Introduction
Traumatic hemorrhagic shock (THS) has a high
mortality rate of up to 40% (1,2). The
pathophysiological process of THS is complex, involving systemic
inflammatory responses and pathological changes, such as
microcirculatory disorders, hypovolemia, hypoxemia and oxidative
stress (3). The most common
complications of THS include systemic inflammatory response
syndrome, sepsis, multiple organ dysfunction syndrome and acute
lung injury (ALI), which are the primary causes of high mortality
(4,5). ALI is characterized by pulmonary edema
and micropulmonary nodules, caused by diffuse alveolar capillary
injury and resulting in acute, progressive hypoxic respiratory
failure (6). Currently, the
characteristics of ALI are believed to be the result of extensive
destruction of pulmonary vascular endothelial cells and alveolar
epithelial cells due to excessive inflammation in the body
(7). Patients with ALI exhibit
significant morbidity and mortality, poor treatment outcomes and
the disease represents a risk factor for clinically critical
illness at all ages (8). A key
factor in THS-induced injury is the inflammatory response (9,10).
Therefore, the majority of studies have focused on the regulation
of pro-inflammatory mediators (11,12),
and anti-inflammatory agents are a treatment for lung injury
induced by THS.
MicroRNAs (miRNAs), a class of small (~22
nucleotides), non-coding, single-stranded and highly-conserved
RNAs, negatively regulate the expression of target genes during
various cellular events, including proliferation, apoptosis and
differentiation, via binding the 3'UTR of target genes (13-16).
MiRNAs have been identified to be involved in various disease
types, including cancer, and cardiovascular, nervous system and
metabolic diseases, as well as numerous diseases caused by trauma
(16-21).
In addition, the role of miRNAs in lung injury has been extensively
studied (22). However, the role of
miRNAs in THS-induced acute lung injury is poorly studied and
further research is needed.
MiR-15a-5p has not previously been well
characterized. Wang et al (23) reported that miR-15a-5p inhibits
endometrial cancer cell growth via regulating the Wnt/β-catenin
signaling pathway. Chen et al (24) reported that miR-15a-5p regulates
cell survival and metastasis of chronic myeloid leukemia by
targeting CXCL10. Long et al (25) demonstrated that miR-15a-5p prevents
cell proliferation and division in human hepatocellular carcinoma
via targeting BDNF. Moreover, miR-15a-5p has been revealed as a
prognostic biomarker for recurrent colorectal adenocarcinoma
(26). In the present study,
bioinformatics software analysis revealed that TNFAIP3-interacting
protein 2 (TNIP2) was a potential target of miR-15a-5p. The TNIP2
gene encodes a protein identified as a suppressor of NF-κB
activation (27). TNIP2 has been
revealed to serve important roles in myocardial injury induced by
acute pancreatitis via regulating the inflammatory response
(28). It is generally believed
that TNIP2 serves an important regulatory role in the NF-κB
signaling pathway (28,29), and NF-κB has been reported to serve
notable roles in the development of lung injury (30,31).
These data indicate that miR-15a-5p/TNIP2 may serve critical roles
in acute lung injury.
Therefore, the purpose of the present study was to
investigate the expression of miR-15a-5p in THS induced acute lung
injury and the molecular mechanism underlying its role.
Materials and methods
Clinical samples
A total of 30 peripheral blood samples (5 ml per
individual) from 30 patients (median age, 38.2; age range, 24-59
years; 25 male; 5 female) with acute lung injury induced by
trauma-hemorrhagic shock (THS), as well as 30 peripheral blood
samples from 30 healthy volunteers (median age, 37.1; age range,
21-58 years old; 25 male; 5 female) were collected at Affiliated
Hospital of Jiangsu University (Zhenjiang, China) between June 2015
and June 2017. Blood samples were collected from patients 24 h
after traumatic hemorrhagic shock and stored at -80˚C. Informed
consent was signed for each patient participating in the study. The
present study was approved by the Ethics Committee of Affiliated
Hospital of Jiangsu University.
Cell culture
293T cells were purchased from American Type Culture
Collection. Cells were grown in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% streptomycin-penicillin
solution and incubated at 37˚C with 5% CO2.
Animals and THS model
establishment
A total of 50 male Sprague-Dawley rats (10-14 weeks
old; 360-400 g) were obtained from Vital River Company (Beijing,
China). Rats were housed at 25±5˚C, 50% humidity and 12 h
dark/light cycle conditions. All rats had free access to food and
water. The present experiments were conducted following the
Recommended Guideline for the Care and Use of Laboratory Animals
issued by Chinese Council on Animal Research. The current study was
approved by Animal Ethics Committee of the Affiliated Hospital of
Jiangsu University.
The rat model of THS was conducted according to a
previous study (32). Rats were
randomly assigned into five groups: Control (Sham; rats underwent
the same anesthetic and surgical procedures, but trauma/hemorrhage
was not induced), THS, THS + inhibitor control (intraperitoneal
injection), THS + miR-15a-5p inhibitor (intraperitoneal injection),
THS + miR-15a-5p inhibitor + TNIP2-siRNA (intraperitoneal
injection). Rats were intraperitoneally injected with inhibitor
control (80 mg/kg/day; 5'-CAGUACUUUUGUGUAGUACAA-3'; Shanghai
GenePharma Co., Ltd.), miR-15a-5p inhibitor (80 mg/kg/day;
5'-CACUGGUACAAGGGUUGGGAGA-3'; Shanghai GenePharma Co., Ltd.) or
miR-15a-5p inhibitor (80 mg/kg/day) + TNIP2-siRNA (80 mg/kg/day;
cat. no. sc-44638; Santa Cruz Biotechnology, Inc.) prior to surgery
using in vivo transfection reagent (EntransterTM-in
vivo; Engreen Biosystem Co., Ltd.). Rats in the Sham and THS
groups were given saline (0.9% NaCl) solution intraperitoneally.
All rats were anesthetized with 30 mg/kg pentobarbital and handled
24 h after TSH induction. After the specified treatments, following
experiments were conducted.
Evan's blue dye (EBD)
EBD was performed to evaluate the lung permeability
according to a previous study (33). In brief, 1 ml of 1% EBD solution was
injected into rats from different groups via the jugular vein.
Then, 1.5 ml blood sample was collected from the femoral artery
catheter. After 20 min, the rats were sacrificed and the lungs were
removed before being washed three times with 5 ml of physiological
saline to collect bronchoalveolar lavage fluid (BALF). The
supernatant was collected via centrifugation (1,000 x g for 10 min
at 4˚C). The concentration of EBD in plasma and BALF was detected
via measuring the absorbance value at 620 nm using a micro-plate
reader (Elx800; BioTek Instruments, Inc.). Finally, the ratio of
EBD in the BALF to EBD in plasma was calculated.
Lung permeability index
To calculate the lung permeability index, the ratio
of the BALF protein concentration to the serum protein
concentration was measured, according to a previous study (34). Briefly, 1 µl of sample supernatant
was mixed with 4 µl normal saline and 250 µl Coomassie brilliant
blue G250 at room temperature for 5 min. The absorbance at 595 nm
was measured using a micro-plate reader (BioTek Instruments, Inc.).
Calculation of protein concentration in BALF and plasma was based
on a standard curve of bovine serum albumin (BSA).
Lung edema evaluation
Lung edema was assessed by measuring lung wet/dry
weight ratio (W/D) of the rats. The rats were anesthetized with 3%
isoflurane. Then the lungs from rats in different groups were
removed and the wet weight (W) of rats were measured. After drying
for 72 h at 100˚C, the dry weight (D) of the lungs from rats in
different groups was also detected. Finally, the ratio of W/D was
calculated.
Measurement of proinflammatory
factors
Peripheral blood samples were centrifuged at 1,000 x
g for 10 min at 4˚C and the serum was collected. Bronchoalveolar
lavage fluid (BALF) was collected by intratracheal instillation of
lungs with sterile PBS three times, and then the BALF supernatant
was harvested via centrifugation at 800 x g for 10 min at 4˚C.
ELISA kits were then used to measure the levels of pro-inflammatory
factors including tumor necrosis factor (TNF)-α (cat. no. PT516;
Beyotime Institute of Biotechnology) and interleukin (IL)-6 (cat.
no. PI328; Beyotime Institute of Biotechnology), in serum or BALF,
according to the manufacturer's instructions of each kit.
Nitric oxide (NO) detection
The lung tissues from rats of different groups were
homogenized, freeze-thawed with liquid nitrogen three times and
then centrifuged (10,000 x g; 10 min; 4˚C) to collect supernatants.
Bicinchoninic Acid Protein Assay kit was used to detect protein
concentrations, according to the manufacturer's instructions. Then,
proteins were diluted to 2 µg/µl in PBS. Finally, total Nitric
Oxide Assay kit (cat. no. S0023; Beyotime Institute of
Biotechnology) was used to measure the concentration of NO,
following the manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from tissues or blood samples was isolated
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and reversely transcribed into cDNA with the PrimeScript™ RT
reagent kit (Takara Bio, Inc.) as per the manufacture's protocol.
SYBR® Premix Ex Taq™ II (Takara Bio Inc.) was used for
qPCR analysis. Amplification conditions for qPCR were as follows:
10 min at 95˚C, followed by 35 cycles of 15 sec at 95˚C and 40 sec
at 55˚C. U6 for miRNA (24) and
GAPDH for mRNA (28) were used as
the endogenous controls. The primer sequences were as follows:
miR-15a-5p forward, 5'-GG GTAGCAGCACATAATGGTTTGTG-3' and reverse,
5'-CAGTGCGTGTCGTGGAGT-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; GAPDH forward,
5'-CTTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'; TNIP2 forward,
5'-CTAAAGAGGCGGCAGGTCCCTC-3' and reverse,
5'-CAAGATGACCTTCCAGTGAC-3'; iNOS forward,
5'-TCTCCGACCACCACTACAGCAA-3' and reverse:
5'-GGGGAACTGGGCAGACTCAA-3'. Relative gene expression was quantified
using the 2-ΔΔCq method (35).
Western blot assay
Total proteins from tissues/blood samples were
extracted using RIPA lysis buffer (50 mM Tris (pH 7.4), 150 mM
NaCl, 1% NP-40, 0.5% sodium deoxycholate) supplemented with PMSF at
a final concentration of 1 mM. BCA protein assay kit was used to
detect the concentrations of proteins. Protein samples (25 µg per
lane) were separated using 12% SDS-PAGE, transferred onto PVDF
membranes (EMD Millipore), and blocked using 5% skimmed milk at
room temperature for 1 h. Then, the membranes were incubated with
primary antibodies: TNIP2, phosphorylated (p-)NF-κB (p-p65),
inducible nitric oxide synthase (iNOS) and β-actin (1:1,000; Cell
Signaling Technology, Inc.) at 4˚C overnight. Subsequently, the
membranes were incubated with the anti-rabbit IgG HRP-linked
antibody (cat. no. 7074; 1:5,000; Cell Signaling Technology, Inc.)
at room temperature for 2 h. Finally, ECL reagents (EMD Millipore)
were used to visualize the corresponding protein bands. The band
densities of p-p65 was analyzed using Gel-Pro-Analyzer software
(Version 6.3; Media Cybernetics, Inc.), and the relative protein
level of p-p65 was presented as fold of the control group.
Dual luciferase reporter assay
In the current study, TargetScan bioinformatics
software version 7.1 (www.targetscan.org/vert_71) was used to predict the
targets of miR-15a-5p, and it was revealed that TNIP2 was a
potential target of miR-15a-5p. To confirm the binding sites
between miR-15a-5p and TNIP2, the wild type (WT-TNIP2) and mutant
(MUT-TNIP2) 3'UTR of TNIP2 were cloned into a pmiR-RB-ReportTM dual
luciferase reporter gene plasmid vector (Guangzhou RiboBio Co.,
Ltd). Subsequently, 293T cells were cotransfected with WT-TNIP2 or
MUT-TNIP2 and miR-15a-5p mimic or mimic control using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), as per the manufacturer's protocols. After 48 h
of cell transfection, luciferase activity was determined using the
dualluciferase assay system (Promega Corporation) in line with the
manufacturer's protocol. Luciferase activity was normalized to
Renilla luciferase activity.
Statistical analysis
Data obtained from the current study was displayed
as the mean ± SD. SPSS 16.0 statistical software (SPSS, Inc.) was
used for all statistical analyses. Unpaired Student's t-tests and
one-way ANOVA followed by Tukey's post hoc test were performed to
analyze the differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
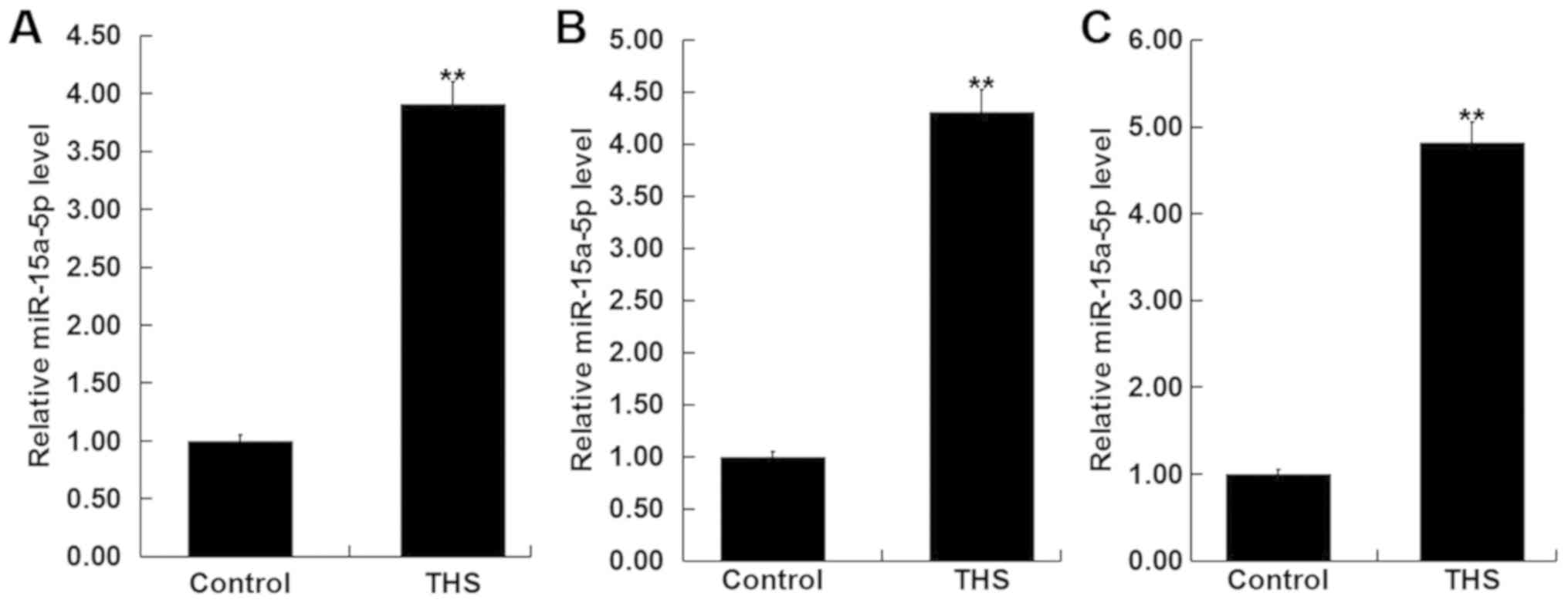
miR-15a-5p is significantly
upregulated in patients with THS and rats
To detect the level of miR-15a-5p in the blood of
patients with THS, RT-qPCR was performed. As revealed in Fig. 1A, compared with the healthy control,
the level of miR-15a-5p was significantly increased in the blood
samples of patients with THS. The findings indicated that
miR-15a-5p may influence the development of THS.
Subsequently, the levels of miR-15a-5p were detected
in the blood and the lung tissues of THS rats, and the results
showed that miR-15a-5p was significantly upregulated in the blood
and the lung tissues of THS rats (Fig.
1B and C).
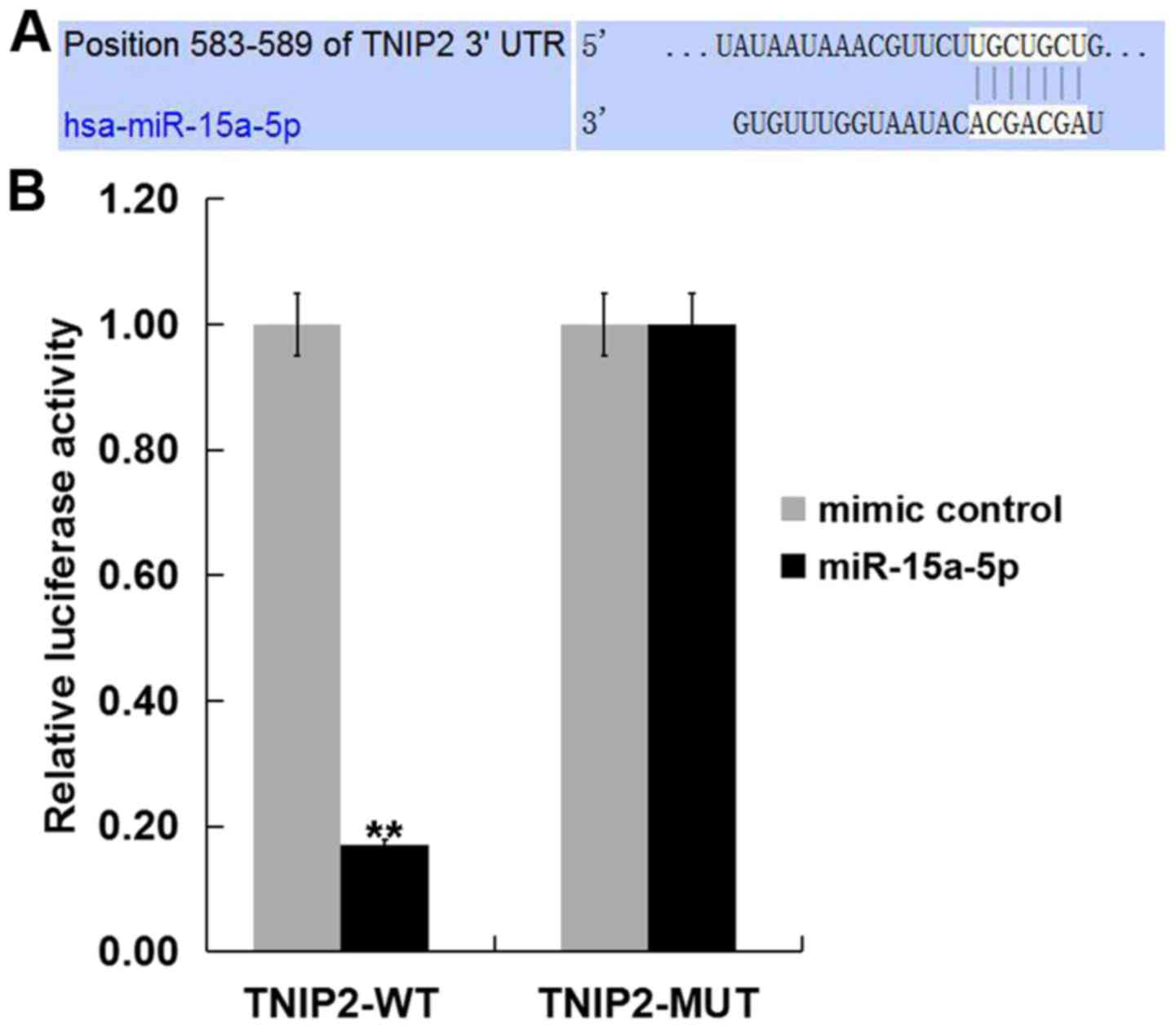
TNIP2 is a target of miR-15a-5p
To investigate the target genes of miR-15a-5p, we
firstly predicted the target gene of miR-15a-5p using TargetScan
(www.targetscan.org/vert_71). The
results indicated that miR-15a-5p has hundreds of potential target
genes, including TNIP2. The role of TNIP2 in THS-induced lung
injury is yet to be fully elucidated. Therefore, TNIP2 was selected
for further study. Subsequently, to reveal whether miR-15a-5p
directly binds TNIP2, a dual luciferase reporter assay was
performed. The miR-15a-5p-TNIP2-WT or miR-15a-5p-TNIP2-MUT reporter
plasmid were co-transfected into 293T cells with miR-15a-5p mimic
or mimic control and it was revealed that the luciferase activity
was significantly decreased in the 293T cells co-transfected with
miR-15a-5p mimic with miR-15a-5p-TNIP2-WT, but not with
miR-15a-5p-TNIP2-MUT (Fig. 2). The
data suggested that TNIP2 represents a target gene of
miR-15a-5p.
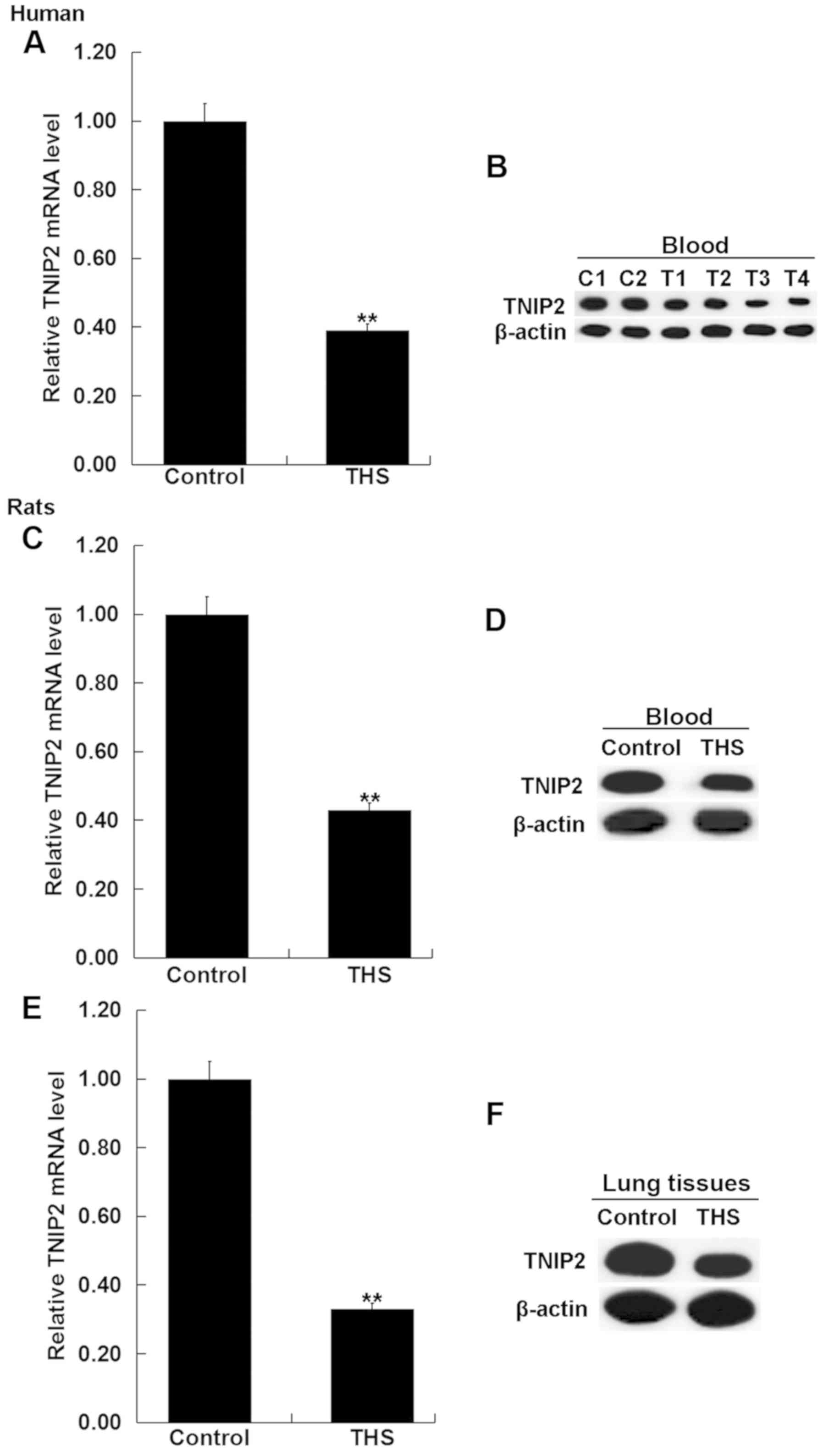
TNIP2 is significantly downregulated
in patients with THS and rats
To detect the expression of TNIP2 in the blood of
patients with THS and rats, RT-qPCR and western blot assay were
performed. As indicated in Fig. 3A
and B, compared with the healthy
control, the mRNA and protein levels of TNIP2 significantly
decreased in the blood samples of patients with THS. Then the mRNA
and protein levels of TNIP2 were detected in the blood and the
pulmonary tissues of rats with THS, and the results revealed that
TNIP2 was significantly downregulated in the blood and the
pulmonary tissues of THS rats (Fig.
3C-E).
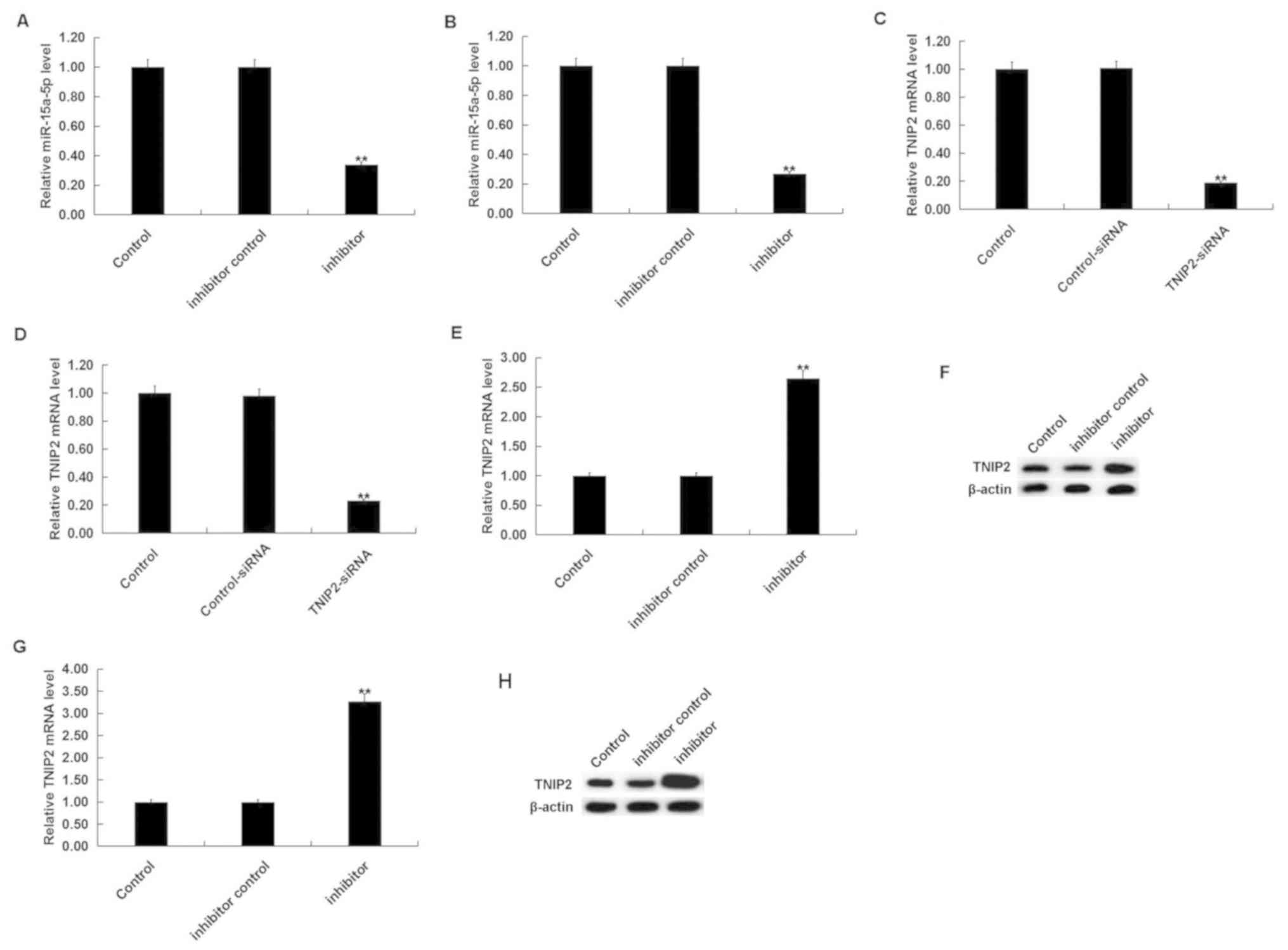
miR-15a-5p inhibitor alleviates lung
injury in THS rats
To investigate the effect of miR-15a-5p on THS rats,
rats were intraperitoneally injected with inhibitor control or
miR-15a-5p inhibitor, prior to THS induction. After 24 h, it was
revealed that the miR-15a-5p inhibitor significantly decreased the
level of miR-15a-5p in the blood and lung tissues of THS rats
(Fig. 4A and B). Moreover, TNIP2-siRNA significantly
decreased the mRNA level of TNIP2 in the blood and lung tissues of
THS rats (Fig. 4C and D). The miR-15a-5p inhibitor significantly
increased both the mRNA and protein levels of TNIP2 in the blood
and the lung tissues of THS rats (Fig.
4E-H).
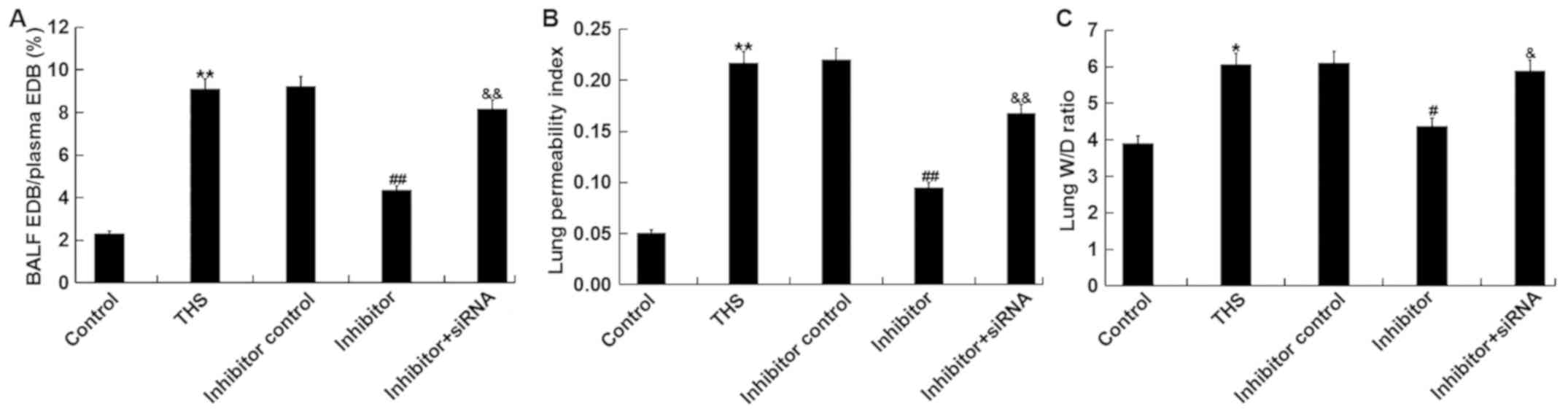
The effects of miR-15a-5p on THS-induced lung injury
were subsequently investigated. The ratio of EBD in the BALF to EBD
in plasma of THS rats, and the lung permeability index (ratio of
the BALF protein concentration to the serum protein concentration)
were measured to determine lung capillary permeability. The results
demonstrated that these two indicators were significantly increased
after THS but were decreased by miR-15a-5p inhibitor treatment.
However, the ratio of EBD in the BALF to EBD in plasma of THS rats
and the lung permeability index were significantly enhanced by
transfection with TNIP2-siRNA compared with the miR-15a-5p
inhibitor treatment group (Fig. 5A
and B). Moreover, the lung W/D
ratio was calculated to determine the lung edema in current study,
and it was revealed that the increased lung wet/dry ratio induced
by THS was decreased by miR-15a-5p inhibitor treatment, and this
reduction was eliminated by TNIP2-siRNA (Fig. 5C). Taken together, the data
indicated that miR-15a-5p inhibitor alleviated lung injury in THS
rats.
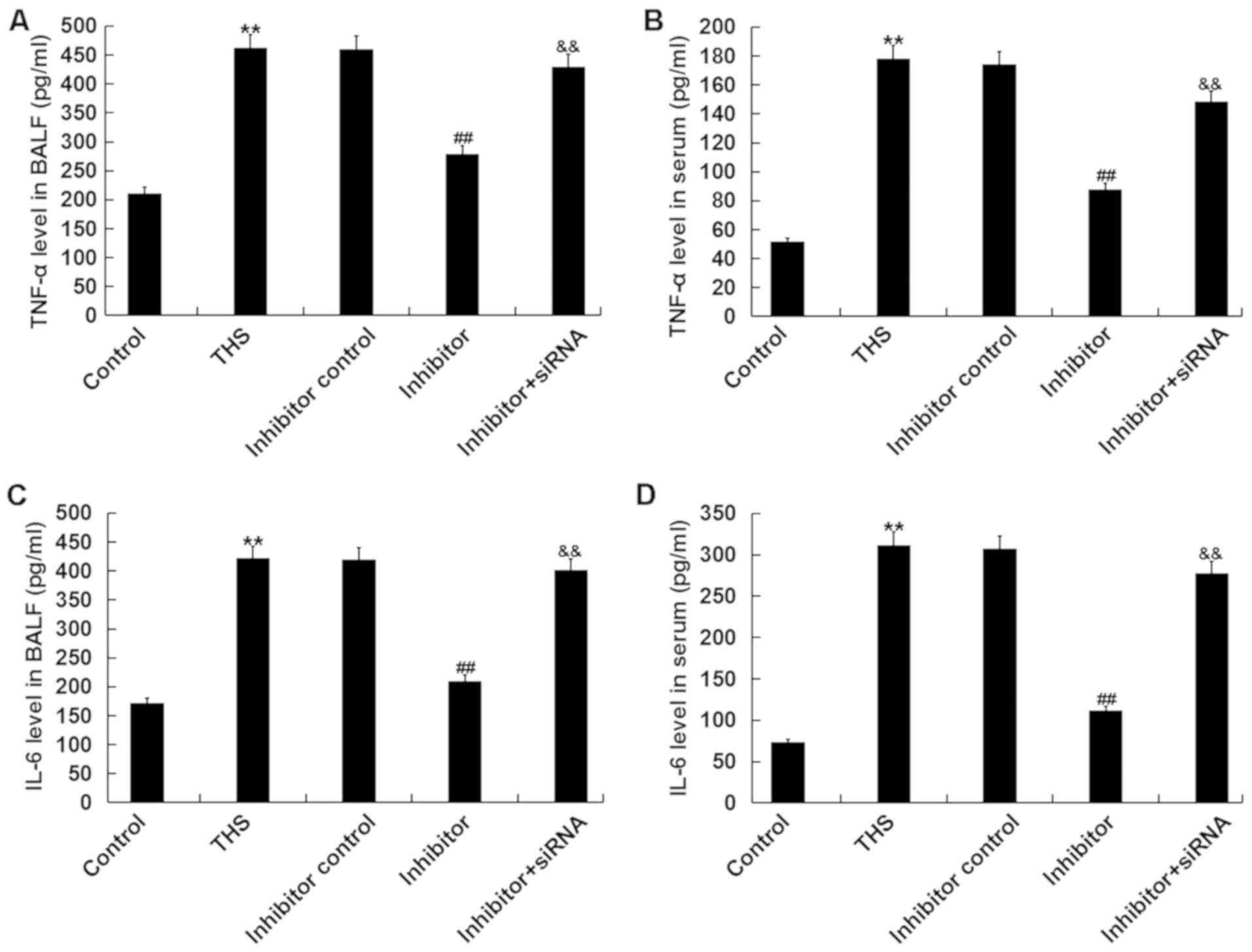
miR-15a-5p inhibitor prevents
inflammatory response in THS rats
It was determined whether the miR-15a-5p inhibitor
had anti-inflammatory effects. As depicted in Fig. 6, the increased levels of TNF-α and
IL-6 in BALF and serum of THS rats were significantly decreased
following miR-15a-5p inhibitor treatment, and these reductions were
eliminated by TNIP2-siRNA.
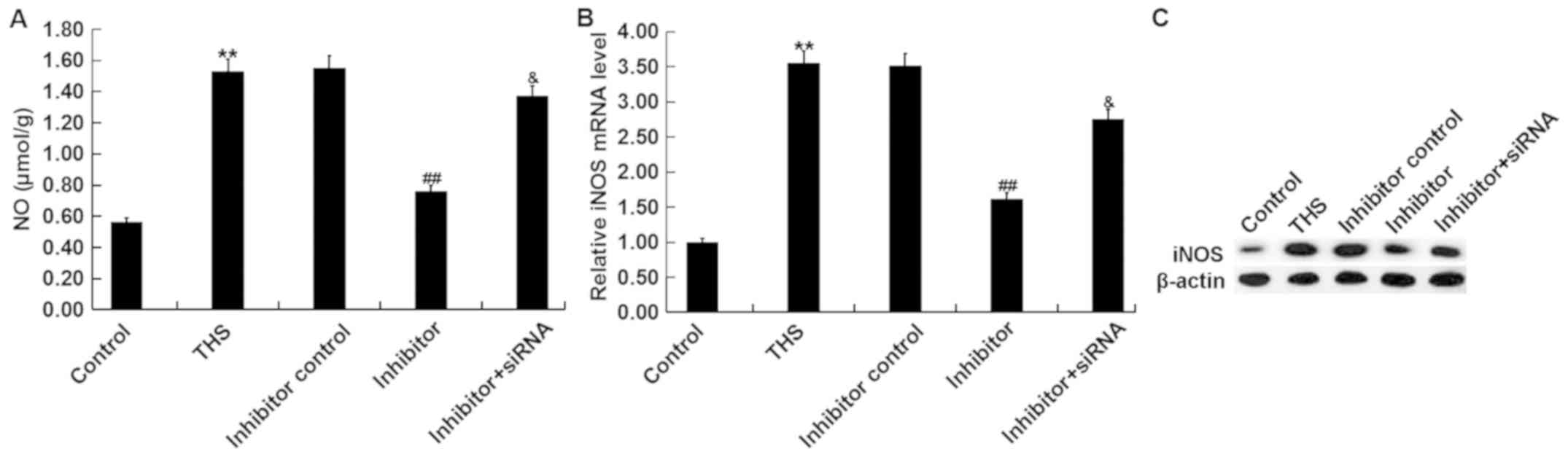
miR-15a-5p inhibitor decreases the
expression of NO and iNOS in THS rats
Overproduction of NO by iNOS influences the
pathogenesis of THS-induced lung injury (36). Thus, the present study detected the
concentration of NO in the lungs of THS rats and the mRNA and
protein levels of iNOS were determined. It was demonstrated that
the concentration of NO and protein and mRNA levels of iNOS in THS
rats significantly increased compared with the sham group. However,
the miR-15a-5p inhibitor notably decreased the expression of NO and
iNOS in the lung tissues of THS rats, and these decreases were
attenuated by TNIP2-siRNA (Fig.
7).
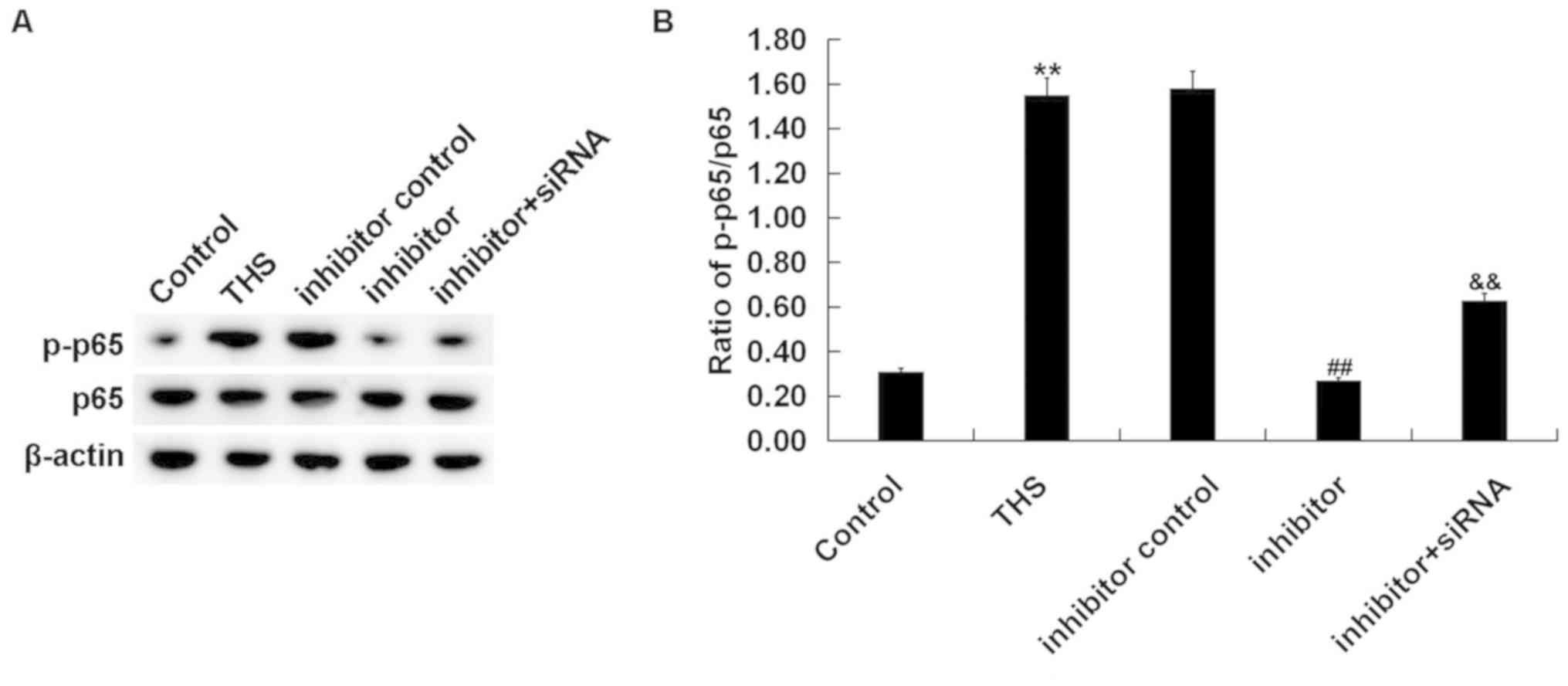
miR-15a-5p inhibitor decreases NF-κB
activation in THS rats
Finally, to investigate the mechanism of the effect
of miR-15a-5p inhibitor on THS rats, the NF-κB pathway was analyzed
by determining p-p65 protein expression and the ratio of p-p65/p65
expression. It was revealed that the enhanced p-p65 protein level
(Fig. 8A) and increased p-p65/p65
ratio (Fig. 8B) in lung tissues of
THS rats was decreased following treatment with the miR-15a-5p
inhibitor, and this reduction was alleviated by TNIP2-siRNA
(Fig. 8).
Discussion
The present study demonstrated that miR-15a-5p was
significantly upregulated in patients and rats with acute lung
injury induced by THS. TNIP2 was revealed as a target of
miR-15a-5p, and it was downregulated in patients and rats with THS.
Further analyses indicated that downregulation of miR-15a-5p
significantly relieved THS-induced acute lung injury and inhibited
the inflammatory response in a rat model of THS via targeting
TNIP2. Moreover, it was revealed that THS-induced NO production,
iNOS expression and NF-κB pathway activation in lung tissues were
all repressed following miR-15a-5p inhibition via targeting TNIP2,
indicating that these pathways may be a part of the mechanisms
responsible for the protective effects of miR-15a-5p downregulation
on lungs in THS rats.
THS has a very high mortality rate, and acute lung
injury is one of the major complications of THS (1,2,5). Since
the molecular mechanisms underlying acute lung injury after THS are
very complicated, there is still no effective method for treating
THS-induced acute lung injury. Previous studies on miRNAs have
provided new directions for the diagnosis and treatment of various
diseases (16-22).
However, there have been few studies on miRNAs in THS-induced acute
lung injury. The present study investigated the functional role of
miR-15a-5p in THS-induced acute lung injury and explored the
underlying molecular mechanism.
miR-15a-5p had not been well characterized but has
been studied in several cancer types, including endometrial cancer,
chronic myeloid leukemia, colorectal adenocarcinoma and
hepatocellular carcinoma (23-26).
The present study investigated whether miR-15a-5p was involved in
THS induced acute lung injury. Firstly, the level of miR-15a-5p was
determined in the blood of patients with THS and in the blood and
the lung tissues of THS rats, and the results revealed that
miR-15a-5p was highly expressed in both the blood of patients with
THS and the blood and lung tissues of THS rats, indicating the
involvement of miR-15a-5p in THS-induced acute lung injury. Then,
to explore the potential roles of miR-15a-5p in THS-induced acute
lung injury, the targets of miR-15a-5p were predicted, and it was
revealed that TNFAIP3-interacting protein 2 (TNIP2) was a direct
target of miR-15a-5p and it was downregulated in patients with THS
and rats.
The protein encoded by the TNIP2 gene inhibits the
activation of the NF-κB pathway (27). It is generally believed that TNIP2
serves an important regulatory role in the NF-κB signaling pathway
(28,29). NF-κB is an important transcription
factor involved in the regulation of survival, immune, oxidative
stress, and inflammatory reaction (37-39).
Therefore, it was hypothesized that miR-15a-5p may serve a role in
THS-induced acute lung injury by regulating the TNIP2/NF-κB
signaling pathway and thereby regulating the inflammatory response.
As expected, the findings of current study indicated that
downregulation of miR-15a-5p significantly relieved THS induced
lung injury, inhibited inflammatory response in lung tissues of THS
rats by targeting TNIP2. Besides, miR-15a-5p inhibitor
significantly reduced the protein level of p-p65 and the ratio of
p-p65/p65 in the lung tissue of THS rats, indicating the inhibition
of NF-κB signaling pathway, and this effect was reversed by
TNIP2-siRNA. Overproduction of NO by iNOS is involved in the
pathogenesis of THS-induced lung injury (37). The current study also revealed the
inhibitory effect of miR-15a-5p inhibitor on NO production and iNOS
expression in the lung tissues of THS rats. However, a group of
miR-15a-5p inhibitor + control-siRNA was not set up in our
experiments, and this may represent a limitation of the present
study.
Taken together, the current results demonstrated
that miR-15a-5p was upregulated in THS-induced acute lung injury,
and its inhibition served a protective role in via repressing the
inflammatory response by regulating the TNIP2/NF-κB signaling
pathway. miR-15a-5p may represent a potential diagnostic marker and
therapeutic target for the treatment of THS-induced acute lung
injury. However, the current study was a preliminary study of the
role of miR-15a-5p in the pathogenesis of acute lung injury induced
by THS, and this topic requires extensive research. It would be
beneficial to determine the correlation of miR-15a-5p expression
with the clinical characteristics and prognosis of patients with
THS in order to elucidate the role of miR-15a-5p in THS-induced
lung injury. Furthermore, there is a need to demonstrate the role
of TNIP2 in THS-induced lung injury in future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhenjiang social
development project (grant no. SH2017025).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ contributed to study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. ZZL, ZJM, FXW and CS contributed to data collection
and statistical analysis. HZC performed experiments, and
contributed to manuscript preparation and literature searching. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was signed for each patient
participating in the study. The present study was approved by the
Ethics Committee of Affiliated Hospital of Jiangsu University.
Patient consent for publication
All patients consented to publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kauvar DS, Lefering R and Wade CE: Impact
of hemorrhage on trauma outcome: An overview of epidemiology,
clinical presentations, and therapeutic considerations. J Trauma.
60 (Suppl 6):S3–S11. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kauvar DS and Wade CE: The epidemiology
and modern management of traumatic hemorrhage: US and international
perspectives. Crit Care. 9 (Suppl 5):S1–S9. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Angele MK, Schneider CP and Chaudry IH:
Bench-to-bedside review: Latest results in hemorrhagic shock. Crit
Care. 12(218)2008.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Cai B, Deitch EA and Ulloa L: Novel
insights for systemic inflammation in sepsis and hemorrhage.
Mediators Inflamm. 2010(642462)2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ananthakrishnan P, Cohen DB, Xu DZ, Lu Q,
Feketeova E and Deitch EA: Sex hormones modulate distant organ
injury in both a trauma/hemorrhagic shock model and a burn model.
Surgery. 137:56–65. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheifetz IM: Year in review 2015:
Pediatric ARDS. Respir Care. 61:980–985. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hoegl S, Brodsky KS, Blackburn MR,
Karmouty-Quintana H, Zwissler B and Eltzschig HK: Alveolar
epithelial A2B adenosine receptors in pulmonary protection during
acute lung injury. J Immunol. 195:1815–1824. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
De Luca D, Piastra M, Tosi F, Pulitanò S,
Mancino A, Genovese O, Pietrini D and Conti G: Pharmacological
therapies for pediatric and neonatal ALI/ARDS: An evidence-based
review. Curr Drug Targets. 13:906–916. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee CC, Chang IJ, Yen ZS, Hsu CY, Chen SY,
Su CP, Chiang WC, Chen SC and Chen WJ: Delayed fluid resuscitation
in hemorrhagic shock induces proinflammatory cytokine response. Ann
Emerg Med. 49:37–44. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Claridge JA, Schulman AM and Young JS:
Improved resuscitation minimizes respiratory dysfunction and blunts
interleukin6 and nuclear factor-kappa B activation after traumatic
hemorrhage. Crit Care Med. 30:1815–1819. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiang H, Huang Y, Xu H, Hu R and Li QF:
Inhibition of hypoxia inducible factor-1α ameliorates lung injury
induced by trauma and hemorrhagic shock in rats. Acta Pharmacol
Sin. 33:635–643. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koscsó B, Trepakov A, Csóka B, Németh ZH,
Pacher P, Eltzschig HK and Haskó G: Stimulation of A2B adenosine
receptors protects against trauma-hemorrhagic shock-induced lung
injury. Purinergic Signal. 9:427–432. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol.
15(429)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vickers KC, Rye KA and Tabet F: MicroRNAs
in the onset and development of cardiovascular disease. Clin Sci
(Lond). 126:183–194. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang J, Liu Y and Lu L: Emerging role of
microRNAs in peripheral nerve system. Life Sci. 207:227–233.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Karolina DS, Tavintharan S, Armugam A,
Sepramaniam S, Pek SLT, Wong MTK, Lim SC, Sum CF and Jeyaseelan K:
Circulating miRNA profiles in patients with metabolic syndrome. J
Clin Endocrinol Metab. 97:E2271–E2276. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang W, Tang S, Li H, Liu R, Su Y, Shen L,
Sun M and Ning B: MicroRNA-21a-5p promotes fibrosis in spinal
fibroblasts after mechanical trauma. Exp Cell Res. 370:24–30.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ferruelo A, Peñuelas Ó and Lorente JA:
MicroRNAs as biomarkers of acute lung injury. Ann Transl Med.
6(34)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang ZM, Wan XH, Sang GY, Zhao JD, Zhu QY
and Wang DM: miR-15a-5p suppresses endometrial cancer cell growth
via Wnt/β-catenin signaling pathway by inhibiting WNT3A. Eur Rev
Med Pharmacol Sci. 21:4810–4818. 2017.PubMed/NCBI
|
|
24
|
Chen D, Wu D, Shao K, Ye B, Huang J and
Gao Y: MiR-15a-5p negatively regulates cell survival and metastasis
by targeting CXCL10 in chronic myeloid leukemia. Am J Transl Res.
9:4308–4316. 2017.PubMed/NCBI
|
|
25
|
Long J, Jiang C, Liu B, Fang S and Kuang
M: MicroRNA-15a-5p suppresses cancer proliferation and division in
human hepatocellular carcinoma by targeting BDNF. Tumour Biol.
37:5821–5828. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kontos CK, Tsiakanikas P, Avgeris M,
Papadopoulos IN and Scorilas A: miR-15a-5p, a novel prognostic
biomarker, predicting recurrent colorectal adenocarcinoma. Mol
Diagn Ther. 21:453–464. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Verstrepen L, Carpentier I, Verhelst K and
Beyaert R: ABINs: A20 binding inhibitors of NF-kappa B and
apoptosis signaling. Biochem Pharmacol. 78:105–114. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xie H, Yang M, Zhang B, Liu M and Han S:
Protective role of TNIP2 in myocardial injury induced by acute
pancreatitis and its mechanism. Med Sci Monit. 23:5650–5656.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang W, Gao J and Wang F:
MiR-663a/MiR-423-5p are involved in the pathogenesis of lupus
nephritis via modulating the activation of NF-κB by targeting
TNIP2. Am J Transl Res. 9:3796–3803. 2017.PubMed/NCBI
|
|
30
|
Guo S, Jiang K, Wu H, Yang C, Yang Y, Yang
J, Zhao G and Deng G: Magnoflorine ameliorates
lipopolysaccharide-induced acute lung injury via suppressing NF-κB
and MAPK activation. Front Pharmacol. 9(982)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yu J, Ni L, Zhang X, Zhang J, Abdel-Razek
O and Wang G: Surfactant protein D dampens lung injury by
suppressing NLRP3 inflammasome activation and NF-κB signaling in
acute pancreatitis. Shock. 51:557–568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang G, Peng X, Hu Y, Lan D, Wu Y, Li T
and Liu L: 4-phenylbutyrate benefits traumatic hemorrhagic shock in
rats by attenuating oxidative stress, not by attenuating
endoplasmic reticulum stress. Crit Care Med. 44:e477–e491.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shi HP, Deitch EA, Da Xu Z, Lu Q and
Hauser CJ: Hypertonic saline improves intestinal mucosa barrier
function and lung injury after trauma-hemorrhagic shock. Shock.
17:496–501. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu WJ, Zhong ZJ, Cao LH, Li HT, Zhang TH
and Lin WQ: Paclitaxel-induced lung injury and its amelioration by
parecoxib sodium. Sci Rep. 5(12977)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Senthil M, Watkins A, Barlos D, Xu DZ, Lu
Q, Abungu B, Caputo F, Feinman R and Deitch EA: Intravenous
injection of trauma-hemorrhagic shock mesenteric lymph causes lung
injury that is dependent upon activation of the inducible nitric
oxide synthase pathway. Ann Surg. 246:822–830. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-κB transcription factors in the immune system.
Annu Rev Immunol. 27:693–733. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mariappan N, Elks CM, Sriramula S,
Guggilam A, Liu Z, Borkhsenious O and Francis J: NF-kappaB-induced
oxidative stress contributes to mitochondrial and cardiac
dysfunction in type II diabetes. Cardiovasc Res. 85:473–483.
2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pateras I, Giaginis C, Tsigris C,
Patsouris E and Theocharis S: NF-κB signaling at the crossroads of
inflammation and atherogenesis: Searching for new therapeutic
links. Expert Opin Ther Targets. 18:1089–1101. 2014.PubMed/NCBI View Article : Google Scholar
|