Introduction
Hydrocephalus is the most common pediatric
neurosurgical diagnosis, and the majority of patients require
implantation of a cerebrospinal fluid diversion device, a shunt.
Prevention of shunt infection is pivotal, since this could cause
shunt malfunction, scarring and ventricular loculation with
potentially devastating consequences for the patient (1-6).
Furthermore, shunt infection is considered one of the most costly
implant-related infections, with a cost of over 50,000 USD per
infection in the United States (7).
The majority of shunt infections result from
colonisation of the device by non-pathogenic gram-positive cocci,
such as Staphylococcus epidermidis and S. aureus, and
are acquired during surgery (1,8,9). One approach to reducing shunt-related
CSF infection is to use antibiotic-impregnated shunt (AIS) shunt
catheters instead of a standard shunt (SS). AIS have been available
for more than a decade, and contain 0.054% rifampin and 0.15%
clindamycin, shown to effectively prevent colonization (10-12).
Several studies have been published to evaluate the
effectiveness of AIS in reducing shunt infection in pediatric
population compared to SS. The most recent meta-analysis by Klimo
et al on this subject was published in 2014(13). However, since then numerous new
trials have been published comparing AIS with SS in pediatric
patients.
A primary objective in the present study is to
update the meta-analysis with systematic search of new studies
comparing the use of AISs and SSs for the prevention of shunt
infections in the pediatric population.
Materials and methods
Study design and search strategy
A search, systematic review and meta-analysis of
eligible studies was conducted in the major databases (PubMed,
EMBASE, Medline, Cochrane Library, TRIP Database, CINAHL and Google
Scholar), using the MeSH or free text terms for all eligible
articles published up to 31st October 2019. The search was
restricted to studies performed on human pediatric subjects. No
restriction on language or publication period was set. The
reference lists from all the included studies were also searched
for any additional eligible articles.
This systematic literature review was performed
following the guidelines of the PRISMA statement (Preferred
Reporting Items for Systematic Reviews and Meta-analyses) and
Cochrane Handbook for Systematic Reviews of Intervention while
using the following MeSH subject headings: (‘cerebrospinal fluid
shunts’ OR (‘cerebrospinal fluid’ AND (shunt* OR catheter*) OR
‘shunt system’) AND (‘antibiotic-impregnated’ OR (antibiotic AND
impregnated) OR Ventriculo-Peritoneal System AND infection.
Selection criteria
All studies comparing AISs and SSs in pediatric
patients (<18 years) were included. Duplicates, case reports,
case series were excluded.
Risk of bias in individual
studies
Missing data were requested from the original
authors through electronic correspondence or mail. We examined the
methodological quality of each study included in our meta-analysis
by data on random assignment, treatment allocation concealment,
group similarity at baseline, eligibility criteria specified,
blinding, lost to follow up percentage and use of intention to
treat analysis by using quality assessment scale. Two authors
independently assessed the quality of included studies.
Inconsistency over the quality scores was resolved by discussion
among all the authors until subsequent agreement was reached.
Missing data were requested from the original authors through
electronic correspondence or mail. Publication bias was assessed by
using the Begg's funnel plot analysis.
Data collection and analysis
The essential information was extracted carefully
and independently from each included study by two authors: the
first author's name, year of publication, age range of
participants, number of participants (Antibiotic Impregnated
Shunt/Conventional Shunt group), number of procedures, number of
infections and Quality Score. Any disagreement was resolved by
discussion.
Statistical analysis
Pooled risk ratio (RR) with 95% confidence intervals
(CIs) and publication bias were calculated using Review Manager
Statistical Software [RevMan, version 5.3; Nordic Cochrane Centre
(Cochrane Collaboration), Copenhagen, Denmark; 2014]. We
investigated the presence of heterogeneity of the included studies
by using the Cochran's Q statistic and I2 metric tests.
If the I2 was <50% then a fixed-effects model was
used, otherwise a random-effects model was used to combine the
results.
Results
Identification of relevant
studies
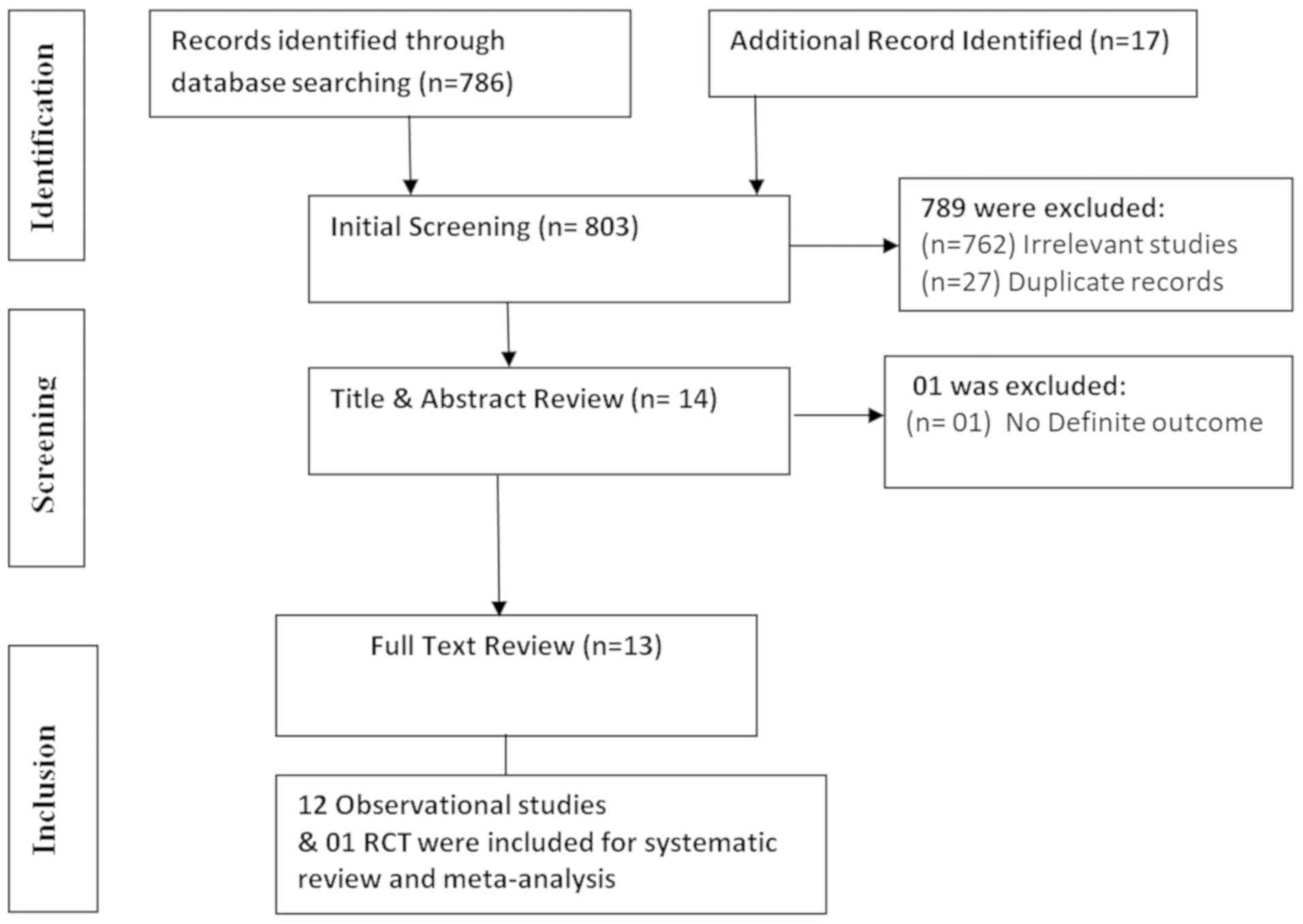
A total of 803 published articles were retrieved
from the systematic search during the initial screening (Fig. 1). Out of this number, 789 studies
were excluded because of irrelevancy (n=762), and duplicate records
(n=27). One additional study was excluded after title and abstract
review due to lack of definite outcome (n=1). Thus, a total of 12
observational studies and one randomized controlled trial (RCT)
were included for systematic review and meta-analysis.
Characteristics of eligible
studies
Table I summarizes
the characteristics of the selected studies, including number of
participants, age group, number of surgical procedures, number of
CSF infections, and study conclusions. There were a total of 2,860
pediatric patients in the AIS stunt group, and 5,092 pediatric
patients in the SS group, with a number of patients in each study
ranging from 11 to 1,963. Two of the studies, by Mallucci et
al (14) and Eymann et al
(18) included, not only pediatric,
but also adult patients, and separate data are reported for these
two groups (13,14). Twelve of the 13 included studies were
single-institution, while one study, by Hayhurst et al was a
retrospective multicentric cohort study (15). Studies were published between January
2005 and October 2019.
 | Table IPrimary study characteristics of the
published studies included in the meta-analysis. |
Table I
Primary study characteristics of the
published studies included in the meta-analysis.
| | Number of
infections | |
|---|
| Study No. | Authors, year
(Ref) | Study design | Country | Study period | No. of participants,
AIS/non-AIS | Age group | No. of procedures
(AIS/non-AIS) | Authors'
conclusion | AIS group | Non-AIS group | Quality score Tooth
et al (35) |
|---|
| 1 | Aryan et al,
2005(16) | Retrospective cohort
study | USA | 2002-2003 | 32/46 | Children (6 months to
17 years) | 78 (32/46) | Lower incidence shunt
infection noted with AIS | 01/32 | 07/46 | 18 |
| 2 | Kan et al,
2007(17) | Retrospective cohort
study | USA | 2003-2004 | 80/80 | Children (0 to 8
years) | 160 (80/80) | Infection rate less
in AIS group but not statistically significant | 04/80 | 07/80 | 13 |
| 3 | Eymann et
al, 2008(18) | Retrospective
cohort study | Germany | 1998-2006 | 26/22 | Adults (18-86
years); children (2 days to 12 years) | Total 317
(197/120); adults 269 (171/98); children 48 (26/22) | AIS reduce
infection rates and cost | 01/26 | 03/22 | 15 |
| 4 | Hayhurst et
al, 2008(15) | Retrospective
multicentric cohort study | UK Ireland | 2002-2006 | 214/77 | Children 1 to 16
years | 291 (214/77) | AIS can reduce
number of shunt infections in certain subgroups, particularly
neonates | 21/214 | 08/77 | 19 |
| 5 | Eymann et
al, 2009(19) | Retrospective
cohort study | Germany | 2002-2007 | 34/22 | Children (1 day to
8 years) | 56 (34/22) | AIS appear to
reduce the number of primary shunt infections in children | 01/34 | 03/22 | 12 |
| 6 | Parker et
al, 2009(20) | Retrospective
cohort study | USA | 1997-2007 | 502/570 | Children (1 day to
20 years) | 1,072
(502/570) | AIS reduced
infections in the high risk population | 16/502 | 64/570 | 11 |
| 7 | Raffa et al,
2015(21) | Retrospective
cohort study | Italy | 2002-2012 | 22/26 | Children 0.1 to 1
years | 48 (22/26) | AICs are effective
in reducing VP shunt infection in highrisk pediatric patients
younger than 1 year old | 1/22 | 5/26 | 13 |
| 8 | van Lindert et
al, 2018(22) | Retrospective
cohort study | Netherland | 2010-2011 | 499/263 | Children >17
years | 762 (499/263) | Adding
intraoperative vancomycin to a shunt infection prevention protocol
significantly reduces CSF shunt infection rate | 15/499 | 18/263 | 15 |
| 9 | James et al,
2014(24) | Retrospective
cohort study | UK | 1993-2009 | 500/1592 | Children 0.1 to 1
years | 2,092
(500/1,592) | Use of AIS tubing
significantly improves shunt infection rates in both general
pediatric and infant populations | 4/500 | 69/1592 | 16 |
| 10 | Jaeger et
al, 2017(23) | Retrospective
cohort study | Australia | 2002-2015 | 24/23 | Children <28
days | 47 (22/23) | AIS catheters and a
48-h perioperative antibiotic regimen may be beneficial in neonatal
hydrocephalus | 1/24 | 2/23 | 12 |
| 11 | Kandasamy et
al, 2011(25) | Retrospective
cohort study | UK | 1997-2007 | 581/1963 | Children 1 to 16
years | 2,544
(581/1,963) | AIS may
significantly reduce infection rates in de novo and clean
revision shunt implants | 30/581 | 155/1963 | 16 |
| 12 | Sciubba et
al, 2005(26) | Retrospective
cohort study | Maryland | 2001-2004 | 145/208 | Children 1 to 16
years | 353 (145/208) | AIS catheter
significantly reduced incidence of CSF shunt infection in children
with hydrocephalus during the early postoperative period (<6
months) | 2/145 | 2/208 | 17 |
| 13 | Mallucci et
al, 2019(14) | Randomized control
trial | UK | 2013-2017 | 538/536 | 1 to 91 years | 401 (201/200) | Provides evidence
to support the adoption of antibiotic shunts in UK patients | 3/201 | 4/200 | 18 |
Meta-analysis: shunt-related CSF
infections
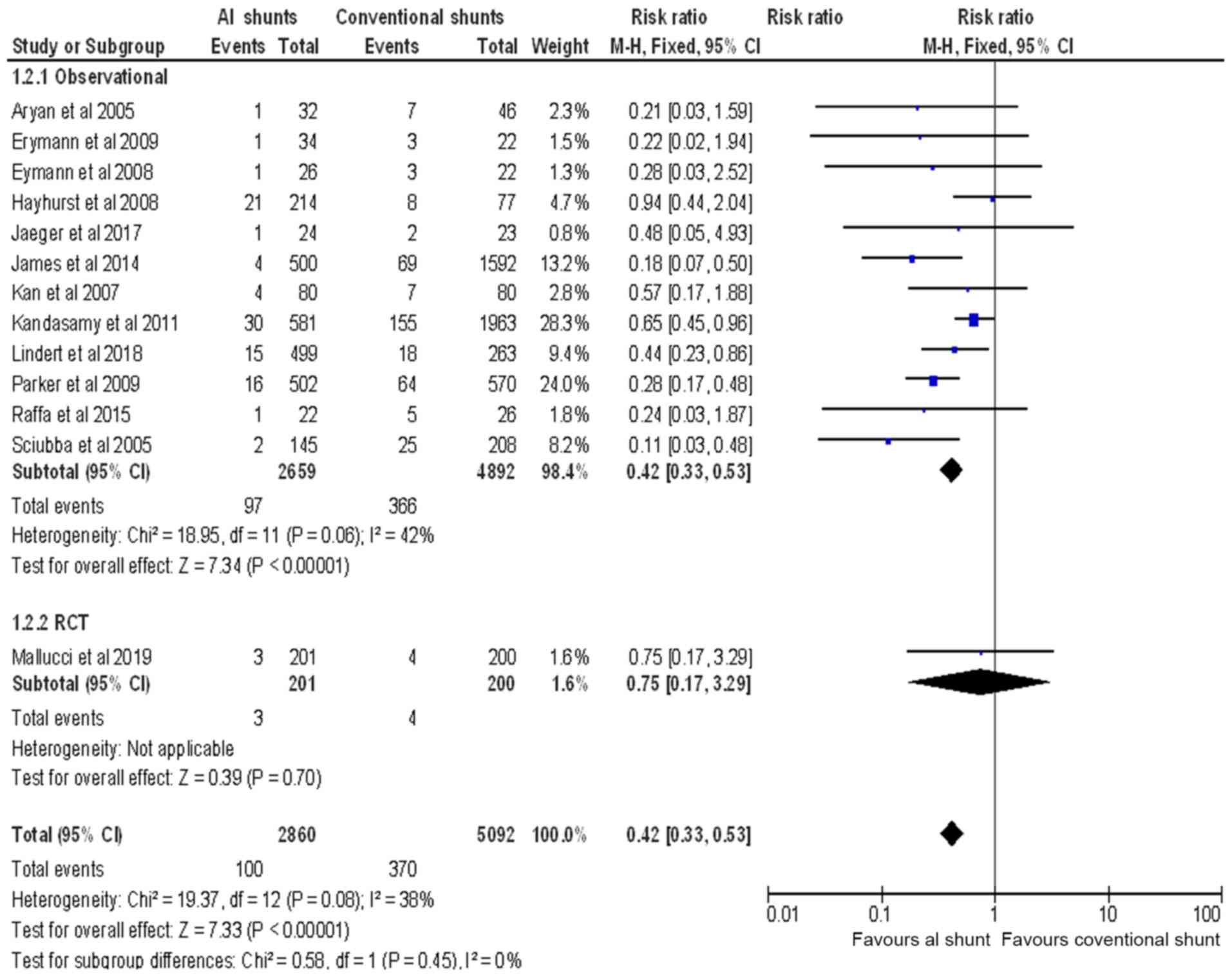
There was a total of 463 CSF infections reported in
the selected observational studies (16-26).
Of these, 366 were associated with the use of SS, yielding an
infection rate of 7.48%. In the pediatric patients receiving AIS,
97 CSF infections were reported, with an infection rate of 3.65%.
Four infection events associated with the use of SS, and three
events, associated with the use of AIS were reported in the
selected RCT (14), yielding an
infection rate of 2- and 1.4%, respectively (Fig. 2). The overall pooled RR of CSF
infection using a fixed effects model was 0.42 (95% CI: 0.33 to
0.53; P<0.00001) with no statistical heterogeneity across the
included studies (I2=42%) (Fig. 2).
These results indicated a significant decrease in
CSF infection rates in the AIS group. All 13 included studies
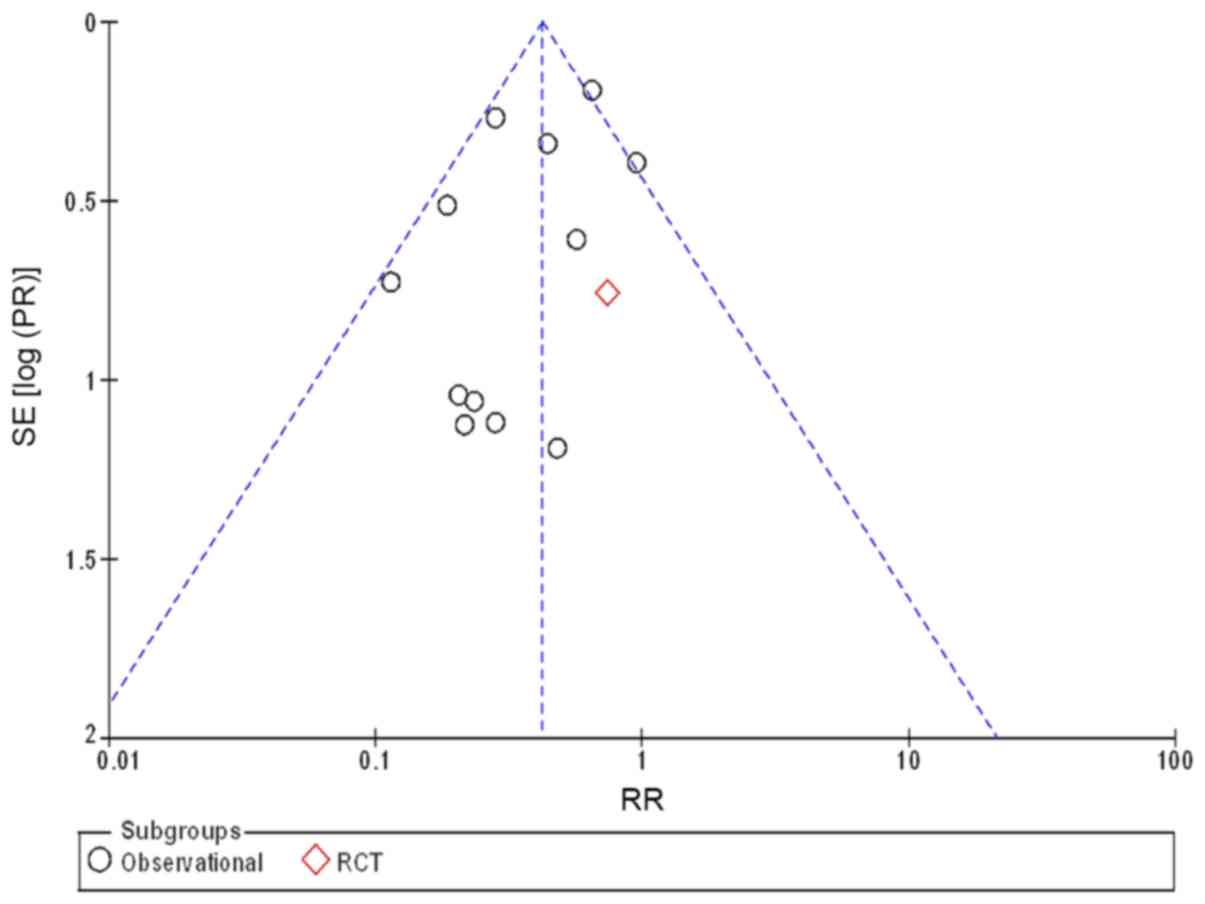
provide sufficient details of an adequate method of allocation
concealment and blinding. Begg's funnel plots were used to assess
the potential selection and performance publication bias. As shown
in Fig. 3, the shape of the funnel
plot suggests the absence of publication bias.
Discussion
In this meta-analysis, we found that the use of AIS
in pediatric patients was associated with a decreased risk of CSF
infection. A SS, consisting of silastic ventricular and peritoneal
catheters, is an effective treatment for hydrocephalus (27). However, due to CSF shunt
complications, such as shunt failure and infection, SS placement
often requires revision surgery, With infection rates approximately
3-fold higher after a first revision and their frequency increasing
with each additional procedure (28). AISs have been introduced to prevent
CSF infections, especially in the early postoperative period
(10-12).
Of the13 studies included in the analysis, 8 reported statistically
significant reduction in CSF infections associated with the use of
AIS (18-22,24-26).
In one of the earliest studies comparing AIS and SS
groups of pediatric patients, Sciubba et al (26) reported that AIS was associated with
significant reduction in CSF infection rates in children with
hydrocephalus during the early postoperative period (<6 months).
This was subsequently confirmed by Klimo et al (13), albeit AIS in the study of Sciubba
et al (26) was independently
associated with a 2.4-fold decreased likelihood of shunt infection.
James et al compared an AIS study group with historical
cohort of SS procedures, and identified a significant reduction in
CSF infection rates in pediatric patients (24). At the same time, they reported an
increased rate of CSF infections in revision surgery in the AIS
group, thus questioning the efficiency of AIS for long-term use
(24).
The study of Eymann et al (18) included both adult and pediatric
patients, and showed that the infection rates were 2.18-fold higher
with SS than with AIS. Similarly, in a subsequent study by the same
group (19), which included 56
pediatric patients, risk score for shunt infections was
significantly higher in the AIS study group than in the SS control
group. In a large multicenter study Kandasamy et al reported
that AIS significantly reduced CSF infection rates in de
novo and clean revision shunt implants, despite an apparent
overlap of confidence intervals in the results from various sites
(25). In a retrospective cohort
study, van Lindert et al used vancomycin antibiotic solution
to drench and flush the shunt and fill the shunt reservoir instead
of using AIS, and reported a significant decrease in infection
rates (an absolute risk reduction of 3.8%, and relative risk
reduction of 56%) in antibiotic-treated shunt group of pediatric
patients, as well as an increase in time from surgery to infection
(22).
In their retrospective study, Parker et al
examined CSF infections in several groups of patients with
high-risk factors [prematurity, post-meningitis, conversion of
external ventricular drains (EVD), prolonged hospital stay]. In all
high-risk groups AIS was associated with significantly reduced
rates of shunt infections (20).
A study by Raffa et al reported for the first
time the effectiveness of AIS in reducing VP shunt infection in
high-risk pediatric patients <1-year-old. Additionally, AIS had
a protective effect against shunt infections in all the specific
high-risk subgroups, such as preterm newborns, children with
post-hemorrhagic or post-infective hydrocephalus, and children with
a previous EVD (21).
Five of the 13 studies included in this
meta-analysis presented negative statistical findings. While Aryan
et al did not identify a statistically significant
difference in infection rates between the AIS and SS groups, they
report protective benefits of AIS compared to SS (16). Similarly, in a study by Kan and
Kestle, there was no difference in the shunt infection rate between
groups, even when adjusted for age, medical history, diagnosis, and
previous infections (17). In a
retrospective cohort study of neonates (<28 days), Jaeger et
al reported a statistically significantly delayed median time
interval between insertion and revision in the AIS group, while an
overall rate of CSF infection was unaffected (23).
In the only included RCT by Mallucci et al,
AIS was associated with a 3-fold decrease in shunt revisions.
Although that study included both pediatric and adult patients, it
strongly supported the use of AIS in patients undergoing their
first shunt insertion (14).
The limitations of the current meta-analysis arise
among others from heterogeneity of the included studies. While most
of the studies included only pediatric patients, others included
both children and adults (14,18), and
a definition of a shunt infection may vary from study to study.
There is also a variety of confounders for shunt infections, such
as age and prematurity (29-31),
gender (28), former shunt and
systemic infections (32,33), previous revisions, extended hospital
stay, positive CSF cultures prior to implantation, and the
preoperative occurrence of CSF leakage or the use of an EVD
(10). While prospective, blinded,
RCT may provide more balanced information, such studies would not
be cost-effective, and would require very large patient numbers,
multicenter cooperation, and development of standard protocols
(34).
Our analysis shows that AISs can substantially
reduce the risk of shunt infections. AIS, therefore, may be
beneficial for all pediatric patients with hydrocephalus who
require a shunt.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQ designed the paper. YQ and YW were involved in
literature search and data interpretation. YQ was responsible for
the data analysis and prepared the manuscript. YW edited the
manuscript. Both authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choux M, Genitori L, Lang D and Lena G:
Shunt implantation: Reducing the incidence of shunt infection. J
Neurosurg. 77:875–880. 1992.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jamjoom AB, Mohammed AA, al-Boukai A,
Jamjoom ZA, Rahman N and Jamjoom HT: Multiloculated hydrocephalus
related to cerebrospinal fluid shunt infection. Acta Neurochir
(Wien). 138:714–719. 1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vanaclocha V, Sáiz-Sapena N and Leiva J:
Shunt malfunction in relation to shunt infection. Acta Neurochir
(Wien). 138:829–834. 1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Walters BC, Hoffman HJ, Hendrick EB and
Humphreys RP: Cerebrospinal fluid shunt infection. Influences on
initial management and subsequent outcome. J Neurosurg.
60:1014–1021. 1984.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chadduck W and Adametz J: Incidence of
seizures in patients with myelomeningocele: A multifactorial
analysis. Surg Neurol. 30:281–285. 1988.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liao F, Li G, Yuan W, Chen Y, Zuo Y,
Rashid K, Zhang JH, Feng H and Liu F: LSKL peptide alleviates
subarachnoid fibrosis and hydrocephalus by inhibiting TSP1-mediated
TGF-β1 signaling activity following subarachnoid hemorrhage in
rats. Exp Ther Med. 12:2537–2543. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Darouiche RO: Treatment of infections
associated with surgical implants. N Engl J Med. 350:1422–1429.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kestle JRW, Hoffman HJ, Soloniuk D,
Humphreys RP, Drake JM and Hendrick EB: A concerted effort to
prevent shunt infection. Childs Nerv Syst. 9:163–165.
1993.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kestle JRW, Riva-Cambrin J, Wellons JC
III, Kulkarni AV, Whitehead WE, Walker ML, Oakes WJ, Drake JM,
Luerssen TG, Simon TD, et al: Hydrocephalus Clinical Research
Network: A standardized protocol to reduce cerebrospinal fluid
shunt infection: The Hydrocephalus Clinical Research Network
Quality Improvement Initiative. J Neurosurg Pediatr. 8:22–29.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pattavilakom A, Kotasnas D, Korman TM,
Xenos C and Danks A: Duration of in vivo antimicrobial activity of
antibiotic-impregnated cerebrospinal fluid catheters. Neurosurgery.
58:930–935; discussion 930-935. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bayston R: Duration of in vivo
antimicrobial activity of antibiotic-impregnated cerebrospinal
fluid catheters. Neurosurgery. 60(E208): author reply E208.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bayston R, Ashraf W and Bhundia C: Mode of
action of an antimicrobial biomaterial for use in hydrocephalus
shunts. J Antimicrob Chemother. 53:778–782. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Klimo P Jr, Van Poppel M, Thompson CJ,
Baird LC, Duhaime AC and Flannery AM: Pediatric Hydrocephalus
Systematic Review and Evidence-Based Guidelines Task Force.
Pediatric hydrocephalus: systematic literature review and
evidence-based guidelines. Part 6: Preoperative antibiotics for
shunt surgery in children with hydrocephalus: a systematic review
and meta-analysis. J Neurosurg Pediatr. 14 (Suppl 1):44–52.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mallucci CL, Jenkinson MD, Conroy EJ,
Hartley JC, Brown M, Dalton J, Kearns T, Moitt T, Griffiths MJ,
Culeddu G, et al: BASICS Study collaborators: Antibiotic or silver
versus standard ventriculoperitoneal shunts (BASICS): A
multicentre, single-blinded, randomised trial and economic
evaluation. Lancet. 394:1530–1539. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hayhurst C, Cooke R, Williams D, Kandasamy
J, O'Brien DF and Mallucci CL: The impact of antibiotic-impregnated
catheters on shunt infection in children and neonates. Childs Nerv
Syst. 24:557–562. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aryan HE, Meltzer HS, Park MS, Bennett RL,
Jandial R and Levy ML: Initial experience with
antibiotic-impregnated silicone catheters for shunting of
cerebrospinal fluid in children. Childs Nerv Syst. 21:56–61.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kan P and Kestle J: Lack of efficacy of
antibiotic-impregnated shunt systems in preventing shunt infections
in children. Childs Nerv Syst. 23:773–777. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eymann R, Chehab S, Strowitzki M, Steudel
W-I and Kiefer M: Clinical and economic consequences of
antibiotic-impregnated cerebrospinal fluid shunt catheters. J
Neurosurg Pediatr. 1:444–450. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eymann R, Steudel W-I and Kiefer M:
Infection rate with application of an antibiotic-impregnated
catheter for shunt implantation in children - a retrospective
analysis. Klin Padiatr. 221:69–73. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Parker SL, Attenello FJ, Sciubba DM,
Garces-Ambrossi GL, Ahn E, Weingart J, Carson B and Jallo GI:
Comparison of shunt infection incidence in high-risk subgroups
receiving antibiotic-impregnated versus standard shunts. Childs
Nerv Syst. 25:77–83; discussion 85. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Raffa G, Marseglia L, Gitto E and Germanò
A: Antibiotic-impregnated catheters reduce ventriculoperitoneal
shunt infection rate in high-risk newborns and infants. Childs Nerv
Syst. 31:1129–1138. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
van Lindert EJ, Bilsen MV, Flier MV,
Kolwijck E, Delye H and Oever JT: Topical vancomycin reduces the
cerebrospinal fluid shunt infection rate: A retrospective cohort
study. PLoS One. 13(e0190249)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jaeger W, Lee S, Vineet D, Keil A, Agarwal
N and Rao S: Ventriculoperitoneal shunts in neonates: A
retrospective study of outcomes with antibiotic-impregnated
catheters and a modified peri-operative antibiotic protocol. Br J
Neurosurg. 31:672–676. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
James G, Hartley JC, Morgan RD and Ternier
J: Effect of introduction of antibiotic-impregnated shunt catheters
on cerebrospinal fluid shunt infection in children: A large
single-center retrospective study. J Neurosurg Pediatr. 13:101–106.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kandasamy J, Dwan K, Hartley JC, Jenkinson
MD, Hayhurst C, Gatscher S, Thompson D, Crimmins D and Mallucci C:
Antibiotic-impregnated ventriculoperitoneal shunts - a multi-centre
British paediatric neurosurgery group (BPNG) study using historical
controls. Childs Nerv Syst. 27:575–581. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sciubba DM, Kretzer RM and Wang PP: Acute
intracranial subdural hematoma following a lumbar CSF leak caused
by spine surgery. Spine. 30:E730–E732. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bondurant CP and Jimenez DF: Epidemiology
of cerebrospinal fluid shunting. Pediatr Neurosurg. 23:254–258;
discussion 259. 1995.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Simon TD, Whitlock KB, Riva-Cambrin J,
Kestle JR, Rosenfeld M, Dean JM, Holubkov R, Langley M and Hamblett
NM: Revision surgeries are associated with significant increased
risk of subsequent cerebrospinal fluid shunt infection. Pediatr
Infect Dis J. 31:551–556. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fulkerson DH, Vachhrajani S, Bohnstedt BN,
Patel NB, Patel AJ, Fox BD, Jea A and Boaz JC: Analysis of the risk
of shunt failure or infection related to cerebrospinal fluid cell
count, protein level, and glucose levels in low-birth-weight
premature infants with posthemorrhagic hydrocephalus. J Neurosurg
Pediatr. 7:147–151. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pople IK, Bayston R and Hayward RD:
Infection of cerebrospinal fluid shunts in infants: A study of
etiological factors. J Neurosurg. 77:29–36. 1992.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Enger PØ, Svendsen F and Wester K: CSF
shunt infections in children: Experiences from a population-based
study. Acta Neurochir (Wien). 145:243–248; discussion 248.
2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ritz R, Roser F, Morgalla M, Dietz K,
Tatagiba M and Will BE: Do antibiotic-impregnated shunts in
hydrocephalus therapy reduce the risk of infection? An
observational study in 258 patients. BMC Infect Dis.
7(38)2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Peng J, Deng X, He F, Omran A, Zhang C,
Yin F and Liu J: Role of ventriculoperitoneal shunt surgery in
grade IV tubercular meningitis with hydrocephalus. Childs Nerv
Syst. 28:209–215. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Richards HK, Seeley HM and Pickard JD:
Efficacy of antibiotic-impregnated shunt catheters in reducing
shunt infection: Data from the United Kingdom Shunt Registry. J
Neurosurg Pediatr. 4:389–393. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tooth L, Ware R, Bain C, Purdie DM and
Dobson A: Quality of reporting of observational longitudinal
research. Am J Epidemiol. 161:280–288. 2005.PubMed/NCBI View Article : Google Scholar
|