Introduction
Ankle fractures, especially in children, are one of
the most common injuries of the bone, with an incidence rate of 187
per 100,000 people (1,2). Delayed diagnosis or treatment of ankle
fractures can lead to deformities and disability (3). Therefore, timely diagnosis and
effective treatment of fractures are essential for relieving pain
and fracture healing. Bone formation is a balanced process between
osteoblast and osteoclast activity (4). Following fracture, the expression of
pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α
and interleukin (IL)-1β, are positively correlated with the number
of osteoclasts whilst a large number of osteoblasts are required
during fracture healing (5).
Therefore, the inflammatory response serves an important role in
the pathogenesis of ankle fractures whilst oxidative stress has
also been implicated in affecting fractures (6).
MicroRNAs (miRNAs or miRs) are a class of small
non-coding single stranded RNA that negatively regulates gene
expression by binding to the 3'untranslated region (UTR) of
multiple target mRNAs (7). miRNAs
are involved in various pathophysiological processes, such as the
immune response, inflammatory response and oxidative stress
(8-10).
During fracture, miRNA levels in bone tissue are significantly
altered, which may be associated with the fracture healing process
affecting osteoblasts and bone growth factors (11). miRNAs serve important roles in
fracture healing (11-13);
however, the underlying molecular mechanisms have not been fully
elucidated.
miR-146a is involved in the development and
progression of several diseases, including cancer, arthritis,
coronary heart disease and diseases of the nervous system (14-18).
Studies have also revealed the important roles of miR-146a in the
regulation of inflammation and oxidative stress (19,20).
To the best of our knowledge, the expression and functional role of
miR-146a in ankle fractures has yet to be fully determined.
The aim of the present study was to investigate the
expression and role of miR-146a in the development of ankle
fractures and to determine the associated underlying molecular
mechanism.
Materials and methods
Clinical samples
A total of 60 patients with ankle fracture (12-53
years old; sex ratio, 1:1) presenting at the Affiliated Drum Tower
Hospital of Nanjing University Medical School from June 2016 to
June 2017 were included in the current study. Peripheral blood
samples (2 ml per individual) were collected from each patient and
60 healthy volunteers (11-55 years old; sex ratio, 1:1) during the
same time period. Each patient provided informed consent and the
present study was approved by the Ethics Committee of the
Affiliated Drum Tower Hospital of Nanjing University Medical
School.
Cell culture and treatment
Human osteoblastic osteosarcoma cell line MG-63 was
obtained from the American Type Culture Collection (cat. no.
CRL-1427). Cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin solution
(Gibco; Thermo Fisher Scientific, Inc.), then incubated at 37˚C
with 5% CO2. The cells were passaged every 2-3 days.
MG-63 cells were then treated with bradykinin (BK, 1 µg/ml;
Sigma-Aldrich, Merck KGaA) for 24 h to establish an in vitro
model of ankle fracture
Cell transfection
MG-63 cells were seeded into 6-well plates
(1x106 cells per well) and cultured at 37˚C for 24 h.
Then 50 nM miR-146a mimic (sense 5'-UGAGAACUGAAUUCCAUGGGUU-3' and
antisense 5'-CCCAUGGAAUUCAGUUCUCAUU-3'; Shanghai GenePharma Co.,
Ltd.), 50 nM mimic control (sense 5'-UUCUCCGAACGUGUCACGUTT-3' and
antisense 5'-ACGUGACACGUUCGGAGAATT-3'; Shanghai GenePharma Co.,
Ltd.), 50 nM miR-146a inhibitor (5'-UGAGAACUGAAUUCCAUGGGUU-3';
Shanghai GenePharma Co., Ltd.) or 50 nM inhibitor control
(5'-CAGUACUUUUGUGUAGUACAA-3'; Shanghai GenePharma Co., Ltd.) was
transfected into MG-63 cells using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Transfection efficiency was measured after
24 h. MG-63 cells were pre-transfected with miR-146a mimic, mimic
control, miR-146a inhibitor or inhibitor control for 2 h and then
treated with 1 µg/ml BK at 37˚C for 24 h, as previously described
(21). Subsequently, the cells were
subjected to following experiments.
Cell viability assay
Cell viability was determined using an MTT assay.
Following transfections and BK treatment, 20 µl MTT solution (5
mg/ml) was added to each well and cells were further incubated for
4 h at 37˚C. To assess cell viability, absorbance at 490 nm was
measured using a microplate reader (Bio-Rad Laboratories,
Inc.).
ELISA
TNF-α, IL-1β and IL-6 levels were measured using
ELISA. Peripheral blood was collected from patients with or without
ankle fracture, after which the serum was isolated via
centrifugation at 3,000 x g at 4˚C for 15 min. Levels of TNF-α
(cat. no. PT518; Beyotime Institute of Biotechnology), IL-1β (cat.
no. PI305; Beyotime Institute of Biotechnology) and IL-6 (cat. no.
PI330; Beyotime Institute of Biotechnology) in serum were detected
using ELISA kits according to the manufacturer's protocol.
Measurement of oxidative
stress-associated indicators
Peripheral blood from patients with or without ankle
fracture was collected and the serum was isolated by centrifugation
at 3,000 x g at 4˚C for 15 min. For in vitro experiments,
MG-63 cells (5x104 cells per well) were plated into
six-well plates and transfected with miR-146a mimic, mimic control,
miR-146a inhibitor or inhibitor control for 2 h, after which cells
were treated with 1 µg/ml BK at 37˚C for 24 h. Subsequently, MG-63
cells were harvested and the supernatants collected via
centrifugation at 1,600 x g for 10 min at 4˚C. malondialdehyde
(MDA; cat. no. S0131M; Beyotime Institute of Biotechnology) levels,
and superoxide dismutase (SOD; cat. no. S0086; Beyotime Institute
of Biotechnology) and catalase (CAT; cat. no. S0082; Beyotime
Institute of Biotechnology) enzymatic activities were determined in
the blood of patients with or without ankle fracture. MG-63 cells
were detected using the appropriate kits as per the manufacturer's
protocol. The enzyme activity of cells was presented as units/mg of
protein as previously described in the literature (22).
Western blot analysis
Protein levels of TNF receptor associated factor 6
(TRAF6), phosphorylated (p)-NF-κB (p-p65), p-65, TNF-α, IL-1β, IL-6
or β-actin in MG63 cells were determined via western blot analysis.
Total protein from cells was extracted using lysis buffer (Cell
Signaling Technology, Inc.). A bicinchoninic acid protein assay
(Thermo Fisher Scientific, Inc.) was used to determine the
concentration of protein samples. An equal quantity of protein (30
µg per lane) were then separated by SDS-PAGE on a 12% gel,
transferred onto polyvinylidene difluoride membranes then blocked
with 5% non-fat milk at room temperature for 2 h. Subsequently, the
membranes were incubated with primary antibodies against TRAF6
(cat. no. 8028), p-NFκB p65 (cat. no. 3033), p65 (cat. no. 8242),
TNF-α (cat. no. 3707), IL-1β (cat. no. 12703), IL-6 (cat. no.
12153) or β-actin (cat. no. 4970; all 1:1,000; all Cell Signaling
Technology, Inc.) overnight at 4˚C. Membranes were then incubated
with an anti-rabbit immunoglobulin G, horseradish peroxidase-linked
antibody (1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.)
at room temperature for 2 h. Finally, protein bands were visualized
using the enhanced chemiluminescence detection system (Super Signal
West Dura Extended Duration Substrate; Pierce Chemical; Thermo
Fisher Scientific, Inc.) and quantified with Image J software
(version 1.48u; National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® regent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract RNA from blood samples and
cells. PrimeScript reverse transcription reagent kit (Takara
Biotechnology Co., Ltd.) was used to synthesize cDNAs as per the
manufacturer's protocol. The temperature protocol for this step was
as follows: 70˚C for 5 min, 37˚C for 5 min and 42˚C for 1 h. qPCR
was subsequently performed using a TaqMan Universal PCR Master Mix
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The following primer sequences were used:
miR-146a forward, 5'-GCGAGGTCAAGTCACTAGTGGT-3' and reverse,
5'-CGAGAAGCTTGCATCACCAGAGAACG-3'; TRAF6 forward,
5'-GCAGTGAAAGATGACAGCGTGA-3' and reverse,
5'-TCCCGTAAAGCCATCAAGCA-3'; TNF-α forward,
5'-GAACTGGCAGAAGAGGCACT-3' and reverse, 5'-GGTCTGGGCCATAGAACTGA-3';
IL-1β forward, 5'-TGTGAAATGCCACCTTTTGA-3' and reverse,
5'-TGAGTGATACTGCCTGCCTG-3'; IL-6 forward,
5'-CCGGAGAGGAGACTTCACAG-3' and reverse, 5'-CAGAATTGCCATTGCACA-3';
GAPDH forward, 5'-GGCATTGCTCTCAATGACAA-3' and reverse,
5'-TGTGAGGGAGATGCTCAGTG-3' and U6 forward, 5'-CTCGCTTCGGCAGCACA-3'
and reverse, 5'-AACGCTTCACGAATTTGCGT-3'. U6 or GAPDH was used as
internal reference genes. mRNA relative expression was quantified
using the 2-ΔΔCq method (23).
Statistical analysis
All data were analyzed using SPSS version 18.0
(SPSS, Inc.) and data were presented as the mean ± standard
deviation. Differences between two groups were determined using a
Student's t test and differences between multiple groups were
determined using one-way ANOVA followed by Student-Neuman-Keuls
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-146a is decreased in patients with
ankle fracture
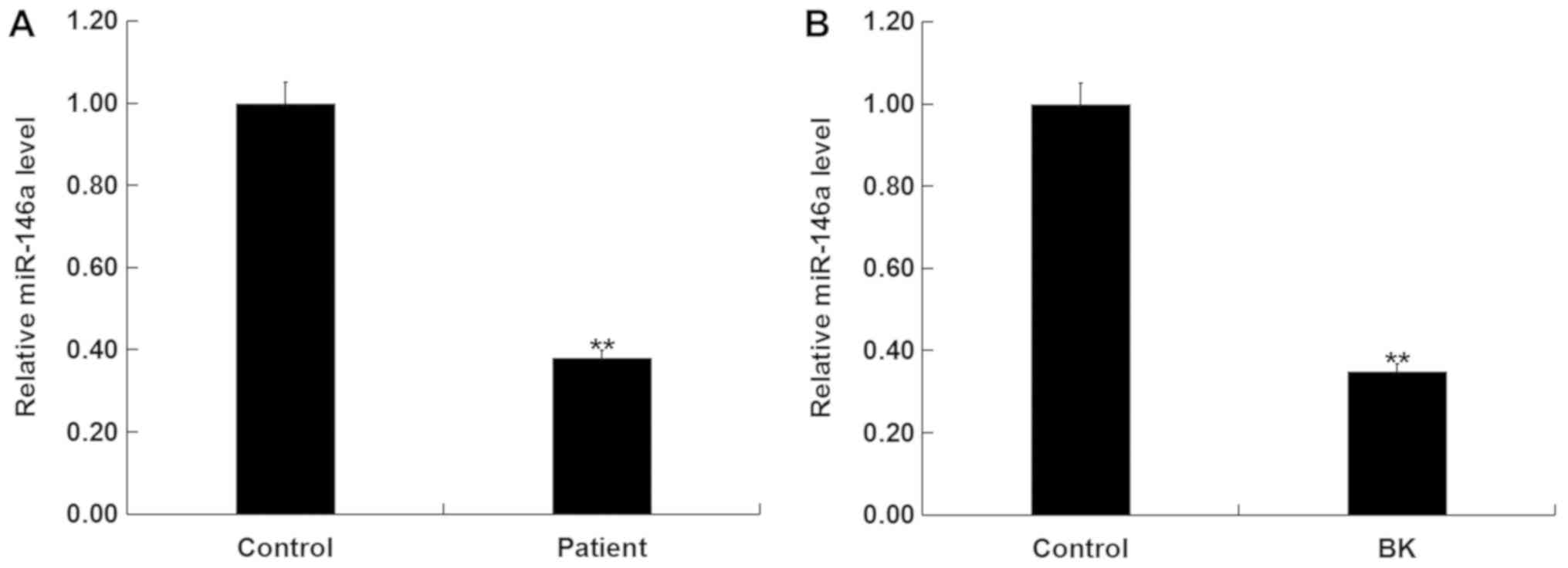
Levels of miR-146a in the blood of patients with or
without ankle fracture were determined via RT-qPCR. Compared with
the healthy control, levels of miR-146a were significantly
decreased in patients with ankle fracture (Fig. 1A). Furthermore, MG-63 cells
stimulated with BK to establish an in vitro model of ankle
fracture demonstrated significantly decreased miR-146a levels
(Fig. 1B).
Inflammatory response and oxidative
stress increases in patients with ankle fracture
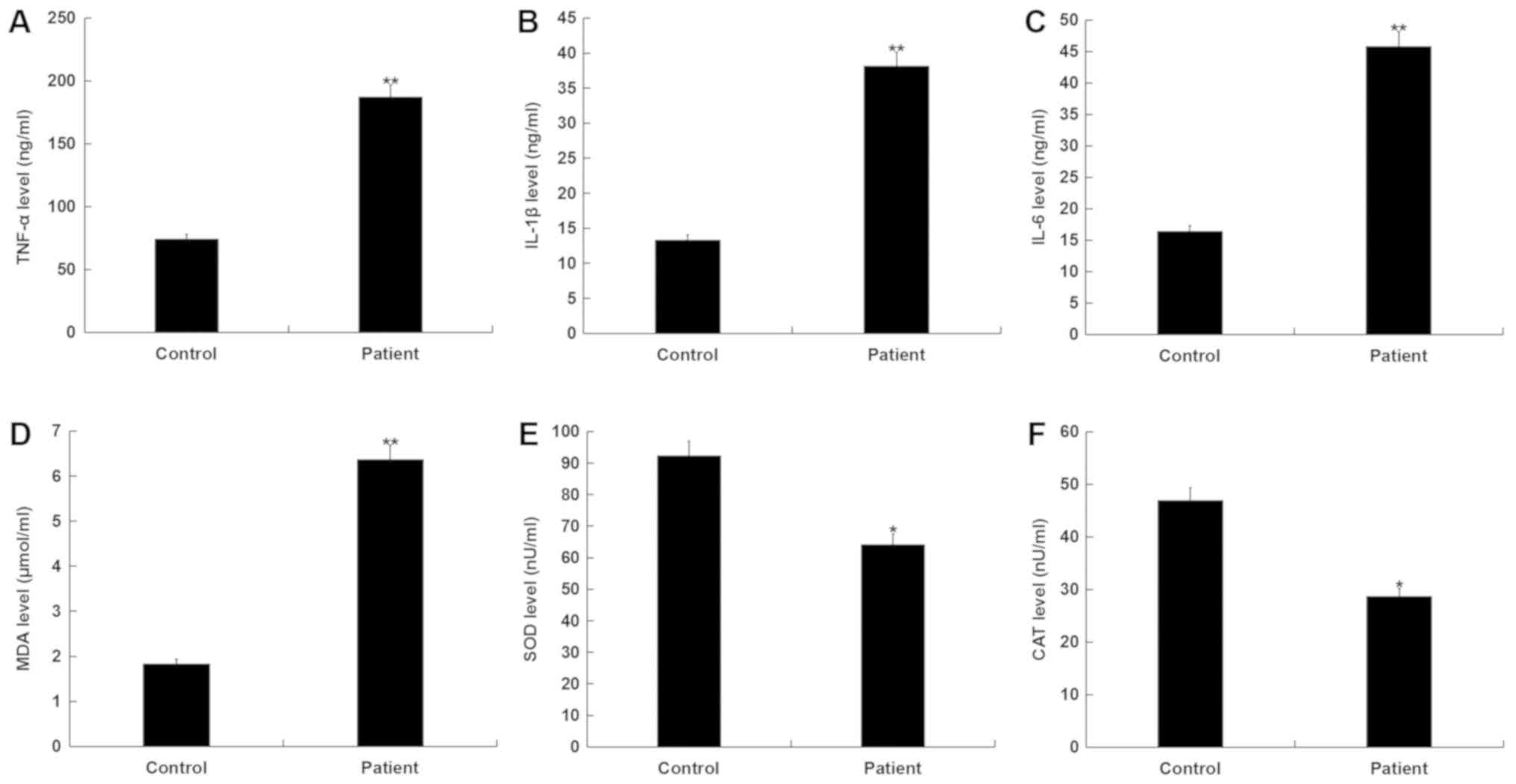
ELISA was used to determine the levels of
pro-inflammatory factors (TNF-α, IL-1β and IL-6) in the blood of
patients with ankle fracture. The results determined that, compared
with the healthy controls, TNF-α, IL-1β and IL-6 levels
significantly increased in patients with ankle fracture (Fig. 2A-C).
In addition, MDA levels significantly increased,
whilst SOD and CAT activities significantly decreased in patients
with ankle fracture compared with the healthy controls (Fig. 2D-F). These results indicated that
there was an enhanced inflammatory response and increased oxidative
stress in patients with ankle fracture.
miR-146a increases cell viability
following BK treatment in vitro
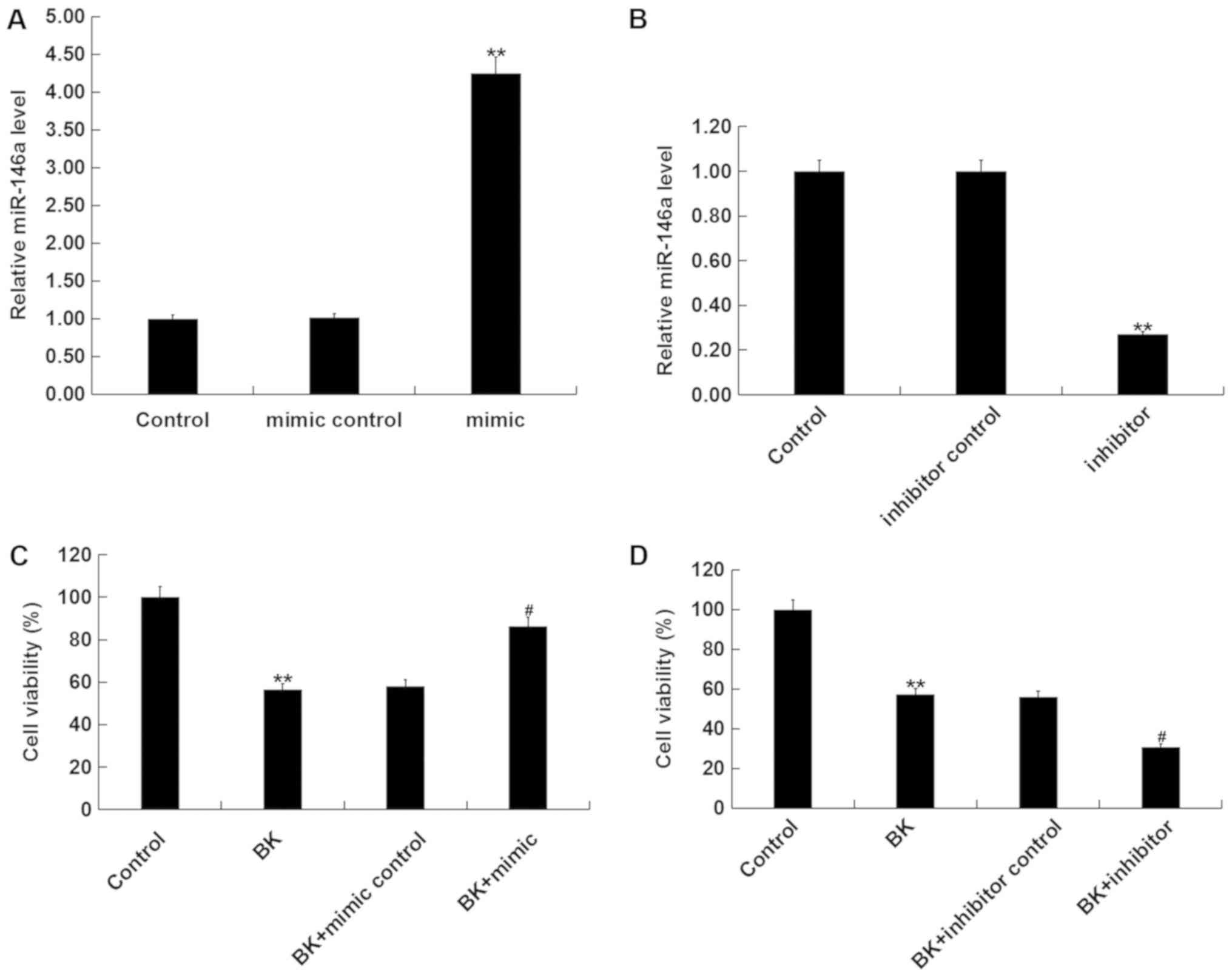
To further elucidate the role of miR-146a in ankle
fracture, MG-63 cells were transfected with miR-146a mimic, mimic
control, miR-146a inhibitor or inhibitor control, after which cells
were treated with 1 µg/ml BK for 24 h. Transfection efficiency was
determined via RT-qPCR 24 h post cell transfection. The results
demonstrated that compared with the control group, miR-146a mimic
significantly enhanced miR-146a expression, whilst miR-146a
inhibitor significantly decreased the level of miR-146a (Fig. 3A and B). Additionally cell viability was
significantly decreased in BK-treated MG-63 cells compared with the
control group. miR-146a mimic significantly promoted cell viability
following BK treatment (Fig. 3C),
while miR-146a inhibitor significantly decreased cell viability
(Fig. 3D).
miR-146a decreases pro-inflammatory
factor expression following BK treatment in vitro
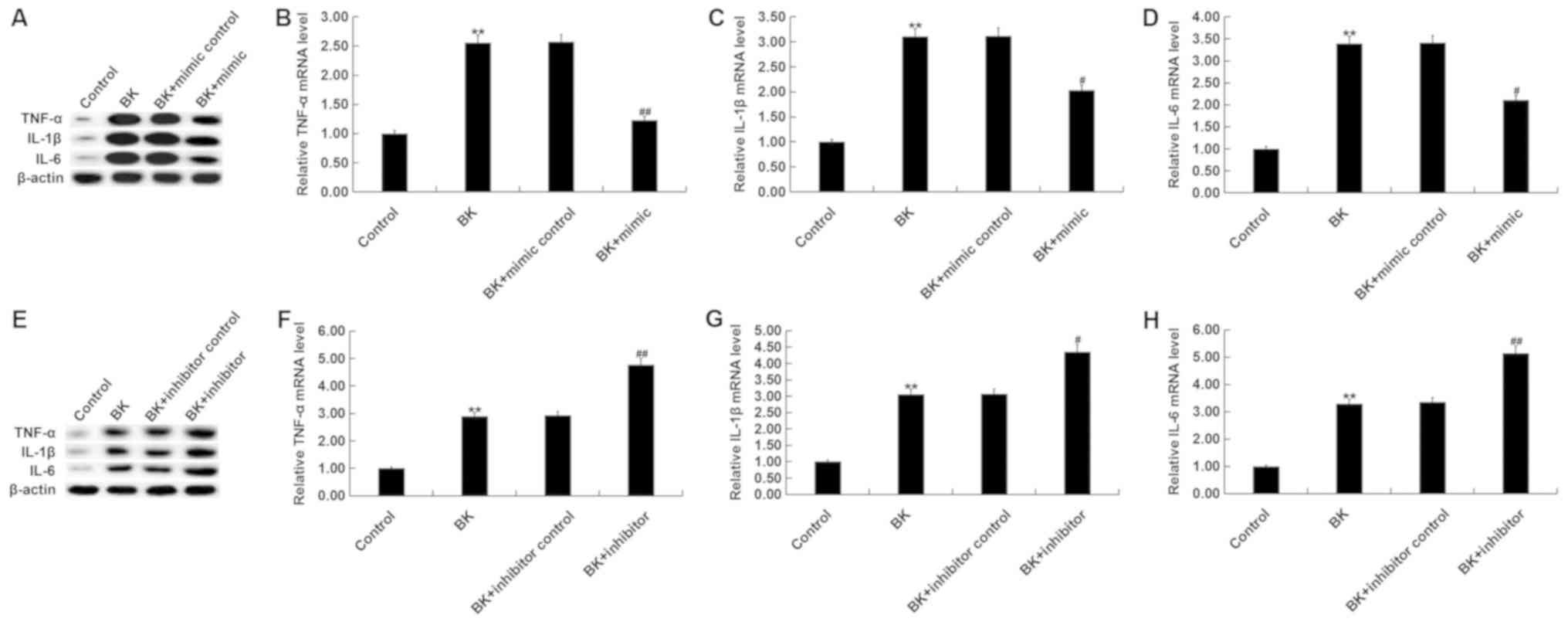
Levels of pro-inflammatory factors (TNF-α, IL-1β and
IL-6) in MG-63 cells were determined via RT-qPCR and western blot
analysis. The results revealed that BK treatment significantly
increased TNF-α, IL-1β and IL-6 mRNA and protein levels (Fig. 4A-D). By contrast, miR-146a mimic
treatment decreased pro-inflammatory factors following BK treatment
(Fig. 4A-D). miR-146a inhibitor
significantly increased TNF-α, IL-1β and IL-6 levels compared with
the controls and BK treatment group (Fig. 4E-H).
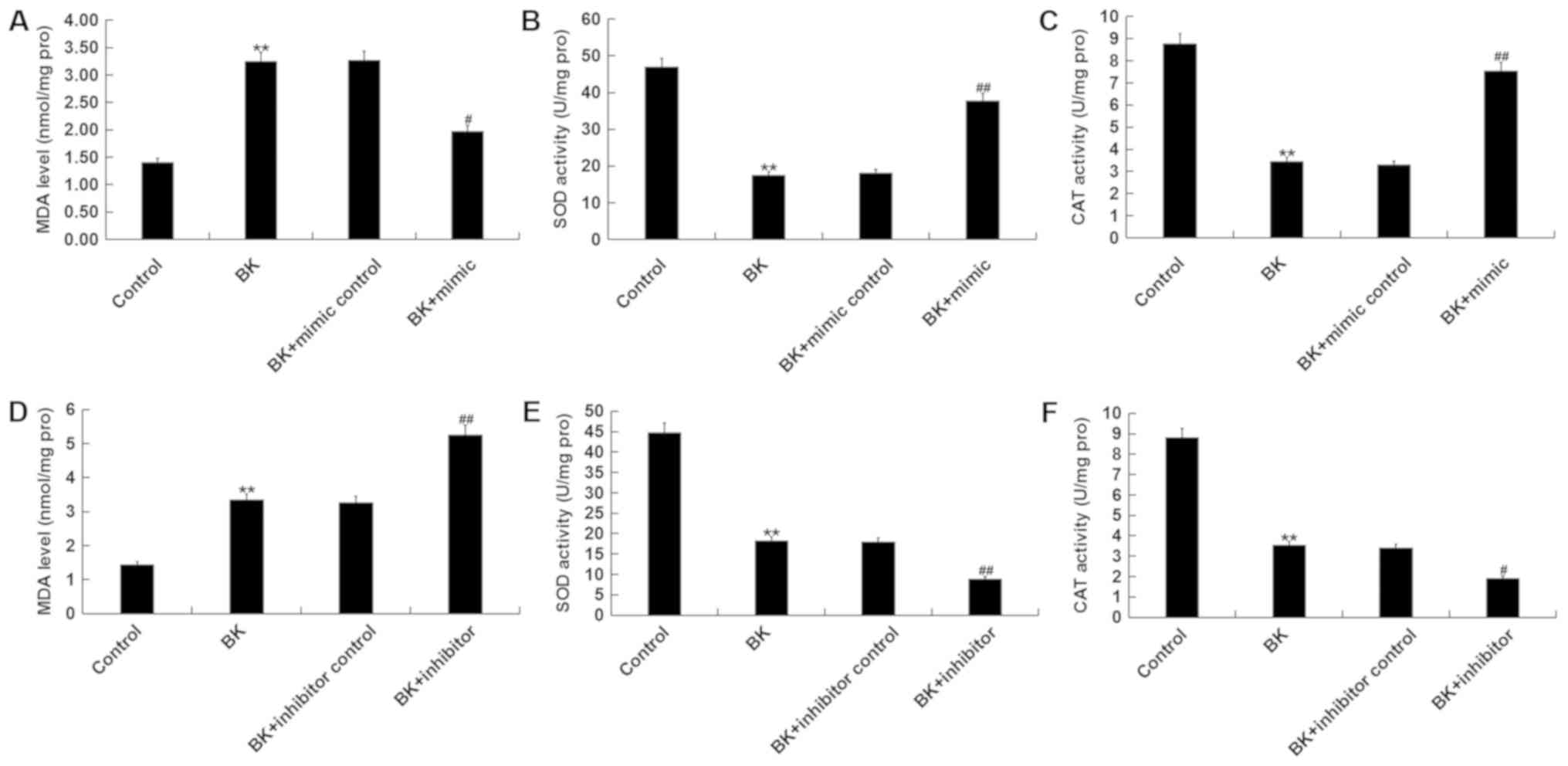
miR-146a attenuates oxidative stress
following BK treatment in vitro
BK treatment significantly increased MDA levels,
while the activities of SOD and CAT decreased compared with the
control (Fig. 5A-C). Additionally,
miR-146a mimic significantly decreased MDA levels, and increased
SOD and CAT activities following BK treatment (Fig. 5A-C). miR-146a inhibitor
significantly increased MDA levels, while the activities of SOD and
CAT decreased compared with the control and BK treatment groups
(Fig. 5D-F).
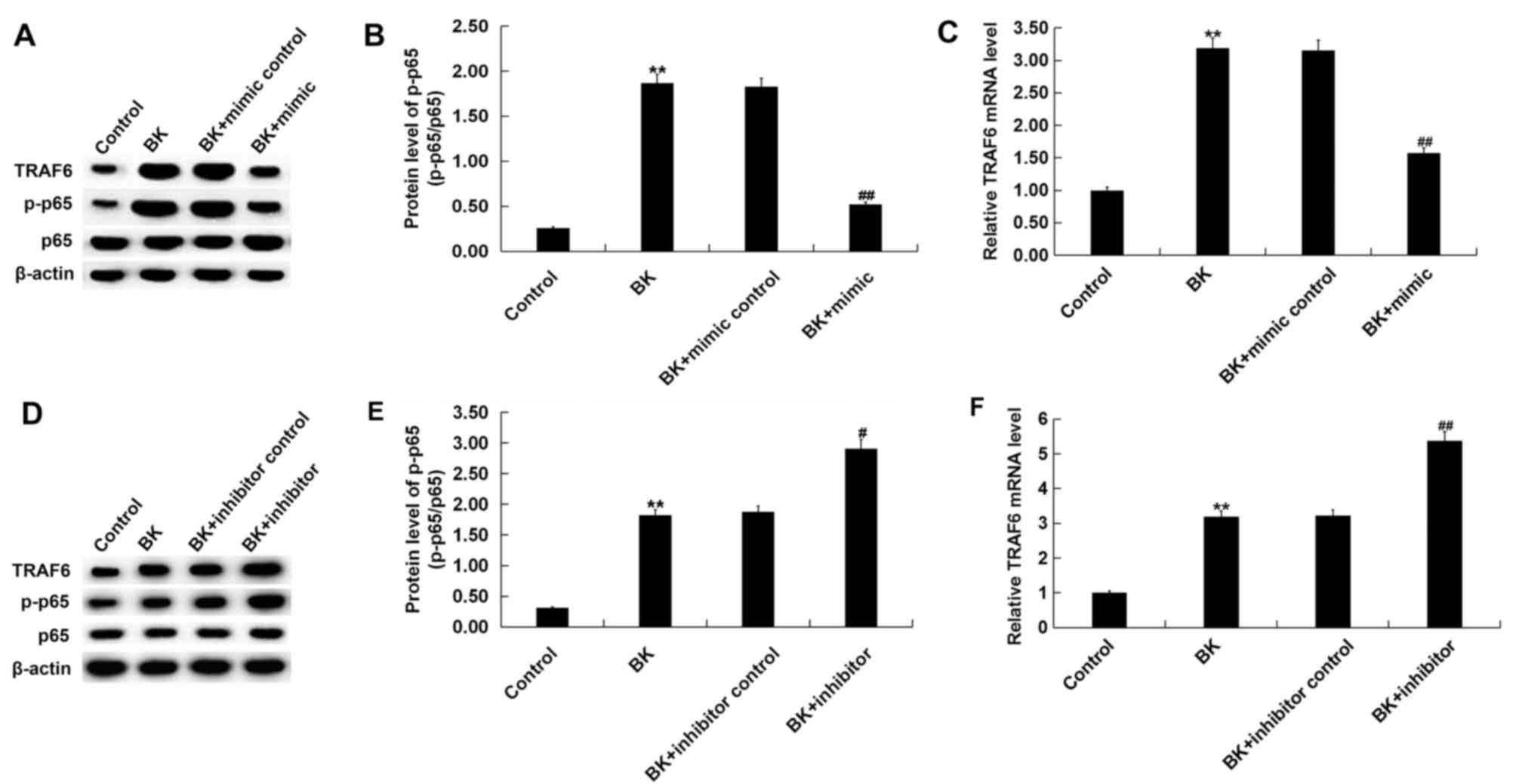
Effect of miR-146a on the TRAF6/NF-κB
pathway following BK treatment in vitro
Previous studies have identified the inhibitory
effect of miR-146a on the TRAF6/NF-κB pathway (14,20,24).
Therefore, the molecular mechanism of miR-146a in ankle fracture
and whether the TRAF6/NF-κB pathway was involved, was assessed in
the present study. The results demonstrated that TRAF6 protein and
mRNA levels, and p-NF-κB p-p-65 protein levels were increased
compared with the control group following BK treatment (Fig. 6). miR-146a mimic significantly
decreased TRAF6 protein and mRNA levels, and p-p-65 protein level
following BK treatment (Fig. 6A and
B). miR-146a inhibitor
significantly increased TRAF6 protein and mRNA levels, and p-NF-κB
p-p-65 protein level compared with the control and BK treatment
(Fig. 6C and D). No significant changes were obtained in
the protein expression of p-65.
Discussion
Fractures, particularly affecting the ankles, are
common. Ankle fractures can have serious consequences without
timely diagnosis and treatment. Therefore, effective treatment
strategies are urgently required. In recent years, an increasing
number of studies have demonstrated the important roles of miRNAs
in the pathogenesis and development of fractures (25-29).
Zou et al (25) identified
that miR-124 polymorphism is associated with fracture healing by
targeting bone morphogenetic protein 6. Yoshizuka et al
(26) reported that miR-222
downregulation accelerated bone healing in rats. Lee et al
(27) determined that miR-29b
promoted bone healing in a mouse fracture model. In addition,
mesenchymal stem cells overexpressing miR-21 accelerated fracture
healing in a rat closed femur fracture model (28). A further study demonstrated that
miR-92a inhibition enhanced fracture healing by promoting
angiogenesis (29). Therefore,
miRNAs may provide a novel approach for the treatment of ankle
fracture.
The present study investigated the expression and
role of miR-146a in the development of fractures. miR-146a is
involved in various diseases, such as numerous types of cancer,
arthritis, coronary heart disease and Alzheimer's disease, and
serves important roles in regulating inflammation and oxidative
stress (14-20).
In the present study, the level of miR-146a in the blood samples of
patients with ankle fracture was lower than those without ankle
fracture. For in vitro experiments, MG-63 cells were treated
with 1 µg/ml BK for 24 h to establish a model of ankle fracture.
The results demonstrated that BK treatment significantly decreased
the level of miR-146a in cells. These results indicated that
miR-146a was significantly decreased during fracture, further
suggesting that it might serve an important role in fracture
development. To confirm the elevated inflammatory response and
oxidative stress during ankle fracture, levels of pro-inflammatory
factors, including TNF-α, IL-1β and IL-6, and bio-markers of
oxidative stress, including MDA, SOD and CAT, were determined. The
results revealed that the inflammatory response and oxidative
stress significantly increased in ankle fracture. miR-146a was then
overexpressed or downregulated in MG-63 cells following BK
treatment. miR-146a overexpression significantly promoted cell
viability and suppressed the BK-induced elevation of the
inflammatory response and oxidative stress. By contrast, treatment
with the miR-146a inhibitor demonstrated the opposite effect on
cell viability, the inflammatory response and oxidative stress.
Previous studies have identified the inhibitory effect of miR-146a
on the TRAF6/NF-κB pathway (30,31).
Therefore, whether the TRAF6/NF-κB pathway was involved in the
molecular mechanism of miR-146a on ankle fracture was investigated
in the present study. The results demonstrated that miR-146a
overexpression markedly inhibited the TRAF6/NF-κB pathway following
BK treatment, whilst miR-146a inhibition promoted the TRAF6/NF-κB
pathway.
The present study is preliminary work into the role
of miR-146a in ankle fracture; therefore, to make the conclusions
more convincing, the correlation between miR-146a expression and
clinical characteristics of patients with ankle fracture will be
investigated in future.
In conclusion, the clinical results of the present
study identified that miR-146a was downregulated during ankle
fracture. For in vitro experiments, miR-146a protected
against inflammation and oxidative stress and inhibited the
TRAF6/NF-κB pathway following establishment of a fracture model.
The present results suggested that miR-146a might promote ankle
fracture healing and thus, may be a potential therapeutic target
for ankle fracture treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or anlayzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM contributed to study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. GX contributed to data collection and statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Each patient provided informed consent and the
current study was approved by the Ethics Committee of the
Affiliated Drum Tower Hospital of Nanjing University Medical
School.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hedström EM, Svensson O, Bergström U and
Michno P: Epidemiology of fractures in children and adolescents.
Acta Orthop. 81:148–153. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
MacIntyre NJ and Dewan N: Epidemiology of
distal radius fractures and factors predicting risk and prognosis.
J Hand Ther. 29:136–145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chevalley T, Bonjour JP, van Rietbergen B,
Rizzoli R and Ferrari S: Fractures in healthy females followed from
childhood to early adulthood are associated with later menarcheal
age and with impaired bone microstructure at peak bone mass. J Clin
Endocrinol Metab. 97:4174–4181. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He Z, Selvamurugan N, Warshaw J and
Partridge NC: Pulsed electromagnetic fields inhibit human
osteoclast formation and gene expression via osteoblasts. Bone.
106:194–203. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J
and Yang P: Dose-specific effects of tumor necrosis factor alpha on
osteogenic differentiation of mesenchymal stem cells. Cell Prolif.
44:420–427. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sheweita SA and Khoshhal KI: Calcium
metabolism and oxidative stress in bone fractures: Role of
antioxidants. Curr Drug Metab. 8:519–525. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Nugent M: MicroRNAs and fracture healing.
Calcif Tissue Int. 101:355–361. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
He B, Zhang ZK, Liu J, He YX, Tang T, Li
J, Guo BS, Lu AP, Zhang BT and Zhang G: Bioinformatics and
microarray analysis of miRNAs in aged female mice model implied new
molecular mechanisms for impaired fracture healing. Int J Mol Sci.
17(E1260)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Waki T, Lee SY, Niikura T, Iwakura T,
Dogaki Y, Okumachi E, Oe K, Kuroda R and Kurosaka M: Profiling
microRNA expression during fracture healing. BMC Musculoskelet
Disord. 17(83)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tan W, Liao Y, Qiu Y, Liu H, Tan D, Wu T,
Tang M, Zhang S and Wang H: miRNA 146a promotes chemotherapy
resistance in lung cancer cells by targeting DNA damage inducible
transcript 3 (CHOP). Cancer Lett. 428:55–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li D, Duan M, Feng Y, Geng L, Li X and
Zhang W: MiR-146a modulates macrophage polarization in systemic
juvenile idiopathic arthritis by targeting INHBA. Mol Immunol.
77:205–212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu ZW, Liu YF, Wang S and Li B: miRNA-146a
induces vascular smooth muscle cell apoptosis in a rat model of
coronary heart disease via NF-κB pathway. Genet Mol Res.
14:18703–18712. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang B, Wang LL, Ren RJ, Dammer EB, Zhang
YF, Huang Y, Chen SD and Wang G: MicroRNA-146a represses LRP2
translation and leads to cell apoptosis in Alzheimer's disease.
FEBS Lett. 590:2190–2200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lukiw WJ, Cui JG, Yuan LY, Bhattacharjee
PS, Corkern M, Clement C, Kammerman EM, Ball MJ, Zhao Y, Sullivan
PM and Hill JM: Acyclovir or Aβ42 peptides attenuate HSV-1-induced
miRNA-146a levels in human primary brain cells. Neuroreport.
21:922–927. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xie Y, Chu A, Feng Y, Chen L, Shao Y, Luo
Q, Deng X, Wu M, Shi X and Chen Y: MicroRNA-146a: A comprehensive
indicator of inflammation and oxidative stress status induced in
the brain of chronic T2DM rats. Front Pharmacol.
9(478)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu L, Wan C, Zhang W, Guan L, Tian G,
Zhang F and Ding W: MiR-146a regulates PM1 -induced inflammation
via NF-κB signaling pathway in BEAS-2B cells. Environ Toxicol.
33:743–751. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhou P, Liu H, Wu Y and Chen D: Propofol
promotes ankle fracture healing in children by inhibiting
inflammatory response. Med Sci Monit. 24:4379–4385. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu W, Kang J, Hu K, Tang S, Zhou X, Yu S,
Li Y and Xu L: Angiotensin-(1-7) inhibits inflammation and
oxidative stress to relieve lung injury induced by chronic
intermittent hypoxia in rats. Braz J Med Biol Res.
49(e5431)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lv F, Huang YZ, Lv WT, Yang L, Li F, Fan J
and Sun J: MicroRNA-146a ameliorates inflammation via TRAF6/NF-κB
pathway in intervertebral disc cells. Med Sci Monit. 23:659–664.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zou L, Zhang G, Liu L, Chen C, Cao X and
Cai J: A MicroRNA-124 polymorphism is associated with fracture
healing via modulating BMP6 expression. Cell Physiol Biochem.
41:2161–2170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yoshizuka M, Nakasa T, Kawanishi Y,
Hachisuka S, Furuta T, Miyaki S, Adachi N and Ochi M: Inhibition of
microRNA-222 expression accelerates bone healing with enhancement
of osteogenesis, chondrogenesis, and angiogenesis in a rat
refractory fracture model. J Orthop Sci. 21:852–858.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee WY, Li N, Lin S, Wang B, Lan HY and Li
G: miRNA-29b improves bone healing in mouse fracture model. Mol
Cell Endocrinol. 430:97–107. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun Y, Xu L, Huang S, Hou Y, Liu Y, Chan
KM, Pan XH and Li G: mir-21 overexpressing mesenchymal stem cells
accelerate fracture healing in a rat closed femur fracture model.
Biomed Res Int. 2015(412327)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Murata K, Ito H, Yoshitomi H, Yamamoto K,
Fukuda A, Yoshikawa J, Furu M, Ishikawa M, Shibuya H and Matsuda S:
Inhibition of miR-92a enhances fracture healing via promoting
angiogenesis in a model of stabilized fracture in young mice. J
Bone Miner Res. 29:316–326. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
He X, Zheng Y, Liu S, Shi S, Liu Y, He Y,
Zhang C and Zhou X: MiR-146a protects small intestine against
ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB
pathway. J Cell Physiol. 233:2476–2488. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhong JH, Li J, Liu CF, Liu N, Bian RX,
Zhao SM, Yan SY and Zhang YB: Effects of microRNA-146a on the
proliferation and apoptosis of human osteoarthritis chondrocytes by
targeting TRAF6 through the NF-κB signalling pathway. Biosci Rep.
37(BSR20160578)2017.PubMed/NCBI View Article : Google Scholar
|