by isoflurane in rats. TMP ameliorates the
isoflurane-induced impairments in learning and memory via the
attenuation of neuroinflammation by promoting the miR-150/AKT
pathway. Moreover, miR-150 may serve as a potential therapeutic
target for the alleviation of isoflurane-induced cognitive
dysfunction.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC and ZX made substantial contributions to
conception and design, acquisition of data, analysis and
interpretation of data. HC, ZX and CQ were involved in animal
experiments, drafting the manuscript and revising it. All authors
read and approved the final manuscript
Ethics approval and consent to
participate
All the animal experiments were in accordance with
the guidelines of the National Institutes of Health and Association
for Assessment and Accreditation of Laboratory Animal Care, and
were approved by the Animal Care and Use Committee of Dongying
Hospital of Traditional Chinese Medicine Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen L, Xie W, Xie W, Zhuang W, Jiang C
and Liu N: Apigenin attenuates isoflurane-induced cognitive
dysfunction via epigenetic regulation and neuroinflammation in aged
rats. Arch Gerontol Geriatr. 73:29–36. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sha HY, Zhao JB, Sha MX and Guo SM:
Effects of vitamin B12 on postoperative cognitive dysfunction
induced by isoflurane anesthesia in rats. Eur Rev Med Pharmacol
Sci. 21:1959–1966. 2017.PubMed/NCBI
|
|
3
|
Bartels K, Li YJ, Li YW, White WD,
Laskowitz DT, Kertai MD, Stafford-Smith M, Podgoreanu MV, Newman MF
and Mathew JP: Apolipoprotein epsilon 4 genotype is associated with
less improvement in cognitive function five years after cardiac
surgery: A retrospective cohort study. Can J Anaesth. 62:618–626.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Song J, Chu S, Cui Y, Qian Y, Li X, Xu F,
Shao X, Ma Z, Xia T and Gu X: Circadian rhythm resynchronization
improved isoflurane-induced cognitive dysfunction in aged mice. Exp
Neurol. 306:45–54. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guo M, Zhu X, Xu H, Li J, Yang S, Zuo Z
and Lin D: Ulinastatin attenuates isoflurane-induced cognitive
dysfunction in aged rats by inhibiting neuroinflammation and
β-amyloid peptide expression in the brain. Neurol Res. 41:1–7.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Callaway JK, Wood C, Jenkins TA, Royse AG
and Royse CF: Isoflurane in the presence or absence of surgery
increases hippocampal cytokines associated with memory deficits and
responses to brain injury in rats. Behav Brain Res. 303:44–52.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang W, Chen X, Zhang J, Zhao Y, Li S, Tan
L, Gao J, Fang X and Luo A: Glycyrrhizin attenuates
isoflurane-induced cognitive deficits in neonatal rats via its
anti-inflammatory activity. Neuroscience. 316:328–336.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shao Z, Wang L, Liu S and Wang X:
Tetramethylpyrazine protects neurons from oxygen-glucose
deprivation-induced death. Med Sci Monit. 23:5277–5282.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu Y, Li X, Jiang S and Ge Q:
Tetramethylpyrazine protects against high glucose-induced vascular
smooth muscle cell injury through inhibiting the phosphorylation of
JNK, p38MAPK, and ERK. J Int Med Res. 46:3318–3326. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shao Z, Wu P, Wang X, Jin M, Liu S, Ma X
and Shi H: Tetramethylpyrazine protects against early brain injury
and inhibits the PERK/Akt pathway in a rat model of subarachnoid
hemorrhage. Neurochem Res. 43:1650–1659. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Michel HE, Tadros MG, Esmat A, Khalifa AE
and Abdel-Tawab AM: Tetramethylpyrazine ameliorates
rotenone-induced Parkinson's disease in rats: Involvement of its
anti-inflammatory and anti-apoptotic actions. Mol Neurobiol.
54:4866–4878. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu SH, Yin MS, Liu B, Chen ML, He GW, Zhou
PP, Cui YJ, Yang D and Wu YL: Tetramethylpyrazine-2'-O-sodium
ferulate attenuates blood-brain barrier disruption and brain oedema
after cerebral ischemia/reperfusion. Hum Exp Toxicol. 36:670–680.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fu S, Wang J, Hao C, Dang H and Jiang S:
Tetramethylpyrazine ameliorates depression by inhibiting TLR4-NLRP3
inflammasome signal pathway in mice. Psychopharmacology (Berl).
236:2173–2185. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Z, Wang Q, Wang C, Xu X and Yu H:
Tetramethylpyrazine attenuates periorbital allodynia and
neuroinflammation in a model of traumatic brain injury. J Inflamm
(Lond). 14(13)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vienberg S, Geiger J, Madsen S and
Dalgaard LT: MicroRNAs in metabolism. Acta Physiol (Oxf).
219:346–361. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rodrigues DC, Kim DS, Yang G, Zaslavsky K,
Ha KC, Mok RS, Ross PJ, Zhao M, Piekna A, Wei W, et al: MECP2 is
post-transcriptionally regulated during human neurodevelopment by
combinatorial action of RNA-binding proteins and miRNAs. Cell Rep.
17:720–734. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gaudet AD, Fonken LK, Watkins LR, Nelson
RJ and Popovich PG: MicroRNAs: Roles in regulating
neuroinflammation. Neuroscientist. 24:221–245. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ji LJ, Shi J, Lu JM and Huang QM: MiR-150
alleviates neuropathic pain via inhibiting toll-like receptor 5. J
Cell Biochem. 119:1017–1026. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cai W, Zhang Y, Liu Y, Liu H, Zhang Z and
Su Z: Effects of miR-150 on neuropathic pain process via targeting
AKT3. Biochem Biophys Res Commun. 517:532–537. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang J, Masika J, Zhou J, Wang J, Zhu M,
Luo H, Hu X, Zhang L, Tang M, Gao L, et al: Traditional Chinese
medicine baicalin suppresses mESCs proliferation through inhibition
of miR-294 expression. Cell Physiol Biochem. 35:1868–1876.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Newcomer CE: The evolution and adoption of
standards used by AAALAC. J Am Assoc Lab Anim Sci. 51:293–297.
2012.PubMed/NCBI
|
|
22
|
Chang CY, Kao TK, Chen WY, Ou YC, Li JR,
Liao SL, Raung SL and Chen CJ: Tetramethylpyrazine inhibits
neutrophil activation following permanent cerebral ischemia in
rats. Biochem Biophys Res Commun. 463:421–427. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shi C, Yi D, Li Z, Zhou Y, Cao Y, Sun Y,
Chui D and Guo X: Anti-RAGE antibody attenuates isoflurane-induced
cognitive dysfunction in aged rats. Behav Brain Res. 322:167–176.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shan L, Ma D, Zhang C, Xiong W and Zhang
Y: miRNAs may regulate GABAergic transmission associated genes in
aged rats with anesthetics-induced recognition and working memory
dysfunction. Brain Res. 1670:191–200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li FS and Weng JK: Demystifying
traditional herbal medicine with modern approach. Nat plants.
3(17109)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang Y, Zhang Z, Wang H, Cai N, Zhou S,
Zhao Y, Chen X, Zheng S, Si Q and Zhang W: Neuroprotective effect
of ginsenoside Rg1 prevents cognitive impairment induced by
isoflurane anesthesia in aged rats via antioxidant,
anti-inflammatory and anti-apoptotic effects mediated by the
PI3K/AKT/GSK-3beta pathway. Mol Med Rep. 14:2778–2784.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen L, Zhang B, Shan S and Zhao X:
Neuroprotective effects of vitexin against isoflurane-induced
neurotoxicity by targeting the TRPV1 and NR2B signaling pathways.
Mol Med Rep. 14:5607–5613. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liang L, Ma Z, Dong M, Ma J, Jiang A and
Sun X: Protective effects of salidroside against isoflurane-induced
cognitive impairment in rats. Hum Exp Toxicol. 36:1295–1302.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhao S, Zhang Z, Yao Z, Shao J, Chen A,
Zhang F and Zheng S: Tetramethylpyrazine attenuates sinusoidal
angiogenesis via inhibition of hedgehog signaling in liver
fibrosis. IUBMB life. 69:115–127. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhou Y, Ji Z, Yan W, Zhou Z, Li H and Xiao
Y: Tetramethylpyrazine inhibits prostate cancer progression by
downregulation of forkhead box M1. Oncol Rep. 38:837–842.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang G, Zhang T, Li N, Wu L, Gu J, Li C,
Zhao C, Liu W, Shan L, Yu P, et al: Tetramethylpyrazine nitrone
activates the BDNF/Akt/CREB pathway to promote post-ischaemic
neuroregeneration and recovery of neurological functions in rats.
Br J Pharmacol. 175:517–531. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zheng B, Lai R, Li J and Zuo Z: Critical
role of P2X7 receptors in the neuroinflammation and cognitive
dysfunction after surgery. Brain Behav Immun. 61:365–374.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Miao HH, Zhang Y, Ding GN, Hong FX, Dong P
and Tian M: Ginsenoside Rb1 attenuates isoflurane/surgery-induced
cognitive dysfunction via inhibiting neuroinflammation and
oxidative stress. Biomed Environ Sci. 30:363–372. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen L, Liu T, Wang Q and Liu J:
Anti-inflammatory effect of combined tetramethylpyrazine,
resveratrol and curcumin in vivo. BMC Complement Altern Med.
17(233)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sorrenti V, Giusti P and Zusso M: A model
of systemic inflammation to study neuroinflammation. Methods Mol
Biol. 1727:361–372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu J, Li M, Wang Y and Luo J: Curcumin
sensitizes prostate cancer cells to radiation partly via epigenetic
activation of miR-143 and miR-143 mediated autophagy inhibition. J
Drug Target. 25:645–652. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fan L, Zhang H, Li X, Yang G, Ru J and Liu
T: Emodin protects hyperglycemia-induced injury in PC-12cells by
up-regulation of miR-9. Mol Cell Endocrinol. 474:194–200.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fan Y and Wu Y: Tetramethylpyrazine
alleviates neural apoptosis in injured spinal cord via the
downregulation of miR-214-3p. Biomed Pharmacother. 94:827–833.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang X, Dong H, Liu Y, Han J, Tang S and
Si J: Tetramethylpyrazine partially relieves hypoxia-caused damage
of cardiomyocytes H9c2 by downregulation of miR-449a. J Cell
Physiol: Feb 15, 2019 (Epub ahead of print). doi:
10.1002/jcp.28151.
|
|
41
|
Dolati S, Aghebati-Maleki L, Ahmadi M,
Marofi F, Babaloo Z, Ayramloo H, Jafarisavari Z, Oskouei H, Afkham
A, Younesi V, et al: Nanocurcumin restores aberrant miRNA
expression profile in multiple sclerosis, randomized, double-blind,
placebo-controlled trial. J Cell Physiol. 233:5222–5230.
2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen X, Li Z, Xu D and Li S: LINC01121
induced intervertebral disc degeneration via modulating
miR-150-5p/MMP16 axis. J Gene Med: May 21, 2020 (Epub ahead of
print). doi: 10.1002/jgm.3231.
|
|
43
|
Zhang T, Shi Z, Wang Y, Wang L, Zhang B,
Chen G, Wan Q and Chen L: Akt3 deletion in mice impairs spatial
cognition and hippocampal CA1 long long-term potentiation through
downregulation of mTOR. Acta Physiol (Oxf).
225(e13167)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Howell KR, Floyd K and Law AJ:
PKBgamma/AKT3 loss-of-function causes learning and memory deficits
and deregulation of AKT/mTORC2 signaling: Relevance for
schizophrenia. PLoS One. 12(e0175993)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
DuBois JC, Ray AK, Gruber RC, Zhang Y,
Aflakpui R, Macian-Juan F and Shafit-Zagardo B: Akt3-mediated
protection against inflammatory demyelinating disease. Front
Immunol. 10(1738)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tang G, Yang H, Chen J, Shi M, Ge L, Ge X
and Zhu G: Metformin ameliorates sepsis-induced brain injury by
inhibiting apoptosis, oxidative stress and neuroinflammation via
the PI3K/Akt signaling pathway. Oncotarget. 8:97977–97989.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yang Q, Huang DD, Li DG, Chen B, Zhang LM,
Yuan CL and Huang HH: Tetramethylpyrazine exerts a protective
effect against injury from acute myocardial ischemia by regulating
the PI3K/Akt/GSK-3beta signaling pathway. Cell Mol Biol Lett.
24(17)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Fan H, Liu X, Zheng WW, Zhuang ZH and Wang
CD: MiR-150 alleviates EMT and cell invasion of colorectal cancer
through targeting Gli1. Eur Rev Med Pharmacol Sci. 21:4853–4859.
2017.PubMed/NCBI
|
|
49
|
Li Y, Su J, Li F, Chen X and Zhang G:
MiR-150 regulates human keratinocyte proliferation in hypoxic
conditions through targeting HIF-1alpha and VEGFA: Implications for
psoriasis treatment. PLoS One. 12(e0175459)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Peng L, Yang C, Yin J, Ge M, Wang S, Zhang
G, Zhang Q, Xu F, Dai Z, Xie L, et al: TGF-β2 induces Gli1 in a
Smad3-dependent manner against cerebral ischemia/reperfusion injury
after isoflurane post-conditioning in rats. Front Neurosci.
13(636)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cao Y, Li Z, Li H, Ni C, Li L, Yang N, Shi
C, Zhong Y, Cui D and Guo X: Hypoxia-inducible factor-1alpha is
involved in isoflurane-induced blood-brain barrier disruption in
aged rats model of POCD. Behav Brain Res. 339:39–46.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mehmood K, Zhang H, Li K, Wang L, Rehman
MU, Nabi F, Iqbal MK, Luo H, Shahzad M and Li J: Effect of
tetramethylpyrazine on tibial dyschondroplasia incidence, tibial
angiogenesis, performance and characteristics via HIF-1alpha/VEGF
signaling pathway in chickens. Sci Rep. 8(2495)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yang C, Xu Y, Zhou H, Yang L, Yu S, Gao Y,
Huang Y, Lu L and Liang X: Tetramethylpyrazine protects
CoCl2-induced apoptosis in human umbilical vein endothelial cells
by regulating the PHD2/HIF/1α-VEGF pathway. Mol Med Rep.
13:1287–1296. 2016.PubMed/NCBI View Article : Google Scholar
|